Abstract
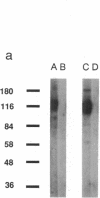
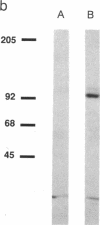
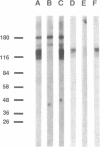
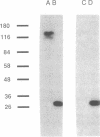
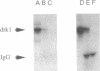
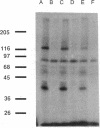
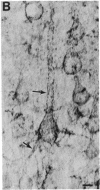
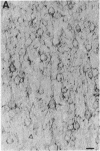
Antibodies specific for the drk1 polypeptide were used to characterize the corresponding protein in rat brain. Recombinant and synthetic immunogens containing fragments of the drk1 polypeptide were produced. Antibodies raised to these immunogens display monospecific reactions with the same 130-kDa polypeptide on immunoblots of adult rat brain membranes. Immunoprecipitation of 125I-labeled brain membranes identifies a 38-kDa peptide in tight association with the drk1 polypeptide. Immunohistochemical staining of sections of adult rat cortex shows that drk1 protein is restricted to neurons, where staining is present on dendrites and cell bodies but not on axons. These studies point to the value of such immunological reagents to the further characterization of the components of this delayed rectifier K+ channel in the mammalian central nervous system.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Christie M. J., Adelman J. P., Douglass J., North R. A. Expression of a cloned rat brain potassium channel in Xenopus oocytes. Science. 1989 Apr 14;244(4901):221–224. doi: 10.1126/science.2539643. [DOI] [PubMed] [Google Scholar]
- FERGUSON K. A., WALLACE A. L. Starch-gel electrophoresis of anterior pituitary hormones. Nature. 1961 May 13;190:629–630. doi: 10.1038/190629a0. [DOI] [PubMed] [Google Scholar]
- Frech G. C., VanDongen A. M., Schuster G., Brown A. M., Joho R. H. A novel potassium channel with delayed rectifier properties isolated from rat brain by expression cloning. Nature. 1989 Aug 24;340(6235):642–645. doi: 10.1038/340642a0. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):449–472. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isacoff E. Y., Jan Y. N., Jan L. Y. Evidence for the formation of heteromultimeric potassium channels in Xenopus oocytes. Nature. 1990 Jun 7;345(6275):530–534. doi: 10.1038/345530a0. [DOI] [PubMed] [Google Scholar]
- Langosch D., Thomas L., Betz H. Conserved quaternary structure of ligand-gated ion channels: the postsynaptic glycine receptor is a pentamer. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7394–7398. doi: 10.1073/pnas.85.19.7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon R. Determination of the subunit stoichiometry of a voltage-activated potassium channel. Nature. 1991 Mar 21;350(6315):232–235. doi: 10.1038/350232a0. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlie J. P., Lindstrom J. Assembly in vivo of mouse muscle acetylcholine receptor: identification of an alpha subunit species that may be an assembly intermediate. Cell. 1983 Oct;34(3):747–757. doi: 10.1016/0092-8674(83)90531-7. [DOI] [PubMed] [Google Scholar]
- Moczydlowski E., Lucchesi K., Ravindran A. An emerging pharmacology of peptide toxins targeted against potassium channels. J Membr Biol. 1988 Oct;105(2):95–111. doi: 10.1007/BF02009164. [DOI] [PubMed] [Google Scholar]
- Oettgen H. C., Pettey C. L., Maloy W. L., Terhorst C. A T3-like protein complex associated with the antigen receptor on murine T cells. Nature. 1986 Mar 20;320(6059):272–275. doi: 10.1038/320272a0. [DOI] [PubMed] [Google Scholar]
- Olmsted J. B. Affinity purification of antibodies from diazotized paper blots of heterogeneous protein samples. J Biol Chem. 1981 Dec 10;256(23):11955–11957. [PubMed] [Google Scholar]
- Rehm H., Lazdunski M. Purification and subunit structure of a putative K+-channel protein identified by its binding properties for dendrotoxin I. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4919–4923. doi: 10.1073/pnas.85.13.4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm H., Newitt R. A., Tempel B. L. Immunological evidence for a relationship between the dendrotoxin-binding protein and the mammalian homologue of the Drosophila Shaker K+ channel. FEBS Lett. 1989 Jun 5;249(2):224–228. doi: 10.1016/0014-5793(89)80628-3. [DOI] [PubMed] [Google Scholar]
- Ruppersberg J. P., Schröter K. H., Sakmann B., Stocker M., Sewing S., Pongs O. Heteromultimeric channels formed by rat brain potassium-channel proteins. Nature. 1990 Jun 7;345(6275):535–537. doi: 10.1038/345535a0. [DOI] [PubMed] [Google Scholar]
- Schwarz T. L., Papazian D. M., Carretto R. C., Jan Y. N., Jan L. Y. Immunological characterization of K+ channel components from the Shaker locus and differential distribution of splicing variants in Drosophila. Neuron. 1990 Jan;4(1):119–127. doi: 10.1016/0896-6273(90)90448-o. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Stühmer W., Ruppersberg J. P., Schröter K. H., Sakmann B., Stocker M., Giese K. P., Perschke A., Baumann A., Pongs O. Molecular basis of functional diversity of voltage-gated potassium channels in mammalian brain. EMBO J. 1989 Nov;8(11):3235–3244. doi: 10.1002/j.1460-2075.1989.tb08483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stühmer W., Stocker M., Sakmann B., Seeburg P., Baumann A., Grupe A., Pongs O. Potassium channels expressed from rat brain cDNA have delayed rectifier properties. FEBS Lett. 1988 Dec 19;242(1):199–206. doi: 10.1016/0014-5793(88)81015-9. [DOI] [PubMed] [Google Scholar]
- Swanson R., Marshall J., Smith J. S., Williams J. B., Boyle M. B., Folander K., Luneau C. J., Antanavage J., Oliva C., Buhrow S. A. Cloning and expression of cDNA and genomic clones encoding three delayed rectifier potassium channels in rat brain. Neuron. 1990 Jun;4(6):929–939. doi: 10.1016/0896-6273(90)90146-7. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama S., Imoto K., Kawamura T., Higashida H., Iwabe N., Miyata T., Numa S. Potassium channels from NG108-15 neuroblastoma-glioma hybrid cells. Primary structure and functional expression from cDNAs. FEBS Lett. 1989 Dec 18;259(1):37–42. doi: 10.1016/0014-5793(89)81488-7. [DOI] [PubMed] [Google Scholar]