Abstract
Vaginal lactobacilli (VLB) spread from the mother to the infant during vaginal delivery. However, the effects of VLB on infant intestinal function remain unclear. We investigated the probiotic function and immune effects of VLB on the human embryonic intestinal epithelial cell line INT-407. VLB survived artificial gastric juice and adhered to INT-407 cells. Exposure of INT-407 cells to VLB attenuated both the lipopolysaccharide (LPS)-induced stimulation of interleukin-8 and tumor necrosis factor alpha production and the LPS-stimulated upregulation of TLR4 expression. These results suggest that specific VLB suppresses the inflammation induced by LPS stimulation through downregulation of TLR4 expression in human embryonic intestinal epithelial cells.
Keywords: vaginal lactobacilli, Toll-like receptor, infant, INT-407 cell
Lactobacilli are predominant in the normal vaginal microbiota [1]. Lactobacilluscrispatus, Lactobacillus gasseri, Lactobacillus jensenii, and Lactobacillus vaginalis are frequently isolated from the vaginal region of healthy women [2,3,4]. These vaginal lactobacilli (VLB) suppress the growth of pathogenic microorganisms by lowering the environmental pH through production of lactic acid or antimicrobial substances such as hydrogen peroxide [4]. Moreover, Rose et al. [5] have reported that VLB modulate the secretion of inflammatory cytokines by vaginal epithelial cell cultures stimulated by bacterial components. Thus, VLB play an important role in preventing pathogenic bacterial infections through modulation of immune systems in the vaginal tract.
In the human newborn infant, colonization by microorganisms begins around birth. It is usually considered that the newborn infant acquires initial intestinal microbiota from the VLB and fecal bifidobacteria of the mother during delivery [6,7,8]. Interestingly, Matsumiya et al. reported that VLB form the intestinal microbiota in the early stage of newborn infants [6]. The gastrointestinal mucosal immune system is quite immature at birth, which puts the newborn infant at risk for a variety of infections [9]. Therefore, VLB spread from the mother’s vagina to the newborn infant may play an important role in the immature immune systems of newborn infants such as those of vaginal tracts. However, the effects of VLB on infant intestinal function remain unclear. In the current study, we investigated the probiotic function of VLB and the immune effect of VLB on human embryonic intestinal epithelial cells.
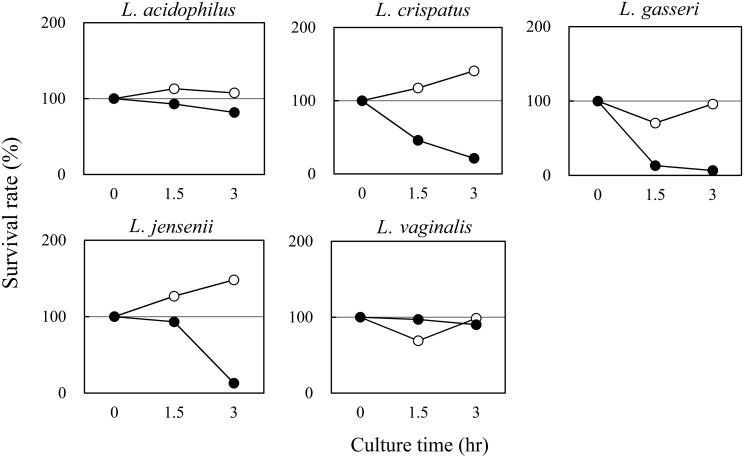
To evaluate the probiotic effects of VLB, we investigated the viability of VLB in artificial gastric juice and the adhesion of VLB to human embryonic intestinal epithelial cells. Lactobacillus acidophilus JCM2124, L. crispatus JCM2009, L. gasseri JCM5344, L. jensenii JCM11034, and L. vaginalis JCM9505, which were used as VLB, were obtained from the Japan Collection of Microorganisms (JCM; Ibaraki, Japan). The sources from which these lactobacilli were separated are shown in Table 1. Lactobacillus rhamnosus, a GG strain that is a known probiotic Lactobacillus [10], was obtained from the American Type Culture Collection (ATCC; Rockville, MD, USA). INT-407 cells were purchased from the European Collection of Authenticated Cell Culture (ECACC; Salisbury, UK). Survival of VLB in artificial gastric juice was tested using the protocol of Takeda et al. [11]. Briefly, VLB were inoculated into de Man, Rogosa and Sharpe (MRS) medium and cultivated for 24 hr at 37°C. Fresh cultures were then inoculated into MRS medium (5.0 × 105 cfu/ml) with 0.04% pepsin (pH 3.0 or pH 4.0) and incubated for 3 hr at 37°C with shaking at 140 rpm. The surviving VLB were enumerated by poured-plate cultivation. Adhesion of VLB to INT-407 cells was tested according to Takeda et al. [11] with modifications. Briefly, INT-407 cells were suspended in Eagle’s minimal essential medium with L-glutamine and phenol red (EMEM) containing 10% defined fetal bovine serum (FBS; Hyclone Laboratories, Logan, UT, USA) without antibiotics, adjusted to a density of 1.0 × 105 viable cells/ml, and were cultured in 48-well cell culture plates at 37°C in a humidified 5% CO2 incubator for 2 hr. The supernatant was then removed. Fresh lactobacilli were suspended at a density of 1.0 × 106 cfu/ml in EMEM containing 10% FBS without antibiotics, seeded on INT-407 cells, and cultured for 2 hr. After incubation, the INT-407 cells were washed three times with 0.01 M phosphate-buffered saline (PBS; pH 7.2), and 0.25% trypsin solution (Wako Pure Chemical Industries, Osaka, Japan) was added to detach the INT-407 cells. The number of VLB adhered to INT-407 cells was enumerated by poured-plate cultivation. Gastric acid plays an important role as a primary bactericidal barrier [12]. The pH in the stomach of adults is between 1.0 and 2.0, whereas the pH in the stomach of infants is higher than 4.0, because gastric secretion is immature [13]. Figure 1 shows the viability of the VLB in the artificial gastric juice. All VLB strains were viable in MRS medium (pH 4.0) with 0.04% pepsin. These results suggest that these VLB tolerate gastric acid and arrive in a live state at the intestinal tract of infants. INT-407 cells are derived from the human embryonic intestinal jejunum and ileum [14]. Table 2 shows the adhesion of VLB to INT-407 cells. The number of VLB that adhered to the cells ranged from 2.0 × 104 to 7.7 × 104 cfu/ml. These results suggest that these VLB adhere to the intestinal cells of infants. Matsumiya et al. [6] reported that VLB spread from the mother to the infant during vaginal delivery and colonized the infant intestine shortly after birth. They also reported that the acquired VLB did not last long-term in the intestine of the infant. L. rhamnosus GG is one of the most studied probiotics [10], and it is well established that L. rhamnosus GG adheres to the intestinal epithelium [10]. As shown in Table 2, the number of probiotic L. rhamnosus GG bacteria that adhered to INT-407 was higher than the number of adhered VLB. These results suggest that VLB cannot adhere as effectively as probiotics to the intestinal epithelium. However, intestinal mucus contains antimicrobial products and secretory IgA and forms a physical and immunological barrier to microbiota [15]. Therefore, our results will need to be further confirmed in the context of in vivo mucosal environments, which are crucial for microbial interaction with (and adhesion to) the gut.
Table 1. Vaginal lactobacilli used in this study.
| Vaginal lactobacilli | Source | |
|---|---|---|
| L. acidophilus | JCM2124 | Vagina |
| L. crispatus | JCM2009 | Urine |
| L. gasseri | JCM5344 | Vaginal tract |
| L. jensenii | JCM11034 | Vaginal swab |
| L. vaginalis | JCM9505 | Vagina |
Fig. 1.
Survival rate of VLB in artificial gastric juice. The VLB were inoculated into MRS medium with 0.04% pepsin (●, pH 3.0: ○, pH 4.0) and were then incubated for 3 hr at 37°C with shaking at 140 rpm. The surviving VLB were enumerated by poured-plate cultivation.
Table 2. Adhesion of lactobacilli to INT-407 cells.
| Lactobacilli | Adhesion to INT-407 cells |
|---|---|
| (×104 cfu/ml) | |
| L. acidophilus | 7.2 |
| L. crispatus | 2.5 |
| L. gasseri | 2.0 |
| L. jensenii | 7.7 |
| L. vaginalis | 4.7 |
| L. rhamnosus GG | 16.0 |
INT-407 cells were incubated with VLB (1.0 × 106 cfu/ml) for 2 hr. L. rhamnosus GG was used as a probiotic strain in this study.
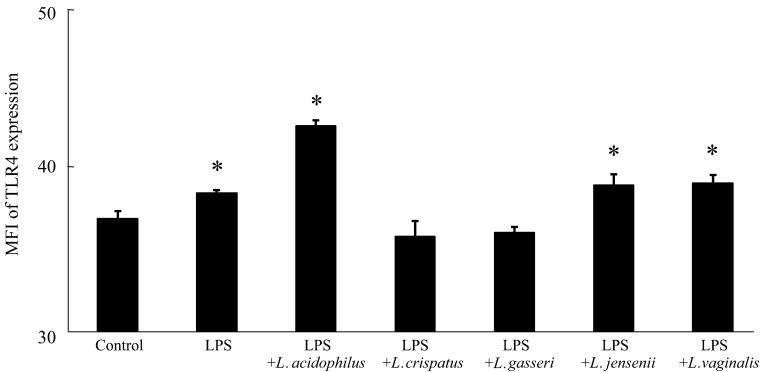
Exogenous mediators such as pathogenic bacteria interact with the intestinal epithelium via pattern-recognition receptors such as the Toll-like receptor (TLR) family [16]. For example, lipopolysaccharide (LPS) derived from gram-negative bacteria binds to TLR4 [17]. Binding to these TLRs activates signals that trigger the induction of inflammatory responses, including the production of inflammatory cytokines [16]. Therefore, we next examined the immune effect of VLB on the LPS-stimulated INT-407 cells. VLB were cultivated in MRS medium for 24 hr at 37°C, collected by centrifugation, washed three times with sterile saline, and lyophilized. INT-407 cells were suspended in EMEM containing 10% FBS and 1% Penicillin-Streptomycin Solution (Wako Pure Chemical Industries) and adjusted to 1.0 × 105 viable cells/ml. The cells were cultured in 48-well cell culture plates in the presence of LPS from Salmonella Typhimurium (Wako Pure Chemical Industries) and VLB (0 or 10 µg/ml) at 37°C in a humidified 5% CO2 incubator for 48 hr. The cell culture supernatants were then stored at −80°C until they were assayed for measurement of interleukin (IL)-8 and tumor necrosis factor alpha (TNFα) levels as inflammatory cytokines. The viability of INT-407 cells was determined with a Guava easyCyte flow cytometry single sample system (Millipore,Hayward,CA,USA) using a Guava ViaCount assay kit (Millipore) according to the manufacturer’s instructions, the IL-8 and TNFα levels in the INT-407 cell culture supernatants were determined using human IL-8 and TNFα ELISA MAXTM standard sets (BioLegend, San Diego, CA, USA) according to the manufacturer’s instructions, and the expression of INT-407 cell surface TLR4 was determined by immunofluorescent labeling according to our previous study [18] with modifications. Briefly, cell surface TLR4 was detected by incubation of the cells with a mouse anti-human TLR4 monoclonal antibody (clone 76B357.1, Imgenex San Diego, CA, USA) for 15 min at 4°C, followed by incubation with a fluorescein isothiocyanate (FITC)-labeled goat anti-mouse IgG monoclonal antibody (sc-3699, Santa Cruz Biotechnology, Dallas, TX, USA) for 15 min at 4°C. The mean fluorescence intensity (MFI) of TLR4-positive cells was then determined using the Guava easyCyte flow cytometry single sample system. Data are presented as the mean ± standard deviation (SD). Statistical analyses were performed using Dunnett’s multiple comparison tests for one-way analysis of variance. Differences were considered significant when p values were less than 0.05. The intestine is exposed to exogenous inflammatory mediators. Table 3 shows the cell survival rate and the IL-8 and TNFα levels in LPS-stimulated INT-407 cell cultures in the presence or absence of specific VLB. The cell survival rate was significantly higher in the cell culture with LPS and L. crispatus than in the control culture (no added LPS and VLB). In addition, there were no significant decreases in cell survival rate between any of the other cell cultures. These results indicate that IL-8 and TNFα production is not influenced by a decrease in the cell survival rate. The IL-8 level was significantly higher in the cell culture with LPS than in the control culture, while the IL-8 level in the cell culture with LPS and L. crispatus and that in the cell culture with LPS and L. gasseri showed no significant difference compared with the control culture. The TNFα level was significantly higher in the cell culture with LPS than in the control culture, while the TNFα level in the cell culture with LPS and VLB, except L. vaginalis, showed no significant difference compared with the control culture. These results suggest that some VLB suppress the IL-8 and TNFα productions that is induced by LPS stimulation in INT-407 cell cultures. Figure 2 shows the MFI of TLR4 expression in INT-407 cells stimulated by LPS in the presence or absence of various VLB. The MFI of TLR4 expression was significantly higher in the cell culture with LPS than in the control culture, while the MFI of TLR4 expression in the cell culture with LPS and L. crispatus, and that in the cell culture with LPS and L. gasseri showed no significant difference compared with the control culture. These results suggest that some VLB suppress the enhancement of TLR4 protein expression in INT-407 cells that is stimulated by LPS. However, the MFI of TLR4 expression was significantly higher in the cells cultured with LPS in the presence of L. acidophilus or L. jensenii than in the control culture. These VLB may suppress the production of TNFα through the inhibition of activation of TLR4 signaling rather than through the suppression of TLR4 protein expression in the cells. Therefore, it will be necessary to obtain further evidence on cellular signaling in this model.
Table 3. Effects of VLB on survival rate and the production of IL-8 and TNFa in cells cultured with LPS.
| Survival rate (%) | IL-8 (pg/ml) | TNFα (pg/ml) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | 56.9 | ± | 10.3 | 285.4 | ± | 37.6 | 1.2 | ± | 0.7 |
| LPS | 57.9 | ± | 0.8 | 405.9 | ± | 59.5* | 5.8 | ± | 1.5* |
| LPS + L. acidophilus | 49.8 | ± | 1.6 | 400.9 | ± | 22.4* | 1.9 | ± | 1.1 |
| LPS + L. crispatus | 67.9 | ± | 1.3* | 375.1 | ± | 28.1 | 1.9 | ± | 1.4 |
| LPS + L. gasseri | 63.6 | ± | 5.8 | 293.4 | ± | 29.0 | 0.2 | ± | 0.3 |
| LPS + L. jensenii | 48.2 | ± | 1.7 | 403.4 | ± | 5.2* | 0.1 | ± | 0.1 |
| LPS + L. vaginalis | 47.8 | ± | 6.4 | 461.8 | ± | 19.5* | 3.7 | ± | 1.5* |
INT-407 cells were cultured with LPS (0 or 10 µg/ml) and VLB (0 or 10 µg/ml) for 48 hr. The IL-8 and TNFa concentrations in the cell cultures were measured using ELISA. The cell survival rate was determined using a Guava easyCyte flow cytometry single sample system. The data are presented as means ± SD (n=3). *p<0.05 (vs. control).
Fig. 2.
MFI of TLR4 expression in LPS-stimulated INT-407 cell cultures in the presence of specific VLB. INT-407 cells were cultured with LPS (0 or 10 µg/ml) and the indicated VLB (0 or 10 µg/ml) for 48 hr. The MFI of TLR4-positive cells was then determined using a Guava easyCyte flow cytometry single sample system. Data are presented as the mean ± SD (n=3). * p<0.05 (compared with control using Dunnett’s multiple comparison test).
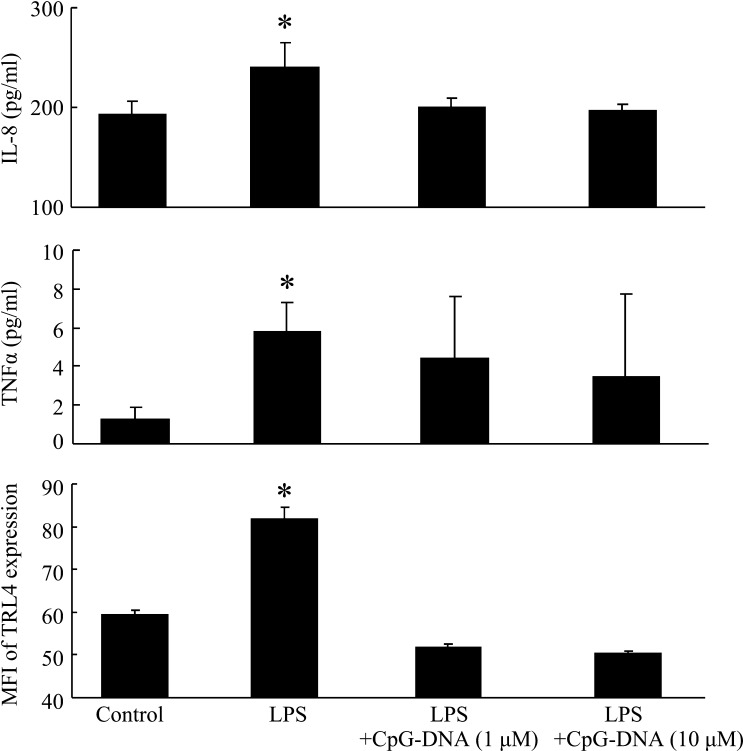
Ren et al. [19] reported that some lactobacilli inhibit S. Typhimurium-induced IL-8 production through competitive adhesion to intestinal epithelial cells. In the present study, we did not observe immunological interaction between the anti-inflammatory effects and the adhesion of VLB to the INT-407 cells. It is known that TLR9 recognizes bacterial deoxyribonucleic acid containing unmethylated CpG dinucleotides (CpG-DNA) [20]. Furthermore, Yu et al. [21] reported that signal activation of TLR9 inhibited LPS-mediated TLR4 signaling and expression via regulation of multiple cyclin-dependent kinase pathways. Moreover, it is known that Lactobacillus strains and the abovementioned bacterial oligodeoxynucleotides bind to TLR9 and induce the innate immune response through TLR9 in human intestinal epithelial cells [22, 23], and Ju et al. [24] reported that INT-407 (Henle 407) cells express the majority of the TLRs (with the exception of TLR8) and that CpG-DNA activated signaling in this cell line. In the present study, we investigated the immune effect of CpG-DNA (Hycult Biotech, Uden, Netherlands), a known TLR9 ligand, on LPS-stimulated INT-407 cells. Figure 3 shows the IL-8 and TNFα levels and the MFI of TLR4 expression in LPS-stimulated INT-407 cell cultures in the presence of 1 or 10 μM CpG-DNA. The levels of these cytokines and TLR4 were significantly higher in the cells cultured with LPS than in the control culture (no added LPS or CpG-DNA). In addition, cytokine levels in cells cultured with both LPS and CpG-DNA showed no significant differences compared with those in the control culture. These results suggested that specific VLB suppress the production of inflammatory cytokines and TLR4 expression that are otherwise induced by LPS stimulation of INT-407 cells through TLR9.
Fig. 3.
The IL-8 and TNFα productions and MFI of TLR4 expression in LPS-stimulated INT-407 cell cultures in the presence of CpG-DNA. INT-407 cells were cultured with LPS (10 µg/ml) and the indicated CpG-DNA (1 or 10 μM) for 48 hr. The IL-8 and TNFα concentrations in the cell cultures were measured using ELISA. The MFI of TLR4-positive cells was then determined using a Guava easyCyte flow cytometry single sample system. Data are presented as the mean ± SD (n=3). *p<0.05 (compared with control using Dunnett’s multiple comparison test).
Necrotizing enterocolitis (NEC) is one of the most common and severe gastrointestinal conditions in preterm newborn infants, and it has a high mortality rate [25]. Premature birth and abnormal bacterial colonization are the predisposing factors for NEC [25]. The pathogenesis of NEC is associated with an excessive inflammatory IL-8 response [26]. In addition, LPS stimulation through TLR-4 signaling leads to NEC development [27]. The present study suggests that specific VLB suppress the inflammation induced by LPS stimulation through the downregulation of TLR4 expression in human intestinal epithelial cells. Thus, these VLB may prevent NEC in infants.
In conclusion, we established the probiotic function and immune effect of VLB in human embryonic intestinal epithelial cells. Our results suggested that specific VLB suppress the production of inflammatory cytokines that are induced by LPS stimulation through the downregulation of TLR4 expression in human intestinal epithelial cells and further indicated that the TLR9 ligand plays an important role in these effects. Thus, these VLB may play an important role in the development of the immature immune system in infants.
References
- 1.Petricevic L, Kaufmann U, Domig KJ, Kraler M, Marschalek J, Kneifel W, Kiss H. 2014. Molecular detection of Lactobacillus species in the neovagina of male-to-female transsexual women. Sci Rep 4: 3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, Brotman RM, Davis CC, Ault K, Peralta L, Forney LJ. 2011. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci USA 108Suppl 1: 4680–4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romero R, Hassan SS, Gajer P, Tarca AL, Fadrosh DW, Nikita L, Galuppi M, Lamont RF, Chaemsaithong P, Miranda J, Chaiworapongsa T, Ravel J. 2014. Correction: the composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome 2: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pendharkar S, Magopane T, Larsson PG, de Bruyn G, Gray GE, Hammarström L, Marcotte H. 2013. Identification and characterisation of vaginal lactobacilli from South African women. BMC Infect Dis 13: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rose WA, 2nd, McGowin CL, Spagnuolo RA, Eaves-Pyles TD, Popov VL, Pyles RB. 2012. Commensal bacteria modulate innate immune responses of vaginal epithelial cell multilayer cultures. PLoS One 7: e32728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsumiya Y, Kato N, Watanabe K, Kato H. 2002. Molecular epidemiological study of vertical transmission of vaginal Lactobacillus species from mothers to newborn infants in Japanese, by arbitrarily primed polymerase chain reaction. J Infect Chemother 8: 43–49. [DOI] [PubMed] [Google Scholar]
- 7.Carlsson J, Gothefors L. 1975. Transmission of Lactobacillus jensenii and Lactobacillus acidophilus from mother to child at time of delivery. J Clin Microbiol 1: 124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Makino H, Kushiro A, Ishikawa E, Kubota H, Gawad A, Sakai T, Oishi K, Martin R, Ben-Amor K, Knol J, Tanaka R. 2013. Mother-to-infant transmission of intestinal bifidobacterial strains has an impact on the early development of vaginally delivered infant’s microbiota. PLoS One 8: e78331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mannick E, Udall JN., Jr1996. Neonatal gastrointestinal mucosal immunity. Clin Perinatol 23: 287–304. [PubMed] [Google Scholar]
- 10.Yan F, Polk DB. 2012. Lactobacillus rhamnosus GG: an updated strategy to use microbial products to promote health. Funct Food Rev 4: 77–84. [PMC free article] [PubMed] [Google Scholar]
- 11.Takeda S, Yamasaki K, Takeshita M, Kikuchi Y, Tsend-Ayush C, Dashnyam B, Ahhmed AM, Kawahara S, Muguruma M. 2011. The investigation of probiotic potential of lactic acid bacteria isolated from traditional Mongolian dairy products. Anim Sci J 82: 571–579. [DOI] [PubMed] [Google Scholar]
- 12.Hunt RH. 1988. The protective role of gastric acid. Scand J Gastroenterol Suppl 146: 34–39. [DOI] [PubMed] [Google Scholar]
- 13.Agunod M, Yamaguchi N, Lopez R, Luhby AL, Glass GB. 1969. Correlative study of hydrochloric acid, pepsin, and intrinsic factor secretion in newborns and infants. Am J Dig Dis 14: 400–414. [DOI] [PubMed] [Google Scholar]
- 14.Henle G, Deinhardt F. 1957. The establishment of strains of human cells in tissue culture. J Immunol 79: 54–59. [PubMed] [Google Scholar]
- 15.Viggiano D, Ianiro G, Vanella G, Bibbò S, Bruno G, Simeone G, Mele G. 2015. Gut barrier in health and disease: focus on childhood. Eur Rev Med Pharmacol Sci 19: 1077–1085. [PubMed] [Google Scholar]
- 16.Srikanth CV, McCormick BA. 2008. Interactions of the intestinal epithelium with the pathogen and the indigenous microbiota: a three-way crosstalk. Interdiscip Perspect Infect Dis 2008: 626827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takeda K, Kaisho T, Akira S. 2003. Toll-like receptors. Annu Rev Immunol 21: 335–376. [DOI] [PubMed] [Google Scholar]
- 18.Tobita K, Otani H. 2015. Lactobacillus crispatus KT-11 strain promotes IL-12 produced from a mouse macrophage-like cell line, J774.1 stimulated with a few lactic acid bacteria and Toll-like receptor 2 ligand. Milk Science 64: 1–6. [Google Scholar]
- 19.Ren DY, Li C, Qin YQ, Yin RL, Du SW, Ye F, Liu HF, Wang MP, Sun Y, Li X, Tian MY, Jin NY. 2013. Lactobacilli reduce chemokine IL-8 production in response to TNF-α and Salmonella challenge of Caco-2 cells. BioMed Res Int 2013: 925219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408: 740–745. [DOI] [PubMed] [Google Scholar]
- 21.Yu X, Lin J, Yu Q, Kawai T, Taubman MA, Han X. 2014. Activation of Toll‐like receptor 9 inhibits lipopolysaccharide‐induced receptor activator of nuclear factor kappa‐ B ligand expression in rat B lymphocytes. Microbiol Immunol 58: 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kingma SD, Li N, Sun F, Valladares RB, Neu J, Lorca GL. 2011. Lactobacillus johnsonii N6.2 stimulates the innate immune response through Toll-like receptor 9 in Caco-2 cells and increases intestinal crypt Paneth cell number in biobreeding diabetes-prone rats. J Nutr 141: 1023–1028. [DOI] [PubMed] [Google Scholar]
- 23.Hiramatsu Y, Satho T, Hyakutake M, Irie K, Mishima K, Miake F, Kashige N. 2014. The anti-inflammatory effects of a high-frequency oligodeoxynucleotide from the genomic DNA of Lactobacillus casei. Int Immunopharmacol 23: 139–147. [DOI] [PubMed] [Google Scholar]
- 24.Ju CH, Chockalingam A, Leifer CA. 2009. Early response of mucosal epithelial cells during Toxoplasma gondii infection. J Immunol 183: 7420–7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neu J, Walker WA. 2011. Necrotizing enterocolitis. N Engl J Med 364: 255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nanthakumar N, Meng D, Goldstein AM, Zhu W, Lu L, Uauy R, Llanos A, Claud EC, Walker WA. 2011. The mechanism of excessive intestinal inflammation in necrotizing enterocolitis: an immature innate immune response. PLoS One 6: e17776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hackam DJ, Good M, Sodhi CP. 2013. Mechanisms of gut barrier failure in the pathogenesis of necrotizing enterocolitis: Toll-like receptors throw the switch. Semin Pediatr Surg 22: 76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]