Abstract
Barrett’s esophagus is characterized by the erosive replacement of esophageal squamous epithelium by a range of metaplastic glandular phenotypes. These glandular phenotypes likely change over time, and their distribution varies along the Barrett’s segment. Although much recent work has addressed Barrett’s esophagus from the genomic viewpoint—its genotype space—the fact that the phenotype of Barrett’s esophagus is nonstatic points to conversion between phenotypes and suggests that Barrett’s esophagus also exists in phenotype space. Here we explore this latter concept, investigating the scope of glandular phenotypes in Barrett’s esophagus and how they exist in physical and temporal space as well as their evolution and their life history. We conclude that individual Barrett’s glands are clonal units; because of this important fact, we propose that it is the Barrett’s gland that is the unit of selection in phenotypic and indeed neoplastic progression. Transition between metaplastic phenotypes may be governed by neutral drift akin to niche turnover in normal and dysplastic niches. In consequence, the phenotype of Barrett’s glands assumes considerable importance, and we make a strong plea for the integration of the Barrett’s gland in both genotype and phenotype space in future work.
Keywords: Barrett’s Esophagus, Neutral Drift, Metaplasia
Abbreviation used in this paper: CCO, cytochrome c oxidase
Summary.
This review addresses the scope of phenotypic diversity within Barrett's esophagus. Although often underemphasized, the authors argue that this diversity may be key to understanding Barrett's initiation and progression.
Barrett’s esophagus occurs when the stratified squamous epithelium at the lower end of the esophagus is replaced with glands composed of columnar cells—columnar-lined esophagus. How and why does this occur? Classically, this is explained by severe acid and bile reflux destroying the stratified squamous epithelium, which is replaced with columnar epithelial glands. Teleologically, these are more resistant to acid/peptic digestion1 and have a selective advantage over the squamous epithelium in the harsh, mutagenic environment associated with reflux.2
Recent progress has seen a step-change in our knowledge of the changes in the genotype of Barrett’s gland with time. Where initially we thought that Barrett’s segments progressed to malignancy through a number of clonal selective sweeps in well-defined genes,3 it has recently become clear that such selective sweeps, where mutations spread clonally throughout the segment and become “fixed,” are rare indeed. Many patients with Barrett’s esophagus maintain an equilibrium level of genetic alterations—stasis—over time, but sometimes with infrequent but significant genetic change or punctuation caused by growth of clones with huge numbers of genetic alterations.2, 4 The clonal dynamics in nonprogressor Barrett’s genomes are thus stable over long periods, but patients who progress develop substantially increased genetic changes in a relatively short time frame: unexpectedly, this occurs within just a few years before the development of cancer.2
Surprisingly, no attempt has been made to map these genotypes to the changing phenotypes seen in Barrett’s mucosa—sometimes referred to as a genotype–phenotype map. Which phenotype(s) is prone to develop such catastrophic changes? Does such a correlation exist? Widening the argument, why do the several phenotypes seen in Barrett’s esophagus occur? Why and when may some phenotypes be favored over others? What is the phylogenetic relationship between these several phenotypes, and what, if anything, does this say about the origin and development of Barrett’s esophagus? Such questions have direct clinical relevance: recent years have seen a sharp disagreement about which phenotype predisposes to cancer development,5, 6 about who should be entered into surveillance and treatment programs for Barrett’s esophagus,7, 8 and indeed about what the very definition is of Barrett’s esophagus itself.9, 10, 11, 12
We investigate the range of glandular phenotypes observed in Barrett’s esophagus and explore their relations in phenotype space, which is formally defined as an abstract space containing every possible phenotype in a given biologic system.13 When we try to think about Barrett’s esophagus and especially Barrett’s glands in evolutionary terms, it is not usual to consider it as a system where the phenotype is defined by a trajectory of characteristics. We usually think of evolution in terms of selection—here, of glands of different phenotypes being selected because of their fitness (or otherwise). Fitness would be defined as the ability of any Barrett’s gland phenotype to both survive and reproduce. Conceptually, for any given ecologic niche, this selection results in an optimal phenotype, which will occupy a point in abstract phenotype space. However, where Barrett’s glands are concerned, we do not know the nature of the fitness characteristic under selection: it could be, for example, its ability to survive acid/peptic digestion,1 mediated through its mucin/trefoil family peptide pattern of expression, stress response phenotype,14 defense capacity,15, 16 or indeed simply its ability to (rapidly) reproduce itself and clonally expand, which indeed has been observed14 and thus is possibly related to a proliferative phenotype.1 Conceivably, intergland competition could play a role: perhaps some glands actively suppress the growth of their neighbors, leading to competitive phenotypes being selected. Thus, a number of characteristics may collectively contribute to fitness. In this review, we put forth a number of questions. What explains the protean manifestation of Barrett’s esophagus? Why are such diverse gland phenotypes selected? Alternatively, does an optimal phenotype exist within Barrett’s esophagus? And finally, where in phenotype space are the various metaplasias observed in Barrett’s esophagus situated?
Gland Phenotypes in Barrett’s Esophagus
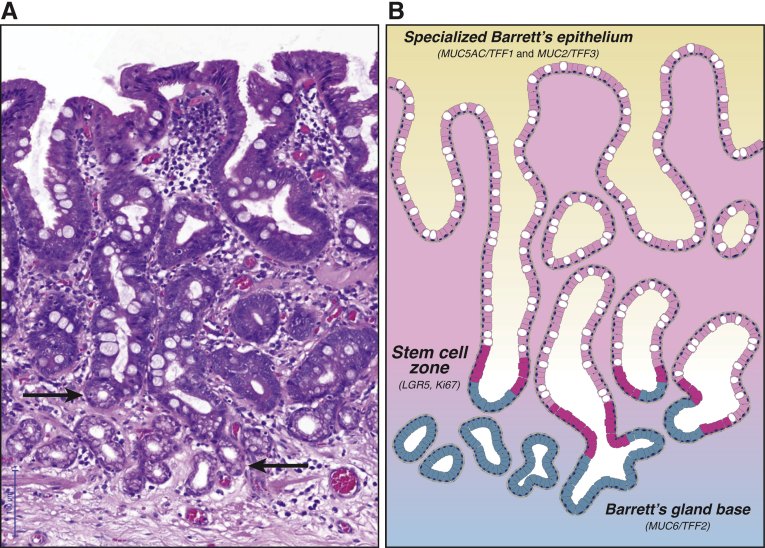
Before we plot possible evolutionary trajectories in Barrett’s phenotype space, we will need to define the phenotypes observed in Barrett’s esophagus. The canonical Barrett’s gland displays so-called specialized metaplasia or specialized epithelium (Figure 1A). Until recently, the unique functional properties of this gland phenotype may not have been properly appreciated. Specialized epithelium displays a rich admixture of goblet cells11, 12 against a background of columnar cells that resemble gastric foveolar cells, which contain acid sialomucin and neutral mucin of normal gastric foveolar cells.17, 18, 19 This combination of differentiated lineages thus resembles type II intestinal metaplasia in the gastric mucosa, sometimes called the incomplete type, which includes types II and III depending on whether the columnar and goblet cells secrete sialomucins (type II) or sulfomucins (type III).20, 21 Accordingly, mucin core proteins commonly used to categorize lesions of gastric or intestinal type are both found in specialized Barrett’s epithelium. Thus, both MUC2 and MUC3, as normally seen in intestinal epithelium, and MUC1, MUC5AC, and MUC6, as characteristic of gastric epithelium,17, 22 are found in specialized Barrett’s epithelium, a pattern also reflected in trefoil peptide expression.17, 23 Important to this discussion is the striking regional localization of these mucin core proteins along the gland axis with MUC5AC/TFF1 and MUC2/TFF3 found in the upper portion of the gland, and MUC6 and TFF2 localized in the mucous cells of the Barrett’s gland base (Figure 1B). This compartmentalization strongly resembles the basic architecture of the pyloric gland in the gastric antrum. Leucine-rich repeat-containing G-protein coupled receptor 5 (LGR5) is thought to locate intestinal stem cells in mice and humans24, 25, 26 and in situ hybridization shows LGR5 mRNA expression at the junction of the MUC5AC+/TFF1+ cells and the MUC6+/TFF2+ cells, indicating the site of the stem cell niche.17 This is also the origin of the bidirectional cell flux seen in these glands and reflects their kinetic organization, with maximum proliferative activity in the midportion as shown by Ki-67.17, 27, 28
Figure 1.
Barrett’s glands show functional compartmentalization.(A) H&E photomicrograph of a series of nondysplastic Barrett’s glands (of canonical, specialized type) demonstrating abundant goblet cells. Note the mucous glands arranged as small acini at the base of these Barrett’s gland (arrows). (B) Illustration of the Barrett’s glands shown in A. The stem cell zone (shown in magenta) demonstrates maximal Ki-67 proliferative activity and LGR5 labeling on in situ hybridization. This proliferative compartment is located about one-third up the glandular axis. Labeling studies demonstrate bidirectional flow from this stem cell compartment. Specifically, specialized epithelium (a combination of MUC5AC+/TFF1+ foveolar cells and MUC2+/TFF3+ goblet cells, both shown in pink) migrates toward the luminal surface, while MUC6+/TFF2+ mucous cells (shown in blue) migrate toward the glandular base at a much slower rate. This functional compartmentalization replicates pyloric-type gland organization.
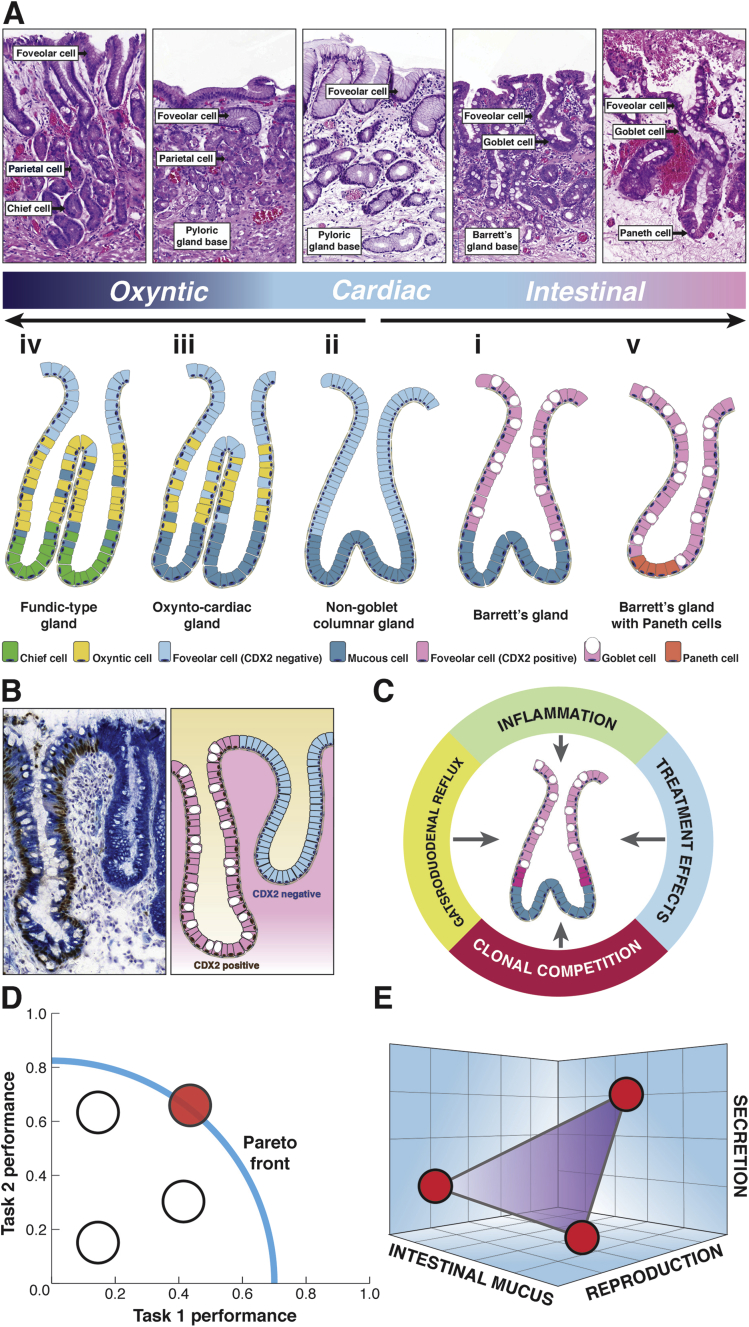
In addition to this canonical specialized gland phenotype of Barrett’s esophagus, the microscopic phenotype of Barrett’s esophagus includes a wide variety of cell lineages and proliferative units (illustrated in Figure 4A). First are the mucinous glands without parietal cells,29, 30 variously called cardiac epithelium by some31 and non-goblet columnar epithelium by others32: in these glands, the superficial glandular epithelium is typically composed of mucinous columnar cells without goblet cells. The base is formed of acini lined by MUC6+/TFF2+ mucinous cells, much like the Barrett’s gland base already discussed. Some of these glands may show early evidence of intestinalization, as demonstrated by villin and CDX-2 expression (reported in 17% and 43% of cases, respectively), although MUC2 is absent.32
Figure 4.
The Barrett’s gland in phenotype space.(A) Morphologic definition of the spectrum of gland phenotypes found in Barrett’s esophagus. The top row shows H&E photomicrographs of the various gland phenotypes, and the second row shows an illustration of these glandular phenotypes (see the article for details). Esophageal derivation (submucosal esophageal glands, double muscularis mucosae) was verified for all examples shown here. The histology panels show labels denoting the differentiated lineage that (often in combination) defines the specific metaplasia. (Further details on immunohistochemical markers for these various differentiated lineages along with references are provided in Table 1.) The glands are numbered in order of appearance in the main text. (i) The canonical goblet-containing Barrett gland is discussed in detail in Figure 1. (ii) The non-goblet columnar gland, or cardiac gland, closely parallels the makeup and functional organization of mature Barrett’s glands, save for the absence of MUC2+ goblet cells. Foveolar cells are shown in this case in light blue because they lack CDX2 expression (see Figure 2B). (iii) The oxyntocardiac gland demonstrates parietal cell differentiation (in yellow) and is functionally equivalent to the pyloric gland. (iv) The fundic-type gland shows chief cell differentiation (light green) and is functionally equivalent to the glandular phenotype of the gastric corpus. (v) Barrett’s glands showing mature intestinal differentiation. Barrett’s glands may show Paneth cell (orange) and enterocyte differentiation (not shown), the latter possibly indicating further intestinal maturation. (B) Frozen Barrett’s material costained for Muc5AC (cytoplasmic labeling, blue substrate) and CDX2 (nuclear labeling, brown substrate). Shown are two neighboring glandular units; the left shows clear goblet cell differentiation and CDX2 expression that are both absent in the right gland (type ii and type i in Figure 2A, respectively). Foveolar differentiation and MUC5AC expression are found in both glands, showing that foveolar maturation is compatible with CDX2 expression. (C) Barrett’s glands evolve genotypically and phenotypically under the continuous Malthusian selection pressures of gastroduodenal reflux, chronic and acute inflammation, clonal competition, and finally treatment effects. (D) Schematic view of the Pareto front in performance space. Phenotypes can be plotted according to performance on two separate tasks. The phenotypes that are on the Pareto front (in red) manage to optimize trade-offs between these tasks. The front therefore represents the set of best compromises. The phenotypes that are behind the front are feasible (white) but not observed, because they are dominated by the phenotype(s) on the Pareto front.13(E) The glandular phenotypes in Barrett’s can be viewed as an integrated biologic system where the phenotype is defined according to a vector of traits within phenotype space. Traits are shown along the axes, which define an abstract three-dimensional space. Note that, in contrast to D, the axes show traits, not task performance. An optimal phenotype with maximum fitness will occupy a point in phenotype space. By studying Barrett’s esophagus from an evolutionary perspective, we may understand the adaptive forces governing transitions between metaplastic phenotypes.
Second, oxyntocardiac glands are found throughout the Barrett’s segment29 and are formally defined as glands containing a mixture of mucous cells and parietal cells,30, 31, 32 which phenocopy normal pyloric epithelium. This gland phenotype also displays a basic ground plan of superficial foveolar glandular epithelium and a mucous base formed of acini lined by MUC6+/TFF2+ mucinous cells. Note that, contrary to common belief, parietal cells are not restricted to corpus-type mucosa and are abundantly found in normal pyloric mucosa.33 Mature fundic-type glands can also be found in Barrett’s mucosa.30 These glands display chief cell and parietal cell differentiation to varying degrees, reminiscent of gastric corpus glands.
Finally, glands showing mature intestinal differentiation may be seen. This can manifest as the complete type of intestinal metaplasia (also designated type I) with enterocytes, Paneth cells, and goblet cells secreting sialomucins and containing MUC2 intestinal mucin, but without gastric mucin core proteins, This phenotype may be localized to areas around the gastroesophageal junction.34 More commonly, canonical specialized Barrett’s glands, as outlined previously, show Paneth cell differentiation—possibly indicating maturation toward the complete intestinal phenotype—while retaining foveolar differentiation and gastric mucin core expression.
Barrett’s Glands in Physical Space
There is good evidence for a spatial distribution of the various phenotypes within the Barrett’s segment: Paull et al.30 mapped their distribution and in some patients showed a zonal distribution with either gastric fundic-type epithelium with parietal and chief cells or cardia type epithelium interposed between the specialized Barrett’s glands and the lower esophagus. Such zonation has been confirmed31, 32, 34, 35: although the specialized metaplasia in the more distal segments is always mixed with oxyntocardiac and cardiac glands, specialized metaplasia is usually present as a contiguous zone abutting the squamocolumnar junction.36, 37 Indeed, there is a gradient of goblet cell density such that there are significantly fewer goblet cells in the distal Barrett’s segment,31 correlated with the more acidic esophageal luminal pH gradient closer to the lower esophageal sphincter.38
Others have found the different phenotypes randomly distributed throughout Barrett’s mucosa,39 but cardiac mucosa is present throughout the segment, with oxyntocardiac mucosa more frequently found distally,31 which was confirmed by Going et al.,29 who also found higher frequencies of cardiac and oxyntocardiac mucosa in the distal Barrett’s segment, with different mucosal phenotypes present as a mosaic that varied in proportion with anatomic site. The evidence strongly suggests that a spatial gradient of phenotypes exists, with specialized (goblet-containing) Barrett’s glands concentrated in the proximal segment.
The Barrett’s Gland in Time
An important question is whether individual glands lining Barrett’s esophagus evolve with time; or, once formed, whether they are phenotypically stable. There are perhaps two lines of thought that allow us to answer these questions. First, studies performed after operations such as esophagectomy with gastric pull-up have found that about 50% of such patients develop columnar epithelium in areas originally (that is, after surgery) covered with squamous mucosa; persuasively, the length of columnar mucosa increases with the period of observation.40, 41, 42, 43, 44, 45, 46, 47 Importantly, the phenotype of the columnar epithelium is initially the cardiac type—mucinous glands lacking parietal cells—which shares the immunophenotype of cardiac mucosa found in control patients who had not been subjected to the procedure: progression to intestinal metaplasia then occurs.43 BMP4 activation, early expression of CDX2, and later expression of CDX1 and MUC2 are seen progressively.48 Although in esophagectomy patients cardiac mucosa develops rapidly (within 1 to 2 years), intestinalization of this columnar epithelium only occurs after a further 3 to 5 years.43, 44, 45, 46, 49 However, in other studies, while sucrase-isomaltase expression appeared within 2 years, specialized intestinal metaplasia took 10 years to appear.49 Second, analysis of non-goblet cell Barrett’s mucosa shows evidence of intestinalization: up to 40% of cases show CDX2 and 17% villin expression,32, 50, 51 with a similar proportion showing sucrase-isomaltase and dipeptidyl peptidase IV expression.52 Moreover, non-goblet cell mucosa is commoner in younger patients.53
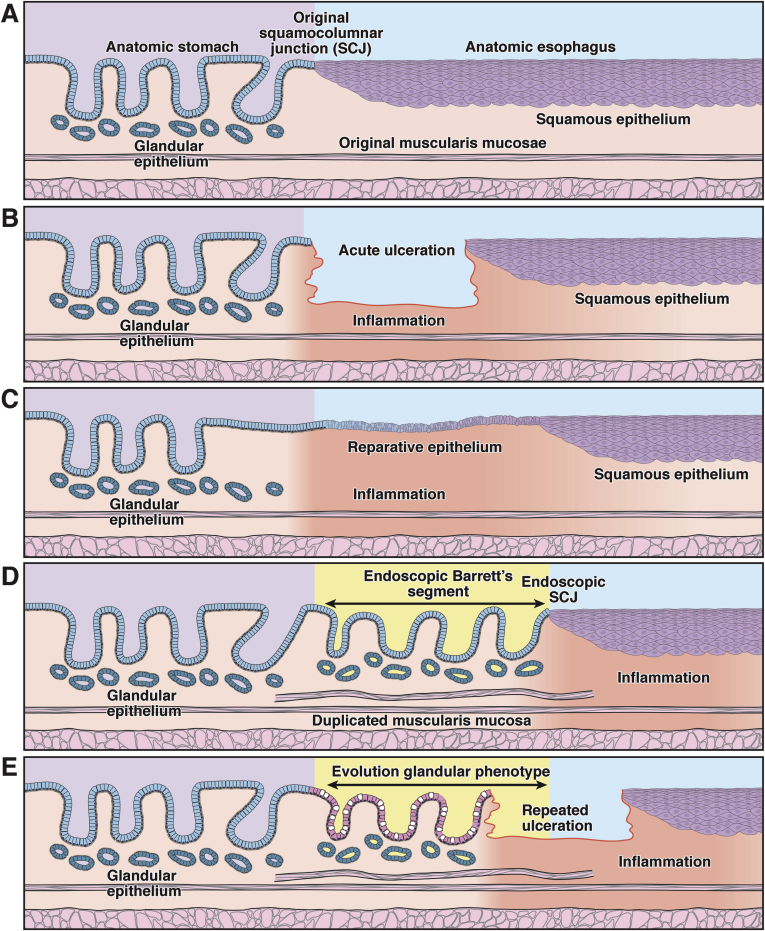
Thus, intestinalization appears to progress with time. It is natural to ask from where the antecedent columnar-lined mucosa without goblet cells derives. Much current thought regards the esophageal stratified squamous epithelium as the precursor,54 but a detailed examination of the Barrett’s lesion itself provides little or no evidence for this view, suggesting that gastric glands themselves are the origin of the columnar-lined mucosa without goblet cells.17 Even in the normal stomach gastric glands with a low number of parietal and peptic cells are seen near the gastroesophageal junction. If, as has been repeatedly claimed, this cardiac mucosa (which is, in effect, columnar epithelium without goblet cells31, 36, 37) is itself metaplastic, then it is conceptually simpler that expansion of Barrett’s follows microerosive trauma and begins with microscopic foci of cardiac mucosa at the gastroesophageal junction.55 With recurrent bouts of reflux and ulceration, the cardiac mucosa expands, replacing the distal esophageal squamous epithelium. This cyclic expansion model is depicted in Figure 2. This is in effect a normal wound-healing response, but characterized by secondary selection for phenotypes best adapted to the local microenvironment. Migration of the squamocolumnar junction proximally occurs because esophageal submucosal glands are seen beneath the cardiac mucosa.31 Patients with microscopic specialized metaplasia at the cardia have been seen to progress to macroscopically evident Barrett’s esophagus.54, 55
Figure 2.
The Barrett’s gland as a unit of selection. This diagram shows the evolution of the glandular Barrett’s phenotype over time in the context of recurrent erosive esophagitis. There are various time points within the evolution of a Barrett’s segment when selection is important: during the establishment of the segment, during its progression to a stable state, and, where it does occur, during the development of dysplasia and carcinoma. At each of these time points, different traits may be under selection. (A) At baseline, the endoscopic squamocolumnar junction coincides with the anatomic gastroesophageal junction. (B) After chronic gastroduodenal reflux, the squamous lining of the esophagus is repeatedly damaged and eventually ulcerates. (C) A breach in the epithelial lining is followed by a stereotypical wound-healing response stimulated by inflammation. In the acute, proliferative stage of wound healing, competition may be dominated by selection for secretory and proliferative traits favoring (mucosal) repair. (D) Repetitive injury leads to cephalad expansion of the glandular lining within the tubular esophagus. As can be seen in other regions of the intestinal tract, remodeling is accompanied by the deposition of a complete mucosal unit, including a newly derived muscularis mucosae. (E) After the initial proliferative and remodeling stage, this simple glandular phenotype may evolve to a complex, specialized glandular phenotype.
The evidence thus strongly suggests that Barrett’s glands show an evolutionary sequence: from columnar-lined mucosa without goblet cells, through glands showing intestinal gene expression, to specialized metaplastic glands.54, 56 (Figure 2, and Figure 4A and B). Solubility of caustic bile salts depends on luminal pH and is greatest at intermediate pH ranges seen most proximally.38 Solubilized duodenal bile salts are a strong inducer of CDX2 expression.57 Combining this with the increased density of goblet cells proximally (see the previous discussion), it is tempting to propose that goblet cell-containing (specialized) glands move proximally in both time and space and that Barrett’s glands therefore form a “marching front” adapted for competition with squamous cells.
Perhaps the erosive microenvironment proximally (characterized by intermediate pH values, but high solubility of caustic bile salts) selects for the specialized phenotype, while the non-goblet cell glands compete well with specialized glands distally, where the pH is more acidic, but bile salts are insoluble.38 Or are specialized glands clonally purified and on a trajectory to dominance over time? What little evidence we have favors the latter hypothesis.
Some evidence for a proximally migrating, marching front of columnar epithelium replacing eroded squamous epithelium can be gleaned from a recent report by the McKeon group.58 The investigators used p63null mice to demonstrate a proximal shift of the squamocolumnar junction. Their data elegantly demonstrate that stomach-derived columnar epithelium can expand and cover a denuded esophagus. However, the differences (anatomically, temporally, and mechanistically) between this transgenic model and the development of Barrett’s esophagus in patients are vast, and future studies will need to address whether the phenotype in these p63null mice can model the multitude of histologic forms that define Barrett’s metaplasia.
Mechanisms of Phenotypic Evolution—Neutral Drift in Metaplasia
We have seen that Barrett’s specialized glands contain multiple differentiated cell lineages, thus fulfilling the requirement for a true metaplasia, the transformation of one tissue type into another.59 This involves change in the potential of stem cells, which undergo transcriptional modification to deliver a whole series of lineages to give the several gland phenotypes we observe. If indeed this were a stem cell change, we would predict that such glands would be clonal populations. Direct visualization of clonal mutations in the mitochondrial DNA-encoded gene cytochrome c oxidase (CCO) shows that specialized Barrett’s glands are clonal60; similarly, the metaplastic crypts of gastric intestinal metaplasia are also clonal.61 What has happened here is that Barrett’s glands have undergone the process of monoclonal conversion, or replacement with a new clone, which incidentally carries a CCO-mutation so that all contained lineages also carry this clonal mark. Such CCO mutations are thought not to confer a fitness advantage,62 so this can be interpreted in terms of a CCO-mutant stem cell drifting to fixation within the stem cell niche.
Can such drift be the cause of the conversion of columnar-lined mucosa to specialized Barrett’s metaplasia? The evidence indicates that specialized Barrett’s glands were originally a pyloric-type gastric gland with TFF1+/MUC5AC+ and TFF2+/MUC6+ lineages. A stem cell expressing CDX2 will derive progeny initially expressing intestinal genes such as villin and sucrase-isomaltase, and a balanced homeostasis within the gland is achieved via competition between symmetrically dividing stem cell cells. Thus, drift governs stem cell dynamics,63, 64 a conclusion now substantiated for human epithelial stem cells.65 Stochastically, a CDX2+ stem cell may survive in the niche, and again may undergo niche succession. Cells that are positive for MUC2 and for TFF3 are not seen in non-goblet columnar glands; however, once a stem cell under the transcriptional control of CDX2 or CDX1 switches on MUC2/TFF3 expression, then by the same process of neutral competition these cells may survive and come to populate the niche, leading to the familiar specialized metaplastic gland. The progressive intestinalization seen in gastric glands with time suggests that such drift may be biased66 toward niche succession—that the metaplastic stem cells have a fitness advantage. Moreover, once phenotypic drift has led to niche succession and clonal conversion to a totally metaplastic gland, the process is likely irreversible,59 possibly associated with the expression of the homeobox gene CDX2 which activates intestinal differentiation67 in the stomach by Helicobacter pylori infection and in Barrett’s glands by bile/acid reflux.57
It is clear that while distinctively gastric (chief cell) and intestinal (Paneth cell) lineages are seen in Barrett’s glandular phenotypes, cells simultaneously expressing gastric and intestinal lineage markers are also present,17 possibly explained as the progeny of stem cells where CDX2 is partially activated, allowing mixed gastric and intestinal gene expression programs in the same cell (Figure 4B). We can thus propose that the evolutionary sequence leading to specialized metaplastic glands is non-goblet cell columnar gland to specialized metaplastic gland.32, 49, 52, 53 This switch may be associated by pSMAD/CDX2 interaction.56 Moreover, as we have seen previously, the distribution of gland phenotypes from Z-line to cardia in the squamo-oxyntic gap37 complies with this proposal. In this respect, we would argue that most of the evidence for a direct squamous epithelial origin of non-goblet cell columnar epithelium is derived from often flawed experimental models68 and not from the study of the lesion itself.55
The Life History of Barrett’s Glands
It is possible that columnar metaplasia develops gradually to involve more of the esophagus36, 55, 69 or rapidly develops to its full extent with little subsequent change.70 Certainly the mean length of columnar epithelium does not increase during prolonged follow-up observation for as long as 7.3 years.70 At diagnosis, columnar metaplasia appears to be a stable state with little evidence of natural progression or regression. However, it is known that structures such as human small intestinal and colonic crypts have a life history: in neonatal life, the early crypts increase their number by the process of crypt fission—a crypt divides to make two daughters.71 But this process continues throughout life—new crypts are continually generated, albeit at a slow rate. The crypt cycle in normal adults (the time between two fission events in a given crypt) has been variously estimated as 17 to 26 years,72 although it may be as long as 36 years.65 The question is, do Barrett’s glands have a similar life history? Are they born by a fission event from a parent? How long do they live, and are they lost from the population? These questions are rarely if ever asked, possibly because we do not know the answers! But what is clear is that gastric glands, which bear a considerable relationship at least to specialized metaplastic glands,17 do have such a life history.
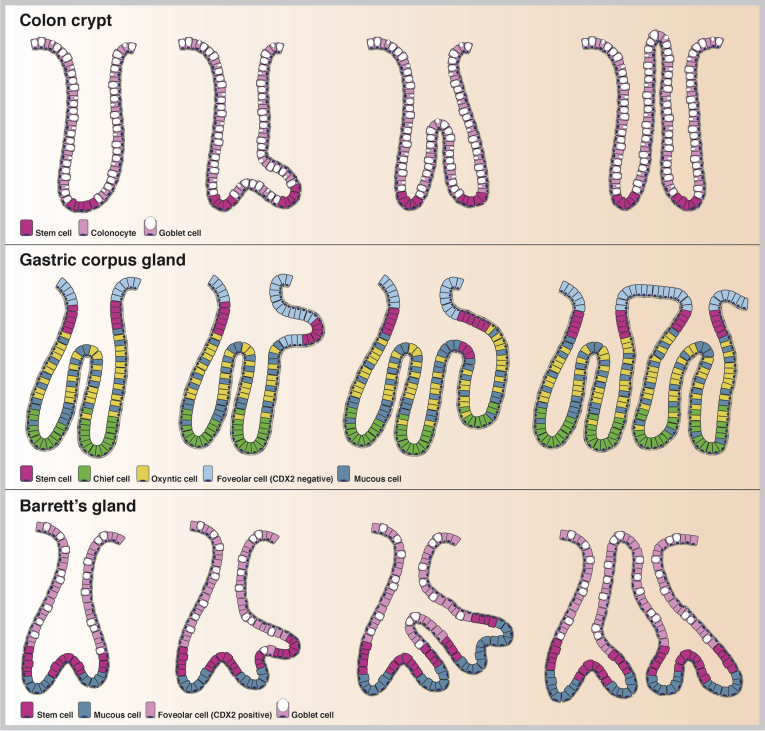
We know little or nothing about the mode of replication of gastric glands, only that it does occur. For example, we reported a patch of entirely CCO-mutated gastric glands (or gastric units) where direct sequencing showed all glands to contained a clonal mutation (G→A transition at position 2593 in the 16S rRNA [MTRNR2] gene) showing that a single, wholly mutated gastric gland has expanded by multiple fission events to form this clonal patch.73 How does this expansion take place? This has only really been studied in the gastric corpus of the hamster in a series of elegant articles by Hattori.74 The process begins with a doubling of the number of cells around the circumference of the neck region of the gland, and some of the proliferating cells appear to become attached to the opposite side of the lumen such that the tubule is asymmetrically divided into two. The smaller tubule then grows to form a new foveolus at the surface, followed by the formation of a bud at the level of the neck, by localized cell division, and by the new gland growing down toward the level of the muscularis mucosae, by which time the new tubule is completely separated by stromal ingrowth. Although the location of stem cells in the human corpus gastric gland is not known, current thought favors the isthmus/neck region.75 As stated, fission events appear to begin in this stem cell zone.74 Similarly, fission begins with a budding event in the base of the colonic crypt where similarly the crypt stem cells reside.76, 77 In the specialized metaplastic glands in Barrett’s esophagus, the LGR5 stem cell zone is found at the junction of the basal MUC6+/TFF2+ cells and the more superficial MUC5AC+/TFF1+ and MUC2+/TFF3+ cells.17 In analogy with the situation in the gastric corpus and colonic crypt where gland fission is initiated from the stem cell zone (at the neck zone and the base of the crypt, respectively), we might speculate that Barrett’s glands clonally expand by fission initiated from the isthmus region (Figure 3).
Figure 3.
Clonal expansion of glandular units in the gastrointestinal tract. Glandular units in the gastrointestinal tract expand as clonal patches through a fission process. Crypts in the normal colon fission on average about every 30 years, forming a small clonal patch.65 Fission is initiated from the stem cell zone at the crypt base (in magenta), after which the crypt unzips toward the luminal surface (top panel). Glands in the stomach fission by forming a bud from the stem cell zone at the level of the neck (in magenta) through localized cell division. The new gland grows down toward the level of the muscularis mucosae (middle panel). We propose that Barrett’s glands form clonal patches in a similar manner by forming a glandular bud at the level of the stem cell zone (again in magenta), which is located just above the MUC6/TFF2+ Barrett’s gland base. This bud extends and unzips toward the luminal surface (bottom panel). Constituent cell types of each glandular unit are indicated per panel.
But what evidence is there that, in Barrett’s segments, fission events occur? Until recently, it was thought that mutations spread through Barrett’s segments in a series of multiple selective sweeps by new, selectively advantageous genotypes that swept to fixation in the whole segment,3 so it was natural to propose that gland fission was the mechanism.78 In fact, one recent study found only one such case of a clone that grew to stably dominate the Barrett’s segment.79 However, early mutations at fragile genomic sites and in genes such as p16 are found throughout the Barrett’s segment,79, 80 and thus, possibly during the establishment of the segment, gland fission is important in the spread of such mutations. However, it is clear that smaller clonal expansions occur in Barrett’s mucosa,2, 80 and thus, as has recently been shown to be the case in the colon, gland fission is the mechanism driving the expansion of clones.
A final question must be, is the Barrett’s segment clonal? This question takes us right back to the beginning of the evolution of the Barrett’s segment: depending on our view of the derivation of Barrett’s mucosa, does it arise from a single (clonal) gland in the cardia, a single squamous epithelial stem cell, or even a single esophageal gland? This is an intriguing concept for which there is little evidence either way. Many Barrett’s segments show early sometimes clonal genetic events,79 which have been interpreted as indications of damage rather than a clonal origin. If the segment is clonal, it implies that a gland with some ancestral phenotype (near-gastric in phenotype?) clonally expanded and filled the segment, then the conversion to other gland types occurred. These conversions either occurred multiple times, so that the metaplastic glands represent multiple distinct clones, or once, and the glands represent a clonal sweep. We propose that phylogenetic analysis coupled with phenotyping would be the way to approach this question and, of course, that evolution between phenotypes would be revealed by this approach, regardless of the underlying clonality of the Barrett’s segment. Although it would be fascinating, an experimental approach may not be feasible at this time.
We have discussed the scope of Barrett’s glandular phenotypes and made the point that they have a distribution in space and time. These metaplastic glands also clonally expand. If, as we propose, the cause of these phenomena is clonal expansion by fission (Figure 3), then it follows that the unit of selection is the gland itself.
The Barrett’s Gland as a Unit of Selection
There are various time points within the evolution of a Barrett’s segment when selection is important: during the establishment of the segment, during its progression to a stable state, and, where it does occur, during the development of dysplasia and carcinoma. The available evidence strongly suggests that the phenotype that is initially selected after erosion is the non-goblet cell columnar gland, which initially forms the cardiac-type mucosa (Figure 4A).36, 49, 53 This is followed by the development of intestinalization32, 52 and the selection of specialized metaplastic glands containing goblet cells. The foveolar cells in these glands coexpress gastric mucin core proteins (MUC5AC) and intestinal marker genes (CDX2), still pointing to this transition (Figure 4B). Importantly, at every point during this sequence the unit of selection is the gland itself, which adapts to a range of locally prevailing selection pressures (Figure 4C). This selection takes place in time and space, taking several years to complete; although all phenotypes are generally seen at all levels in the segment, specialized metaplastic glands are concentrated in the proximal part of the segment.29 Finally, mutations occur within Barrett’s glands, leading to the selection of the dysplastic phenotype, which if low-grade may remain stable for many years.13, 81 Even high-grade dysplasia can remain histologically stable for up to 20 months,82 although recent studies have shown that sudden, punctuated evolutionary events lead rapid progression to cancer within a period as short as 4 years,2 implying that very strong selection can occur for an optimal phenotype. However, the adaptations that this strongly selected phenotype has developed are unclear—perhaps they are just fast replicators? Further research into the functional properties of the dysplastic gland phenotype may clarify this issue.
We might therefore ask, why is such a broad array of metaplastic gland phenotypes observed in Barrett’s esophagus to begin with? What explains its protean manifestation? The concept of selection among different gland phenotypes is easiest to understand within the context of malignant progression, where clonal mutations within growth-promoting genes would be expected to increase the fitness of mutant glands. These dysplastic glands have migrated up a fitness landscape to reach an area of higher fitness. This fitness landscape is much more difficult to conceptualize for metaplastic Barrett’s esophagus glands. In the first place, although we know their distribution in general terms, we have no hard data on the scale of such variations in phenotype in the stable Barrett’s segment. For example, we are not informed whether metaplastic patches vary in size along the Barrett’s segment. Nonetheless, gland-to-gland changes in metaplastic phenotype can be seen in Barrett’s esophagus (Figure 4B). On an H&E level, the clonal transition from a non-goblet columnar phenotype to a specialized Barrett’s gland phenotype (compare phenotypes ii and i in Figure 4A, respectively) is limited to the acquisition of a few goblet cells. As a result, clonal transitions between neighboring glands do not stand out as clearly as, for example, intestinal metaplasia in chronically inflamed corpus mucosa or pseudopyloric glands in Crohn’s ileitis. Nonetheless, a topographic gland-by-gland comparison of the heterogeneity of metaplastic phenotypes along the Barrett’s segment (within and between patients) could reinforce the concept that Barrett’s esophagus is best viewed as a patchwork of related, but phenotypically distinct, clonal units.
Following the above hypothesis that the early Barrett’s segment is occupied by non-goblet cell Barrett’s, we might propose that there is strong selection in the toxic gastroduodenal reflux environment of Barrett’s esophagus initially for this phenotype.1 After some time, possibly years, the specialized metaplastic glands appear; however, these do not replace the non-goblet cell columnar glands, which remain as ancestral clones at all levels in the segment while the specialized glands set up their gradient within the segment. Because the specialized metaplastic glands rarely if ever monopolize the segment, selection for them over the non-goblet cell Barrett’s glands is relatively weak. Clearly, we lack an exact quantitative formulation of the rate of clonal expansion of metaplastic clones in the esophagus. Future studies should address this gap in our understanding. Only through detailed integration of genotype and phenotype can we resolve this matter and compare the rate of clonal expansion of the various metaplastic phenotypes (Figure 4C).
It remains uncertain whether the traits under selection in the different gland types are the ability to survive acid/peptic digestion or to reproduce by fission. Certainly Barrett’s mucosa actively secretes anions and HCO3−, which buffers acid,83 elaborates a thick mucus coat which protects against bile and acid,84 and expresses a series of claudins that resists acid permeation.85 Additionally, genes that control mucosal repair are up-regulated in Barrett’s mucosa.14, 15, 16 Indeed, there may be multiple other properties of Barrett’s glands that we have failed to identify but nevertheless all contribute to fitness. Fitness can thus be viewed as an aggregate parameter that relates to the all-around performance of a specific gland (or gland phenotype) relative to the other glands in the segment. Naturally, the best phenotype for one function is usually not the best for others—a so-called trade-off,86, 87 as seen in nature with fertility versus offspring survival, or performance measures such as speed versus endurance, or growth versus shell robustness in snails. Barrett’s esophagus can similarly be understood as one ecologic system where the observed phenotype represents an optimal trade-off between performances on various tasks (Figure 4D). An optimal phenotype with maximum fitness will occupy a point in phenotype space although here, as in other cases,13 we do not know the nature of the fitness, and of course Barrett’s epithelium performs multiple functions, or tasks, that contribute to its fitness. It thus becomes important to understand how such trade-offs affect the phenotypes we find; Pareto optimality can be used to understand such trade-offs that decide the range of phenotypes (Figure 4E).13, 86
We need much more detailed quantitative information about the distribution of gland phenotypes, and particularly how the distribution of gland phenotypes evolve over time within a Barrett’s segment, before developing these arguments further. Of note, patients treated with proton pump inhibitors versus those without perhaps offer a natural “experimental system” whereby the effects of reflux on gland evolution can be studied in vivo.
The Barrett’s Gland Phenotype and Selection for Dysplasia/Malignancy
There are few more contentious topics: what is the phenotype of the Barrett’s gland that is selected for progression to malignancy? Here, thought is sharply divided, with one school firmly believing that without intestinal metaplasia (ie, the presence of goblet cells) there is no proven risk of neoplastic progression,88 while the other avers that columnar non-goblet epithelium is fully capable of progression89, 90 and that there are thus multiple pathways of carcinogenesis in Barrett’s esophagus.91 If we accept the former case, we should of course ask why intestinal metaplasia progresses and the non-goblet cell phenotype does not. Goblet cells are usually regarded as terminally differentiated cells and as such are not strong candidates for a cell of origin; indeed, it has been suggested that the induction of goblet cell differentiation in Barrett’s mucosa might be an effective therapeutic strategy.92 Could it be that goblet cells are merely a marker for malignant predisposition?8 Goblet cells are effectively the most extreme marker of intestinalization, so could it also be that the more extreme the expression of the intestinal phenotype the more likely is selection for neoplastic progression, possibly due to an intrinsically higher mutation rate or because the intragland clonal dynamics are different, with fewer stem cells?
Could those cases of non-goblet cell phenotype that progress be the fraction of the non-goblet cell phenotype that also shows evidence of intestinal differentiation,9, 32 expressing markers such as MUC2, DAS-1, villin, and CDX2? It is possible, although carcinomas of complete gastric immunophenotype have been associated with complete gastric (foveolar) dysplasia and the gastric (non-goblet cell) metaplastic immunophenotype.91 The metaplastic non-goblet cell phenotype can be associated with chromosomal instability90 and DNA abnormalities,93 but, then again, possibly not with the same high frequency as metaplasia with goblet cells.6 Naturally, this discussion is predicated on the goblet cell lineage being terminally differentiated: there is evidence that goblet cell populations in gastric intestinal metaplasia can retain proliferative capacity,94 in which case this assumption may need revisiting.
However, from the evolutionary standpoint, specialized epithelium has on average gone through at least one additional bottleneck event compared to nonspecialized epithelium. Because the number of bottleneck events may relate to cancer risk, and every clonal patch of specialized epithelium represents one separate ecologic bottleneck event, this may explain the relatively increased risk of cancer in stretches of goblet-containing specialized epithelium. Thus, while nonspecialized epithelium is not risk free, if indeed the gland is the unit of selection and there is directional evolution, then specialized epithelium may be placed one bottleneck farther down the road to progression. Perhaps a reasonable standpoint would be that any Barrett’s metaplasia can be selected for malignant progression but that the selection is biased toward the intestinal phenotype. Again, quantitative information on the life histories of glandular phenotypes would be a first step toward resolving this issue.
The Barrett’s Gland in Regression
The fact that Barrett’s mucosa in regression after acid-inhibition or radiofrequency ablation apparently reverts directly to a stratified squamous phenotype without any intermediary has elicited little comment. However, it is clearly not the Barrett’s mucosa itself that differentiates back to squamous epithelium,95 but rather the squamous epithelium emanates from an alternative clone source from within esophageal gland ducts.96 The evolutionary mechanics surrounding this change are obscure—why the squamous progeny of stem cells located in gland ducts should have a fitness advantage over cardiac or Barrett’s mucosa is as yet unknown. One recent study in fact indicates that after complete endoluminal eradication of Barrett’s mucosa and a documented return to squamous epithelium, nearly half of all patients show a return of Barrett’s mucosa, in some cases with high-grade lesions.97 Clearly this is an area for future research, in particular in relation to the initiation and progression of Barrett’s in a high-risk cohort.
Conclusions
Increasingly, we look at genomics for the answers we need concerning the evolution of Barrett’s esophagus, often to the total exclusion of phenotype considerations.2 However, it is clear from this discussion that such an approach is likely to be ultimately incomplete, and that only by the integration of Barrett’s glands in genotype and phenotype space can we expect to answer the many unanswered questions that surround this enigmatic lesion.
Table 1.
Spectrum of Gland Phenotypes in Barrett's Esophagus
| Gland Phenotype | Lineage Differentiation | Marker | References |
|---|---|---|---|
| Fundic-type gland | Foveolar cell | MUC5AC | Vieth and Montgomery 201498 |
| Parietal cell | H+/K+-ATPase | Choi et al 201433 | |
| Chief cell | MIST1 | Lennerz et al 201099 | |
| Oxyntocardiac gland | Foveolar cell | MUC5AC | Vieth and Montgomery 201498 |
| Parietal cell | H+/K+-ATPase | Choi et al 201433 | |
| Pyloric gland base | MUC6 | Choi et al 201433 | |
| Non-goblet columnar gland | Foveolar cell | MUC5AC | Lavery et al 201417 |
| Pyloric gland base | MUC6 | Lavery et al 201417 | |
| Barrett’s gland | Foveolar cell | MUC5AC | Lavery et al 201417 |
| Goblet cell | MUC2 | Lavery et al 201417 | |
| Barrett’s gland base | MUC6 | Lavery et al 201417 | |
| Barrett’s gland with Paneth cells | Foveolar cell | MUC5AC | Lavery et al 201417 |
| Goblet cell | MUC2 | Lavery et al 201417 | |
| Paneth cell | CD24 | Lennerz et al 201099 |
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding Funded by Cancer Research UK (to NAW), the Dutch Cancer Society (to MJ), and by CORE, the MRC, and Barts Charity (to SACM). The funders had no influence on the design or practice of this study.
References
- 1.Reid B.J., Kostadinov R., Maley C.C. New strategies in Barrett’s esophagus: integrating clonal evolutionary theory with clinical management. Clin Cancer Res. 2011;17:3512–3519. doi: 10.1158/1078-0432.CCR-09-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li X., Blount P.L., Reid B.J. Quantification of population benefit in evaluation of biomarkers: practical implications for disease detection and prevention. BMC Med Inform Decis Mak. 2014;14:15. doi: 10.1186/1472-6947-14-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maley C.C., Galipeau P.C., Li X. Selectively advantageous mutations and hitchhikers in neoplasms: p16 lesions are selected in Barrett’s esophagus. Cancer Res. 2004;64:3414–3427. doi: 10.1158/0008-5472.CAN-03-3249. [DOI] [PubMed] [Google Scholar]
- 4.Kostadinov R.L., Kuhner M.K., Li X. NSAIDs modulate clonal evolution in Barrett’s esophagus. PLoS Genet. 2013;9:e1003553. doi: 10.1371/journal.pgen.1003553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bansal A., McGregor D.H., Anand O. Presence or absence of intestinal metaplasia but not its burden is associated with prevalent high-grade dysplasia and cancer in Barrett’s esophagus. Dis Esophagus. 2014;27:751–756. doi: 10.1111/dote.12151. [DOI] [PubMed] [Google Scholar]
- 6.Bandla S., Peters J.H., Ruff D. Comparison of cancer-associated genetic abnormalities in columnar-lined esophagus tissues with and without goblet cells. Ann Surg. 2014;260:72–80. doi: 10.1097/SLA.0000000000000424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Westerhoff M., Hovan L., Lee C. Effects of dropping the requirement for goblet cells from the diagnosis of Barrett’s esophagus. Clin Gastroenterol Hepatol. 2012;10:1232–1236. doi: 10.1016/j.cgh.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Spechler S.J. Barrett’s esophagus: is the goblet half empty? Clin Gastroenterol Hepatol. 2012;10:1237–1238. doi: 10.1016/j.cgh.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riddell R., Odze R. Definition of Barrett’s esophagus: time for a rethink—is intestinal metaplasia dead? Am J Gastroenterol. 2009;104:2588–2594. doi: 10.1038/ajg.2009.390. [DOI] [PubMed] [Google Scholar]
- 10.Fitzgerald R.C., di Pietro M., Ragunath K. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett’s oesophagus. Gut. 2014;63:7–42. doi: 10.1136/gutjnl-2013-305372. [DOI] [PubMed] [Google Scholar]
- 11.Weinstein W.M., Ippoliti A.F. The diagnosis of Barrett’s esophagus: goblets, goblets, goblets. Gastrointest Endosc. 1996;44:91–95. doi: 10.1016/s0016-5107(96)70239-0. [DOI] [PubMed] [Google Scholar]
- 12.Sampliner R.E. Practice guidelines on the diagnosis, surveillance, and therapy of Barrett’s esophagus. Practice Parameters Committee of the American College of Gastroenterology. Am J Gastroenterol. 1998;93:1028–1032. doi: 10.1111/j.1572-0241.1998.00362.x. [DOI] [PubMed] [Google Scholar]
- 13.Shoval O., Sheftel H., Shinar G. Evolutionary trade-offs, Pareto optimality, and the geometry of phenotype space. Science. 2012;336:1157–1160. doi: 10.1126/science.1217405. [DOI] [PubMed] [Google Scholar]
- 14.Ostrowski J., Mikula M., Karczmarski J. Molecular defense mechanisms of Barrett’s metaplasia estimated by an integrative genomics. J Mol Med (Berl) 2007;85:733–743. doi: 10.1007/s00109-007-0176-3. [DOI] [PubMed] [Google Scholar]
- 15.Nancarrow D.J., Clouston A.D., Smithers B.M. Whole genome expression array profiling highlights differences in mucosal defense genes in Barrett’s esophagus and esophageal adenocarcinoma. PLoS One. 2011;6:e22513. doi: 10.1371/journal.pone.0022513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orlando R.C. The integrity of the esophageal mucosa: balance between offensive and defensive mechanisms. Best Pract Res Clin Gastroenterol. 2010;24:873–882. doi: 10.1016/j.bpg.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lavery D.L., Nicholson A.M., Poulsom R. The stem cell organisation, and the proliferative and gene expression profile of Barrett’s epithelium, replicates pyloric-type gastric glands. Gut. 2014;63:1854–1863. doi: 10.1136/gutjnl-2013-306508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee R.G. Mucins in Barrett’s esophagus: a histochemical study. Am J Clin Pathol. 1984;81:500–503. doi: 10.1093/ajcp/81.4.500. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y.Y., Wang H.H., Antonioli D.A. Significance of acid-mucin-positive nongoblet columnar cells in the distal esophagus and gastroesophageal junction. Hum Pathol. 1999;30:1488–1495. doi: 10.1016/s0046-8177(99)90172-7. [DOI] [PubMed] [Google Scholar]
- 20.Jauregui H.O., Davessar K., Hale J.H. Mucin histochemistry of intestinal metaplasia in Barrett’s esophagus. Mod Pathol. 1988;1:188–192. [PubMed] [Google Scholar]
- 21.Lapertosa G., Baracchini P., Fulcheri E. Mucin histochemical analysis in the interpretation of Barrett’s esophagus: results of a multicenter study. Operative Group for the Study of Esophageal Precancer. Am J Clin Pathol. 1992;98:61–66. doi: 10.1093/ajcp/98.1.61. [DOI] [PubMed] [Google Scholar]
- 22.Glickman J.N., Blount P.L., Sanchez C.A. Mucin core polypeptide expression in the progression of neoplasia in Barrett’s esophagus. Hum Pathol. 2006;37:1304–1315. doi: 10.1016/j.humpath.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 23.Van De Bovenkamp J.H.B., Korteland-Van Male A.M., Warson C. Gastric-type mucin and TFF-peptide expression in Barrett’s oesophagus is disturbed during increased expression of MUC2. Histopathology. 2003;42:555–565. doi: 10.1046/j.1365-2559.2003.01619.x. [DOI] [PubMed] [Google Scholar]
- 24.Barker N., Huch M., Kujala P. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6:25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 25.Schepers A.G., Snippert H.J., Stange D.E. Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science. 2012;337:730–735. doi: 10.1126/science.1224676. [DOI] [PubMed] [Google Scholar]
- 26.Merlos-Suarez A., Barriga F.M., Jung P. The intestinal stem cell signature identifies colorectal cancer stem cells and predicts disease relapse. Cell Stem Cell. 2011;8:511–524. doi: 10.1016/j.stem.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 27.Montgomery E. What can be expected from Ki-67 nuclear antigen expression in the follow-up of patients with dysplasia? In: Siewert J.R., Couturier D., Scarpignato C., editors. Barrett’s esophagus. OESO; Paris: 2003. http://www.hon.ch/OESO/books/Vol_6_Barrett_s_Esophagus/Articles/vol1/art133.html [Google Scholar]
- 28.Moyes L.H., McEwan H., Radulescu S. Activation of Wnt signalling promotes development of dysplasia in Barrett’s oesophagus. J Pathol. 2012;228:99–112. doi: 10.1002/path.4058. [DOI] [PubMed] [Google Scholar]
- 29.Going J.J., Fletcher-Monaghan A.J., Neilson L. Zoning of mucosal phenotype, dysplasia, and telomerase activity measured by telomerase repeat assay protocol in Barrett’s esophagus. Neoplasia. 2004;6:85–92. [PMC free article] [PubMed] [Google Scholar]
- 30.Paull A., Trier J.S., Dalton M.D. The histologic spectrum of Barrett’s esophagus. N Engl J Med. 1976;295:476–480. doi: 10.1056/NEJM197608262950904. [DOI] [PubMed] [Google Scholar]
- 31.Chandrasoma P.T., Lokuhetty D.M., Demeester T.R. Definition of histopathologic changes in gastroesophageal reflux disease. Am J Surg Pathol. 2000;24:344–351. doi: 10.1097/00000478-200003000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Hahn H.P., Blount P.L., Ayub K. Intestinal differentiation in metaplastic, nongoblet columnar epithelium in the esophagus. Am J Surg Pathol. 2009;33:1006–1015. doi: 10.1097/PAS.0b013e31819f57e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi E., Roland J.T., Barlow B.J. Cell lineage distribution atlas of the human stomach reveals heterogeneous gland populations in the gastric antrum. Gut. 2014;63:1711–1720. doi: 10.1136/gutjnl-2013-305964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Voutilainen M., Farkkila M., Juhola M. Complete and incomplete intestinal metaplasia at the oesophagogastric junction: prevalences and associations with endoscopic erosive oesophagitis and gastritis. Gut. 1999;45:644–648. doi: 10.1136/gut.45.5.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gottfried M.R., McClave S.A., Boyce H.W. Incomplete intestinal metaplasia in the diagnosis of columnar lined esophagus (Barrett’s esophagus) Am J Clin Pathol. 1989;92:741–746. doi: 10.1093/ajcp/92.6.741. [DOI] [PubMed] [Google Scholar]
- 36.Chandrasoma P., Wijetunge S., Demeester S.R. The histologic squamo-oxyntic gap: an accurate and reproducible diagnostic marker of gastroesophageal reflux disease. Am J Surg Pathol. 2010;34:1574–1581. doi: 10.1097/PAS.0b013e3181f06990. [DOI] [PubMed] [Google Scholar]
- 37.Chandrasoma P., Wijetunge S., Ma Y. The dilated distal esophagus: a new entity that is the pathologic basis of early gastroesophageal reflux disease. Am J Surg Pathol. 2011;35:1873–1881. doi: 10.1097/PAS.0b013e31822b78e8. [DOI] [PubMed] [Google Scholar]
- 38.Theodorou D., Ayazi S., DeMeester S.R. Intraluminal pH and goblet cell density in Barrett’s esophagus. J Gastrointest Surg. 2012;16:469–474. doi: 10.1007/s11605-011-1776-3. [DOI] [PubMed] [Google Scholar]
- 39.Thompson J.J., Zinsser K.R., Enterline H.T. Barrett’s metaplasia and adenocarcinoma of the esophagus and gastroesophageal junction. Hum Pathol. 1983;14:42–61. doi: 10.1016/s0046-8177(83)80045-8. [DOI] [PubMed] [Google Scholar]
- 40.Dunn LJ, Shenfine J, Griffin SM. Columnar metaplasia in the esophageal remnant after esophagectomy: a systematic review. Dis Esophagus. Published online November 13, 2013, DOI: 10.1111/dote.12129. [DOI] [PubMed]
- 41.Meyer W., Vollmar F., Bär W. Barrett-esophagus following total gastrectomy. A contribution to it’s [sic] pathogenesis. Endoscopy. 1979;11:121–126. doi: 10.1055/s-0028-1098335. [DOI] [PubMed] [Google Scholar]
- 42.Oberg S., Johansson J., Wenner J. Metaplastic columnar mucosa in the cervical esophagus after esophagectomy. Ann Surg. 2002;235:338–345. doi: 10.1097/00000658-200203000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lord R.V.N., Wickramasinghe K., Johansson J.J. Cardiac mucosa in the remnant esophagus after esophagectomy is an acquired epithelium with Barrett’s-like features. Surgery. 2004;136:633–640. doi: 10.1016/j.surg.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 44.Dresner S.M., Griffin S.M., Wayman J. Human model of duodenogastro-oesophageal reflux in the development of Barrett’s metaplasia. Br J Surg. 2003;90:1120–1128. doi: 10.1002/bjs.4169. [DOI] [PubMed] [Google Scholar]
- 45.Lindahl H., Rintala R., Sariola H. Cervical Barrett’s esophagus: a common complication of gastric tube reconstruction. J Pediatr Surg. 1990;25:446–448. doi: 10.1016/0022-3468(90)90391-l. [DOI] [PubMed] [Google Scholar]
- 46.O’Riordan J.M., Tucker O.N., Byrne P.J. Factors influencing the development of Barrett’s epithelium in the esophageal remnant postesophagectomy. Am J Gastroenterol. 2004;99:205–211. doi: 10.1111/j.1572-0241.2004.04057.x. [DOI] [PubMed] [Google Scholar]
- 47.Hamilton S.R., Yardley J.H. Regnerative of cardiac type mucosa and acquisition of Barrett mucosa after esophagogastrostomy. Gastroenterology. 1977;72:669–675. [PubMed] [Google Scholar]
- 48.Castillo D., Puig S., Iglesias M. Activation of the BMP4 pathway and early expression of CDX2 characterize non-specialized columnar metaplasia in a human model of Barrett’s esophagus. J Gastrointest Surg. 2012;16:227–237. doi: 10.1007/s11605-011-1758-5. [DOI] [PubMed] [Google Scholar]
- 49.Chaves P., Pereira A.D., Cruz C. Recurrent columnar-lined esophageal segments–study of the phenotypic characteristics using intestinal markers. Dis Esophagus. 2002;15:282–286. doi: 10.1046/j.1442-2050.2002.00264.x. [DOI] [PubMed] [Google Scholar]
- 50.Groisman G.M., Amar M., Meir A. Expression of the intestinal marker Cdx2 in the columnar-lined esophagus with and without intestinal (Barrett’s) metaplasia. Mod Pathol. 2004;17:1282–1288. doi: 10.1038/modpathol.3800182. [DOI] [PubMed] [Google Scholar]
- 51.Phillips R.W., Frierson H.F., Jr., Moskaluk C.A. Cdx2 as a marker of epithelial intestinal differentiation in the esophagus. Am J Surg Pathol. 2003;27:1442–1447. doi: 10.1097/00000478-200311000-00006. [DOI] [PubMed] [Google Scholar]
- 52.Chaves P., Cardoso P., de Almeida J.C. Non-goblet cell population of Barrett’s esophagus: an immunohistochemical demonstration of intestinal differentiation. Hum Pathol. 1999;30:1291–1295. doi: 10.1016/s0046-8177(99)90058-8. [DOI] [PubMed] [Google Scholar]
- 53.Dias Pereira A., Chaves P. Columnar-lined oesophagus without intestinal metaplasia: results from a cohort with a mean follow-up of 7 years. Aliment Pharmacol Ther. 2012;36:282–289. doi: 10.1111/j.1365-2036.2012.05170.x. [DOI] [PubMed] [Google Scholar]
- 54.Parekh D., Clark G.W.B., DeMeester T.R. Are all different types of Barrett’s epithelium originally present, or do they appear only after some period of evolution? In: Giuli R., Tytgat G.N.J., DeMeester T.R., Galmiche J.-P., editors. The esophageal mucosa. OESO; Paris: 1994. http://www.hon.ch/OESO/books/Vol_3_Eso_Mucosa/Articles/ART213.HTML [Google Scholar]
- 55.Leodolter A., Nocon M., Vieth M. Progression of specialized intestinal metaplasia at the cardia to macroscopically evident Barrett’s esophagus: an entity of concern in the ProGERD study. Scand J Gastroenterol. 2012;47:1429–1435. doi: 10.3109/00365521.2012.733952. [DOI] [PubMed] [Google Scholar]
- 56.Mari L., Milano F., Parikh K. A pSMAD/CDX2 complex is essential for the intestinalization of epithelial metaplasia. Cell Rep. 2014;7:1197–1210. doi: 10.1016/j.celrep.2014.03.074. [DOI] [PubMed] [Google Scholar]
- 57.Souza R.F., Krishnan K., Spechler S.J. Acid, bile, and CDX: the ABCs of making Barrett’s metaplasia. Am J Physiol Gastrointest Liver Physiol. 2008;295:211–218. doi: 10.1152/ajpgi.90250.2008. [DOI] [PubMed] [Google Scholar]
- 58.Wang X., Ouyang H., Yamamoto Y. Residual embryonic cells as precursors of a Barrett’s-like metaplasia. Cell. 2011;145:1023–1035. doi: 10.1016/j.cell.2011.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Slack J.M.W. Metaplasia and transdifferentiation: from pure biology to the clinic. Nat Rev Mol Cell Biol. 2007;8:369–378. doi: 10.1038/nrm2146. [DOI] [PubMed] [Google Scholar]
- 60.Nicholson A.M., Graham T.A., Simpson A. Barrett’s metaplasia glands are clonal, contain multiple stem cells and share a common squamous progenitor. Gut. 2012;61:1380–1389. doi: 10.1136/gutjnl-2011-301174. [DOI] [PubMed] [Google Scholar]
- 61.Gutierrez-Gonzalez L., Graham T.A., Rodriguez-Justo M. The clonal origins of dysplasia from intestinal metaplasia in the human stomach. Gastroenterology. 2011;140:1251–1260. doi: 10.1053/j.gastro.2010.12.051. e1–6. [DOI] [PubMed] [Google Scholar]
- 62.Nooteboom M., Johnson R., Taylor R.W. Age-associated mitochondrial DNA mutations lead to small but significant changes in cell proliferation and apoptosis in human colonic crypts. Aging Cell. 2010;9:96–99. doi: 10.1111/j.1474-9726.2009.00531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leushacke M., Ng A., Galle J. Lgr5(+) gastric stem cells divide symmetrically to effect epithelial homeostasis in the pylorus. Cell Rep. 2013;5:349–356. doi: 10.1016/j.celrep.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 64.Snippert H.J., van der Flier L.G., Sato T. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell. 2010;143:134–144. doi: 10.1016/j.cell.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 65.Baker A., Cereser B., Melton S. Quantification of crypt and stem cell evolution in the normal and neoplastic human colon. Cell Rep. 2014;8:940–947. doi: 10.1016/j.celrep.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Snippert H.J., Schepers A.G., van Es J.H. Biased competition between Lgr5 intestinal stem cells driven by oncogenic mutation induces clonal expansion. EMBO Rep. 2014;15:62–69. doi: 10.1002/embr.201337799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guo R.-J., Suh E.R., Lynch J.P. The role of Cdx proteins in intestinal development and cancer. Cancer Biol Ther. 2004;3:593–601. doi: 10.4161/cbt.3.7.913. [DOI] [PubMed] [Google Scholar]
- 68.Garman K.S., Orlando R.C., Chen X. Review: Experimental models for Barrett’s esophagus and esophageal adenocarcinoma. Am J Physiol Gastrointest Liver Physiol. 2012;302:1231–1243. doi: 10.1152/ajpgi.00509.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nandurkar S., Talley N.J. Barrett’s esophagus: the long and the short of it. Am J Gastroenterol. 1999;94:30–40. doi: 10.1111/j.1572-0241.1999.00768.x. [DOI] [PubMed] [Google Scholar]
- 70.Cameron A.J., Lomboy C.T. Barrett’s esophagus: age, prevalence, and extent of columnar epithelium. Gastroenterology. 1992;103:1241–1245. doi: 10.1016/0016-5085(92)91510-b. [DOI] [PubMed] [Google Scholar]
- 71.Cummins A.G., Catto-Smith A.G., Cameron D.J. Crypt fission peaks early during infancy and crypt hyperplasia broadly peaks during infancy and childhood in the small intestine of humans. J Pediatr Gastroenterol Nutr. 2008;47:153–157. doi: 10.1097/MPG.0b013e3181604d27. [DOI] [PubMed] [Google Scholar]
- 72.Graham T.A., Humphries A., Sanders T. Use of methylation patterns to determine expansion of stem cell clones in human colon tissue. Gastroenterology. 2011;140:1241–1250. doi: 10.1053/j.gastro.2010.12.036. e1–9. [DOI] [PubMed] [Google Scholar]
- 73.McDonald S.A., Greaves L.C., Gutierrez-Gonzalez L. Mechanisms of field cancerization in the human stomach: the expansion and spread of mutated gastric stem cells. Gastroenterology. 2008;134:500–510. doi: 10.1053/j.gastro.2007.11.035. [DOI] [PubMed] [Google Scholar]
- 74.Hattori T. On cell proliferation and differentiation of the fundic mucosa of the golden hamster. Fractographic study combined with microscopy and 3H-thymidine autoradiography. Cell Tissue Res. 1974;148:213–226. doi: 10.1007/BF00224583. [DOI] [PubMed] [Google Scholar]
- 75.Karam S.M., Straiton T., Hassan W.M. Defining epithelial cell progenitors in the human oxyntic mucosa. Stem Cells. 2003;21:322–336. doi: 10.1634/stemcells.21-3-322. [DOI] [PubMed] [Google Scholar]
- 76.Maskens A.P. Histogenesis of adenomatous polyps in the human large intestine. Gastroenterology. 1979;77:1245–1251. [PubMed] [Google Scholar]
- 77.Greaves L.C., Preston S.L., Tadrous P.J. Mitochondrial DNA mutations are established in human colonic stem cells, and mutated clones expand by crypt fission. Proc Natl Acad Sci USA. 2006;103:714–719. doi: 10.1073/pnas.0505903103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Garcia S.B., Park H.S., Novelli M. Field cancerization, clonality, and epithelial stem cells: the spread of mutated clones in epithelial sheets. J Pathol. 1999;187:61–81. doi: 10.1002/(SICI)1096-9896(199901)187:1<61::AID-PATH247>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 79.Lai L.A., Kostadinov R., Barrett M.T. Deletion at fragile sites is a common and early event in Barrett’s esophagus. Mol Cancer Res. 2010;8:1084–1094. doi: 10.1158/1541-7786.MCR-09-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Leedham S.J., Preston S.L., McDonald S.A. Individual crypt genetic heterogeneity and the origin of metaplastic glandular epithelium in human Barrett’s oesophagus. Gut. 2008;57:1041–1048. doi: 10.1136/gut.2007.143339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wani S., Falk G.W., Post J. Risk factors for progression of low-grade dysplasia in patients with Barrett’s esophagus. Gastroenterology. 2011;141:1179–1186.e1. doi: 10.1053/j.gastro.2011.06.055. [DOI] [PubMed] [Google Scholar]
- 82.Reid B.J., Blount P.L., Rubin C.E. Flow-cytometric and histological progression to malignancy in Barrett’s esophagus: prospective endoscopic surveillance of a cohort. Gastroenterology. 1992;102:1212–1219. [PubMed] [Google Scholar]
- 83.Tobey N.A., Argote C.M., Awayda M.S. Effect of luminal acidity on the apical cation channel in rabbit esophageal epithelium. Am J Physiol Gastrointest Liver Physiol. 2007;292:G796–G805. doi: 10.1152/ajpgi.00385.2005. [DOI] [PubMed] [Google Scholar]
- 84.Dixon J., Strugala V., Griffin S.M. Esophageal mucin: an adherent mucus gel barrier is absent in the normal esophagus but present in columnar-lined Barrett’s esophagus. Am J Gastroenterol. 2001;96:2575–2583. doi: 10.1111/j.1572-0241.2001.04159.x. [DOI] [PubMed] [Google Scholar]
- 85.Jovov B., Van Itallie C.M., Shaheen N.J. Claudin-18: a dominant tight junction protein in Barrett’s esophagus and likely contributor to its acid resistance. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1106–G1113. doi: 10.1152/ajpgi.00158.2007. [DOI] [PubMed] [Google Scholar]
- 86.Aktipis C.A., Boddy A.M., Gatenby R.A. Life history trade-offs in cancer evolution. Nat Rev Cancer. 2013;13:883–892. doi: 10.1038/nrc3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sheftel H., Shoval O., Mayo A. The geometry of the Pareto front in biological phenotype space. Ecol Evol. 2013;3:1471–1483. doi: 10.1002/ece3.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chandrasoma P., Wijetunge S., DeMeester S. Columnar-lined esophagus without intestinal metaplasia has no proven risk of adenocarcinoma. Am J Surg Pathol. 2012;36:1–7. doi: 10.1097/PAS.0b013e31822a5a2c. [DOI] [PubMed] [Google Scholar]
- 89.Kelty C.J., Gough M.D., Van Wyk Q. Barrett’s oesophagus: intestinal metaplasia is not essential for cancer risk. Scand J Gastroenterol. 2007;42:1271–1274. doi: 10.1080/00365520701420735. [DOI] [PubMed] [Google Scholar]
- 90.Chaves P., Crespo M., Ribeiro C. Chromosomal analysis of Barrett’s cells: demonstration of instability and detection of the metaplastic lineage involved. Mod Pathol. 2007;20:788–796. doi: 10.1038/modpathol.3800787. [DOI] [PubMed] [Google Scholar]
- 91.Khor T.S., Alfaro E.E., Ooi E.M. Divergent expression of MUC5AC, MUC6, MUC2, CD10, and CDX-2 in dysplasia and intramucosal adenocarcinomas with intestinal and foveolar morphology: is this evidence of distinct gastric and intestinal pathways to carcinogenesis in Barrett esophagus? Am J Surg Pathol. 2012;36:331–342. doi: 10.1097/PAS.0b013e31823d08d6. [DOI] [PubMed] [Google Scholar]
- 92.Menke V., van Es J.H., de Lau W. Conversion of metaplastic Barrett’s epithelium into post-mitotic goblet cells by gamma-secretase inhibition. Dis Model Mech. 2010;3:104–110. doi: 10.1242/dmm.003012. [DOI] [PubMed] [Google Scholar]
- 93.Liu G.-S., Gong J., Cheng P. Distinction between short-segment Barrett’s esophageal and cardiac intestinal metaplasia. World J Gastroenterol. 2005;11:6360–6365. doi: 10.3748/wjg.v11.i40.6360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wong W.M., Stamp G.W., Elia G. Proliferative populations in intestinal metaplasia: evidence of deregulation in Paneth and goblet cells, but not endocrine cells. J Pathol. 2000;190:107–113. doi: 10.1002/(SICI)1096-9896(200001)190:1<107::AID-PATH504>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 95.Paulson T.G., Xu L., Sanchez C. Neosquamous epithelium does not typically arise from Barrett’s epithelium. Clin Cancer Res. 2006;12:1701–1706. doi: 10.1158/1078-0432.CCR-05-1810. [DOI] [PubMed] [Google Scholar]
- 96.Lorinc E., Öberg S. Submucosal glands in the columnar-lined oesophagus: evidence of an association with metaplasia and neosquamous epithelium. Histopathology. 2012;61:53–58. doi: 10.1111/j.1365-2559.2012.04180.x. [DOI] [PubMed] [Google Scholar]
- 97.Anders M., Bahr C., El-Masry M.A. Long-term recurrence of neoplasia and Barrett’s epithelium after complete endoscopic resection. Gut. 2014;63:1535–1543. doi: 10.1136/gutjnl-2013-305538. [DOI] [PubMed] [Google Scholar]
- 98.Vieth M., Montgomery E.A. Some observations on pyloric gland adenoma: an uncommon and long ignored entity! J Clin Pathol. 2014;67:883–890. doi: 10.1136/jclinpath-2014-202553. [DOI] [PubMed] [Google Scholar]
- 99.Lennerz J.K., Kim S.H., Oates E.L. The transcription factor MIST1 is a novel human gastric chief cell marker whose expression is lost in metaplasia, dysplasia, and carcinoma. Am J Pathol. 2010;177:1514–1533. doi: 10.2353/ajpath.2010.100328. [DOI] [PMC free article] [PubMed] [Google Scholar]