Discoidin domain receptor (DDR)1 is a tyrosine kinase-receptor, which is activated by various types of collagens.1 Several studies showed DDR1 expression in human cancers, and reported its role in cell proliferation, epithelial to mesenchymal transition, migration and invasiveness.2 In addition, high DDR1 expression has been related to adverse prognosis in some human solid tumours. As far as haematological malignancies are concerned, DDR1 gene is highly expressed in adult B-ALL cases without ALL1/AF4 and E2A/PBX1 molecular rearrangements, as well as in B-cell receptor/ABL-positive cases.3 Also, acute myeloid leukaemia (AML) blasts express DDR1, which provides a supportive stimulus in the bone marrow microenvironment.4 Moreover, activation of DDR1 protects Hodgkin lymphoma cells from apoptosis. Although Hodgkin and Reed–Sternberg cells express DDR1, it is not detected in their normal counterpart, the germinal centre B cells of reactive tonsils.5 The microenvironment of the bone marrow and secondary lymphoid organs has an essential role in chronic lymphocytic leukemia (CLL) pathogenesis and resistance to treatment.6 Indeed, stimuli deriving from the B-cell receptor, cell-to-cell contacts with nurse-like cells or activated T cells, and several chemokines and cytokines promote CLL proliferation and survival.6, 7 In a previous study, we reported that IL-21, which regulates CLL B-cell survival in a context-dependent fashion, modulates the expression of several mRNAs, among which DDR1 was one of the most downregulated genes.8 These data suggested that DDR1 may be expressed in CLL cells but, to the best of our knowledge, no other studies have specifically addressed this issue. Since CLL is a clinically heterogeneous disease, here we studied the expression of DDR1 gene in independent retrospective series of CLL in relationship to time to first treatment (TTFT), known prognostic markers, and miRNA expression.
To analyse DDR1 mRNA expression we used three public data sets of CLL gene expression.9, 10, 11 The raw gene and miRNA expression data were retrieved from the NCBI Gene Expression Omnibus repository (http://www.ncbi.nlm.nih.gov/geo/) through GEO Series accession numbers GSE22762 (GPL570), GSE39671 and GSE40571. The raw intensity expression values were processed by Robust Multi-array Average procedure with the re-annotated Chip Definition Files from BrainArray libraries version 18.0.0 available at http://brainarray.mbni.med.umich.edu. The statistical procedures were standard functions in base R package (Pearson's product–moment correlations and Wilcoxon rank-sum tests). TTFT analysis was performed using the Kaplan–Meier method. A value of P<0.05 was considered significant. To test DDR1 protein expression, blood samples were obtained with approval of the Institutional Review Board and informed written consent of the patients, in accordance with the declaration of Helsinki. Indirect immunofluorescence with anti-DDR1 murine IgG1 mAb (clone 7A9)12 and Western blot analysis of DDR1 expression were performed as detailed in the Supplementary information.
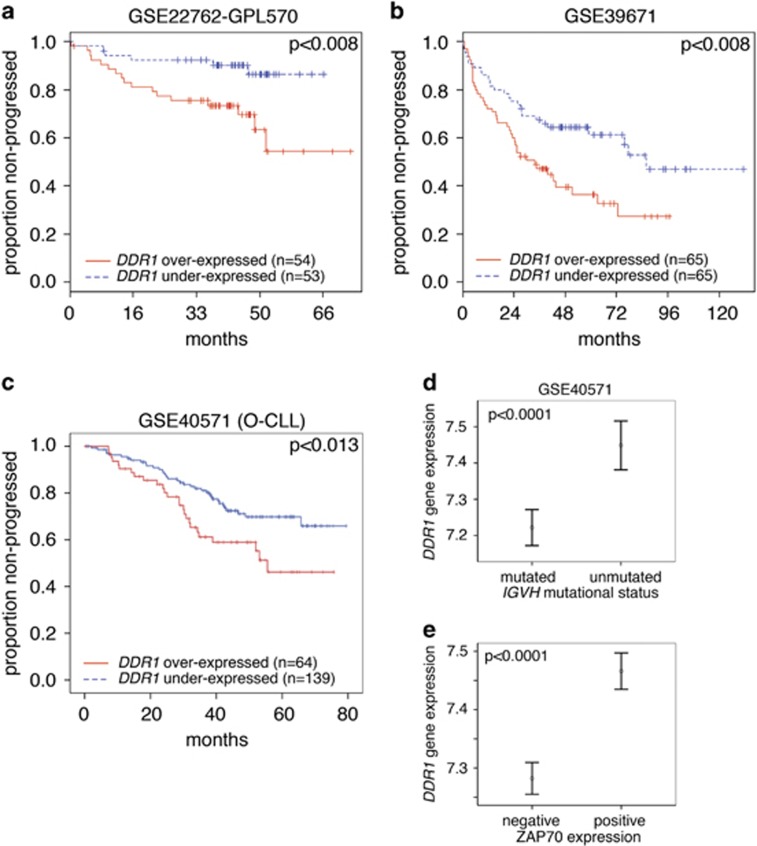
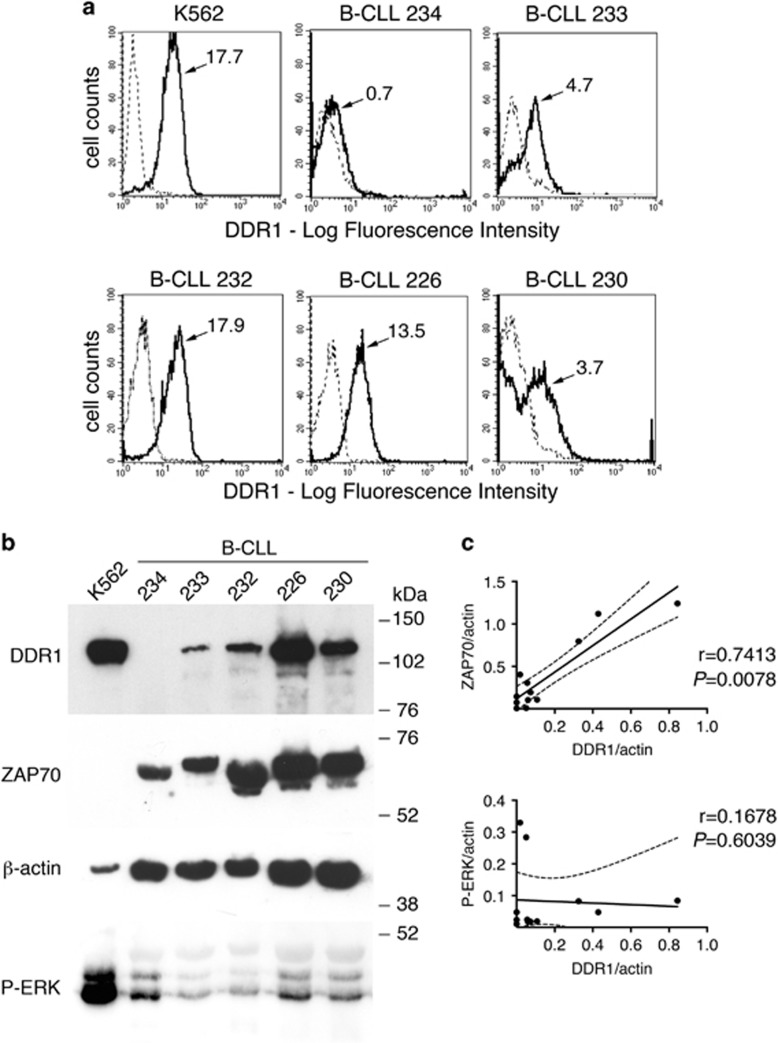
Since DDR1 expression correlates with disease progression in different types of cancers, we first analysed DDR1 mRNA in three public data sets of CLL gene expression. The analysis of these CLL cohorts, stratified according to DDR1 cut-off levels, showed that high DDR1 gene expression correlates with a shorter TTFT (Figures 1a–c). Next, we analysed whether DDR1 expression had any relationship with known prognostic markers of CLL, such as ZAP70, CD38 and IGVH mutational status.13 A significant, albeit borderline, correlation between DDR1 and ZAP70 mRNA levels was found in the GSE22762-GPL570 (r=0.24, P=0.0064), GSE39671 (r=0.31, P=1.6e-04) and GSE40571 (r=0.29, P=4.674e-06) data sets. Concerning CD38 expression, a weak correlation was found in the GSE39671 (r=0.19, P=0.015) and in the GSE40571 (r=0.17, P=0.0098) data sets. Furthermore, cases with high ZAP70 protein expression or with unmutated IGVH genes showed higher DDR1 gene expression, in the GSE40571 data set (Figures 1d and e). To further investigate the DDR1 surface expression and its molecular form we performed immunofluorescence and western blot analysis on CLL cells from a small cohort (Supplementary Table S1). Peripheral blood leukaemia cells express surface DDR1 at variable levels, in different CLL cases, as detected by immunofluorescence (representative cases are shown in Figure 2a and Supplementary Figure S1A). Out of 34 CLL tested (Supplementary Table S2), 11 showed a DDR1 low/negative phenotype (<30% of DDR1+ cells), 13 were DDR1 bright (>65%) and 10 showed intermediate levels, indicating heterogeneity. Of note, DDR1 is virtually absent in circulating mature B cells (CD20+) from healthy donors, whereas DDR1 and CD20 double staining confirmed expression on leukaemia B cells (Supplementary Figure S1B). Western blot analysis with an anti-DDR1 antibody showed a predominant band of ~130 kDa, under reducing conditions, which displayed variable intensity in the different CLL samples (figure 2B and Supplementary Figure S2). As control, the K562 erythroleukaemia cell line, known to express full length DDR1,4 showed a band of the same size. On the basis of the correlation of ZAP70 and DDR1 gene expression, we verified whether such relationship also exists at the protein level. To this end, we re-probed the same blots with an anti-ZAP70 antibody. Densitometry analyses, normalised to the β-actin content, confirmed the correlation of ZAP70 and DDR1 at the protein level, in a small independent cohort (n=12, r=0.7413, CI: 0.2733–0.9255, P=0.0078; Figure 2c). Since DDR1 expression in activated T cells has been related to ERK1/2 phosphorylation,14 we also analysed phosphorylated ERK1/2, but no correlation with DDR1 was found (Figure 2c and Supplementary Figure S2). Finally, our previous data indicated that miR663b may downregulate DDR1 expression in response to IL-21 stimulation of CLL cells, in vitro.8 However, we could not find a significant anti-correlation between miR663b and DDR1 (data not shown) by an integrated analysis of gene expression profiling and miRNAome in the proprietary database GSE40571.11 Also, miR199b-5p, which was reported to downregulate DDR1 expression in AML,4 showed a good, albeit non-significant, reverse correlation with DDR1 mRNA levels (data not shown).
Figure 1.
(a–c) High DDR1 gene expression correlates with a shorter time-to-treatment in CLL in three public-access data sets. Cut-off was median DDR1 value in a and b. In c the best cut-off point for DDR1 gene expression discriminating cases with immunoglobulin variable heavy (IGVH) mutated from those unmutated was sought by constructing receiver operating character (ROC) analysis. Curves were constructed using the Kaplan–Meier method and compared with the Wilcoxon log-rank test. (d, e) DDR1 gene expression is higher in CLL with unmutated IGVH relative to mutated cases (d) and in ZAP70-positive cases relative to negative ones (e) (mean±s.e.m.).
Figure 2.
(a) Peripheral blood mononuclear cells from CLL cases express variable levels of surface DDR1 as detected by immunofluorescence and fluorescence-activated cell sorter analysis, gated on lymphoid cells; (b) western blot analysis of DDR1, ZAP70 and pERK1/2: DDR1 showed a predominant band of an apparent mw of ~130 kDa. K562 erythroleukemia cell line is shown as positive control (c) Densitometry analyses of ZAP70 and DDR1, relative to actin control, showed a significant correlation, whereas no correlation with phosphorylated ERK1/2 (P-ERK) was found (analysis includes also western blots shown in Supplementary Figure S2).
In this study, we show for the first time, that the tyrosine kinase DDR1 is expressed at the cell surface of circulating B-CLL cells, although with a remarkable heterogeneity among individual cases. In addition, DDR1 gene expression correlates with that of the ZAP70 tyrosine kinase and with the IGVH mutational status, which are regarded as prognostic markers in CLL.13 Accordingly, high DDR1 gene expression shows a significant relationship with ZAP70 gene and TTFT in independent CLL cohorts. A similar correlation between high DDR1 expression and worse prognosis has been reported in different solid tumours, where DDR1 has been involved in interaction between stroma and tumour cells. Indeed, DDR1 acts as a sensor for various types of collagen of the extracellular matrix, and mediates enhanced tumour cell migration, survival, proliferation and matrix remodelling.2 Therefore, DDR1 has a pro-invasive role in different tumours and is studied as a target for kinase inhibitors. In AML, DDR1 responds to remodelled type IV collagen present in the stroma of the bone marrow microenvironment.4 It is conceivable that DDR1 may act as a sensor for stromal collagen of the bone marrow and lymphoid tissues also in CLL and provide a supportive stimulus, acting in concert with other environmental signals. Indeed, DDR1 may act in concert with other receptor systems. For example, the Insulin-like growth factor-I (IGF-I)/IGF-I receptor system cooperates with DDR1 and the G-protein oestrogen receptor to support progression, in mesothelioma and lung cancer cells.15 In normal T cells, DDR1 is expressed only upon cell activation through mechanisms, which involve ERK1/2 signalling. Similarly, normal circulating B cells or tonsil germinal centre B cells do not express DDR1,5 suggesting that stimuli activating the ERK/1/2 pathway may induce DDR1 expression in CLL cells. However, the mechanisms controlling DDR1 expression in CLL remain elusive, since we could not find any correlation between DDR1 expression and constitutive activation of the ERK1/2 pathway. Also, different miRNA have been reported to regulate DDR1 expression, such as miR199b-5p in AML cells,4 or IL-21-induced miR663b in B-CLL cells.8 Nonetheless, the combined analysis of DDR1 gene and miRNA expression profiles failed to identify significant anti-correlations suggestive of miRNA regulating DDR1 expression, in B-CLL cells. In conclusion, the correlation of DDR1 mRNA levels with CLL outcome and other biomarkers of progression suggests a potential role of DDR1 in CLL biology and lends support to further studies to address the functional role of DDR1 in this disease.
Acknowledgments
Associazione Italiana Ricerca sul Cancro (AIRC) grant 5xmille no. 9980, 2010-15 to M.F., AN and FM, AIRC IG10492 to M.F, AIRC IG13518 to SF and Italian Ministry of Health 5x1000 Funds 2013. AIRC and Fondazione CaRiCal co-financed Multi Unit Regional Grant n.16695, 2015/2018 to FM. We wish to thank Dr. Silvana Canevari for helpful comments.
Author contributions
MM, FM, LDC, MG and AN performed data analysis and interpretation. GC, SZ collected patient samples and clinical data for protein validation study. GB and MFa performed immunofluorescence and WB analyses. BL provided essential reagents and discussion. MFe revised data and manuscript. SF designed experiments and wrote the paper.
Footnotes
Supplementary Information accompanies this paper on Blood Cancer Journal website (http://www.nature.com/bcj)
The authors declare no conflict of interest.
Supplementary Material
References
- Vogel W, Gish GD, Alves F, Pawson T. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell 1997; 1: 13–23. [DOI] [PubMed] [Google Scholar]
- Valiathan RR, Marco M, Leitinger B, Kleer CG, Fridman R. Discoidin domain receptor tyrosine kinases: new players in cancer progression. Cancer Metastasis Rev 2012; 31: 295–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaretti S, Li X, Gentleman R, Vitale A, Wang KS, Mandelli F et al. Gene expression profiles of B-lineage adult acute lymphocytic leukemia reveal genetic patterns that identify lineage derivation and distinct mechanisms of transformation. Clin Cancer Res 2005; 11: 7209–7219. [DOI] [PubMed] [Google Scholar]
- Favreau AJ, Cross EL, Sathyanarayana P. miR-199b-5p directly targets PODXL and DDR1 and decreased levels of miR-199b-5p correlate with elevated expressions of PODXL and DDR1 in acute myeloid leukemia. Am J Hematol 2012; 87: 442–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cader FZ, Vockerodt M, Bose S, Nagy E, Brundler MA, Kearns P et al. The EBV oncogene LMP1 protects lymphoma cells from cell death through the collagen-mediated activation of DDR1. Blood 2013; 122: 4237–4245. [DOI] [PubMed] [Google Scholar]
- Burger JA, Montserrat E. Coming full circle: 70 years of chronic lymphocytic leukemia cell redistribution, from glucocorticoids to inhibitors of B-cell receptor signaling. Blood 2013; 121: 1501–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiorazzi N, Ferrarini M. B cell chronic lymphocytic leukemia: lessons learned from studies of the B cell antigen receptor. Annu Rev Immunol 2003; 21: 841–894. [DOI] [PubMed] [Google Scholar]
- De Cecco L, Capaia M, Zupo S, Cutrona G, Matis S, Brizzolara A et al. Interleukin 21 controls mRNA and microRNA expression in CD40-activated chronic lymphocytic leukemia cells. PLoS One 2015; 10: e0134706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold T, Jurinovic V, Metzeler KH, Boulesteix AL, Bergmann M, Seiler T et al. An eight-gene expression signature for the prediction of survival and time to treatment in chronic lymphocytic leukemia. Leukemia 2011; 25: 1639–1645. [DOI] [PubMed] [Google Scholar]
- Chuang HY, Rassenti L, Salcedo M, Licon K, Kohlmann A, Haferlach T et al. Subnetwork-based analysis of chronic lymphocytic leukemia identifies pathways that associate with disease progression. Blood 2012; 120: 2639–2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morabito F, Mosca L, Cutrona G, Agnelli L, Tuana G, Ferracin M et al. Clinical monoclonal B lymphocytosis versus Rai 0 chronic lymphocytic leukemia: a comparison of cellular, cytogenetic, molecular, and clinical features. Clin Cancer Res 2013; 19: 5890–5900. [DOI] [PubMed] [Google Scholar]
- Carafoli F, Mayer MC, Shiraishi K, Pecheva MA, Chan LY, Nan R et al. Structure of the discoidin domain receptor 1 extracellular region bound to an inhibitory Fab fragment reveals features important for signaling. Structure 2012; 20: 688–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh SA, Shanafelt TD. Prognostic factors and risk stratification in chronic lymphocytic leukemia. Semin Oncol 2016; 43: 233–240. [DOI] [PubMed] [Google Scholar]
- Chetoui N, El Azreq MA, Boisvert M, Bergeron ME, Aoudjit F. Discoidin domain receptor 1 expression in activated T cells is regulated by the ERK MAP kinase signaling pathway. J Cell Biochem 2011; 112: 3666–3674. [DOI] [PubMed] [Google Scholar]
- Avino S, De Marco P, Cirillo F, Santolla MF, De Francesco EM, Perri MG et al. Stimulatory actions of IGF-I are mediated by IGF-IR cross-talk with GPER and DDR1 in mesothelioma and lung cancer cells. Oncotarget 2016, e-pub ahead of print 30 June 2016 doi:10.18632/oncotarget.10348. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.