Abstract
Decidual natural killer (dNK) cells express an array of activation receptors to regulate placental immunity and development during early pregnancy. We investigated the functional character of human dNK cells during the first and second trimester of gestation and the interaction between dNK and trophoblast cells. Although the frequency of CD56+CD16− dNK among the total CD45+ leukocytes did not change over this period, the expression of the activating receptors, NKp80 and NKG2D, was greatly upregulated. We observed a significantly higher number of extravillous trophoblast cells in proximity to the dNK cells in the first trimester in comparison with the second trimester decidua. NKG2D expression by first trimester dNK cells was decreased when co-cultured with the HTR-8 trophoblast cell line. In the second trimester, functional markers of dNK activation, i.e., angiogenic factor production (e.g., vascular endothelial growth factor, interleukin-8, interferon-gamma), remained stable despite an increase in NKp80 or NKG2D surface expression. Furthermore, the degranulation capacity of dNK cells, as assessed by CD107a, was decreased in the second trimester. We suggest that in the first trimester, trophoblast–dNK interactions generate a population of dNK cells with a suppressed activating phenotype. In the second trimester, the loss of trophoblast–dNK interactions led to the inhibition of dNK cell function, although their activating receptor expression was increased. We speculate that during pregnancy, two mechanisms operate to modulate the dNK cell activation:suppression of activating receptor levels in the first trimester by trophoblasts and disengagement of receptor–ligand coupling in the second trimester.
Keywords: decidual NK cells, IFN-gamma, NKG2D, NKp80, pregnancy
Introduction
During human pregnancy, CD56brightCD16− decidual natural killer (dNK) cells accumulate in decidualizing endometrium and become the dominant leukocyte population.1,2 Immunohistochemical assays have largely confirmed the distribution of human dNK cells during the first trimester, and numerous studies have investigated the contribution of dNK cells to trophoblast-associated decidual spiral artery remodeling during this period.3,4 Although human dNK cells are thought to play a key role in regulating placental development in normal and pathological pregnancy,5,6,7 there is very limited information regarding dNK cell phenotype and function after the first trimester.8,9 Thus, an advanced analysis of the surface markers and functional capacity of dNK cells across different phases of human pregnancy is necessary.
NK cells are known to express an array of different activating receptors to maintain their homeostasis and function.10 In comparison with peripheral NK cells, dNK represent a specified NK population with limited cytolytic activity.11,12 During early gestational stage, dNK cells express NKp46 (CD335/NCR1), NKp44 (CD336/NCR2), and NKp30 (CD337/NCR3), activating receptors NKG2D (CD314) and NKp80 to regulate their immune reactions and angiogenesis ability.13,14 In addition to intra-cellular signaling pathways, dNK cell function can be modulated by these receptors through inter-cellular communication.12,15 Inhibition of dNK activation has been observed following the interaction of the dNK receptor, 2B4 (CD244), with decidual stromal cells.16 Moreover, a recent study demonstrated that dNK cytotoxicity toward trophoblasts is downregulated by decidual macrophages via transforming growth factor beta signaling.17 Collectively, these results indicate that the immunoregulatory function of dNK cells might be modulated by activating receptors and cell–cell interactions within the decidual microenvironment.
Because dNK cells are incapable of forming effective immunological synapses with target cells, their angiogenic function rather than cytotoxic capability has been the focus of most studies.5,12,18 Human dNK cells can regulate extravillous trophoblast (EVT) invasion and spiral artery angiogenesis via various soluble factors, including vascular endothelial growth factor (VEGF), interferon-gamma (IFN-γ), interleukin 8 (IL-8), and placental growth factor (PlGF).5,19 Conversely, dNK cell reactivity can be affected by interactions with trophoblast cells that express ligands for NK cell activating receptors.18,20 Although dNK cells are central components in uterine vascular remodeling and decidual immune surveillance during pregnancy,7,21 their precise function in these processes is unclear. Abnormal frequencies and functions of dNK cells have been reported in human gestational syndromes, such as recurrent miscarriage, preeclampsia, intrauterine growth restriction, and preterm birth.6,22,23,24 Furthermore, the receptor expression and angiogenesis function of dNK cells are altered in early pregnancies with impaired vascular remodeling and a higher risk of preeclampsia.25,26 Recent studies indicate that due to microenvironmental stimulation, the character of uterine natural killer cells are distinct from the conventional spleen, liver, and thymus NK cells;27,28 therefore, to define dNK heterogeneity, it is imperative to identify the phenotypic and functional capabilities of dNK cells throughout pregnancy.
In this study, we applied 9-color flow cytometry to investigate the contribution of activating receptors to human dNK cell degranulation and cytokine expression over the first and second trimester of pregnancy. Our data demonstrate changes in the activating receptor repertoire of dNK cells from early (6th week) to mid (20th week) pregnancy and dissociation between these receptors and dNK cell angiogenic function and degranulation capacity. Our study also identified the importance of dNK–trophoblast interactions for dNK cell proliferation and function.
Materials and methods
Primary tissues
Decidual samples were obtained following informed consent from healthy women undergoing elective pregnancy termination between 6 and 20 weeks of gestation between 2010 and 2013. The Morgentaler Clinic and the Research Ethics Board of Mount Sinai Hospital approved this study (Toronto, Canada).
Flow cytometry
Cell isolation and surface markers staining
Decidual samples were first macroscopically identified and rinsed with Hank's Balanced Salt Solution (HBSS). Tissue was cut into small pieces (∼1mm3) and shaken for 30 minutes at 37 °C in a rotating incubator (140 times/minutes) in pre-warmed Ca, Mg-free HBSS (25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 1 mM dithiothreitol, 1 mM ethylenediaminetetraacetic acid). The dissociated cell suspension was filtered, and decidual leukocytes were isolated by Ficoll gradient (2000 rpm for 30 minutes) (GE Healthcare, Uppsala, Sweden). Red blood cells were lysed with BD Pharm Lyse buffer, and the cells were then treated with a LIVE/DEAD Fixable Cell Stain kit (L/D-violet; Invitrogen, Eugene, OR, USA). After non-specific blocking with serum-free protein block (Dako, Glostrup, Denmark), the cells were stained with following antibodies for 30 minutes at 4 °C to investigate their dNK phenotype: mouse anti-human CD45-APC/Cy7, CD56-PE/Cy7, CD3-Alexa Fluor 700 (BD Pharmingen, San Jose, CA, USA) and CD16-Krome Orangeplus combinations of either NKp30/CD337-PE, NKp46/CD335-PE/Cy5, and NKp44/CD336-Alexa647 or NKp80-PE, 2B4/CD244-PE/Cy5, and NKG2D/CD314-APC (Beckman Coulter, San Jose, CA, USA).
Intracellular staining
Decidual leukocytes were incubated with a commercial cell stimulation cocktail containing phorbol 12-myristate 13-acetate (PMA) and ionomycin (eBioscience, San Diego, CA, USA) for 4 hours at 37 °C to challenge their functional potential. The cells were then collected and stained with L/D-violet dye and the dNK surface markers described above. Then, the cells were incubated with fixation/permeabilization buffer for 30 minutes (BD Cytofix/Cytoperm Plus Kit; BD Biosciences, San Jose, CA, USA) and subsequently stained with anti-human IFN-γ-FITC (BD Pharmingen), VEGF-APC, and IL-8-PE (R&D Systems, Minneapolis, MN, USA) for 30 minutes. In the degranulation assays, freshly isolated decidual leukocytes were treated with a cell stimulation cocktail in the presence of an anti-human CD107a-PE/Cy5 antibody (BD Pharmingen) for 4 hours before staining.
Flow cytometric data were acquired with a Gallios Flow Cytometer (Beckman Coulter). Offline data analyses were performed on live cells (L/D-violet dye negative population). The viable CD45+CD56+CD16− cells were further investigated for dNK surface markers and intracellular functional analysis. Data were analyzed with FlowJo, Version 7.6 (Tree Star, Ashland, OR, USA) or the Kaluza 1.2 software (Beckman Coulter).
Immunohistochemistry assay
Immunohistochemistry staining
Human decidual samples were fixed in 4% paraformaldehyde and embedded in paraffin. The blocks were serial sectioned at 5 μm and deparaffinized in xylene followed by rehydration through an ethanol gradient. Endogenous peroxidase activity was blocked by incubation of the sections in 3% hydrogen peroxide (Fisher Scientific, Pittsburgh, PA, USA) for 30 minutes. Antigen retrieval was performed with a microwave using the Target Retrieval Solution (Dako), followed by three phosphate-buffered saline (PBS) washes. After a 30-minute incubation with Dako protein blocking solution, the sections were incubated overnight at 4 °C with primary antibodies, including monoclonal mouse anti-human CD45, CD56 (Dako; 1:200), HLA-G (Exbio, Vestec, Czech Republic; 1:800), and cytokeratin-7 (CK7; Dako; 1:400). The sections were then washed with PBS and incubated with biotinylated rabbit anti-mouse IgG (Dako; 1:200). Subsequently, the sections were incubated with horseradish peroxidase substrate (Universal LSAB-HRP kit, Dako), developed with diaminobenzidine (Dako) and counterstained with Gill's Hematoxylin (Sigma-Aldrich, St. Louis, MO, USA). To conduct dual immunofluorescence co-staining, CK7 staining was first detected with donkey anti-mouse Alexa Fluor546 (Invitrogen) followed by incubation with a CD56 antibody and detection with a goat anti-mouse FITC antibody (Invitrogen). The slides were mounted using ProLong Gold antifade reagent (Invitrogen). Photomicrographs were obtained with a Leica DMIL LED microscope.
Image analysis
Serial decidual sections were stained for CD45 and CD56 to quantify the number of leukocytes and dNK cells in the first (n = 19) and second (n = 18) trimester of pregnancy. Cell counts were performed using a standard protocol that assigned random counting frames covering 5% of the total masked tissue area. A positively stained ratio was generated by dividing the numbers of CD56+ dNK by the total CD45+ leukocytes. All of the data were acquired with the new CAST software (Visiopharm) with an Olympus BX61 microscope.
Cell stimulation assays
Effect of trophoblasts on the dNK phenotype
The human trophoblast cell line, HTR-8/SVneo29 (obtained from Dr. Charles Graham, Queen's University, Canada), was cultured in RPMI 1640 medium that was supplemented with 10% fetal bovine serum (FBS), 100 IU mL−1 of penicillin, and 100 μg mL−1 of streptomycin (10% FBS/RPMI; Invitrogen) at 37 °C with 5% CO2. After reaching confluence, the cells were incubated in fresh 10% FBS/RPMI for another 24 hours; then, conditioned media (CM) was collected and spun at 4000 rpm for 5 minutes to collect the supernatants, which were stored at −20 °C before use. To test whether cell–cell contact affected the dNK character, freshly prepared decidual leukocytes (5 × 105) from first or second trimester subjects were mixed with HTR-8 trophoblasts (1:1 ratio) and then seeded onto 24-well culture plates in 1 mL 10% FBS/RPMI. After 16 hours of culture, the cells were further incubated with a cell stimulation cocktail and a CD107a antibody for 4 hours at 37 °C. Flow cytometric staining was then conducted to examine the CD56+CD16− dNK phenotype and function as described above. Matched decidual leukocytes (5 × 105) were cultured in 1 mL control medium (10% FBS/RPMI) or HTR-8 CM.
CFSE proliferation assay
Freshly isolated decidual leukocytes were stained with 5 μM cell tracker dye, carboxyfluorescein succinimidyl ester (CFSE; Invitrogen). Then, 2 × 106 leukocytes were cultured for 6 days in the presence of (i) HTR-8 cells (1 × 105); (ii) HTR-8 CM (1 mL); and (iii) rhIL-2 (5 ng mL−1; R&D Systems) and rhIL-15 (10 ng mL−1; R&D Systems) at 37 °C. The proliferation of the CD56+ dNK cells was examined with a Gallios Flow Cytometer.
Statistical analysis
Normal distribution of the data was examined using the SPSS17.0 software (IBM Corporation, 2008; Armonk, NY, USA). Statistically significant differences between experimental treatments/groups were determined with independent t-tests or ANOVA with Tukey's HSD tests. The data are presented as the mean ± SD. p < 0.05 was considered significant.
Results
The dNK cell frequency was stable between 6 and 20 weeks of pregnancy
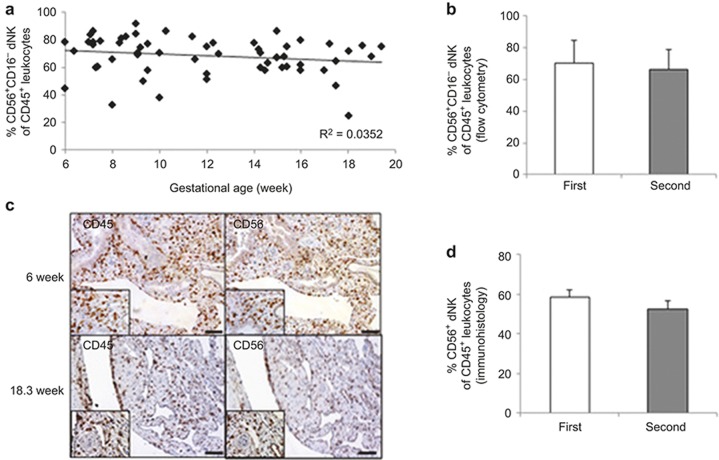
We employed multi-color flow cytometry to examine the dynamics of the dNK cells in the first (6–12 weeks) and second (13–20 weeks) trimester deciduae. To exclude confounding fluorescent signals from dead cells, live/dead staining was applied and only viable CD45+ lymphocytes were examined (Supplementary Figure 1A). Of these cells, no differences in the CD56+CD3−, CD56+CD3+ and CD56−CD3+ subsets were found between the first and second trimester samples (Supplementary Figure 1B). The percentage of CD45+ CD56+CD16− dNK cells remained stable from the 6th to 20th week of pregnancy (70 ± 14% in the first trimester and 66±13% in the second trimester; Figure 1a and 1b). To verify the flow cytometric results, immunohistochemical staining and image analysis of the decidual samples were conducted. As seen in Figure 1c and 1d, the first trimester decidua had similar CD56+ dNK numbers to those of the second trimester samples (58 ± 3.5% vs. 53 ± 4.2%), and no significant difference was detected.
Figure 1.
Quantification of the dNK cell population throughout the first to second trimester of pregnancy. (a) Decidual leukocytes were isolated from the 6- to 20-week pregnancy period. The percentage of viable CD45+CD56+CD16− dNK cells at different gestational ages was illustrated in a scatterplot. Regression trend lines and R2 value are included. n = 65. (b) Histograms summarize the average proportion of first and second trimester dNK cells based on flow cytometric results. n = 34 (first trimester; 9 ± 1.9 week) or n = 31(second trimester; 16 ± 1.5 week). (c) Representative photographs of CD45 and CD56 immunohistochemical staining of serial sections of first and second trimester decidual tissues. High power images were inserted. Scale bar = 100 μm. (d) Summary data of the CD56+ dNK cell proportion amongst the CD45+ leukocytes from immunohistological analysis. n = 19 (first trimester) and 18 (second trimester).
Phenotypic modification of dNK cells in the first and second trimester decidua
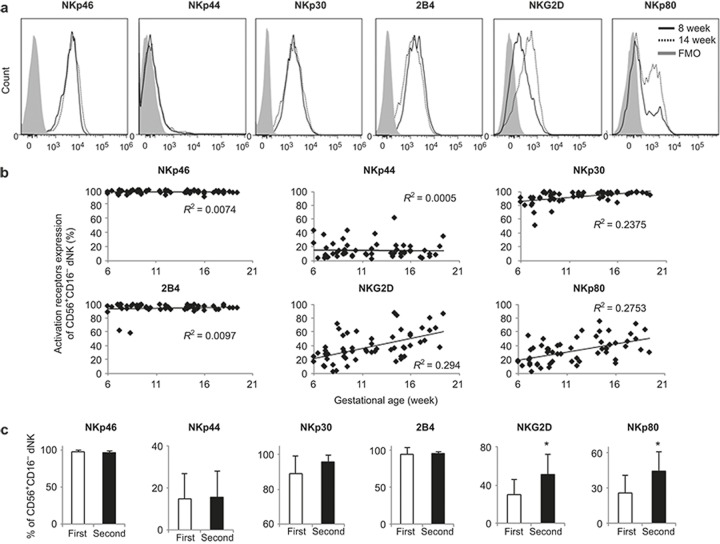
To characterize the phenotype of the dNK cells across the early to mid-gestation periods, we studied the expression of NK cell-activating receptors (Figure 2a and 2b). From the 6th to 20th weeks, the majority of the dNK cells expressed NKp46 (96 ± 2.1%–97 ± 2.1%), NKp30 (89 ± 10%–96 ± 4.1%), and 2B4 (94 ± 9.1%–95 ± 2.9%). There was no change in the NKp46, NKp30, and 2B4 expression levels between the first and second trimester dNK cells (Figure 2c). Only 15 ± 11.9% of the first trimester dNK cells were positive for NKp44, and this proportion did not change significantly in the second trimester pregnancy (16 ± 12.5%). Interestingly, a significant increase of NKG2D+ (50 ± 21%) and NKp80+ (46 ± 16%) dNK cells were detected in the second trimester, compared with that in the first trimester (30 ± 16% NKG2D+, 26 ± 15% NKp80+; p < 0.05; Figure 2b and 2c). Further analysis of the mean fluorescence intensity (MFI) revealed that the second trimester dNK cells had significantly higher NKG2D and NKp80 expression levels than the first trimester dNK cells (p < 0.05; Supplementary Figure 2).
Figure 2.
NKp80 and NKG2D expression levels were upregulated on second trimester dNK cells. (a) Representative histograms of multiple dNK cell activation marker expression from the 8th (first trimester; solid line) and 14th (second trimester; dotted line) week deciduae. Viable CD45+CD56+CD16− dNK cells were gated. A fluorescence minus one (FMO) control is shown in gray. (b) NKp46, NKp44, NKp30, 2B4, NKG2D, and NKp80 expression on dNK cells from 6- to 20-week decidual samples (n = 65). (c) The average percentage of these activation markers between the first and second trimester dNK cells. n = 34 (first trimester; 9 ± 1.9 week) or n = 31 (second trimester; 16 ± 1.5 week). *p < 0.05.
Functional difference between the first and second trimester dNK cells
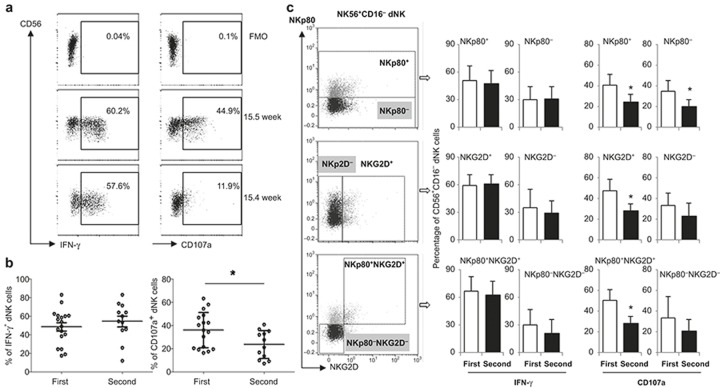
To evaluate functional changes in the dNK cells with advancing gestation, IFN-γ staining and a CD107a degranulation assay were performed (Figure 3a). No significant differences in IFN-γ expression were found between the first and second trimester dNK cells, whereas CD107a expression was significantly decreased in the second trimester dNK cells compared with that of the first trimester cells (p < 0.05; Figure 3b). To further evaluate the dNK cell heterogeneity, IFN-γ and CD107a expression was assessed and compared according to their NKp80 and NKG2D profile. As shown in Figure 3c, both first and second trimester dNK cells had similar IFN-γ expression levels, which were independent of their NKp80 and/or NKG2D expression levels (Figure 3C, left panel). However, a significant decrease in CD107a expression was observed in the second trimester NKp80+/NKp80−, NKG2D+and NKp80+ NKG2D+ dNK sub-populations compared with the first trimester dNK cells (p < 0.05; Figure 3C, right panel).
Figure 3.
Functional changes of the dNK subsets at different gestation stage. (a) Representative dot plots of IFN-γ and CD107a expression by dNK cells from 10.5 and 15.4 weeks of gestation. Decidual leukocytes were cultured for 4 hours with a cell stimulation cocktail and CD107a antibody, then the cells were collected and processed with intracellular IFN-γ staining. Viable CD45+CD56+CD16− dNK cells were gated and studied. (b) Percentages of IFN-γ, CD107a positive dNK cells at different gestational stages of pregnancy. n = 18 (first trimester; 9 ± 1.6 week) or n = 12 (second trimester; 16 ± 1.3 week). The representative plots were from a 14th week decidua. (c) IFN-γ and CD107a expression of different dNK subsets based on their NKp80, NKG2D profile. *p < 0.05.
VEGF and IL-8 expression in first and second trimester dNK cells
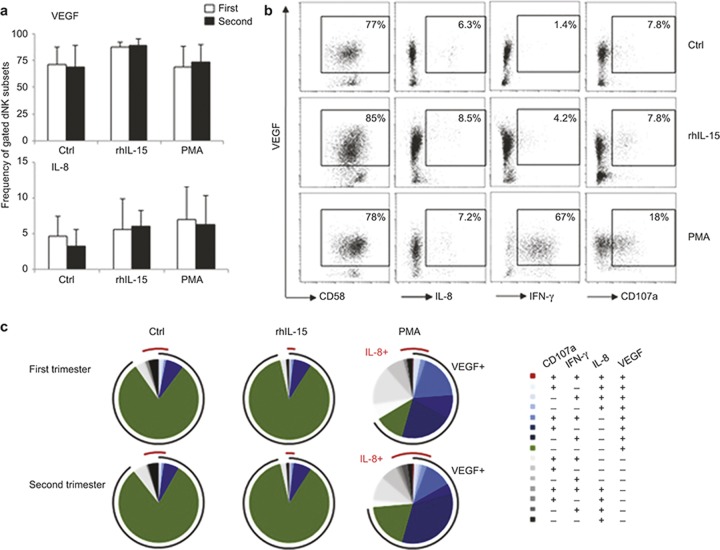
Next, we sought to determine whether the gestational age dependent changes in dNK cell activation resulted in alterations in their angiogenic function. Although first and second trimester dNK cells differed in their activating receptor expression levels (Figure 2), their VEGF and IL-8 expression levels were similar in response to either specific (rhIL-15) or unspecific (PMA) stimulation and did not increase above control (Figure 4a). Moreover, PMA activation enhanced the IFN-γ and CD107a expression by the dNK cells; however, it did not alter their VEGF and IL-8 profiles (Figure 4b).
Figure 4.
dNK cell angiogenic growth factor expression during the first and second pregnancy trimesters. (a) Decidual leukocytes were stimulated with or without rhIL-15 (10 ng mL−1) and PMA for 16 hours, followed by intracellular cytokine staining. The VEGF and IL-8 expression levels from first and second trimester CD56+CD16− dNK cells were analyzed. (b) Representative dot plots of VEGF dual responsiveness with IL-8+, IFN-γ+, and CD107a+ from CD56+CD16− dNK cells are shown. (c) The functional heterogeneity of dNK cells is illustrated by Boolean gating. The arcs indicate the relative frequency of IL-8 or VEGF positive dNK cells in response to different stimuli. n = 12 from first trimester (9 ± 1.6 week); n = 8 from second trimester (16 ± 1.9 week).
Boolean gating analysis revealed that the functional heterogeneity of dNK cells was greatly improved by PMA but not by rhIL-15 stimulation. There was no significant difference in the VEGF+IL-8+ dNK cell frequency between the first and second trimester, indicating that rhIL-15 or PMA stimulation did not change the co-expression of these two angiogenic factors (Figure 4b and 4c).
The dNK cell phenotype and function were altered following interactions with trophoblasts
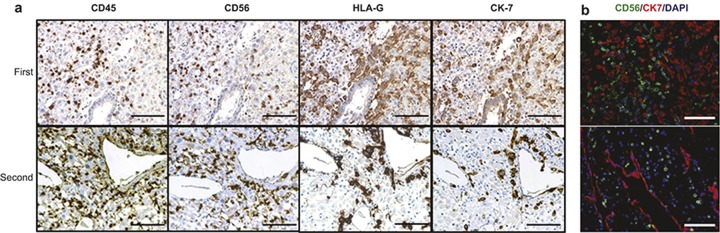
We next sought to determine the mechanism(s) that modulates dNK function between the first and second trimester. In the first trimester of pregnancy, EVTs penetrate into the differentiated decidua and interact with dNK cells to remodel uterine spiral arteries. As illustrated by immunohistochemical analysis (Figure 5a and 5b), the first trimester samples had significant numbers of invasive trophoblasts (expressing HLA-G+ and CK-7+) in proximity to the CD56+ dNK cells within the decidual stroma, but this was rare in the second trimester.
Figure 5.
Immunological staining of dNK cells and trophoblast during the first and second pregnancy trimesters. (a) Representative immunohistochemistry of CD45, CD56, HLA-G, and CK7 to visualize the localization of CD45+ decidual leukocytes, CD56+ dNK and HLA-G+ EVT, CK7+ trophoblasts. (b) Co-staining images of dNK (CD56+; green) and trophoblast (CK7+; red) cells. Scale bar = 100 μm. n = 22 (first trimester; 9 ± 2.0 week) or n = 14 (second trimester; 15 ± 1.7 week).
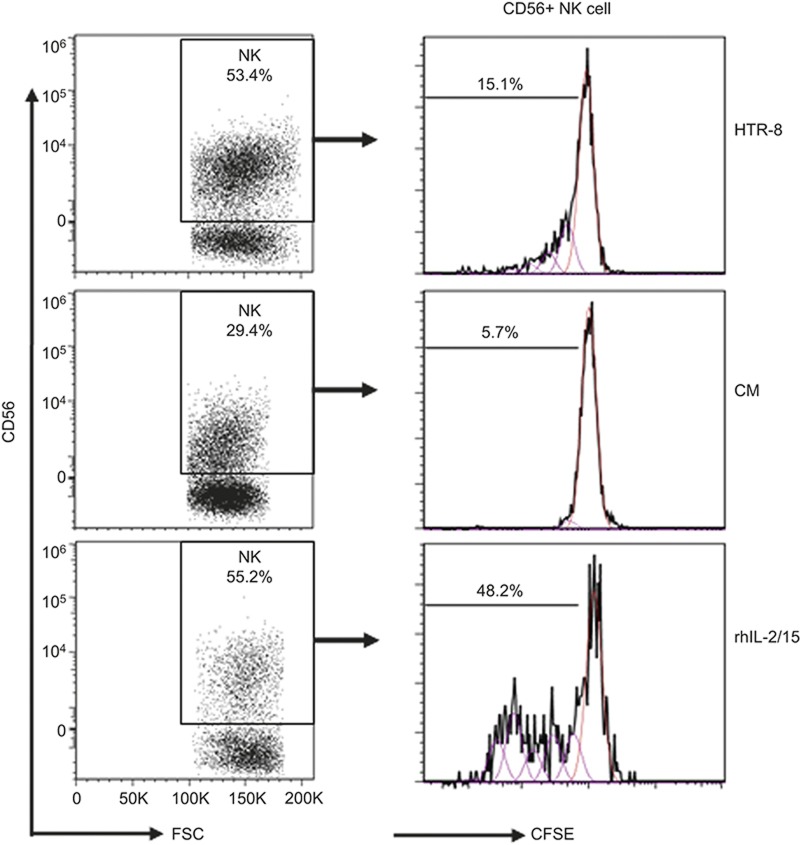
Therefore, we hypothesized that the interaction between the dNK and trophoblast cells might influence dNK homeostasis and function. We mimicked the in vivo condition by culturing dNK cells with an HTR-8 trophoblast cell line. To study a potential role of soluble factors that were secreted by trophoblasts, we also stimulated dNK cells with HTR-8 CM. After 6 days of culture, direct contact with the HTR-8 trophoblasts greatly improved the percentage of viable CD56+ dNK cells, which was similar to that observed following rhIL-2/15 treatment (53 ± 13.4% vs. 55.2 ± 17.5% Figure 6). However, the HTR-8 CM treatment significantly decreased the percentage of dNK cells (29 ± 4.1%) in comparison with direct HTR-8 coculture (p < 0.05). Furthermore, direct cellular interactions with the HTR-8 trophoblasts significantly increased the proliferation of CD56+ dNK cells compared with the CM treatment (15 ± 9.8% vs. 5.7 ± 3.6% p < 0.05; Figure 6).
Figure 6.
Trophoblast engagement drives dNK survival and proliferation. Decidual leukocytes were stained with CFSE and cultured for 6 days in complete RPMI 1640 medium with 10% FBS in the presence of (i) trophoblastic HTR-8 cells; (ii) HTR-8 CM; (iii) cytokine mixture of rhIL-2 (2 ng mL−1) and rhIL-15 (10 ng mL−1). No additional stimulatory cytokines were added to the HTR-8 or CM groups. Viable CD45+CD56+ dNK cells were gated, and their proliferation was assessed. n = 10 with average an gestational age of 11 ± 2.7 week.
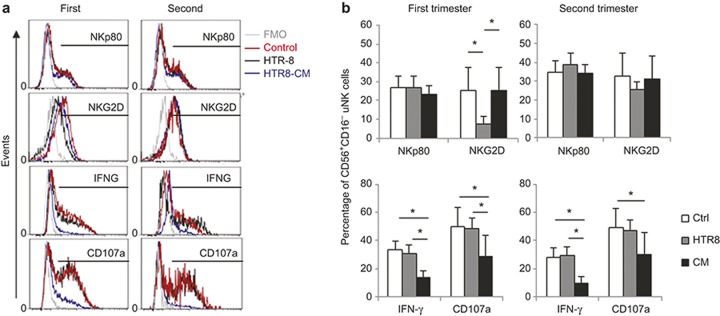
Following co-culture with the HTR-8 trophoblasts or their CM, there was no change in NKp80+ dNK cells (27.3 ± 6.2% vs. 24.2 ± 4.7% in the first and 39.6 ± 12.9% vs. 34.0 ± 14% in the second trimester) in comparison with that of the control groups (27.6 ± 6.7% in the first trimester and 36.4 ± 12.7% in the second trimester, Figure 7). In contrast, cellular contact with the HTR-8 cells significantly decreased the NKG2D+ dNK cell frequency in the first (p < 0.05) but not second trimester compared with the control or CM groups (Figure 7a and 7b). Interestingly, the frequency of IFN-γ+ and CD107a+ dNK cells from either the first or second trimester of pregnancy was not altered by contact with HTR-8 cells; however, it was significantly decreased by the HTR-8 CM (p < 0.05; Figure 7a and 7b).Together, these data suggest that first and second trimester dNK cells have different sensitivities to invasive trophoblasts.
Figure 7.
Trophoblast interactions can change the dNK phenotype and function. (a) first and second trimester decidual leukocytes were cultured alone in RPMI 1640/10% FBS (control; red lines), with HTR-8 trophoblast cells (black lines) or HTR-8 CM (blue lines) for 16 hours, and then re-stimulated with cell stimulation cocktail (PMA/ionomycin/brefeldin A) and CD107a antibody for 4 hours before being processed with intracellular cytokine staining. Viable CD56+CD16− dNK cells were gated to assess their NKp80, NKG2D, IFN-γ, and CD107a expression levels. An FMO control was included (gray line). (b) The summarized results of the dNK cell NKp80, NKG2D, IFN-γ, and CD107a expression levels during the first and second pregnancy trimesters. n = 15 from the first trimester (9 ± 1.7 week); n = 11 from the second trimester (16 ± 1.8 week). *p < 0.05.
Discussion
To our knowledge, this is the first report that has applied multi-parameter flow cytometry to investigate the phenotypic and functional dynamics of dNK cells throughout the first half of pregnancy (6–20weeks). We characterized dNK cells from the first to second trimester of human pregnancy by focusing on the expression of their activation receptors, degranulation capacity, cytokine expression, proliferation, and outcome of interaction with a trophoblast cell line. Our data suggest that during human pregnancy, two mechanisms are operable that modulate dNK cell activity: (i) receptor-mediated regulation in the first trimester and (ii) disengagement of receptor–ligand coupling in the second trimester.
Our data show that from the 6th to 20th weeks of pregnancy, CD56+CD16− dNK cells remain the dominant leukocyte population at the maternal–fetal interface. The presence of dNK cells in the second trimester of pregnancy may support ongoing EVT invasion and transformation of uterine spiral arteries.19,30 Although previous studies have indicated that NKp80 and NKG2D can stimulate cytotoxicity of circulating NK by their engagement with related ligands,31,32 our results demonstrate that in the second trimester, the increase in the number of NKp80+ and NKG2D+ dNK cells does not directly correlate with decreased CD107a expression. Moreover, either blockade or upregulation of NKG2D/NKp80 has no significant effect on IFN-γ or CD107a expression by dNK cells, indicating that they are not sufficient on their own to induce dNK cell immune responses (JH Zhang and SJ Lye et al., unpublished data). Thus our data are consistent with other reports on peripheral NK cells33,34 and suggest that in vivo dNK cytotoxicity and pro-inflammatory reactivity are controlled by multiple receptor–ligand interactions.
In normal pregnancy, dNK cells express CD107a; however, this does not cause direct apoptosis of invasive EVT.35 Such protection is partly achieved by a balanced interaction between CD158 (KIR2D) on dNK cells and HLA-C on trophoblast cells.13 Additionally, because CD107a reactivity may protect peripheral NK cells from degranulation-associated damage,36 we suggest that dNK may similarly adopt this strategy to limit self-destruction and to maintain their presence and angiogenic capability at the maternal–fetal interface. Furthermore, we demonstrated that the maintenance of dNK function (IFN-γ/CD107a expression) was dependent upon cellular interactions between dNK cells and HTR-8 trophoblast cells (irrespective of the presence of activating receptors NKG2D or NKp80). Interestingly, our data also show that HTR-8 CM (but not direct contact with trophoblasts) was able to reduce the percentage of dNK cell expressing IFN-γ and CD107a in both the first and second trimesters. This suggests that soluble factors produced by HTR-8 trophoblast cells can suppress dNK cell function (IFN-γ and CD107a expression); however, this effect is compromised by direct cellular interactions, as reported in studies regarding tumor-infiltrating NK cells.33,37,38 Because the IFN-γ signaling blockade can improve the EVT expansion in vitro,39 the suppressed IFN-γ production of dNK cells by soluble trophoblastic factors may create a unique microenvironment to facilitate the further invasion of trophoblast cells. However, the in vivo situation is much more difficult to examine due to existing complex molecular networks and cellular interactions.40 For example, we did not find IFN-γ changes at the protein level when dNK cells were co-cultured with HTR-8 trophoblast cells, although N. Sotnikova et al. reported that trophoblast cells increased the mRNA expression of IFN-γ by dNK cells.41 These results suggest that dNK cells have tightly controlled thresholds for the transcription and translation of reactive cytokines.
Our data (Figure 4) and those of other studies42,43 demonstrate that during early pregnancy, dNK cells are frequently found in close proximity to fetal trophoblasts/EVT and can modulate their migration. These specialized dNK cells can partner with EVT and remodel uterine spiral arteries in early pregnancy.3,30,44,45 In this study, we also found that direct cellular contact with the HTR-8 trophoblast cell line enhanced the survival and proliferation of dNK cells. Because HTR-8 trophoblasts are positive for major histocompatibility complex class I-related chain A/B (MICA/B) expression (data not shown), we speculate that in the first trimester of pregnancy, ligand-induced internalization might decrease NKG2D expression by dNK cells when they encounter invasive trophoblasts that are positive for NKG2D ligands.46,47 Therefore, the reduced EVT–dNK cell interactions in the second trimester might contribute to an increased proportion of NKG2D+ dNK cells.
During pregnancy, there is a decrease in the expression of the NK inhibitory receptor, KIR2DL1, on dNK cells, which should further enhance the reactivity of dNK cells with trophoblasts.14,48 However, our data indicate that paradoxically, second trimester dNK cells have a lower degranulation capability, indicating that other mechanisms (e.g., soluble factors or receptor uncoupling) may dominate the regulation of dNK functions during this pregnancy period. For example, chronic exposure of NK cells to the NKG2D ligands could impair their NKG2D-dependent cytotoxicity but maintain constitutive IFN-γ production.49,50,51 Moreover, other studies suggest that NKG2D expression by dNK cells is more likely involved in the cross-talk with maternal cells in pathological placentation as opposed to normal trophoblast recognition.46,52 Thus, in line with previous studies documenting the calibration of NK functions by multiple receptor–ligand interactions,10,53,54,55 our data indicate that dNK cells leverage their NKG2D expression as an adaptive strategy to dynamic changes in the decidual environment.
Finally, the decrease in NKG2D expression but not NKp80 expression on first trimester dNK cells following interaction with HTR-8 trophoblast (Figure 7) indicates that these two receptors may use different signaling pathways to regulate dNK cells during their interaction with trophoblasts. This is supported by studies showing that NKG2D acts through the PI3K signaling pathway and that NKp80 acts via the Syk kinase pathway.31,33,56 Interestingly, although NKG2D expression on dNK cells was downregulated by their interaction with trophoblast, the angiogenic function of dNK cells (VEGF and IL-8 expression) remained stable between the first and second trimester. This disengagement of the link between activating receptor expression and dNK cell function suggests that the angiogenic capacity of dNK cells is not directly linked to NKp80 and/or NKG2D expression; rather, it could be related to other dNK receptors not investigated in this study. For instance, we have previously reported that S1PR (sphingosine-1 phosphate receptor) is involved in the regulation of dNK cell angiogenic capacity.45
In summary, our data suggest that interactions between trophoblast and dNK cells during the first trimester suppress dNK cell functions by inhibiting the expression of their activating receptors. However, during the second trimester, although NKG2D- and NKp80-activating receptor expression is increased, dNK cell activation remains suppressed through a different pathway involving dNK cell receptor–ligand disengagement.
Authorship
Jianhong Zhang, Caroline E. Dunk, and Stephen J. Lye designed the experiments. Jianhong Zhang performed the in vitro experiments and the flow cytometric data acquisition and analysis. Melissa Kwan conducted the immunohistological cell counting. The manuscript was drafted by Jianhong Zhang and revised by Caroline E. Dunk, Melissa Kwan, Rebecca L. Jones, Lynda K. Harris, Sarah Keating and Stephen J. Lye. The work was supervised by Stephen J. Lye.
Acknowledgments
This study was supported by the Canadian Institutes of Health Research (CIHR) MOP82811 and MOP130550 for Dr. Stephen J. Lye. Dr. Lynda K. Harris is a BBSRC David Phillips Research Fellow. We thank the donors, the Research Centre for Women's and Infants' Health BioBank Program of Lunenfeld-Tanenbaum Research Institute (LTRI) and the Mount Sinai Hospital/University Health Network (Toronto, Canada) for providing the human specimens. We appreciate the work of Dr. Oksana Shynlova (LTRI) in critical reviewing. We thank Dr. John Kingdom and Dora Baczyk (Mount Sinai Hospital, Toronto) for kindly providing the anti-human CK7 antibody. We thank Ms. Annie Bang and Mr. Michael Parsons (LTRI, Mount Sinai Hospital) for the flow cytometric technical support.
Footnotes
Supplementary information of this article can be found on Cellular & Molecular Immunology website: http://www.nature.com/cmi.
The authors declare no conflicts of interest.
Supplementary Information
References
- King A, Burrows T, Verma S, Hiby S, Loke YW. Human uterine lymphocytes. Hum Reprod Update 1998; 4: 480–485. [DOI] [PubMed] [Google Scholar]
- Moffett A, Regan L, Braude P. Natural killer cells, miscarriage, and infertility. BMJ 2004; 329: 1283–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SD, Dunk CE, Aplin JD, Harris LK, Jones RL. Evidence for immune cell involvement in decidual spiral arteriole remodeling in early human pregnancy. Am J Pathol 2009; 174: 1959–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulmer JN, Lash GE. Human uterine natural killer cells: a reappraisal. Mol Immunol 2005; 42: 511–521. [DOI] [PubMed] [Google Scholar]
- Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med 2006; 12: 1065–1074. [DOI] [PubMed] [Google Scholar]
- Moffett A, Colucci F. Uterine NK cells: active regulators at the maternal-fetal interface. J Clin Invest 2014; 124: 1872–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu B, Li X, Sun R, Tong X, Ling B, Tian Z et al. Natural killer cells promote immune tolerance by regulating inflammatory TH17 cells at the human maternal–fetal interface. Proc Natl Acad Sci USA 2013; 110: E231–E240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PJ, Searle RF, Robson SC, Innes BA, Bulmer JN. Decidual leucocyte populations in early to late gestation normal human pregnancy. J Reprod Immunol 2009; 82: 24–31. [DOI] [PubMed] [Google Scholar]
- Bulmer JN, Williams PJ, Lash GE. Immune cells in the placental bed. Int J Dev Biol 2010; 54: 281–294. [DOI] [PubMed] [Google Scholar]
- Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL et al. Innate or adaptive immunity? The example of natural killer cells. Science 2011; 331: 44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Zhou Y, Fu B, Wu Y, Zhang R, Sun R et al. Molecular signatures and transcriptional regulatory networks of human immature decidual NK and mature peripheral NK cells. Eur J Immunol 2014; 44: 2771–2784. [DOI] [PubMed] [Google Scholar]
- Koopman LA, Kopcow HD, Rybalov B, Boyson JE, Orange JS, Schatz F et al. Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J Exp Med 2003; 198: 1201–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Male V, Sharkey A, Masters L, Kennedy PR, Farrell LE, Moffett A. The effect of pregnancy on the uterine NK cell KIR repertoire. Eur J Immunol 2011; 41: 3017–3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlin R, Duriez M, Berkane N, de Truchis C, Madec Y, Rey-Cuille M-A et al. Dynamic shift from CD85j/ILT-2 to NKG2D NK receptor expression pattern on human decidual NK during the first trimester of pregnancy. PLoS One 2012; 7: e30017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Costa H, Casemayou A, Aguerre-Girr M, Rabot M, Berrebi A, Parant O et al. Critical and differential roles of NKp46- and NKp30-activating receptors expressed by uterine NK cells in early pregnancy. J Immunol 2008; 181: 3009–3017. [DOI] [PubMed] [Google Scholar]
- Vacca P, Pietra G, Falco M, Romeo E, Bottino C, Bellora F et al. Analysis of natural killer cells isolated from human decidua: evidence that 2B4 (CD244) functions as an inhibitory receptor and blocks NK-cell function. Blood 2006; 108: 4078–4085. [DOI] [PubMed] [Google Scholar]
- Co EC, Gormley M, Kapidzic M, Rosen DB, Scott MA, Stolp HAR et al. Maternal decidual macrophages inhibit NK cell killing of invasive cytotrophoblasts during human pregnancy. Biol Reprod 2013; 88: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacca P, Moretta L, Moretta A, Mingari MC. Origin, phenotype and function of human natural killer cells in pregnancy. Trends Immunol 2011; 32: 517–523. [DOI] [PubMed] [Google Scholar]
- Robson A, Harris LK, Innes BA, Lash GE, Aljunaidy MM, Aplin JD et al. Uterine natural killer cells initiate spiral artery remodeling in human pregnancy. FASEB J 2012; 26: 4876–4885. [DOI] [PubMed] [Google Scholar]
- Vacca P, Cantoni C, Prato C, Fulcheri E, Moretta A, Moretta L et al. Regulatory role of NKp44, NKp46, DNAM-1 and NKG2D receptors in the interaction between NK cells and trophoblast cells. Evidence for divergent functional profiles of decidual versus peripheral NK cells. Int Immunol 2008; 20: 1395–1405. [DOI] [PubMed] [Google Scholar]
- Karimi K, Arck PC. Natural Killer cells: keepers of pregnancy in the turnstile of the environment. Brain Behav Immun 2010; 24: 339–347. [DOI] [PubMed] [Google Scholar]
- Zhang J, Chen Z, Smith GN, Croy BA. Natural killer cell-triggered vascular transformation: maternal care before birth? Cell Mol Immunol 2011; 8: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosens I, Pijnenborg R, Vercruysse L, Romero R. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol 2011; 204: 193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Kusanovic JP, Kim CJ. Placental bed disorders in the genesis of the great obstetrical syndromes. In: Pijnenborg R, Brosens I, Romero R (eds.) Placental Bed Disorders. Cambridge: Cambridge University Press, 2010: 271–289. [Google Scholar]
- Wallace AE, Whitley GS, Thilaganathan B, Cartwright JE. Decidual natural killer cell receptor expression is altered in pregnancies with impaired vascular remodeling and a higher risk of pre-eclampsia. J Leukoc Biol 2015; 97: 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace AE, Fraser R, Gurung S, Goulwara SS, Whitley GS, Johnstone AP et al. Increased angiogenic factor secretion by decidual natural killer cells from pregnancies with high uterine artery resistance alters trophoblast function. Hum Reprod 2014; 29: 652–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama WM, Sojka DK, Peng H, Tian Z. Tissue-resident natural killer cells. Cold Spring Harb Symp Quant Biol 2014; 78: 149–156. [DOI] [PubMed] [Google Scholar]
- Sojka DK, Plougastel-Douglas B, Yang L, Pak-Wittel MA, Artyomov MN, Ivanova Y et al. Tissue-resident natural killer (NK) cells are cell lineages distinct from thymic and conventional splenic NK cells. eLife 2014; 3: e01659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham CH, Hawley TS, Hawley RC, MacDougall JR, Kerbel RS, Khoo N et al. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp Cell Res 1993; 206: 204–211. [DOI] [PubMed] [Google Scholar]
- Wallace A, Fraser R, Cartwright J. Extravillous trophoblast and decidual natural killer cells: a remodelling partnership. Hum Reprod Update 2012; 18: 458–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennehy KM, Klimosch SN, Steinle A. Cutting edge: NKp80 uses an atypical hemi-ITAM to trigger NK cytotoxicity. J Immunol 2011; 186: 657–661. [DOI] [PubMed] [Google Scholar]
- Deguine J, Breart B, Lemaître F, Bousso P. Cutting edge: tumor-targeting antibodies enhance NKG2D-mediated NK cell cytotoxicity by stabilizing NK cell–tumor cell interactions. J Immunol 2012; 189: 5493–5497. [DOI] [PubMed] [Google Scholar]
- Long EO, Sik Kim H, Liu D, Peterson ME, Rajagopalan S. Controlling natural killer cell responses: integration of signals for activation and inhibition. Annu Rev Immunol 2013; 31: 227–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimosch SN, Bartel Y, Wiemann S, Steinle A. Genetically coupled receptor–ligand pair NKp80-AICL enables autonomous control of human NK cell responses. Blood 2013; 122: 2380–2389. [DOI] [PubMed] [Google Scholar]
- Apps R, Sharkey A, Gardner L, Male V, Kennedy P, Masters L et al. Ex vivo functional responses to HLA-G differ between blood and decidual NK cells. Mol Hum Reprod 2011; 17: 577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohnen A, Chiang SC, Stojanovic A, Schmidt H, Claus M, Saftig P et al. Surface CD107a/LAMP-1 protects natural killer cells from degranulation-associated damage. Blood 2013; 122: 1411–1418. [DOI] [PubMed] [Google Scholar]
- Brenner CD, King S, Przewoznik M, Wolters I, Adam C, Bornkamm GW et al. Requirements for control of B-cell lymphoma by NK cells. Eur J Immunol 2010; 40: 494–504. [DOI] [PubMed] [Google Scholar]
- Reiners KS, Topolar D, Henke A, Simhadri VR, Kessler J, Sauer M et al. Soluble ligands for NK cell receptors promote evasion of chronic lymphocytic leukemia cells from NK cell anti-tumor activity. Blood 2013; 121: 3658–3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Dutz JP, MacCalman CD, Yong P, Tan R, von Dadelszen P. Decidual NK cells alter in vitro first trimester extravillous cytotrophoblast migration: a role for IFN-γ. J Immunol 2006; 177: 8522–8530. [DOI] [PubMed] [Google Scholar]
- Wang F, Zhou Y, Fu B, Wu Y, Zhang R, Sun R et al. Molecular signatures and transcriptional regulatory networks of human immature decidual NK and mature peripheral NK cells. Eur J Immunol 2014; 44: 2771–2784. [DOI] [PubMed] [Google Scholar]
- Sotnikova N, Voronin D, Antsiferova Y, Bukina E. Interaction of decidual CD56+ NK with trophoblast cells during normal pregnancy and recurrent spontaneous abortion at early term of gestation. Scand J Immunol 2014; 80: 198–208. [DOI] [PubMed] [Google Scholar]
- Arck PC, Hecher K. Fetomaternal immune cross-talk and its consequences for maternal and offspring's health. Nat Med 2013; 99: 548–556. [DOI] [PubMed] [Google Scholar]
- Hazan AD, Smith SD, Jones RL, Whittle W, Lye SJ, Dunk CE. Vascular-leukocyte interactions: mechanisms of human decidual spiral artery remodeling in vitro. Am J Pathol 2010; 177: 1017–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta 2006; 27: 939–958. [DOI] [PubMed] [Google Scholar]
- Zhang J, Dunk CE, Lye SJ. Sphingosine signalling regulates decidual NK cell angiogenic phenotype and trophoblast migration. Hum Reprod 2013; 28: 3026–3037. [DOI] [PubMed] [Google Scholar]
- Apps R, Gardner L, Traherne J, Male V, Moffett A. Natural-killer cell ligands at the maternal–fetal interface: UL-16 binding proteins, MHC class-I chain related molecules, HLA-F and CD48. Hum Reprod 2008; 23: 2535–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund M, Stenqvist A-C, Nagaeva O, Kjellberg L, Wulff M, Baranov V et al. Human placenta expresses and secretes NKG2D ligands via exosomes that down-modulate the cognate receptor expression: evidence for immunosuppressive function. J Immunol 2009; 183: 340–351. [DOI] [PubMed] [Google Scholar]
- Xiong S, Sharkey AM, Kennedy PR, Gardner L, Farrell LE, Chazara O et al. Maternal uterine NK cell-activating receptor KIR2DS1 enhances placentation. J Clin Invest 2013; 123: 4264–4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coudert JD, Zimmer J, Tomasello E, Cebecauer M, Colonna M, Vivier E et al. Altered NKG2D function in NK cells induced by chronic exposure to NKG2D ligand-expressing tumor cells. Blood 2005; 106: 1711–1717. [DOI] [PubMed] [Google Scholar]
- Manaster I, Mandelboim O. The unique properties of uterine NK cells. Am J Reprod Immunol 2010; 63: 434–444. [DOI] [PubMed] [Google Scholar]
- Coudert JD, Scarpellino L, Gros F, Vivier E, Held W. Sustained NKG2D engagement induces cross-tolerance of multiple distinct NK cell activation pathways. Blood 2008; 111: 3571–3578. [DOI] [PubMed] [Google Scholar]
- Le Bouteiller P. Human decidual NK cells: unique and tightly regulated effector functions in healthy and pathogen-infected pregnancies. Front Immunol 2013; 4: 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauriat C, Long EO, Ljunggren H-G, Bryceson YT. Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood 2010; 115: 2167–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman KC, Riley EM. Whatever turns you on: accessory-cell-dependent activation of NK cells by pathogens. Nat Rev Immunol 2007; 7: 279–291. [DOI] [PubMed] [Google Scholar]
- Sojka DK, Tian Z, Yokoyama WM. Tissue-resident natural killer cells and their potential diversity. Semin Immunol 2014; 26: 127–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raulet DH, Gasser S, Gowen BG, Deng W, Jung H. Regulation of ligands for the NKG2D activating receptor. Annu Rev Immunol 2013; 31: 413–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.