Abstract
Situs inversus totalis (SIT) is a rare congenital anomaly that refers to a completely reversed location of the abdominal and thoracic organs. We report the case of 50-year-old man with gastric cancer and SIT who was diagnosed during a screening esophagogastroduodenoscopy. A chest X-ray, abdominopelvic computed tomography, and 18F-fluoro2-deoxyglucose-D-glucose-positron emission tomography scans revealed SIT. We performed a radical subtotal gastrectomy with D2 lymph node dissection. Advanced surgical skill is required to perform a precise lymphadenectomy in a patient with SIT by visualizing the exact mirror image of the anatomy during the operation. The patient had an uneventful intra- and postoperative course and was followed up at the outpatient department without any evidence of recurrence. In conclusion, surgery in a patient with gastric cancer and SIT can be safely performed by paying attention to the inverted anatomic structures during the operation.
Keywords: Stomach cancer, Situs inversus totalis, Congenital anomaly
Introduction
Situs inversus totalis (SIT) is a rare congenital anomaly with an incidence of 1 in 10,000–50,000 persons [1]. SIT refers to complete right-left transformation or a “mirror image” of the normal arrangement of body organs. The anatomic arrangement in a patient with SIT is complete transposition of all viscera in the thoracic and abdominal cavities. Although this condition does not affect normal health or longevity, its recognition is very important for treating many diseases, particularly those requiring surgical intervention [2]. Surgical procedures can be difficult due to the totally different anatomic position of organs. Herein, we report a patient with gastric cancer and SIT who was treated with radical subtotal gastrectomy.
Case Presentation
A 50-year-old man was admitted to our hospital for treatment of early gastric cancer (EGC), which was diagnosed during a screening esophagogastroduodenoscopy (EGD) at a local clinic. He was known to have SIT since early childhood. EGD revealed EGC type IIc located at the gastric angle (Fig. 1), and the pathological report was moderately differentiated adenocarcinoma. His height was 171 cm, weight was 64.8 kg, and his body mass index was 22.2. He had no anemia (red blood cell count, 4.25 × 1012/L; hemoglobin, 13.4 g/dL; hematocrit, 43.6%). The serum carcinoembryonic antigen concentration was not elevated (3.16 ng/ml; reference range, 0–4.3). A chest radiograph showed dextrocardia and a right subphrenic gas pattern in the stomach (Fig. 2). Abdominopelvic computed tomography (CT) showed complete transposition of the abdominal viscera, confirming SIT (Fig. 3). An 18F-fluoro2-deoxyglucose-D-glucose-positron emission tomography (FDG-PET) scan revealed no abnormal uptake and also noted a complete mirror image of the intrathoracic and abdominal viscera (Fig. 4). We decided to perform a laparotomy and found complete transposition of the viscera; the stomach and spleen were located on the right side of the abdomen, and the gall bladder, liver, cecum, and appendix were located on the left side. We performed a radical subtotal gastrectomy with D2 lymph node dissection. Total operation time was 180 min with no specific intraoperative complications. The macroscopic biopsy report revealed a 2.0 × 1-cm EGC type IIc located at the gastric angle with 2 and 6.8 cm proximal and distal safety margins, respectively. Pathological examination showed tubular adenocarcinoma, moderately differentiated with depth of invasion into the muscularis mucosa. There was no cancer metastasis out of the 53 resected lymph nodes. The patient was discharged 10 days after the operation with no specific complications. We followed the patient for 78 months in the outpatient department without recurrence of cancer.
Fig. 1.
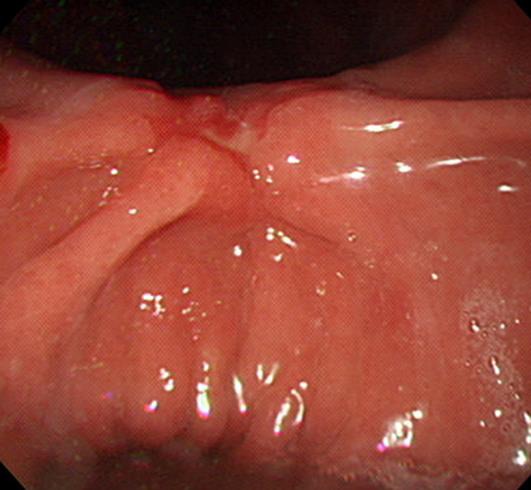
EGD showing type IIc EGC located at the gastric angle. EGD, esophagogastroduodenoscopy; EGC, early gastric cancer.
Fig. 2.
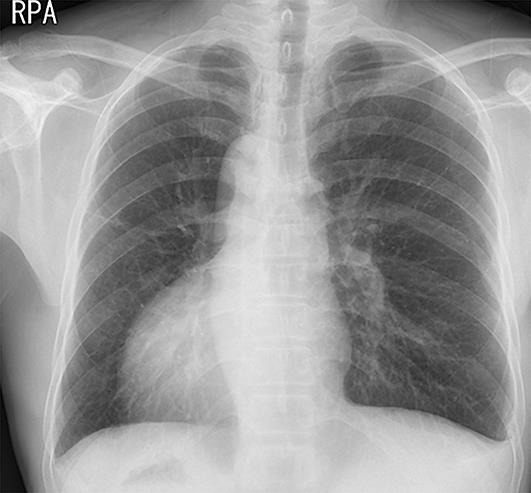
Chest X-ray showing dextrocardia and the right subphrenic gas pattern in the stomach.
Fig. 3.
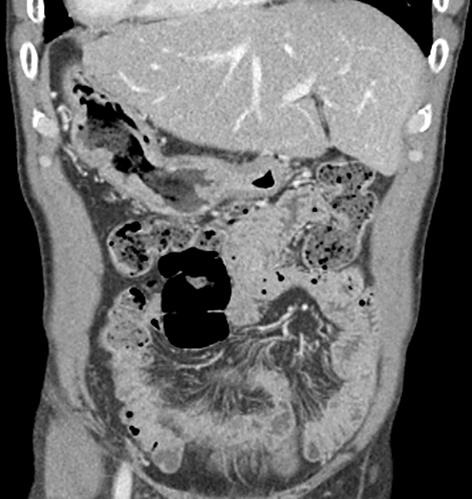
Abdominopelvic CT scan showing the reverse location of the abdominal organs.
Fig. 4.
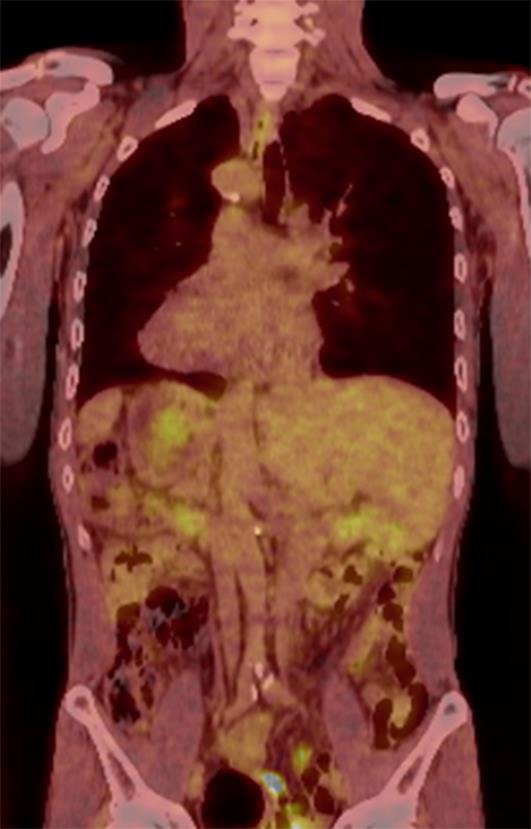
FDG positron emission tomography (PET-CT) showing the reverse locations of the thoracic and abdominal organs or a “mirror image”. FDG, 18F-fluoro2-deoxyglucose-D-glucose; PET, positron emission tomography.
Discussion
SIT is transmitted as an autosomal recessive trait, but the exact genetic cause remains unknown. Shigenori et al. [3] suggested that immobility of nodal cilia inhibits the flow of extraembryonic fluid during embryogenesis, leading to the development of SIT. SIT has various manifestations; dextrocardia with complete reversal of the heart chambers can be seen. The stomach and spleen are located in the right side and the liver and gallbladder are located in the left side of the midline. Cardiovascular abnormalities (septal defects, pulmonary arterial stenosis, tetralogy of Fallot, or transposition of great arteries) and problems with the alimentary tract (atresia or stenosis of the duodenum) can also occur. SIT with normocardia (situs inversus incomplete) is associated with cardiac abnormalities [4]. No significant cardiac or respiratory abnormalities were detected in our patient at the preoperative screening. About 60% of patients with SIT have other congenital anomalies of the gastrointestinal tract, including rotational anomalies, biliary atresia, splenic agenesis, small bowel atresia, duplication, and colon aganglionosis [5]. The initial diagnosis and surgical intervention for diseases, such as cholelithiasis, might be confusing because of the anatomical differences. Reversed side liver dullness, an apical beat in the right fifth intercostal space, a chest radiograph revealing dextrocardia, and abdominal X-ray film demonstrating stomach gas on the right side can be diagnostic clues of SIT [6]. SIT is often incidentally recognized during screening. SIT was recognized in the present patient during adolescence from a screening chest roentgenography. An FDG-PET scan was obtained as a whole body image in a single picture for the stomach cancer stage workup. The locations of all viscera were easily identified on the scan in our case. The transposed heart and liver made it easy to identify SIT [7]. Many different cancers have been reported in patients with SIT, including pancreatic, hepatocellular, colorectal, and gastric cancers. Fabricus reported the first case of mirror image transposition in a man in 1600 [8, 9]. Allen [10] first reported a gastrectomy on a 30-year-old patient with gastric cancer and SIT who died 3 weeks after surgery in 1936. The symptoms of Kartagener's syndrome, including bronchiectasis, chronic sinusitis, and male infertility is a disease that is associated with SIT [11]. Surgery in a patient with SIT and gastric cancer could be difficult due to the anatomic anomalies, including perigastric major vessels. Although the incidence of intra-abdominal malignancies in a patient with SIT is very rare, the surgeon must anticipate the complexity of the surgical procedure in patients with SIT and cancer. Advanced surgical skill is required to perform a precise lymphadenectomy in a patient with SIT by visualizing the exact mirror image of the anatomy during the operation. Visualizing the mirror image of the anatomy was helpful when we performed the lymph node dissection around the named vessels. Therefore, preoperative recognition of the anatomic variations might be needed when operating on a patient with SIT. It might be helpful to perform a three-dimensional CT angiography reconstruction prior to the operation. In conclusion, surgery in a patient with gastric cancer and SIT can be safely performed by paying attention to the inverted anatomic structures during the operation.
Statement of Ethics
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Disclosure Statement
The authors declare that they have no competing interests.
References
- 1.Huh JW, Kim HR, Cho SH, et al. Laparoscopic total mesorectal excision in a rectal cancer patient with situs inversus totalis. J Korean Med Sci. 2010;25:790–793. doi: 10.3346/jkms.2010.25.5.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blegen HM. Surgery in situs inversus. Ann Surg. 1949;129:244–259. doi: 10.1097/00000658-194902000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nonaka S, Tanaka Y, Okada Y, et al. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell. 1998;95:829–837. doi: 10.1016/s0092-8674(00)81705-5. [DOI] [PubMed] [Google Scholar]
- 4.Samaan M, Ratnasingham A, Pittathankal A, Hashemi M. Laparoscopic adjustable gastric banding for morbid obesity in a patient with situs inversus totalis. Obesity Surg. 2008;18:898–901. doi: 10.1007/s11695-008-9445-7. [DOI] [PubMed] [Google Scholar]
- 5.Lee SE, Kim H, Jung S, Lee S, Park K, Kim W. Situs anomalies and gastrointestinal abnormalities. J Pediatr Surg. 2006;41:1237–1242. doi: 10.1016/j.jpedsurg.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 6.Yaghan RJ, Gharaibeh KI, Hammori S. Feasibility of laparoscopic cholecystectomy in situs inversus. J Laparoendosc Adv Surg Tech. 2001;11:233–237. doi: 10.1089/109264201750539763. [DOI] [PubMed] [Google Scholar]
- 7.Chang M, Tsai S, Lin W. Incidental FDG PET finding of situs inversus: a case report. Ann Nucl Med Sci. 2008;21:43–47. [Google Scholar]
- 8.Machado NO, Chopra P. Laparoscopic cholecystectomy in a patient with situs inversus totalis: feasibility and technical difficulties. JSLS. 2006;10:386–391. [PMC free article] [PubMed] [Google Scholar]
- 9.Akbulut S, Caliskan A, Ekin A, Yagmur Y. Left-sided acute appendicitis with situs inversus totalis: review of 63 published cases and report of two cases. J Gastrointest Surg. 2010;14:1422–1428. doi: 10.1007/s11605-010-1210-2. [DOI] [PubMed] [Google Scholar]
- 10.Allen F. A case of malignant tumor of the stomach in a male with transposition of the viscera. Indian Med Gaz. 1936;71:32. [PMC free article] [PubMed] [Google Scholar]
- 11.Haruki T, Maeta Y, Nakamura S, et al. Advanced cancer with situs inversus totalis associated with KIF3 complex deficiency: report of two cases. Surg Today. 2010;40:162–166. doi: 10.1007/s00595-009-4005-x. [DOI] [PubMed] [Google Scholar]


