Abstract
Almost every experimental treatment strategy using non-autologous cell, tissue or organ transplantation is tested in small and large animal models before clinical translation. Because these strategies require immunosuppression in most cases, immunosuppressive protocols are a key element in transplantation experiments. However, standard immunosuppressive protocols are often applied without detailed knowledge regarding their efficacy within the particular experimental setting and in the chosen model species. Optimization of such protocols is pertinent to the translation of experimental results to human patients and thus warrants further investigation. This review summarizes current knowledge regarding immunosuppressive drug classes as well as their dosages and application regimens with consideration of species-specific drug metabolization and side effects. It also summarizes contemporary knowledge of novel immunomodulatory strategies, such as the use of mesenchymal stem cells or antibodies. Thus, this review is intended to serve as a state-of-the-art compendium for researchers to refine applied experimental immunosuppression and immunomodulation strategies to enhance the predictive value of preclinical transplantation studies.
Keywords: animal models, immunomodulation, immunosuppression, transplantation, translational research
Introduction
Immunosuppressive treatments are routinely applied to prevent immune-borne transplant damage or rejection. These interventions continuously regain importance given the increasing need for solid organ and tissue replacement. In many countries, there has actually been a decline in the availability of appropriate transplants.1
In parallel, the development, optimization and implementation of emerging cell- and tissue-based regenerative therapies are underway to compensate for the paucity of transplantable organs. Regenerative therapies also provide a perspective on causal treatments for degenerative diseases that have been considered untreatable for decades.2 Once proven effective, such regenerative therapies will most likely rely on non-autologous ‘off-the-shelf' cell or tissue preparations to meet huge clinical demand. This will in turn require highly developed and effective immunosuppression or immunomodulation protocols to prevent graft rejection. Importantly, regenerative medicine approaches should be assessed in animal studies before clinical translation.
However, immunosuppressive regimens applied in preclinical research are often adopted from basic protocols that already exist in human medicine and often rely on simple body weight-adjusted dose translation. In many cases, they are even restricted to cyclosporin A (CsA) monotherapy, which is frequently reported in experimental transplantation protocols.3 There may be some room for improvement by taking species-specific differences as well as pharmacodynamics and pharmacokinetics into full consideration, as these differences may significantly reduce the biological activity of a particular drug.4 Neglecting these considerations not only leaves many important research questions unsolved (for example, regarding the necessity of graft survival for maximum therapeutic effect, or mitigating the effects emerging from graft decline) but may also severely bias the translatability of preclinical findings themselves. On the other hand, virtually all immunosuppressive strategies are accompanied by undesirable side effects and thus imperatively require a well-balanced, recipient- and species-specific design, including a combination of available protocols or even considering options currently under development.
This review provides a comprehensive, state-of-the-art overview of immunosuppressive treatments in relevant animal model species (that is, non-human primates (NHPs), rodents, dogs, pigs and sheep) and frequently investigated transplantation scenarios. Relevant physiological differences between animal species and humans as well as important differences in pharmacodynamics and pharmacokinetics are emphasized. In addition, we review promising experimental immunosuppressive protocols in terms of their potential roles in experimental cell/tissue transplantation studies. Our compendium thus aims to enable researchers to apply tailored immunosuppressive strategies with respect to the experimental question and/or the particular treatment subject under consideration, with the goal of ultimately ensuring a high predictive value of preclinical study results with respect to the clinical situation.
Immunosuppressive drug classes
Efficient immunosuppression can be achieved via numerous mechanisms, which may be addressed synergistically. However, factors such as species physiology, age, concurrent medication, comorbidities and pharmacology can significantly affect efficacy, half-life and side effects of immunosuppressive agents.5 The following paragraphs review relevant classes of immunosuppressive agents under current clinical or experimental use. Supplementary Table 1 summarizes the most relevant fields of application, whereas Supplementary Table 2 provides a detailed overview of side effects.
Glucocorticoids
Glucocorticoids (GCs) possess strong immunosuppressive properties and are widely used in human and veterinary medicine owing to their broad, although nonspecific, anti-inflammatory and anti-allergenic effects.6 Common applications include the treatment of rheumatoid arthritis and asthma and concomitant administration in solid organ transplantation.7, 8 They inhibit T-lymphocytes and antigen-presenting cells (APCs) and induce a downregulation of proinflammatory cytokines.8 The physiologically active form of GCs is cortisol (11-hydroxycortisol), whereas cortisone is a far less active 11-keto form.9 In the blood, 80% of cortisol is inactive (bound to transcortin), whereas 20% is bound to albumin and can diffuse into tissues. The regulation of GC bioactivity is controlled hepatically by the 11β-hydroxysteroid dehydrogenase, which converts cortisone to cortisol and back (the ‘cortisone-cortisol-shuttle').9 Clinically used GCs mainly include synthetic cortisol analogs. The most common preparation is (methyl)prednisolone, but others such as dexamethasone, triamcinolone, betamethasone and paramethasone exhibit similar effects.8, 10 Synthetic GCs exclusively bind to albumin and therefore have a much higher bioavailability.10 They also have significantly longer biological half-lives than cortisol.10
GCs reach the nuclei of many cell types by forming a complex after binding to a specific cytoplasmic receptor protein (Figure 1). There, they induce the synthesis of tyrosine-aminotransferase and tryptophan pyrrolase,11 which exerts a number of tissue-specific and systemic effects as follows:
reduction of chemotaxis, a pivotal process in inflammatory reactions and neutrophil activity,12
reduction of vessel wall permeability, leading to less edema formation, exudation and migration of inflammatory cells, 10
reduction of antigen phagocytosis, 10
increase in hepatic gluconeogenesis, 10
downregulation of peripheral protein metabolism but enhanced hepatic protein synthesis, 10
alanine release from the musculature (increased plasma levels); this gluconeogenesis substrate surplus induces pancreatic glucagon secretion (from A cells) and subsequent hyperglucagonemia, 10 and
fatty acid mobilization from subcutaneous storage (mainly in the extremities) and blockage of lipogenesis at these sites. In contrast, lipogenesis is increased in abdominal fat tissue. 13
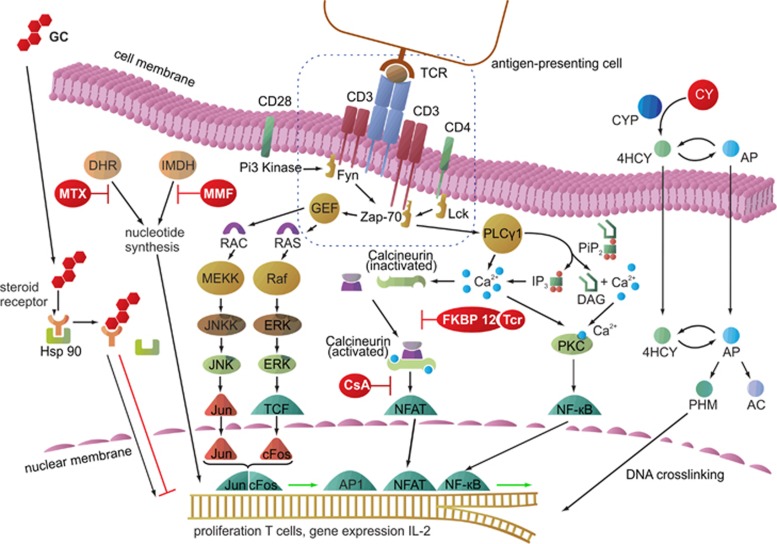
Figure 1.
Cellular pathways of commonly used clinical immunosuppressive agents. GCs reach the nucleus via diffusion through the cell membrane and form a complex after binding to a steroid receptor protein following separation from Hsp 90. The complex binds to specific DNA sequences and affects the transcription of a variety of genes. MTX inhibits DHR, which is necessary for nucleotide synthesis, thereby constraining cell division. MMF blocks the IMDH, which is also required for nucleotide synthesis. CY is metabolized by CYP450 to 4HCY, which interconverts to AP. Both tautomers are able to passively diffuse into cells. Then, AP is converted to AC and PHM, which possesses DNA-crosslinking properties. CsA binds to an intracellular immunophilin and blocks calcineurin to enable NFATs, whereas tacrolimus (Tcr) binds to the intracellular FK506 binding protein (FKBP) and also inhibits NFAT activation, ultimately preventing cell proliferation. AC, acrolein; AP, aldophosphamide; CsA, cyclosporin A; CY, cyclophosphamide; CYP450, cytochrome P450; DAG, diacylglycerol; DHR, dihydrofolate reductase; ERK, extracellular signal-regulated kinase; Fyn, tyrosine-protein kinase; GEF, guanine-nucleotide exchanging factor; GH, glucocorticoid; 4HCY, 4-hydroxyphosphamide; Hsp 90, heat-shock protein 90; IP3, inositol triphosphate; IMDH, inosine monophosphate dehydrogenase; JNK, c-Jun N-terminal kinase; JNKK, c-Jun N-terminal kinase kinase; RAC, guanosine triphosphate; RAS, guanosine-nucleotide-binding protein; Lck, lymphocyte-specific protein tyrosine kinase; MEK, mitogen-activated protein kinase kinase; MEKK, serine/threonine-specific protein kinase; MMF, mycophenolate mofetil; MTX, methotrexate; NF-κB, nuclear factor 'κ-light-chain enhancer' of activated B cells; NFAT, nuclear factor of activated T cell; PHM, phosphoramide mustard; Pip2, phosphatidyl inositol bisphosphate; PKC, protein kinase C; PLCγ, phospholipase C-γ RAF, serine/threonine-specific protein kinase; TCF, transcription factor; TCR, T-cell receptor; Zap-70, zeta-chain-associated protein kinase 70.
The preferred routes of administration and dosages of GCs are highly divergent across species and also depend on the condition being treated. Perioperative intravenous application of 30–500 mg/kg methylprednisolone reduced ischemia–reperfusion injuries by 24% after liver transplantation in humans without increasing susceptibility to infectious complications.14 In the mouse, 50 mg/kg prednisolone was given intraperitoneally after allogeneic skin transplantation,15 while rejection of a corneal xenotransplant was prevented by topical administration of 0.06 mg of dexamethasone over 8 weeks via a drug delivery system.16 In contrast, oral application of 12.5 mg/kg prednisolone (twice a day, starting 5 days before transplantation and continuing until postoperative day (POD) 32) did not prevent graft rejection after allogeneic bone marrow transplantation in canines.17 However, effective immunosuppression, and thereby enhanced survival of allogeneic hindlimb transplants, was achieved with a combination of CsA, mycophenolate mofetil (MMF) and GCs. This protocol was successfully transferred to a porcine model of allogeneic skin transplantation, in which 40 mg/kg per day CsA (whole blood trough levels between 100 and 300 ng/ml), 500 mg per day MMF and 2 mg/kg per day prednisolone were applied. The initial prednisolone dose, given at POD 1, could be reduced by 0.5 mg/kg per day in 3-day intervals to a final concentration of 0.1 mg/kg per day, avoiding side effects. Transplant survival was 19 to 90 days.18, 19 This protocol benefits from the perioperative administration of 500 mg of methylprednisolone, which was also confirmed for sheep in which 30 mg/kg methylprednisolone supported successful engraftment of cardiac transplants.20 Table 1 summarizes recommendations for GC dosing in different species.
Table 1. Glucocorticoids and their uses in experimental transplantation studies.
| Mechanism of action | Species | Plasma half-life (h) | Pharmacokinetics bioavailability/VD | Application form | Dosage examples | |
|---|---|---|---|---|---|---|
| Dexamethasone (DM) | ||||||
| Intracellular receptor binding Building a complex Translocation into the cell nucleus Influence transcription201 | Human | 4.7±0.4,38 5.010 | Oral bioavailability of >80%202 Protein binding: 65–70% VD: 0.8 L/kg203 | Conjunctival, intra-articular, i.m., i.v., optic, p.o.204 | • 16 mg per day, maintenance: <1.5 mg per day205 | |
| Mouse | n.s.i. | n.s.i. | i.m., s.c.,203 i.p.206 | • 1 mg of DM/kg per day, q.d. tapered off towards 48 and 72 h206 | ||
| Rat | 1.4–3.9207 | Bioavailability of 86% CL: 0.23 L/kg/h VD: 0.78 L/kg208 Protein binding: 84.7±0.7%209 | i.m., s.c.,203 injection into the Tx region210 | • 2 μl DM per animal, Tx of 4 × 106 fetal ventral mesencephalic cells210 | ||
| Dog | 4.2±0.6211 | Biologic activity can persist for 48 h or more212 Protein binding: 72.7±1.4%209 VD: 1.2 L/kg213 | Conjunctival, i.m., intra-articular, i.v., p.o., s.c.,203 i.v. dissolved in cardioplegic solution214 | • 250 mg of BM/L cardioplegic solution214 | ||
| Pig | i.v.: 0.8, i.m.: 1.1215 | Bioavailability after i.m.: 131±26.05% CL after i.v.: 2.39±0.57 L/kg/h VD after i.v.: 2.78±0.88 L/kg215 | i.m, intra-articular, i.v., s.c.203 | • 0.04–0.08 mg/kg203, 213 • 1–10 mg per animal203, 216 | ||
| Sheep | 10.0−12.0217 | n.s.i. | i.m, intra-articular, i.v.203 | • 2–20 mg/kg203 | ||
| Prednisolone (P) | ||||||
| Intracellular receptor binding Building a complex Translocation into the cell nucleus Influence on transcription | Human | 3.3 and more10 4.1±0.8218 | Protein binding: 75–90%203 Systemic availability: 79±13% CL: 0.14±0.03 L/kg/h VD: 0.7±0.1 L/kg218 | i.m., intra-articular, i.v., topical,219 ophthalmic, p.o.204 | • 15 mg of P per day10 • 30–500 mg of MP/kg i.v. perioperatively14 • 4 mg of MP per day, 105.2 mg of CsA per day or 4.1 mg of Tcr per day220 | |
| Mouse | 0.8 (at 10 mg/kg i.p. or p.o.)221 | n.s.i. | i.p.,15 p.o.203 | • 50 mg of P/kg15 | ||
| Rat | 0.5 (at 10 mg/kg)222 | CL: 2.3±0.9 L/kg/h VD: 0.82±0.46 L/kg (both higher in obese rats)223 | Bronchoalveolar lavage,224 i.p.,225 i.v.,226 p.o., s.c.,203 topical16 | • 0.06 mg of P for 8 weeks, topical via drug delivery system16 • 100 mg of P application by bronchoalveolar lavage224 • single dose: 5 mg of MP/kg i.v.226 • 10 mg of MP/kg per day, 0.2 mg of Rpm/kg per day, 20 mg of MMF/kg per day i.p. from POD −30 to 100225 | ||
| Dog | 1.3227 | Plasma half-life not meaningful because of intermediate acting, biologic half-life 12–36 h212 CL after p.o.: 0.43–0.58 L/kg/h228 | i.m.,229 i.v.,203 p.o.47 | • 20 mg of MMF/kg per day, 5 mg of CsA/kg per day, 0.1 mg of MP/kg per day p.o.47 • 15 mg of CsA per day, 5 mg of Mz per day, 1 mg of P per day p.o., i.m. from POD −5 to 20229 | ||
| Pig | 0.73±0.15218 | Systemic availability: 27±10% CL: 1.54±0.13 L/kg/h VD: 1.2±0.2 L/kg In general: requires 10–30 times higher i.v. or oral dose of steroids than humans218 | i.v.,230 p.o.18, 19 | • 40 mg of CsA/kg per day, 500 mg of MMF per day, 2 mg of P/kg per day (tapered off towards to 0.1 mg of P/kg per day)18, 19 • Initial dose: 100 mg of CsA, 50 mg of Az, 125 mg of MP, q.d. p.o.:10 mg/kg CsA, 2 mg/kg Az, 20 mg of MP231 • 15 mg of CsA/kg per day, 2 mg of Az/kg per day, 20 mg of P per day or 1 mg of Tcr/kg per day, 2 mg of Az/kg per day, 20 mg of P per day p.o.232 • 500 mg of MP per animal i.v. perioperatively230 | ||
| Sheep | 0.4±0.1 (at 0.5 mg/kg i.v.)233 | CL: 0.93±0.13 L/kg/h VD: 0.45±0.086 L/kg234 | i.m., i.v.203 | • 30 mg of MP/kg perioperatively20 | ||
Abbreviations: Az, azathioprine; BM, betamethasone; CL, total plasma clearance; CsA, cyclosporin A; i.m., intramuscular; i.p., intraperitoneal; i.v., intravenous; MMF, mycophenolate mofetil; MP, methylprednisolone; Mz, mizorbin; n.s.i., no sufficient information available; P, prednisolone; p.o., oral; q.d., once a day; Rpm, rapamycin; s.d., subcutaneous; Tcr, tacrolimus; Tx, transplantation; VD, volume of distribution.
A number of GC side effects in human patients and animals have been reported since the late 1970s. These comprise musculoskeletal (myopathy, osteoporosis), gastrointestinal (ulceration), central nervous system and ophthalmic (glaucoma), cardiovascular (hypertension, peripheral edema formation), renal, metabolic (hyperlipidemia, ketoacidosis), endocrinal (reduced growth, suppression of the hypothalamic-pituitary axis) and fibroblastic (wound healing disturbance) side effects, as well as exaggerated immunosuppression.21 Interspecies differences have also been reported in terms of fetotoxicity. GC application was shown to induce palatoschisis in rats, but no such effects have been observed in humans.10, 22 A species-specific overview of the most relevant applications and common adverse events is provided in Supplementary Tables 1 and 2.
Cytostatics
Cytostatics impair mitosis by acting as purine analogs to inhibit DNA synthesis or inosine monophosphate dehydrogenase. One relevant member of each category is described below.
Azathioprine: a purine analog
Azathioprine (Az) is a prodrug that is non-enzymatically converted in the liver into its active metabolite, 6-mercaptopurine (6-MP).23 6-MP is metabolized via three metabolic pathways. It can be inactivated by thiopurine methyltransferase to 6-methylmercaptopurine or by xanthine oxidase to 6-thiouric, or it can be activated to 6-thioguanine nucleotide.24 The enzyme thiopurine S-methyltransferase is important for Az metabolism. Approximately 90% of human patients present a highly active form, while the enzyme is only moderately active in the remaining 10%, which explains the phenomenon of therapeutic non-responsers.25 The active metabolite 6-thioguanine nucleotide is inserted as a base analog into nucleic acids, inhibiting DNA repair and replication. Az reaches a metabolic steady state only 4–6 months after the initiation of therapy. Hence, treatment must be initiated in a timely manner or initially supported with the use of other immunsuppressants.26 Az causes minor, dose-independent side effects, such as nausea, diarrhea and elevated liver enzymes. Side effects can be reduced by administering 6-MP instead of Az.27 Dose-dependent side effects include leukopenia, thrombocytopenia and hepatitis. Az was used at dosages of 2–3 mg/kg to treat acute renal allograft rejection28 (for more details, see Supplementary Tables 1 and 2).
Cyclophosphamide and methotrexate: DNA synthesis inhibitors
DNA synthesis inhibitors comprise alkylating substances, such as cyclophosphamide (CY), and anti-metabolites, such as methotrexate (MTX).29, 30 CY, a commonly used antitumor agent, exerts cytotoxic and immunosuppressive effects and is also applied as an immunosuppressant.29 Cytochrome P450 (CYP450) metabolizes CY to 4-hydroxyphosphamide, which interconverts to aldophosphamide.31 Both tautomers are able to passively diffuse into target cells (for example, tumor cells). Then, aldophosphamide is converted to phosphoramide mustard, which exerts DNA-crosslinking properties (Figure 1).32 The effects of cytostatic drugs are highly dependent on their impact at a particular phase of the cell cycle.33 CY does not specifically influence a certain cell cycle phase and is thus toxic for all dividing cells, particularly rapidly proliferating cells.33 Its primary effects involve the impairment and depletion of B-lymphocytes,34 observed in mice, guinea-pigs and humans.35, 36 T-suppressor cells are less sensitive, whereas T-helper cells are mostly resistant to the effects of CY.37
There are significant interspecies differences regarding the application and dosing of cytostatic agents (Table 2; for an overview of relevant indications, see Supplementary Table 1). Immunosuppressive effects have been reported for the intramuscular administration of CY at 28.5–33.3 mg/kg twice a day in rabbits.34 Single doses of 250–400 mg/kg were effective in mice, inducing tolerance against ovine erythrocytes.34 In general, typical side effects of immunosuppressants, such as nephrotoxicity or hepatotoxicity, are less frequent when using cytostatic agents compared with immunophilin drugs such as CsA (Supplementary Table 2).
Table 2. Common cytostatic drugs and their uses in experimental transplantation studies.
| Mechanism of action | Species | Plasma half-life (h) | Pharmacokinetics bioavailability/VD | Application form | Dosage examples | |
|---|---|---|---|---|---|---|
| Azathioprine (Az) | ||||||
| Metabolized to 6-thioguanine Base analog in DNA or RNA Inhibits DNA repair and replication235 | Human | Of the active metabolite 6-MP: 1.9±0.6236 | Corticosteroid-sparing effect: 4.3 mg per day237 | p.o.235 | • 1.5 mg/kg per day • Children: 3–5 mg/kg per day • 1 mg/kg per day • 25–100 mg per day235 | |
| Rabbit | n.s.i. | n.s.i. | p.o. in the drinking water238 | • 1.0 mg/kg dissolved in 40 ml of water in combination with CY238 | ||
| Dog | n.s.i. | n.s.i. | p.o.239 | • 3 mg/kg per day for 28 or 56 day after Tx in combination with prednisolone239 | ||
| Pig | n.s.i. | n.s.i. | n.s.i. | • Daily up to 5 mg/kg in combination with prednisolone240 • up to 5 mg of Az/kg daily240 | ||
| Cyclophosphamide (CY) | ||||||
| Is metabolized to 4-hydroxyphosphamide Connects to aldophosphamide Translocation into the cell Release of acrolin Transformation of aldophosphamide to phosphoramide mustard Cytotoxic effect31 | Human | i.v.: 8.9241 7.7±3.6242 Children: 2.5–6.5243 | CL: 0.04–0.18 L/kg/h VD: 0.31–1.05 L/kg244 | i.v., p.o.38 | • 10–15 mg of CY/kg three times per week38 • 1–5 mg of CY/kg per day38 • >1.8 mg of CY/kg per day38 | |
| NHP | n.s.i. | n.s.i. | i.v. as infusion245 | • 180 mg of CY/kg245 | ||
| Mouse | 0.2246 | n.s.i. | i.p. or i.v.34, 247 | • Single dose: 250–400 mg of CY/kg34, 247 | ||
| Rat | 0.1248 | n.s.i. | i.p.249 | • 150 mg of CY/kg249 | ||
| Rabbit | 0.4±0.1250 | n.s.i. | i.m.34 | • 28.5–33.3 mg of CY/kg every second day34 | ||
| Dog | 4–12 h, measurable up to 72 h after administration212 | Pharmacokinetics not detailed for dogs, but supposedly similar to humans good oral absorption with peak levels after 1 h, distribution throughout the body, including CSF (in subtherapeutic levels); minimally protein bound; CY crosses the placenta212 | i.v., s.c.,251 p.o.252 | • 2 mg of CY/kg for 15 days252 | ||
| Pig | n.s.i. | n.s.i. | i.p.253 | • 50 mg of CY/kg253 | ||
| Sheep | n.s.i. | n.s.i. | i.v 254 | • 25 mg of CY/kg254 | ||
| Methotrexate (MTX) | ||||||
| Inhibition of dihydrofolate reductase Inhibition of purine synthesis42 | Human | 1.8–8.5 (at 50–200 mg/kg i.v.)255 | Peak levels 4 h after oral dosing and 30 min to 2 h after i.m. injection212 | p.o.256 | • 7.5 mg of MTX per patient per week256 • 7.5–12.5 mg of MTX per week257 | |
| Mouse | 1.42±0.65258 0.5, up to 100 (Depo-MTX)259 | CL after i.v.: 2.63±0.76 L/kg/h VD: 2.19±0.55 L/kg crosses the blood–brain barrier258 | i.p.260 | • 0.5–5 mg of MTX/kg260 | ||
| Rat | i.v.: 4.2261 | Unbound fraction: 58.1% VD after i.v.: 0.27 L/kg262 | Intrathecally and around the spinal root,263 i.p.264 | • 3 mg of MTX/kg per week264 • 1 mg of MTX/kg263 | ||
| Dog | 7.6 (intracisternal injection)265 <10 h (2–4 h)212 | Good gastrointestinal absorption after oral administration (<30 mg/m2 with bioavailability ~60%), wide distribution in the body, active transport across cell membranes, no therapeutic levels in the CSF after oral or parenteral administration, 50% bound to plasma proteins, crosses the placenta212 | i.m., i.v., p.o,251 s.c.266 | • 10 mg of MTX/kg b.i.d.266 | ||
| Pig | n.s.i. | n.s.i. | Into the 4. cerebral ventricle267 | • 2 mg of MTX for 5 days267 | ||
| Sheep | n.s.i. | n.s.i. | i.a.268 | • 0.25 mg of MTX/kg per day for 28 days268 | ||
Abbreviations: b.i.d., twice a day; CL, total plasma clearance; CSF, cerebrospinal fluid; i.a., intra-arterial; i.m., intramuscular; i.p., intraperitoneal; i.v., intravenous; MP, 6-mercaptopurine; NHP, non-human primate; n.s.i., no sufficient information available; p.o., oral; s.c., subcutaneous; Tx, transplantation; VD, volume of distribution.
The side effects of CY include hemorrhagic cystitis, bone marrow suppression, alopecia and gonadal dysfunction,38 whereas teratogenic, carcinogenic and mutagenic effects have been explained by DNA crosslinking.29, 38 In humans, single doses significantly reduce leukocyte counts to 1400/mm3 (60 mg/kg) and 1000 per mm3 (100 mg/kg for 5 consecutive days).39 Reduced thrombocyte counts were observed after the application of 2000 mg of CY per m2 body surface.40 Gastrointestinal complications, including nausea and vomiting, have been reported in two-thirds of all human patients at doses >50 mg/kg and 6–12 h after a 1-h infusion.41 Similarly, diarrhea and weight loss are observed in rabbits following the intramuscular application of CY.34
The anti-metabolite MTX also reduces purine synthesis by inhibiting dihydrofolate reductase42 (Figure 1). Administration of the anti-metabolite MTX (for dosage regimen, see Table 2; for an overview of relevant indications, see Supplementary Table 1) may result in hepatotoxicity, pneumonitis and bone marrow suppression30 (Supplementary Table 2).
MMF: an inosine monophosphate dehydrogenase inhibitor
MMF inhibits nucleic acid formation,43 presents high bioavailability after oral application and diminishes transplant rejection after tissue or solid organ transplantation.44 This agent is a potent, competitive and reversible inhibitor of inosine-5'-monophosphate dehydrogenase, inhibiting purine synthesis and thus cell proliferation43, 44 (Figure 1). In vivo, MMF is rapidly converted into its active metabolite, mycophenolic acid (MPA), which is excreted renally to avoid the enterohepatic circulation.44 MMF selectively inhibits cytotoxic T cells but does not reduce proliferation of fibroblasts and endothelial cells below a concentration of 100 nm.44 MMF was applied successfully as a stand-alone treatment after pancreatic islet transplantation in mice45 and has been used to treat acute transplant rejection in rats (cardiac allografts), dogs (renal allografts) and humans.46 It also prevents proliferative angiopathies (which are related to chronic rejections) after allotransplantations in rats and NHPs.44 Daily oral application of 30 mg/kg MMF reduced antibody formation against ovine erythrocytes in rats, whereas 40 mg/kg per day effectively prevented graft rejection in mice (cardiac allografts), rats and dogs (renal allografts).44 MMF application twice a day resulted in effective long-term plasma concentrations.44 Moreover, the combination of MMF and CsA exerts an additive effect without known toxicity at common concentrations.44 For example, the combination of 20 mg/kg per day MMF, 5 mg/kg per day CsA and 0.1 mg/kg per day methylprednisolone has been applied in dogs without signs of nephrotoxicity and hepatotoxicity or bone marrow suppression.47 Immunosuppressive protocols using MMF and the reduction of CsA doses by up to 50% resulted in improved renal transplant function in human patients.48, 49 CsA dose reduction is required because CsA inhibits the active transport of MPA-glucuronide in bile, leading to reduced enterohepatic recirculation and thereby reduced MPA exposure.50, 51 Please refer to Table 3 for species- and indication-specific dosing and applications. For an overview of relevant indications, see Supplementary Table 1.
Table 3. MMF and its use in experimental transplantation studies.
| Mechanism of action | Species | Plasma half-life (h) | Pharmacokinetics bioavailability/volume of distribution | Application form | Dosage examples |
|---|---|---|---|---|---|
| Mycophenolate mofetil (MMF) | |||||
| Is metabolized to glucuronide44 Competitive and reversible inhibition of iosin-5'-monophosphate dehydrogenase43 Inhibition of purine synthesis Inhibition of cell proliferation44 | Human | 16.0269 | Oral bioavailability 94%, food reduces peak levels up to 40%212 | p.o.52 | • 2 g of MMF per day52 • 20 mg of MMF/kg per day, gradually increase the dose up to 40 mg/kg per day270 |
| Mouse | n.s.i. | n.s.i. | p.o.271 | • 40 mg of MMF/kg271 | |
| Rat | 4.7±0.3272 | n.s.i. | p.o.273 | • 30 mg of MMF/kg273 • 40 mg of MMF/kg in combination with CsA187 | |
| Dog | 8 (±4)212 | Wide interpatient and interdose variability: bioavailability of 54% (10 mg/kg), 65% (15 mg/kg), 87% (20 mg/kg),212 VD: 5.0±4.5 L/kg with wide interpatient variability212 CL after p.o. (40 mg/kg): 0.14±0.12 L/kg/h262 | p.o.47 | • 20 mg of MMF/kg per day, 5 mg of CsA/kg per day, 0.1 mg of MP/kg per day47 • Dosing in 8-h intervals recommended for optimal immunosuppression212 | |
| Pig | n.s.i. | n.s.i. | i.v., p.o.274 | • 25–100 mg of MMF/kg b.i.d., Tcr274 • 1.5 g of MMF per animal b.i.d., low-dose Tcr275 • 20 mg of MMF/kg i.v. before Tx, Tcr276 | |
| Sheep | n.s.i. | n.s.i. | p.o.277 | • 1.5 g of MMF per day, Tcr, MP277 | |
Abbreviations: b.i.d., twice a day; CL, total plasma clearance; CsA, cyclosporin A; i.v., intravenous; MP, methylprednisolone; n.s.i., no sufficient information available; p.o., oral; Tcr, tacrolimus; Tx, transplantation; VD, volume of distribution.
Most reported side effects of MMF are gastrointestinal complications, which have been noted in humans (2000 mg per day)52 and dogs, but side effects have not included intestinal ulcerations.47 Moreover, the long-term application of MMF even at doses exceeding clinically common levels does not lead to toxicity in dogs or NHPs44 (Supplementary Table 2).
Calcineurin inhibitors
Calcineurin controls the transcription of interleukins (ILs) in T-lymphocytes. Binding of calcineurin inhibitors to intracellular immunophilins decreases IL-2 production and, therefore, T-cell proliferation.53 CsA and tacrolimus are the most relevant calcineurin inhibitors.
Cyclosporin A
CsA has been very widely used both experimentally and clinically since 1978.54 CsA is a lipophilic molecule, isolated from Tolypocladium inflatum,55 that can easily penetrate the blood–brain barrier (BBB).56, 57 Major applications include stem cell and solid organ transplantations,58 as well as the prevention and suppression of graft-versus-host-disease (GvHD).59 Because the drug is often used nonspecifically in experimental studies, the following paragraphs provide detailed information about its mechanisms and side effects. Application regimens will also be recommended.
CsA is a calcineurin inhibitor that reversibly and selectively inhibits T-cell proliferation.60 Its effects are mediated by the formation of intracellular complexes with immunophilin, effectively blocking both the activation of calcineurin and, subsequently, nuclear factor of activated T cells (NFATs; Figure 1).60 Because NFAT is a key regulatory element in the transcription of proinflammatory cytokines such as IL-2, IL-3, IL-4, IL-5, IL-8, IL-13, interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α), application of CsA effectively inhibits proinflammatory immune reactions.61 Reduction of IL-2 is of particular importance among the effects of CsA, as IL-2 is a potent T-cell activator both in humans and canines.62 Because CsA is able to penetrate the BBB,56, 57 positive effects have been reported for central nervous system applications: CsA increases the proliferation and cell survival of neural precursor cells both in vitro and in vivo and is frequently used in neurological studies.63
CsA is metabolized hepatically via CYP450 3A.58, 64 CYP inhibitors such as ketoconazole or diltiazem can decelerate CsA metabolism,64 whereas CYP induction by phenytoin can reduce CsA whole blood levels by up to 50%.65 CsA pharmacokinetics depend on recipient age (younger individuals present enhanced metabolic clearance), the transplanted tissue, CYP-competitive medications and liver function, differences in CsA clearance, apparent volume of distribution and half-life with regard to age, health status and transplantation procedure have also been reported5 (Table 4).
Table 4. Differences in CsA clearance, volume of distribution and half-lifea.
|
Kidney Tx |
Heart Tx | Healthy controls | ||
|---|---|---|---|---|
| Adults | Children | Adults | Adults | |
| Clearance (ml/min/kg) | 5.7 (0.6–23.9) | 11.8 (9.8–15.5) | 4.0 (2.1–5.39) | 3.9 (2.8–4.2) |
| Volume of distribution (l/kg) | 4.5±3.6 | 4.7±1.5 | 1.3±0.2 | 1.2±0.2 |
| Plasma half-life (h) | 10.7 (4.3–53.4) | 7.3 (6.11–16.6) | 6.4 (5.2–9.3) | 6.3 (4.7–9.5) |
Abbreviations: CsA, cyclosporin A; Tx, transplantation.
In relation to age, health and transplantation procedure according to Ptachcinski et al.5
Important effects of individual CsA pharmacokinetics have been shown for CYP 3A5 polymorphisms: individuals presenting the CYP 3A5*3 allele require lower CsA doses to reach desired whole blood levels compared with those individuals with the CYP 3A5*1 variant.66 An interspecies-specific comparison of CsA pharmacokinetics between humans, rats, rabbits and dogs revealed a direct proportional correlation between clearance and volume of distribution in relation to body weight. The capacity to metabolize CsA in dogs is twice as large compared with the capacities of humans, rats and rabbits.67
The bioavailability of CsA demonstrates relatively large intraindividual fluctuations, primarily due to reduced oral absorption in some individuals.68 Thus, some transplantation centers have applied CsA intravenously in human patients for the first 21 days post-transplantation.69 However, oral application is much more common. Importantly, CsA microemulsions for oral delivery reduced rejection frequencies after organ transplantation and have replaced intravenous preparations.70 Oral application of CsA is also common for dogs71 and pigs,72 whereas intravenous injection is preferred in sheep models.73 Intravenous application is also advantageous in species for which good compliance for oral application cannot be ensured and if high blood concentrations must be reached rapidly.
CsA is typically applied for GvHD prevention in humans, and different treatment regimens have been reported: CsA may be applied as a continuous infusion, once per day or twice a day with extended infusion times (2–13 h per day). Applied dosages vary between 1 and 20 mg/kg per day and are generally adopted to reach target whole blood levels, which are usually reported between 100 and 1000 ng/ml.69 Application of CsA requires a careful balance between intended treatment and adverse side effects. Bacigalupo et al.74 compared daily doses of 1 and 5 mg/kg following bone marrow transplantation to treat leukemia. Patients receiving 1 mg/kg per day presented adverse effects less frequently but had a significantly enhanced risk of GvHD.74 In turn, long-term CsA treatments can clearly reduce the overall risk of developing GvHD.75 Prevention of rejection was achieved with whole blood trough levels of 400–800 ng/ml CsA after experimental kidney transplantation in pigs (neonatal: 4 mg/kg per day oral; juvenile: 30 mg/kg per day).72 Rose et al.73 recommended 2–3 mg/kg CsA in combination with 10 mg/kg ketoconazole (twice a day each) as the optimal CsA dosage in a sheep model of xenologous skin transplantation73 (for an overview of relevant indications, see Supplementary Table 1). CsA whole blood levels ranged between 1600 and 2500 ng/ml CsA in this model, and the steady state was reached after 17 days.73 On the other hand, application of 3 mg/kg per day twice a day revealed considerable intraindividual differences and fluctuations in CsA whole blood levels (Diehl et al., 2016, unpublished data). Thus, moderate dose escalation may be required to reach appropriate CsA whole blood levels. This can be considered safe because adverse effects have not been observed after the administration of relatively high doses, including 12 mg/kg per day CsA for 5 days in sheep.76 Inhibition of calcineurin positively correlates with CsA whole blood levels and is therefore the most important indicator of CsA efficacy.60 However, CsA whole blood levels and bioavailability may differ significantly between individuals receiving the same CsA doses58 or even intraindividually.68 The latter finding was corroborated by sheep studies, which strongly pointed to the necessity of thorough CsA whole blood level monitoring in CsA-based immunosuppressive paradigms (Diehl et al., 2016, unpublished data). Commonly used dosages in different species are shown in Table 5.
Table 5. CsA and tacrolimus and their uses in experimental transplantation studies.
| Mechanism of action | Species | Plasma half-life (h) | Pharmacokinetics bioavailability/VD | Application form | Dosage examples | |
|---|---|---|---|---|---|---|
| Ciclosporine A (CsA) | ||||||
| Direct inhibition of calcineurin Selective inhibition of T-cell proliferation60 | Human | 24.0–93.0278 | Bioavailability: 61.9% CL: 29.6 L/h VD: 605 L279 | i.v., p.o.69 | • Initial dose: p.o. 2–12.5 (10.0) mg of P/kg per day, i.v. 1–20 (3.0) mg of CsA/kg per day q.d. or b.i.d. for 2–13 h, after 7–40 (21) days change to p.o. administration for 150–365 days, i.v. 15 mg of MTX/m2 on POD 1 and 10 mg of MTX/m2 on POD 3, 6 and 1169 | |
| Mouse | 4.1±0.6280 | At 2.6 mg/kg i.v.: CL: 0.015 L/kg/h VD: 0.07 L/kg280 | c.p.281 | • 35 mg of CsA/kg per day281 | ||
| Rat | 6.0–10.0282 19.4967 | CL: 0.20±0.04 L/kg/h MRT: 23.20±5.46 h VD: 4.54±0.68 L/kg67 | Implantation of CsA collagen matrix around the homograft,283 s.c.284, 285 | • 10 mg of CsA/kg per day284 • 1 mg of CsA/kg per day bound in collagen matrix283 | ||
| Dog | 8.567 | Poor oral absorption and bioavailability, preparations such as Neoral, Atopica and Sanimmune are not bioequivalent, Neoral achieves much higher blood levels than Atopica (veterinary-labeled oral product): rapid absorption, bioavailability of 23–45%, filled gastrointestinal tract reduces bioavailability by ~20%, high distribution in the liver, fat and lymphocytes212 CL: 6.96 L/h VD: 4.3 L/kg67 | conjunctival, p.o., topical,203 i.m., inhalation, i.v.251 | • 10 mg of CsA/kg/12 h, 2–3 mg of Az/kg/48 h, 0.5 mg of P/kg/12 h (then tapered off)286 • 10–25 mg of CsA/kg/12 h212 • Neoral: 5–10 mg of CsA/kg/12 h212 • Target trough levels: 100–500 ng/ml212 | ||
| Pig | 7.7±2.6287 8.1±1.5218 | Systemic availability: 18±6% CL: 0.87±0.11 L/kg/h VD: 6.5±1.7 L/kg In general: requires 2–4 times higher i.v. or oral dose of CsA than humans218 | i.m., i.v.,288, 289 p.o.72 | • 20 mg of CsA/kg per day288, 289 • 10 mg of CsA/kg per day288, 289 • Neonatal: 4 mg of CsA/kg per day, juvenile: 30 mg of CsA/kg per day72 | ||
| Sheep | 12.1±3.1290 | Abomasal bioavailability (6.4 mg/kg): 0.26±0.09 CL: 0.45±0.05 L/kg/h MRT: 9.6±4.1 h MAT (6.4 mg/kg): 4.7±11.1 h VD: 4.4±2.0 L/kg290 | i.v.,73 p.o. 290 | • 2–3 mg of CsA/kg per day b.i.d., 10 mg of Kc/kg b.i.d.73 | ||
| Tacrolimus (Tcr) | ||||||
| Indirect inhibition of calcineurin Binds to intracellular FK-binding protein Selective inhibition of T cell proliferation98, 99 | Human | In liver-transplanted patients: 12.1±4.7,291 12.0,292 5.5–16.6293 | Accumulation index during chronic therapy: 1.3 CL after p.o.: 0.21±0.08 L/kg/h VD after p.o.: 2.4±0.8 L/kg294 | p.o.295 | • 0.05 mg of Tcr/kg per day b.i.d.200 | |
| NHP | Baboon: 9.6±2.0 (at 10 mg/kg p.o.)293 | 10 mg/kg p.o.: peak plasma concentration of 8.1±1.0 ng/ml reached after 3.8±1.4 h293 | i.v.,296 p.o.104, 297 | • 4 mg of Tcr/kg per day104, 297 • 1 mg of Tcr/kg per day, 2 or 5 mg of ASKP1240/kg on POD 0, 3, 7, 11 and 14 and on POD 28–168 0.51 mg of Tcr/kg per day, 1 or 2.5 mg of ASKP1240/kg two times per week296 | ||
| Rat | 2.4±0.3 (at 1 mg), 2.5±0.3 (at 5 mg)298 | 100 times more potent than CsA at 1 mg/kg: CL: 0.42 L/kg/h VD: 4.62±0.03 L/kg298 | i.m.,293 i.v.,298 p.o.299 | • 0.1 mg of Tcr/kg per day or 1 mg of Tcr/kg per day299 | ||
| Dog | 9.0–13.2 (at 0.2 mg/kg i.v.), 5.6–7.9 (at 1 mg/kg p.o.)293 | Bioavailability after 1 mg/kg p.o.: 5–12% Peak plasma concentration (1.9–4.9 ng/ml) after 1–2 h at 0.2 mg kg i.v.: CL: 0.12–0.19 L/h i.m.: slow absorption, steady-state after 4.0–12.0 (continued up to 24.0)293 | i.m., i.v.,293 p.o.300 | • 0.5 mg of Tcr/kg per day, 5 mg of FTY720/kg per day300 | ||
| Pig | n.s.i. | n.s.i. | i.v. as continuous infusion,301 i.v.302 | • 0.1–0.2 mg of Tcr/kg for 12 days301 • 0.04 mg of Tcr/kg per day207 • 0.15 mg of Tcr/kg per day for 12 days103 | ||
| Sheep | n.s.i. | n.s.i. | i.v., p.o277 | • 2 days prior Tx: 0.02–0.15 mg of Tcr/kg per day p.o., 1.5 g of MMF per day p.o., perioperatively: 50 mg of ATG i.v. (DDI), 500 mg of MP i.v. 40 mg of MP per day p.o. on POD 1–15 (tapered off towards to 16 mg of MP per day) p.o.277 | ||
Abbreviations: ATG, antithymocyte globulin; Az, azathioprine; b.i.d., twice a day; CL, total plasma clearance; DDI, duration drip infusion; i.m., intramuscular; i.p., intraperitoneal; i.v., intravenous; Kc, ketoconazole; MAT, mean absorption time after oral dosing; MP, methylprednisolone; MRT, mean residence time; MTX, methotrexate; NHP, non-human primate; n.s.i., no sufficient information available; P, prednisolone; p.o., oral; POD, postoperative day; q.d., once a day; s.c., subcutaneous; Tx, transplantation; VD, volume of distribution.
The combination of CsA with adjuvant drugs permits dose reduction, leading to less frequent or mitigated adverse effects without compromised treatment efficacy.77, 78 Optimal combination regimens have been investigated in numerous clinical trials. It was shown that the combination of CsA (or tacrolimus (Tcr)) with MTX is highly efficient for preventing GvHD.79 Such combination treatments do not only impact the frequency of acute GvHD but also improve long-term survival in human patients following bone marrow transplantation and therefore have become a standard approach.69 The more recently studied combination of CsA with MMF accelerates bone marrow engraftment while reducing side effects and the frequency of mucositis compared with the CsA/MTX combination.80 The combination of CsA with ketoconazole, a CYP inhibitor, reduces CsA clearance and thus the risk for opportunistic fungal infections.77 This allows the reduction of CsA doses by up to 77% in humans when increasing ketoconazole to 200 mg per day.77
A transient increase in ovine serum bilirubin from 1 to 29±11 μm has been described during CsA treatment at 2–3 mg/kg (twice a day in combination with 10 mg/kg ketoconazole) for 9 weeks73 (Supplementary Table 2). Two weeks after the termination of CsA treatment, serum bilirubin levels returned to 2±0.3 μm.73 Moreover, albumin decreased from 32 to 22 g/l during the study.73 Other studies reported a slight reduction in serum potassium from 4.3 to 4.0 mmol/l when 12 mg/kg per day CsA was applied.76 CsA and prednisolone can further affect hemodynamic parameters such as blood pressure and heart rate (tachycardia), decrease the heart index and both decrease and enhance peripheral resistance in human patients.81 In sheep, a heart rate increase from 58 to 75 beats per min and a concomitant decrease in the cardiac stroke volume from 86 to 68 ml per beat have been observed.76 Mean arterial blood pressure increased from 73 to 90 mm Hg and peripheral resistance from 16 to 21 mm Hg, respectively, whereas renal function remained unaltered.76 Those results have been confirmed by a number of studies in human patients.82, 83 Notably, the blood pressure effects in humans are the opposite of those observed in rats at 20 mg/kg but are not altered when 10 mg/kg is given.84 When investigating the cause of the increase in peripheral resistance, Whitworth et al.76 hypothesized that CsA may directly stimulate the sympathetic nervous system. This assumption was based on the observation that kidney function was normal in the sheep studies, providing no evidence for a renal cause underlying the increase in blood pressure.76 However, more recent work confirms activation of the renin–angiotensin system in sheep.85 A number of urogenital CsA complications have been observed in both humans and other animals. In particular, nephrotoxicity is frequently observed in human patients at 5 mg/kg per day CsA given orally.74 Nephrotoxicity is not restricted to kidney transplantation but is also observed with the transplantation of other solid organs.85 Frequencies of renal dysfunction at CsA trough levels between 150 and >250 ng/ml after bone marrow transplantation range between 73 and 100% in humans.86 Consequences include fluid retention87 and anuria.88 The situation is similar in rats, in which reduced renal inulin clearance (at dosages of 25 and 50 mg/kg CsA as a single dose) and reduced filtration have been observed.89 Again, this stands in opposition to the situation in sheep models, in which these effects have not been observed. Despite a distinct reduction in potassium and sodium excretion, effective plasma clearance increased, and no alteration of the glomerular filtration rate or aldosterone concentration have been observed.73, 76 This led to the assumption that CsA may exert direct effects on the renal tubular system and its epithelia,76 and, in fact, chronic tubular necrosis has been described histologically in sheep.73 Other studies have indicated that high-dose CsA application may decrease TNF-α levels,90 whereas gastrointestinal side effects were reported at 5 mg/kg per day in dogs.3 Similar results have not been reported for sheep during dermal grafting.73
Monitoring of the CsA concentration in the peripheral blood was clinically implemented in the early 1980s to reduce the risk of inappropriate individual dosing and since then has been considered decisive.91 The gold standard for monitoring CsA levels is high-performance liquid chromatography because alternative methods have proven fault-prone, particularly at higher doses.73
A number of studies have investigated appropriate methods to monitor the biological effects of applied CsA doses in clinical settings. For example, a positive correlation between calcineurin inhibition and the maximum CsA blood concentration has been described.92 The assessment of CsA trough levels as the indicative parameter represents a relatively old but reliable approach.1 Thus, measuring trough levels is the current gold standard in clinics, but there is some evidence that the correlation between CsA trough levels and adverse events or effects is not very strong.91 A more precise method to monitor CsA levels is the area under the concentration time curve (AUC) approach. However, this is relatively time-consuming and requires that a high number of blood samples be obtained and is thus less practical in the clinical setting.58 Some studies have shown that CsA trough levels are inferior to AUC0–4h for predicting clinical manifestations and side effects.93 A more practical approach may be the measurement of CsA levels 2 h after oral application as this parameter correlates well with the AUC0–4 h.93
Tacrolimus
Tcr (or FK506) is a potent macrolide antibiotic with similar pharmacokinetic properties to CsA but with a 10– 100-fold therapeutic potency.94, 95, 96 Tcr forms a complex with the intracellular FK-binding protein, which in turn binds to calcineurin, preventing the NFAT activation cascade at a step that is more upstream compared with CsA (Figure 1).97, 98, 99 Similar to CsA, Tcr is primarily metabolized by CYP450.97
Tcr is commonly used following kidney transplantation in humans and is widely applied in experimental transplantation studies.97 It replaced CsA in experimental kidney transplantation in cynomolgus monkeys, and complication-free survival (>90 days) was reported at 2 mg/kg per day.100 Dose escalation to 10 mg/kg per day oral administration was shown to significantly improve the health of experimental subjects without affecting relevant biochemical parameters, such as potassium, calcium, urea and creatinine, and without causing histopathological alterations in the liver, kidney and brain.101 In turn, reduction of the Tcr dose to 0.5 or 1 mg/kg per day ensured mean survival of the grafts until POD 11 or 21 after kidney transplantation, respectively.100 Target blood levels of Tcr in baboon xenotransplantation models (50% successful engraftment) were 30–40 ng/ml and were reached with dosages of 0.15–0.3 mg/kg per day.102 In pigs, the target blood level for successful immunosuppression after renal allograft transplantation was 35 ng/ml, reached using a dosage of 0.15 mg/kg per day103 (Table 6; for an overview of relevant indications, see Supplementary Table 1).
Table 6. Rapamycin and Ev and their uses in experimental transplantation studies.
| Mechanism of action | Species | Plasma half-life (h) | Pharmacokinetics bioavailability/VD | Application form | Dosage examples | |
|---|---|---|---|---|---|---|
| Rapamycin (Rpm) | ||||||
| Binds to intracellular protein FK506 Resulting complex inhibits mTOR Downregulation of protein synthesis and cell proliferation108 | Human | 62.0,303 43.8–86.5304 | Large inter- and intrasubject variability in oral dose clearance Low systemic availability: 14% rapid absorption: 1–2 h VD: 1.7 L/kg305 | p.o.117 | • 24–48 h after Tx 6 mg of Rpm per day, followed by 2 mg of Rpm per day, CsA (trough level: 200–400 ng/ml), 250 mg of MP per day (tapered off to 5–10 mg of MP per day)117 | |
| Mouse | 2.1–4.8 (at 10–100 mg/kg)306 | At 15 mg/kg: serum levels higher for s.c. than for p.o.307 | i.p.,308 p.o., s.c.307 | • 50 000 IU per day recombinant human IL-2 starting 6 days prior Tx, 0.25 mg of anti-CD25 antibody per mouse on POD 0, 0.5 mg of anti-CD8 antibody per day on POD 0, 2, 4, 7, 1 mg of Rpm/kg p.o. starting on POD 0308 | ||
| Rat | 25.0121 | Poor oral bioavailability: <4%309 | Inhalation,309 p.o.307 | • 1 mg of Rpm/kg per day for 24 weeks113 | ||
| Dog | >60.0310 99.5±89.5 h (at 0.1 mg/kg p.o.)311 | At 0.1 mg of p.o.: AUC0–48 h: 140±23.9 ng/h/ml Maximum plasma concentration: 8.39±1.73 ng/ml311 | p.o., s.c.115 | • 15 mg of CsA/kg b.i.d., 0.05 mg of Rpm/kg per day115 | ||
| Pig | 7.3±0.6312 | n.s.i. | p.o.114 | • 1.5 mg of Rpm/kg, 15 mg of CsA/kg114 | ||
| Everolimus (Ev) | ||||||
| Similar to Rpm313 | Human | 28.1±8.4314 | CL: 15.3±11.6 L/h VD: 250±103 L/m2 314 | p.o. 313 | • 0.75 mg of b.i.d.313 | |
| Mouse | 3.0–6.0315 9.8 (at 0.9 mg/kg)316 | At 0.9 mg/kg i.v.: CL: 0.05 L/kg/h VD: 0.42 L/kg316 | i.v.,316 p.o.315 | • 1.5 mg/kg b.i.d.315 | ||
| Rat | 14.3316 | At 1 mg/kg i.v.: CL: 1.26 L/kg/h VD: 53 L/kg316 | i.v., p.o.316 | • 1.5–15 mg/kg per day p.o., 1–10 mg/kg per day i.v.316 | ||
| Pig | n.s.i. | Mean 24-h trough concentration: 16.3±6.6 ng/ml317 | p.o.317 | • 1.5 mg/kg per day317 | ||
| Sheep | n.s.i. | n.s.i. | p.o.317 | • 1.5 mg/kg per day; starting 4 days preoperatively317 | ||
Abbreviations: BID, twice a day; CL, total plasma clearance; CsA, cyclosporin A; i.m., intramuscular; i.p., intraperitoneal; i.v., intravenous; IL-2, interleukin-2; IU, international units; MMF, mycophenolate mofetil; MP, methylprednisolone; mTOR, target of Rpm; NHP, non-human primate; n.s.i., no sufficient information available; p.o., oral; POD, postoperative day; s.c., subcutaneous; Tcr, tacrolimus; Tx, transplantation; VD, volume of distribution.
A common and severe adverse effect of Tcr is nephrotoxicity.97 Lower doses of Tcr (1.0 mg/kg per day orally) could be achieved by concomitant application of the synergistically acting rapamycin (Rpm; 0.5 mg/kg per day orally) in rats and vervet monkeys, significantly reducing the frequency of nephrotoxicity.104 Specific side effects in different species are shown in Supplementary Table 2.
mTOR inhibitors
Rpm and everolimus: an example for mTOR inhibitors
Rpm is a lipophilic substance that binds to FK-binding protein (FKBP12).105, 106, 107 The resulting complex inhibits a protein named mTOR (Target of Rapamycin), which leads to decreased protein synthesis.105, 106, 107 In particular, Rpm arrests mitosis in the G1 phase, while binding of transcription factors to the proliferating cell nuclear antigen is also blocked, together inhibiting cell proliferation.108 Rpm also impedes B-cell functionality via decreased levels of B-cell-activating factor.109 The drug further inhibits T-cell activation by APCs by inhibiting antigen endocytosis into dendritic cells.110 Moreover, Rpm increases growth factor expression (TGF-β1), which enhances fibrogenesis and leads to long-term allograft function.111 The agent is metabolized by CYP3A in the liver and gut.112
Rpm can be applied to prevent and to counter the rejection of transplanted tissue, while various studies have reported antitumorigenic effects and reduced rates of infection with post-transplantation immune suppression110, 111 (Supplementary Table 1). Monotherapy in a rodent renal allograft transplantation model (1 mg/kg per day for 24 weeks) has been described,113 but the combination of Rpm and CsA is frequently reported for canine and swine transplantation models.114, 115 Detailed dosage regimens in different species are shown in Table 6. Side effects of Rpm application include elevated liver enzymes, thrombocytopenia and mild diarrhea.116, 117, 118 These side effects positively correlate with the applied dose117 (Supplementary Table 2).
Everolimus (Ev) is a semisynthetic macrolide from the family of mTOR inhibitors.119 It is structurally similar to Rpm with similar immunosuppressive properties120 but has better bioavailability.121 Ev is more hydrophilic, has a shorter elimination half-life (~30 h, half of Rpm) and requires less time (4 days vs 6 for Rpm) to reach a steady state.119 Similar to the calcineurin inhibitors and Rpm, Ev is biotransformed by CYP3A.122 In clinical trials, Ev was used at two doses (1.5 and 3.0 mg per day) in combination with CsA and steroids in de novo renal transplant recipients.119 The adverse events of Ev are, for the most part, manageable. One common side effect is hyperlipidemia, with increased serum cholesterol and triglyceride levels (in 30–50% of patients).123
Unfortunately, the high frequency of adverse Rpm effects calls for alternative approaches. Recently, the coapplication of Rpm with regulatory T cells (Tregs) has been investigated. This combination enables donor-specific tolerance after transplantation while reducing the side effects of RPM.124 In this combination, Rpm stimulates Tregs and selectively blocks effector T cells (Teffs), efficiently preventing graft rejection.124 Synergistic effects of Rpm and Tregs were obtained via the depletion of a negative regulator of the mTOR pathway through Tregs.125 Given the exceptional benefits and the favorable therapeutic profile of this combination, some authors even expect the treatment regimen to ultimately achieve long-term, donor-specific tolerance after transplantation,124 one of the ‘holy grails' of clinical immunology.126
EXPERIMENTAL APPROACHES
The arsenal of ‘classic' immunosuppressants, including CsA, Tcr and Rpm (including adjuvants), has been increasingly amended by novel, sometimes experimental approaches, including the use of Tregs and anti-CD4 antibodies, which will be reviewed in the following paragraphs.
Regulatory T cells
In vitro studies have identified a sub-population of CD4+CD25+ Tregs that selectively inhibits the proliferative responses of both Teffs and naive T cells127 (see Figure 2). These Tregs have also been observed in vivo, accounting for ~1–10% of all T cells, but are unable to completely prevent graft rejection on their own.128 Nevertheless, some studies have shown a positive correlation between the number of circulating Tregs and transplant survival.129
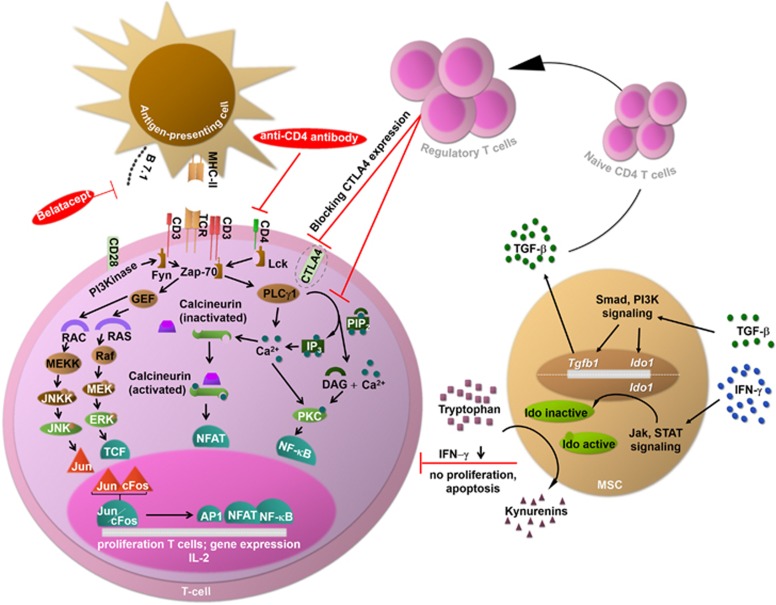
Figure 2.
Mechanisms of action of the experimental approaches. Experimental approaches for immunosuppression comprise MSCs, Tregs, anti-CD4 antibodies and substances blocking costimulatory pathways. MSCs act on CD4+ T cells via IFN-γ and TGF-β. First, the IFN-γ concentration is reduced by MSCs, inhibiting proliferation and inducing the apoptosis of T cells. Second, the TGF-β concentration is increased by MSCs, driving the differentiation of naive CD4+ T cells into Tregs. In turn, Tregs directly inhibit CD4+ T-cell proliferation via the suppression of Ca2+-dependent pathways and indirectly act by downregulating costimulatory molecules such as CTLA4. Finally, anti-CD4 antibodies and substances blocking costimulatory pathways (belatacept) impair T-cell activation. CTLA4, cytotoxic T-lymphocyte-associated protein 4; DAG, diacylglycerol; ERK, extracellular signal-regulated kinase; Fyn, tyrosine-protein kinase; Ido, indolamine-2,3-dioxygenase; GEF, guanine-nucleotide exchanging factor; IFN-γ, interferon-γ IP3, inositol triphosphate; Jak, Janus kinase; JNK, c-Jun N-terminal kinase; JNKK, c-Jun N-terminal kinase kinase; Lck, lymphocyte-specific protein tyrosine kinase; MEK, mitogen-activated protein kinase kinase; MEKK, serine/threonine-specific protein kinase; MSC, mesenchymal stem cell; NF-κB, nuclear factor 'κ light-chain enhancer' of activated B cells; Pip2, phosphatidyl inositol bisphosphate; PI3K/Pi3 kinase, phosphoinositide 3-kinase; PLCγ, phospholipase; PKC, protein kinase C; RAC, guanosine triphosphate; RAF, serine/threonine-specific protein kinase; RAS, guanosine-nucleotide-binding protein; STAT, signal transducer and activator of transcription; TCF, transcription factor; TCR, T-cell receptor; TGF-β, tumor growth factor-β Tregs, regulatory T cells; Zap-70, zeta-chain-associated protein kinase 70.
The immunological impact of Tregs can be tremendous. Recently, syngeneic i.v. transplantation of 2 × 106 bone marrow-derived hematopoietic stem cells with 1 × 106 syngeneic Tregs resulted in a 100% survival rate among transplanted animals and full donor chimerism130 (Table 7; for an overview of relevant indications, see Supplementary Table 1). Other studies demonstrated the successful introduction of Tregs in several animal models, which may lead to a scientific breakthrough in transplantation medicine and the treatment of autoimmune diseases.131, 132, 133 Adverse effects after the application of Tregs have been reported in patients, mice and dogs, ranging from alterations in liver function to cytotoxicity and tumor induction134, 135 (Supplementary Table 2).
Table 7. Cell-based strategies for immunosuppression and immunomodulation.
| Mechanism of action | Species | Application form | Dosage examples | |
|---|---|---|---|---|
| Regulatory T cells (Tregs) | ||||
| Inhibit proliferative response of Teffs and naive T cells127 More effective in combination with Rpm125 | Mouse | i.v.130 | • 2 × 106 BMSCs, 1 × 106 Tregs130 | |
| Rat | i.v.318 | • 0.5 ml (~11.7 μg) Tregs exosomes318 | ||
| Dog | i.v. as infusion135 | • 0.6–13.7 × 108/kg donor peripheral blood lymphocytes135 | ||
| Pig | i.v.319 | • Transfusion of 15 × 106 irradiated donor-specific peripheral blood leukocytes/kg, 10 mg of CTLA4lgG4/kg after reperfusion, 10–13 mg of CsA/kg per day for 12 days319 | ||
| Mesenchymal stem cells (MSCs) | ||||
| Avoid host immunoreactions by not expressing MHC-II139 Immunomodulation via secretion of various mediators143 Regulation of T- and B-cell proliferation142, 145 | Human | i.v. as infusion150 | • 1.4 × 106/kg MSCs (0.4–9 × 106)141 • 0.6 × 106/kg MSCs150 | |
| Mouse | i.v. (Fricke, 2014, unpubl. data) | • 5 × 104 MSCs (Fricke, 2014, unpubl. data) | ||
| Rat | i.a.320 | • 1 × 104 MSCs 24 h after stroke (occlusion and reperfusion)320 | ||
| Dog | i.v. as infusion321 | • 1–30 × 106 MSCs/kg per day 2–5 times per week321 | ||
| Pig | i.m. around the graft322 | • 3 × 106 MSCs322 | ||
| Sheep | i.v.323 | • 2 × 106 MSCs/kg323 | ||
Abbreviations: BMSCs, bone marrow stem cells; CL, total plasma clearance; CsA, cyclosporin A; CTLA4, cytotoxic T-lymphocyte-associated antigen 4; i.a., intra-arterial; Ig, immunoglobulin; i.m., intramuscular; i.v., intravenous; MHC, major histocompatibility complex; Rpm, rapamycin; Teff, effector T cell; Tx, transplantation; VD, volume of distribution.
Mesenchymal stem cells
Mesenchymal stem cell (MSCs) are pluripotent stromal cells that can differentiate into mesenchymal tissues such as bone, cartilage or adipose tissue.136 MSCs can easily be derived from numerous sources and expanded in vitro.136, 137 The cells are primarily obtained from bone marrow specimens, in which their frequency ranges between 0.001 and 0.01%.138
In addition to their role in regenerative medicine as a therapeutic cell population that has been experimentally tested for a wide range of indications, MSCs also possess considerable immunomodulatory properties. Because MSCs do not express major histocompatibility complex class II (MHC-II) and many costimulatory molecules, including CD80, CD86 and CD40, they can passively avoid host immunoreactions.139 Moreover, they can actively suppress or modulate immune responses, including complex interactions with T- and B-lymphocytes, dendritic and NK cells.140 T-cell inhibition by MSCs is not solely MHC-mediated and thus MSCs can suppress both allogeneic and autologous T-lymphocytes.141, 142
Their immunosuppressive effects are primarily based on soluble mediators but also direct cell interactions via vascular cell adhesion molecule, intercellular adhesion molecule-1, activated leukocyte cell adhesion molecule, lymphocyte function associated and integrins.143 MSCs can further induce Tregs144 and inhibit B-lymphocyte proliferation by IFN-γ145 (see Figure 2). MSCs can also reduce antigen presentation on dendritic cells146 and lead to the increased expression of a number of hematopoietic factors, such as stem cell factor, Flt3-ligand, thrombopoietin, leukemia inhibitory factor, IL-6 and IL-11.147 MSCs are used both experimentally and clinically to support the engraftment of hematopoietic stem cells.148
Significantly enhanced survival rates were observed after the administration of 1.4 (0.4–9.0 × 106) MSCs/kg in a phase II clinical trial enrolling patients with severe GvHD.149 Interestingly, the results were independent of the MSC donor, indicating a reproducible and strong effect that could be used even in patients with steroid-resistant chronic excessive GvHD, resulting in partial or complete remission in 14 of 19 patients150 (Table 7).
MSCs have been experimentally applied in models of multiple sclerosis, myocardial infarction and stroke, solid organ transplantation, liver cirrhosis and chronic inflammatory bowel diseases151 (for an overview of relevant indications, see Supplementary Table 1). Owing to their distinct immunomodulatory properties, MSCs can be applied relatively safely in non-autologous approaches. However, the large size of MSCs can lead to complications after intravascular injection.152 Clotting of pulmonary capillaries has been described after intravenous application,153 whereas small cerebral infarcts were observed after intra-arterial (common carotid artery) injection154 (Supplementary Table 2).
Antibodies
In addition to conventional immunosuppression, poly- and monoclonal antibodies can lead to severe immunosuppression in recipient animals. Anti-lymphocyte globulin (ALG) and anti-thymocyte globulin (ATG) are used to prevent graft rejection155 and GvHD.156 These anti-human T-cell xeno-antisera target lymphocyte sub-populations from peripheral blood, lymph nodes, thymus and some T-cell tumors.155 Animals (rabbit, sheep, horses, donkeys and calves) can be immunized to produce ATG and ALG. ATG and ALG are immunosuppressive drugs that exert immunomodulatory effects such as T- and B-cell depletion, modulation of leukocyte and endothelium interactions and the induction of Tregs and natural killer cells.157 Furthermore, some authors have observed dysfunctions in antibody production and macrophage activity, as well as defective antibody opsonization.155 ALG and ATG are commonly used in transplantation animal models, with dosage and application regimens shown in Table 8 (for an overview of relevant indications, see Supplementary Table 1). Side effects such as anaphylaxis, leukopenia, thrombocytopenia and granulocytopenia may occur158 (Supplementary Table 2). When establishing a new antibody therapy, it should be ensured that the antibody only binds to the desired antigen. If the therapeutic antibodies also recognize other targets, this can lead to serious side effects such as the induction of cytokine release syndrome, a life-threatening systemic release of cytokines.159 Nonspecific antibody binding can be avoided by ex vivo treatment of the graft or by performing prior testing in a humanized mouse model.160
Table 8. Antibodies for immunosuppression and immunomodulation.
| Mechanism of action | Species | Plasma half-life (h) | Pharmacokinetics Bioavailability/VD | Application form | Dosage example |
|---|---|---|---|---|---|
| Anti-lymphocyte globulin (ALG) | |||||
| Immunomodulatory effects on T and B cells, leucocytes and endothelium cells T and B cell depletion Induction of Tregs157 | Human | 25.5 days324 | VD: 2.0 l CL: 0.017 l/h 324 | i.v.325 | • 3 mg of CsA/kg on POD 1–180, 10 mg of MTX per m2 on POD 1,3,6, total dose: 30 (20–90) mg of ALG/kg on POD 1–3326 • 15 and 30 mg of ALG per kg per day 325 |
| NHP | n.s.i. | n.s.i. | s.c.327 | • 40 mg of ALG/kg on POD −1,1327 | |
| Mouse | n.s.i. | n.s.i. | i.p.328 | • 0.1 mg of ALG per mouse per day on POD 0,1328 | |
| Rat | n.s.i. | n.s.i. | i.p.329 | • 1 ml ALG-serum per rat, 2 × 107 donor splenocytes as intrathymic injection329 | |
| Dog | n.s.i. | n.s.i. | p.o., s.c.330 | • 2 ml ALG-Serumper kg per day on POD −5 to −1, 20 mg of CsAper kg per day on POD 2–120330 | |
| Anti-thymocyte globulin (ATG) | |||||
| Human | 14.3–45 days331 36 h–18 days332 | VD: 0.07–0.17 l/kg 331 Maximum serum levels reached 1–3 days after final dose332 | n.s.i.333 | • 1 mg of ATG/kg on POD 1 and 0.5 mg of ATG/kg on POD 2–4333 | |
| NHP | n.s.i. | n.s.i. | i.v.102 | • Splenectomy and TBI on POD −7, 4 mg of LoCD2b/kg on POD −5, −4, i.v. 20 mg of ATG-serum/kg on POD −3, TI with 700 cGy on POD −2, i.v. 20 ng PC/kg/h On POD −2 to 4, 0.15–0.3 mg of Tcrper kg per day on POD −6 to 28102 | |
| Mouse | n.s.i. | n.s.i. | i.p.334 | • 0.1 mg of ATGper mouse twice per week, 0.5 mg of CTLA4-Igper mouse on POD 0, 0.25 mg of CTLA4-Igper mouse on POD 2,4,6,8,10334 | |
| Rat | n.s.i. | n.s.i. | i.v.335 | • Single dose of 10 mg of ATG/kg at the time of engraftment335 | |
| Dog | n.s.i. | n.s.i. | p.o., s.c.336 | • 5 mg of CsA/kg BID from 1 day prior to 12 weeks after infection, 7.5 mg of MMF/kg BID starting on day of infection for 4 weeks, 1 mg of ATGper kg per day. Starting 2 days prior to 2 days after infection336 | |
| Pig | n.s.i. | n.s.i. | n.s.i.337 | • CsA (target blood level:>200 ng/ml), 50 mg of Az per animal BID, 50 mg of P per animal per day tapered, 115 mg of ATG per animal BID337 | |
| Sheep | n.s.i. | n.s.i. | i.v., p.o.277 | • 0.02–0.15 mg of Tcrper kg per day on POD −2, 1.5 g of MMF per day, 40 mg of MP per day on POD 1–15 (tapered off towards to 16 mg of MP per day), perioperatively: 50 mg of ATG (DDI), 500 mg of MP277 | |
| Rituximab (RTB) | |||||
| Anti-CD20-antibody which binds human complement lysis of lymphoid B Cells289 | Human | 88.0 (at 20–30 mg)338 445.0±361.0 (at 250–375 mg per m2)339 | CL: 0.044±0.064 l/h MRT: 516.7±247.7 h VD: 11.16±3.20 L327 | n.s.i.162 | • 375 mg of RTB per m2 weekly162 |
| NHP | Cynomolgus monkey: ~168.0 (at 20 mg/kg s.c.)340 | At 20 mg/kg s.c.: maximum plasma concentration of 300 μg/ml at day 2 At 10 mg/kg i.v.: Maximum plasma concentration of 328.5±34.4 μg/ml 1 h after administration328 | i.v.163, s.c.341 | • 0.4–6.4 mg of RTB/kg, for 40 days163 • 1.5 mg of ATG/kg, 19 mg of RTB/kg weekly starting on POD 7, Tcr at dosages to reach blood levels of 20–30 ng/ml, splenectomy on POD 7341 | |
| Mouse | 192.0 and 264.0 (at 1 and 40 mg/kg i.v.)342 | At 1 mg/kg i.v: CL: 8.3 ml/kg/h VD: 0.12 l/kg 330 | i.v., s.c.342 | • 1–40 mg of RTB/kg 342 | |
| CD28/B7 | |||||
| Plays an important role in T cell activation, proliferation, and differentiation331 | Human | 196.6±57.2 331 | CL: 0.51±0.14 ml/kg/hVD: 0.12±0.03 l/kg 331 | i.v.343 | • 5 mg/kg in a 4 or 8 week interval343 |
Abbreviations: Az, azathioprine; BID, twice a day; cGy, centigray; CL, total plasma clearance; CsA, cyclosporin A; DDI, duration drip infusion; GvHD, graft-versus-host-disease; h, hour; i.a., intraarterial; i.v., intravenous; LoCD2b, rat-anti-primate CD2 IgG2b; MMF, mycophenolate mofetil; MP, methylprednisolone; MRT, mean residence time; MTX, methotrexate; NHP, non-human primate; n.s.i., no sufficient information available; P, prednisolone; p.o., oral; PC, prostacyclin; POD, postoperative day; s.c., subcutaneous; TBI, total body irradiation; Tcr, tacrolimus; TI, thymic irradiation; Tregs, regulatory T cells; Tx, transplantation; VD, volume of distribution.
Rituximab is a human immunoglobulin G1 (IgG1) kappa monoclonal antibody.155 Its variable regions were isolated from a murine anti-CD20 antibody (IDEC-2B8).155, 161 Rituximab binds to malignant and normal B cells expressing the CD20 molecule with high affinity162 but not to other normal cell types.155, 163 The antibody binds to human complement, lyses lymphoid B cells and other CD20+ cells through antibody-dependent cellular cytotoxicity, induces apoptosis in human lymphoma cells164 and inhibits cell proliferation via the induction of tyrosine phosphorylation.155, 162 This antibody was tested in preclinical studies using cynomolgus monkeys (Supplementary Table 1). One model used Rituximab administration at 0.4–6.4 mg/kg163 (Table 8). Cells that bound the rituximab molecule disappeared completely, and full B-cell reconstitution was observed within 40 days.163 High dose studies performed with rituximab at 16.8 mg/kg lead to the depletion of all B cells in the lymph nodes in cynomolgus monkeys.163 Specific toxicities were not observed in recipient animals163 (Supplementary Table 2). The first ex vivo studies also showed no effects (binding/depletion) on canine B cells, and Rituximab failed in canine lymphoma treatment.165 In human patients, a weekly Rituximab dose of 375 mg/m2 is safe and shows significant clinical activity in many lymphoma patients.155, 162
Furthermore, it is experimentally possible to reduce GvHD severity without conventional immunosuppression (one of the most important requirements for transplantation immunology126) via ex vivo treatment of the graft with anti-human CD4 antibodies; notably, the antitumor effects of the graft are not minimized166, 167 (one of the most important requirements for allogeneic hematopoietic stem cell transplantation168).
Blocking costimulatory pathways
Costimulatory signals have an important role in T-cell activation, proliferation and differentiation.169 The CD28/B7 costimulatory pathway is one of the best characterized pathways. CD28 is constitutively expressed on all T-cell subsets in mice and on 95% of CD4+ T cells as well as on 50% of CD8+ T cells in humans.170 B7 comprises in two subforms, B7.1 (CD80) and B7.2 (CD86), and is constitutively expressed on the surface of APCs.171 It is also found in T cells.172 The following three signals are required for complete T-cell activation: (i) interaction of the bound antigen with a T-cell receptor (TCR), (ii) binding of CD80 and CD86 molecules on an APC to the CD28 receptor on T cells and (iii) binding of CD28 and B7 in the presence of TCR stimulation, leading to IL-2 expression,173, 174, 175 cytokine transcription176, 177, 178, 179 and T-cell proliferation.180, 181, 182 Failure of costimulation leads to T-cell anergy, that is, reduced proliferation, differentiation and cytokine production.183 Inhibition of the costimulatory CD28/B7 pathway is one approach to prevent graft rejection, for example, by administering belatacept.184, 185 Belatacept binds to B7.1 and B7.2 on APCs, prevents CD28-mediated costimulation and thus impairs T-cell activation185 (see Figure 2). Typical dosing ranges between 5 and 10 mg/kg, and belatacept serum concentrations between 136 and 238 μg/ml have been described after the first application. The median elimination half-life is 8–9 days.185 Studies using other costimulatory inhibitors are currently ongoing but thus far have revealed mixed results and some safety concerns.186
Recommendations for immunosuppressive treatments after experimental tissue and solid organ transplantation
Sufficient immunosuppression is required for the successful realization of experimental transplantation in animals. In principal, long-term survival after transplantation can be ensured in the following two different ways: by the administration of immunosuppressive medication or by the induction of immunological tolerance against the donor tissue.
The classical immunosuppressive protocols have been established for years and usually include a mono- or combination therapy of immunosuppressive drugs targeting immune cells. Based on synergistic effects between different immunosuppressants,187 a combined approach using conventional agents such as CsA, MMF and prednisolone is recommended, especially for long-term immunosuppression.18, 19 This also allows for the dose reduction of single immunosuppressants, ideally leading to less frequent and less severe side effects.77
The induction of immunological tolerance can be initiated by newly developed cellular therapy approaches.131, 132, 133 One decisive factor for long-term tolerance is hematopoietic chimerism after transplantation.188 This can be achieved, for example, by the infusion of donor lymphocytes and concurrent high-dose CY treatment after transplantation.189 However, valid data on cell-based therapies are missing for several animal models, despite very intensive research in recent years. For this reason, successfully established immunosuppressive and immunomodulatory protocols are listed with respect to model and species (Tables 9 and 10).
Table 9. Model-based recommendations for immunosuppressive and/or immunomodulatory protocols after cell or tissue transplantation.
| Species | Indication | Strategy | Survival | Immunosuppressive/immunomodulatory protocol | Target serum levels |
|---|---|---|---|---|---|
| NHP | Bone marrow xenoTx102 | Immunopharmacotherapy | >187 days | Splenectomy and TBI on POD −7, 4 mg of LoCD2b/kg on POD −5,−4, i.v. 20 mg of ATG-serum/kg on POD −3, TI with 700 cGy on POD −2, i.v. 20 ng PC/kg/h on POD −2 to 4, 0.15–0.3 mg of Tcr/kg per day on POD −6 to 28102 | • 30–40 ng of Tcr/ml102 |
| Facial alloTx344 | Pharmacological monotherapy | >177 days | 0.5–1 mg of Tcr/kg per day i.v. as continuous infusion on POD −1 to 26, following i.m Tcr. QD344 | • On POD −1 to 26: 30–50 ng of Tcr/ml following: 10–20 ng of Tcr/ml344 | |
| Islet xenoTx345 | Immunopharmacotherapy | >158 days | Intraportal Tx of wild-type porcine islets, anti-CD25 mAb, FTY720, Ev or Tcr, anti-CD154 mAb, leflunomide345 | • 10.8±8.2 ng of FTY720/ml, 22.1±13.7 ng of Ev/ml or 6.7±4.1 ng of Tcr/ml, 1 000±280 μl of anti-CD154 mAb/ml, 12–40 μg of leflunomide/ml345 | |
| Mouse | Bone marrow xenoTx346 | Immunotherapy | >233 days | i.p. on POD −6,−1: 0.1 ml of rat anti-mouse CD4, 0.1 ml of rat anti-mouse CD8, 500 μg of rat anti-mouse Thy-1.2, 400 μg of murine anti-NK1.1 mAbs, TBI (3 Gy) and TI (7 Gy) on POD 0, 60 × 106 rat BMCs346 | • n.s.i.346 |
| Skin alloTx after successful liver alloTx347 | Strain combination | >100 days (45–100% survival) | No immunosuppression necessary using various strain combinations, for example, liver donor: B10.AKM, recipient: B10.BR, skin donor: B10.AKM or liver donor: B10, recipient: C3H, skin donor: B10; skin alloTx was performed 3 months after successful liver alloTx347 | ||
| Skin alloTx348 | Immunopharmacotherapy and cell-based therapy | >120 days | C3H/HeN mice were primed with i.v. injection of 5 × 107 viable AKR/J spleen cells on POD −9, 150 mg of CP/kg i.p. on POD −7, skin allograft Tx from AKR/J donors on POD 0348 | • n.s.i.348 | |
| Bone marrow/splenocyte alloTx166 | Ex vivo immunotherapy and cell-based GvHD prevention | >30 days | Single incubation and washing of 1.4 × 108 donor bone marrow cells and 1.4 × 108 splenocytes from human CD4+/+, murine CD4 knockout, HLA-DR3+/+—(TTG)-C57Bl/6 mice with 800 μg of anti-human CD4 antibodies (MAX.16H5 IgG1) in 15 ml of Dulbecco's modified Eagle's medium for 2 h at room temperature in the dark. TBI of Balb/cwt recipient mice with 8 Gy (X-ray)166 | • n.s.i.166 | |
| Islet alloTx in combination with autologous MSCs Tx349 | Cell-based therapy | >100 days | 5 × 105 recipient-derived MSCs were administered intragraft (local) with BALB/c islets into C57BL/6 kidney Tx349 | • n.s.i.349 | |
| Rat | Stem cell xenoTx after MCAo350 | Pharmacological monotherapy | Robust survival 30 days after Tx | Tx of 3 × 105 hCNS stem cells 7 days post-dMCAo, 10 mg of CsA/kg per day i.p. on POD −1 to 28350 | • n.s.i.350 |
| Skin alloTx351 | Pharmacological monotherapy | >120 days | 3.2 mg of Tcr/kg i.m. 5 days per week for 2 weeks, starting on POD 0, afterwards maintenance with 0.32 mg of Tcr/kg × 2/week 351 | • n.s.i.351 | |
| Neural xenoTx187 | Pharmacological combination therapy | >84 days (100% survival) | i.m. 1 mg of Tcr/kg per day for 12 weeks, p.o. 20 mg of P/kg per day or 40 mg of MMF/kg per day for 2 weeks187 | • n.s.i.187 | |
| Dog | Bone marrow Tx352 | Pharmacological combination therapy | >730 days | TBI with a single dose (28.5 cGy/min) of 200 cGy, within 4 h after TBI 2.5 × 108 BMCs/kg, p.o. 15 mg of CsA/kg b.i.d. and 10 mg of MMF/kg b.i.d. on POD −1 to 63, then tapered off352 | • n.s.i.352 |
| Skin alloTx in combination with donor bone marrow Tx188 | Pharmacological combination therapy and cell-based therapy | Induced long-term tolerance | i.v. infusion of ~2 × 108 donor mononuclear BMCs within 8 h of TBI (400 cGy), 15 mg of CsA/kg b.i.d. p.o. on POD 1–63, then tapered (20–30% per month), 10 mg of MMF/kg b.i.d. p.o. on POD 0–63, then tapered (20–30% per month), donor skin Tx were performed 4 months after BMC Tx188 | • n.s.i.188 | |
| Islet alloTx353 | Pharmacological monotherapy | >175 days graft survival | 10 mg of CsA/kg per day on POD −2 to 11, following 5 mg of CsA/kg per day, on POD 0 microencapsulated islet alloTx was performed353 | • Low-dose CsA, drug levels were below detectable limits: <30 ng of CsA/ml353 | |
| Pig | Bone marrow alloTx354 | Immunopharmacotherapy | Induced long-term tolerance | TI (700 cGy) and 0.05 mg of pCD3-CRM9/kg i.v. on POD −2, 15–30 mg of CsA/kg per day p.o. on POD −1 to 30, Tx of 100–200 × 108 PBSCs on POD 0354 | • 300–800 ng of CsA/ml354 |
| Tissue alloTx/skin xenoTx18, 19 | Pharmacological combination therapy | >90 days | P.o. 40 mg of CsA/kg per day, 500 mg of MMF per day, 2 mg of P/kg per day (tapered off towards to 0.1 mg of P/kg per day)18, 19 | • 100–300 ng of CsA/ml18 | |
| Skin alloTx with donor bone marrow Tx354 | Pharmacological combination therapy and cell-based therapy | >500 days | TI (700 cGy) and 0.05 mg of pCD3-CRM9/kg i.v. on POD −2, 15–30 mg of CsA/kg per day p.o. on POD −1 to 30, Tx of 100–200 × 108 PBSCs on POD 0, donor skin alloTx on POD 60354 | • 300–800 ng of CsA/ml354 | |
| Islet alloTx337 | Immunopharmacotherapy | Up to 41 days | CsA, 50 mg of Az per animal b.i.d., 50 mg of P per animal per day tapered, 100 mg of DSG per animalper day, 115 mg of ATG per animal b.i.d. (animals weighted 48–80 kg)337 | • >200 ng of CsA/ml337 | |
| Sheep | HSC xenoTx in early gestational age fetus355 | Cell-based therapy | Long-term chimerism for several years | XenoTx of human HSCs in 50–60-day-old sheep fetus355 | |
| MPCs alloTx for posterolateral lumbar spine fusion356 | >270 days | Hydroxyapatite: tricalcium phosphate porous ceramic graft with 25–225 × 106 MPCs356 | |||
| Skin xenoTx73 | Pharmacological monotherapy | >60 days | i.v. 2–3 mg of CsA/kg per day b.i.d., 10 mg of Kc/kg b.i.d.73 | • Maintenance around 1500 ng of CsA/ml73 |
Abbreviations: ATG, antithymocyte globulin;Az, azathioprine; b.i.d., twice a day; BMCs, bone marrow cells; cGy, centiGray; CsA, cyclosporin A; dMCAo, distal middle cerebral artery occlusion; DSG, 15-deoxyspergualin; Ev, everolimus; hCNS, human central nervous system; HSC, hematopoietic stem cell; i.m., intramuscular; i.v., intravenous; Kc, ketoconazole; LoCD2b, rat anti-primate CD2 IgG2b; mAbs, monoclonal antibodies; MMF, mycophenolate mofetil; MPCs, mesenchymal progenitor cells; MTX, methotrexate; NHP, non-human primate; n.s.i., no sufficient information available; P, prednisolone; p.o., oral; PBSCs, peripheral blood stem cells; PC, prostacyclin; POD, postoperative day; q.d., once a day; Rpm, rapamycin; TBI, total body irradiation; Tcr, tacrolimus; TI, thymic irradiation; TTG, triple transgenic; Tx, transplantation.
Table 10. Model-based recommendations for immunosuppressive and/or immunomodulatory protocols after solid organ transplantation.
| Species | Indication | Strategy | Survival | Immunosuppressive/immunomodulatory protocol | Target serum levels |
|---|---|---|---|---|---|
| NHP | Heterotopic cardiac xenoTx357 | Immunopharmacotherapy | >365 days | 50 mg of anti-CD40 antibody/kg per week, 19 mg of anti-CD20 antibody on POD −14, −7, 0 and 7, 5 mg of ATG/kg on POD −2 and −1, 20 mg of MMF/kg b.i.d., 2 mg of steroids/kg tapered off in 4–6 weeks357 | • n.s.i.357 |
| Liver xenoTx358 | Immunopharmacotherapy | 9 days | Induction therapy: Three doses of thymoglobulin on POD −3, LoCD2bn, CVF, 25 mg/kg anti-CD154 on POD −1, 0 and 5, Az on POD −1 and 0, Maintenance therapy: Started on POD −1 with Tcr and 10 mg of MP/kg on POD 0 then tapered off358 | • 10–25 ng of Tcr/ml358 | |
| Renal xenoTx359 | Immunopharmacotherapy | >125 days | Single dose of 50 mg of anti-CD4/kg i.v. between POD −3 and −1, 50 mg of anti-CD8/kg i.v. on POD 0, 20 mg of anti-CD154/kg i.v. on POD 0, 7 and 14 and then biweekly, MMF (applied dosage in previous studies:360 15 mg/kg s.c. b.i.d. on POD 0–14, then q.d.) and steroids359 (applied dosage in previous studies:360 20 mg of MP on POD 0, 16 mg of MP on POD 1, 12 mg of MP on POD 2, 8 mg of MP on POD 3, 4 mg of MP on POD 4, 3 mg of MP on POD 5–14, then 1 mg per day) | • n.s.i.359 | |
| Renal xenoTx361 | Immunopharmacotherapy | 136 days | 10 mg of ATG/kg on POD −3, 10 mg of RTB/kg on POD −2 and 50 mg of RTB/kg on POD −1, 0, 4, 7 and 14 and weekly, 100 U CVF on POD −1 and 0, 0.01 mg of Rpm/kg b.i.d. from POD −3, 5 mg of MP/kg per day tapering to 0.25/kg per day, 10 mg of tocilizumab/kg on POD −1, 7, 14 and every 2 weeks, 0.5 mg of etanercept/kg on POD 0, 3, 7, 28 and 40361 | • 8–12 ng of Rpm/ml361 | |
| Renal alloTx362 | Immunopharmacotherapy | >3478 days | Splenectomy, TBI (1.5 Gy), TI (7 Gy) on POD −1, 15 mg of CsA/kg per day until POD 28, i.v. 0.3–3.5 × 108/kg donor bone marrow cells on POD 0362 | • >300 ng of CsA/ml362 | |
| Mouse | Cardiac alloTx and pretreated donor spleen cells363 | Cell-based therapy | 35±14.4 days | Pretreatment of donor spleen cells with mitomycin C, on POD −8 transfusion of donor spleen cells, on POD −7 to −1 1.5 mg of Rpm/kg per day p.o.363 | • n.s.i.363 |
| Cardiac alloTx in combination with liver alloTx347 | Strain combination | >100 days (45–100% survival) | No immunosuppression necessary using various strain combinations, for example, B10 with C3H, A.TH with A.TL, B10.BR with C3H; simultaneous heterotopic cardiac alloTx of donor origin in recipients of liver allografts on the same day347 | ||
| Liver alloTx347 | Strain combination | >100 days up to >375 days | No immunosuppression necessary using various strain combinations, for example, B10 with C3H, DBA2 with B10.D2, B10.BR with C3H, B10.D2 with B10JHTG, A.SW with A.TH, B10.BR with B10347 | ||
| Renal alloTx in combination with DCs and CHO cells364 | Cell-based therapy | >80 days (60% survival) | i.p. injection of 10 × 106 CHO cells (transfected with a vector encoding murine OX-2, which leads to an overexpression of OX-2 on the surface), infusion of 5 × 106 DCs (1:1 mixture of DCs cytokine-transduced with either Ad-IL-10 or Ad-TGF-β) into the portal vein, 36 h later renal alloTx were performed364 | ||
| Rat | Cardiac alloTx271 | Pharmacological monotherapy | >200 days | p.o. 40 mg of MMF/kg per day271 | • n.s.i.271 |
| Liver alloTx365 | Strain combination | >100 days | No immunosuppression necessary using various strain combinations, for example, donor: DA, DA-P or DA-L, recipient: PVG365 | ||
| Renal alloTx and pretreated donor spleen cells366 | Cell-based therapy | >200 days | i.v. infusion of 1 × 108 pretreated spleen cells on POD −7 and 1, 2 mg of AAT per rat i.p. on POD −1, pretreatment of spleen cells: incubation with 150 mg of ECDI per 3.2 × 108 spleen cells366 | • n.s.i.366 | |
| Dog | Heterotopic cardiac alloTx367 | Immunopharmacotherapy | >538 days | TLI (total cumulative dose of 1800 rad over 4 weeks), cardiac Tx 72 h after completion of radiotherapy, 0.25–2.0 mg of Az/kg per day i.m. for 90 days, 4 mg of ATG/kg i.m. on POD 0, 2, 4, 6, 8 and 10367 | • n.s.i.367 |
| Orthotopic liver alloTx368, 369 | Pharmacological monotherapy | >241 days | 20 mg of CsA/kg per day on POD 1–30, then reduced to 15 mg of CsA/kg per day until POD 90369 | • >200 ng of CsA/ml369 | |
| Renal alloTx286, 370 | Pharmacological combination therapy | Up to 1440 days | p.o. 10 mg of CsA/kg/12 h, 2–3 mg of Az/kg/48 h, 0.5 mg of P/kg/12 h (then tapered)286, 370 | • 400–500 ng of CsA/ml for the first 6 months after Tx, 350–450 ng of CsA/ml thereafter,286, 370 decrease of CsA dosage ~32.9±13.9% via usage of 5 mg of Fcz/kg per day371 | |
| Renal alloTx in combination with donor bone marrow Tx188 | Pharmacological combination therapy and cell-based therapy | >2078 days | i.v. infusion of ~2 × 108 donor mononuclear BMCs within 8 h of TBI (200 cGy), 15 mg of CsA/kg b.i.d. p.o. on POD 1–63, then tapered (20–30%/month), 10 mg of MMF/kg b.i.d. p.o. on POD 0–63, then tapered (20–30%/month), donor renal Tx was performed following TBI but before BMC Tx188 | • n.s.i.188 | |
| Pig | Cardiac alloTx in combination with renal alloTx372 | Pharmacological monotherapy | >269 days | i.v. 10–13 mg of CsA/kg per day on POD 0–11372 | • 400–800 ng of CsA/ml372 |
| Liver alloTx289 | Pharmacological monotherapy | >126 days | i.m. 20 mg of CsA/kg per day on POD −1 to 19289 | • n.s.i.289 | |
| Renal alloTx in combination with donor bone Tx354 | Pharmacological combination therapy and cell-based therapy | Induced long-term tolerance | TI (700 cGy) and 0.05 mg of pCD3-CRM9/kg i.v. on POD −2, 15–30 mg of CsA/kg per day p.o. on POD −1 to 30, Tx of 100–200 × 108 PBSCs on POD 0, donor-matched renal alloTx on POD 98 or 190354 | • 300–800 ng of CsA/ml354 | |
| Renal alloTx288 | Pharmacological monotherapy | >100 days >730 days373 | i.v. 10 mg of CsA/kg per day for 12 days288 | • Reached serum levels: 701±40 ng of CsA/ml288 | |
| Sheep | Uterus alloTx | Pharmacological combination therapy | >180 days | p.o. 8 mg of CsA/kg per day, i.m. 5 mg of P/kg per day on POD −2 to 14374 | • 150 ng of CsA/ml was reached after 5 days of CsA administration374 |
| Immunopharmacotherapy | >118 days, showed signs of estrus | p.o. 0.02–0.15 mg of Tcr/kg per day and p.o. 1.5 g of MMF per day on POD −2, maintain until the end of the trial, p.o. 40 mg of MP per day on POD 1–15 (tapered off towards to 16 mg of MP per day), perioperatively i.v.: 50 mg of ATG (DDI), 500 mg of MP277 | • 3000–6000 ng of Tcr/ml277 |
Abbreviations: AAT, α1-antitrypsin (key serine protease inhibitor); ATG, antithymocyte globulin; Az, azathioprine; b.i.d., twice a day; cGy, centiGray; CHO, Chinese hamster ovary; CsA, cyclosporin A; CVF, cobra venom factor; DCs, dendritic cells, DDI, duration drip infusion, ECDI, 1-ethyl-3-(3′-dimethylaminopropyl)-carbodiimide (induces apoptosis of the spleen cells); Fcz, fluconazole; GCSF, granulocyte colony-stimulating factor; i.m., intramuscular; i.p., intraperitoneal; i.v., intravenous; LoCD2b, monoclonal mouse anti-human CD2b antibody; MMF, mycophenolate mofetil; MP, methylprednisolone; MSCs, mesenchymal stem cells; NHP, non-human primate; n.s.i., no sufficient information available; P, prednisolone; p.o., oral; POD, postoperative day; q.d., once a day; Rpm, rapamycin; RTB, Rituximab; s.c., subcutaneous; TPC, α-Gal-polyethylene; Tcr, tacrolimus; TI, thymic irradiation; TLI, total lymphoid irradiation; Tx, transplantation; U, unit.
General recommendations for the application of immunosuppressive treatments in experimental studies
A number of factors should be carefully considered when planning and carrying out immunosuppressive interventions. These comprise, but are not limited to, the following: bioavailability and metabolization of the immunosuppressant in the host species, the treated condition, including its pathophysiological profile, and the applied transplantation strategy. All of these factors might exert a direct influence on the effectivity of a particular immunosuppressive regimen. Thus, substantial inter- and even longitudinal intraindividual differences in immunosuppression efficacy have been reported, usually requiring multiple immunosuppressive medications as well as continuous monitoring and protocol adoption in human patients.
On the other hand, experimental studies often rely on fixed dosing schemes for immunosuppression and often do not consider interindividual disparities in pharmacokinetics and pharmacodynamics, as observed in both humans58 and animals. Such differences may also emerge based on age, sex, comorbidities and concurrent medications (polypharmacy).5 Importantly, a plethora of potential confounding factors may explain why it is so challenging to provide general and clear guidelines for immunosuppression in experimental studies. To cope with this complexity, exploratory pilot studies, including trough and peak level measurements of effective blood concentrations in individual species and transplantation models before the main experiment, are recommended. In the latter case, appropriate controls for immunosuppression safety/efficacy and, if possible, individualized dosing adjustment protocols should be considered to ensure the reproducibility, transferability and validity of results with respect to the clinical situation.
In addition to fixed dosing schemes, the application of single-drug immunosuppressive treatments, primarily targeting the adaptive immune system, is often reported in experimental protocols. The adaptive immune system is the most powerful instrument to damage and reject allo- and xenografts, but elements of the innate immune system, such as dendritic cells190 and defensins,191 also have the capacity to damage the graft and ‘flare-up' immunological responses. Thus, key elements of the innate immune system (for example, Toll-like receptors triggered by pathogens) can also contribute to the (re-)activation of adaptive immune cells. Moreover, the innate immune system significantly differs with respect to its configuration, localization and activation. This is exemplified by the very complex, and thus far only partially understood, activation of microglia in the brain. CsA application, often performed after non-autologous intracerebral cell transplantation, effectively blocks T-cell proliferation and activation, but its influence on the microglia in the brain is minimal. Thus, thorough immunosuppression in experimental setups should ideally target both the adaptive and the innate immune systems in the targeted transplantation area. Therefore, to increase the security and applicability of cell and organ transplantations, appropriate preclinical and clinical trials should be performed to investigate combination therapies with the active pharmaceutical ingredients discussed in this review.
Another important factor is the targeted transplantation area. The existence of so-called ‘immunoprivileged' domains within the adult mammalian body has been postulated, and immunosuppression is often reduced or omitted upon transplantation into these areas. It has been shown that transplantation of progenitor or mature cells into ‘immunoprivileged' areas shielded by biological barriers, such as the intact BBB or the renal capsule, can prevent strong immuno reaction and graft rejection.192, 193 However, this notion may not be entirely correct as ‘immunoprivileged' areas are not characterized by the complete absence of immunoreactions but rather by different mechanisms of immune system activation, locally maintained mechanisms of immunotolerance and/or limited vascular egress of immunocytes into these areas. However, graft rejection can occur after a breakdown of barrier function and the cessation of processes mediating immunotolerance, for example, following injury and inflammation. Furthermore, the death of mature cells within the transplant and subsequent processing of cell debris by local phagocytes and antigen processing can pave the way for an adaptive immune response.190 In the case of blood vessel ingrowth into the area, recognition of the graft by the immune system can then induce delayed but strong immunoreactions.194
Another important consideration is the transformation of therapeutic drug doses between species of different sizes. In particular, therapeutic dose extrapolation between animals and humans via a linear, body weight-based conversion scheme (applying a fixed dose of mg/kg body weight (BW)) can lead to severe dose underestimation in smaller animals. A common example is metoprolol, which requires a 7.5-d to 25-fold human dose to induce comparable effects in rats.195, 196 In turn, unexpected but relevant side effects after BW-based dose conversion have been reported for psychomimetic197 and immunomodulatory applications.198 The body surface area (BSA) normalization method has been suggested for proper allometric dose translation. BSA correlates well with a number of relevant physiological parameters among mammals, including basal metabolism, blood volume and renal function.199 However, BSA-based dose conversion does not prevent interindividual and intraspecies pharmacokinetic variability.200 Thus, individual monitoring of effective blood concentrations and continuous dose adjustment remain highly recommended. This also permits compensation for size-independent, interspecies and interindividual differences,195 which cannot be prevented by BSA-based dose transformation. Additionally, it should be determined whether biological effects can be achieved via the ex vivo treatment of grafts with immunomodulatory substances. Such a gentle and more clinically applicable method has the potential to decrease dosages and side effects by avoiding systemic applications.
Taken together, the plethora of relevant influential factors faced when planning immunosuppressive treatments or interventions requires that very precise information be available when choosing the optimal treatment protocol for a specific scenario. To obtain this information and to ensure validity as well as translational relevance of the obtained data, we recommend the following measures:
(1) Choose an appropriate combination of immunosuppressants with respect to the pharmacokinetics and pharmacodynamics in the target species. Single-drug protocols should be limited to verified exemptions.
(2) Confirm the protocol's safety (including side effects) and achievement of appropriate target blood levels in the target species and model in exploratory pilot trials. Record potential blood level fluctuations.
(3) If necessary, verify the protocol's efficacy in a pilot experiment.
(4) Define interventional measures or protocol adjustments to optimize immunosuppression in the main trial. This includes the definition of circumstances under which an adjustment is necessary as well as those under which a particular adjustment might compromise the experimental results.
(5) Precisely define transplantation study end points as well as readout measures, including the expected effect size of primary study end points and standard deviation (derived from pilot trials). This will help to plan the main trial with appropriate statistical power.
(6) Strictly apply randomization and blinding protocols in the main trial, planning with appropriate negative and, if possible, positive controls.
(7) Perform thorough post hoc macro- and micropathological assessment of experimental subjects to exclude undetected side effects of immunosuppression.
(8) Publish neutral or negative results, including suboptimal immunosuppression protocols, in the scientific community.
Acknowledgments
We thank Hanka Hahnentritt for her thorough and expert graphic artwork in Figure 1. This work was only supported by intramural funds.
Footnotes
Supplementary Information for this article can be found on the Cellular & Molecular Immunology website (http://www.nature.com/cmi)
The authors declare no conflict of interest.
Supplementary Material
References
- Kahan BD. Cyclosporine. N Engl J Med 1989; 321: 1725–1738. [DOI] [PubMed] [Google Scholar]
- Borlongan CV, Chopp M, Steinberg GK, Bliss TM, Li Y, Lu M et al. Potential of stem/progenitor cells in treating stroke: the missing steps in translating cell therapy from laboratory to clinic. Regen Med 2008; 3: 249–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffan J, Favrot C, Mueller R. A systematic review and meta-analysis of the efficacy and safety of cyclosporin for the treatment of atopic dermatitis in dogs. Vet Dermatol 2006; 17: 3–16. [DOI] [PubMed] [Google Scholar]
- Kirk AD. Crossing the bridge: large animal models in translational transplantation research. Immunol Rev 2003; 196: 176–196. [DOI] [PubMed] [Google Scholar]
- Ptachcinski RJ, Burckart GJ, Venkataramanan R. Cyclosporine concentration determinations for monitoring and pharmacokinetic studies. J Clin Pharmacol 1986; 26: 358–366. [DOI] [PubMed] [Google Scholar]
- Latif T, Morris JCMonoclonal antibody therapy of T-cell leukemia and lymphoma. In:Tomita M (ed.). T-Cell Leukemia: Characteristics, Treatment and Prevention. InTech: Rijeka, Croatia. 2013. [Google Scholar]
- Lee S, Kim H, Song I, Youm J, Dizon LA, Jeong S et al. Glucocorticoids and their receptors: insights into specific roles in mitochondria. Prog Biophys Mol Biol 2013; 112: 44–54. [DOI] [PubMed] [Google Scholar]
- van Kooten C, Stax AS, Woltman AM, Gelderman KA. Handbook of experimental pharmacology ‘dendritic cells': the use of dexamethasone in the induction of tolerogenic DCs. Handb Exp Pharmacol 2009; 2009: 233–249. [DOI] [PubMed] [Google Scholar]
- Sternberg EM, Chrousos GP, Wilder RL, Gold PW. The stress response and the regulation of inflammatory disease. Ann Intern Med 1992; 117: 854–866. [DOI] [PubMed] [Google Scholar]
- Melby JC. Clinical pharmacology of systemic corticosteroids. Annu Rev Pharmacol Toxicol 1977; 17: 511–527. [DOI] [PubMed] [Google Scholar]
- Julian JA, Chytil F. A two-step mechanism for the regulation of tryptophan pyrrolase. Biochem Biophys Res Commun 1969; 35: 734–740. [DOI] [PubMed] [Google Scholar]
- Wiener SL, Wiener R, Urivetzky M, Shafer S, Isenberg HD, Janov C et al. The mechanism of action of a single dose of methylprednisolone on acute inflammation in vivo. J Clin Invest 1975; 56: 679–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krotkiewski M, Blohmé B, Lindholm N, Björntorp P. The effects of adrenal corticosteroids on regional adipocyte size in man. J Clin Endocrinol Metab 1976; 42: 91–97. [DOI] [PubMed] [Google Scholar]
- Orci LA, Toso C, Mentha G, Morel P, Majno PE. Systematic review and meta-analysis of the effect of perioperative steroids on ischaemia–reperfusion injury and surgical stress response in patients undergoing liver resection. Br J Surg 2013; 100: 600–609. [DOI] [PubMed] [Google Scholar]
- Nishihira T, Ishii H, Kasai M, Tachibana T. Prolonged survival time of allogeneic skin grafts in host pretreated with somatic cell hybrids and prednisolone. Tohoku J Exp Med 1979; 129: 71–74. [DOI] [PubMed] [Google Scholar]
- Kagaya F, Usui T, Kamiya K, Ishii Y, Tanaka S, Amano S et al. Intraocular dexamethasone delivery system for corneal transplantation in an animal model. Cornea 2002; 21: 200–202. [DOI] [PubMed] [Google Scholar]
- Yu C, Storb R, Mathey B, Deeg HJ, Schuening FG, Graham TC et al. DLA-identical bone marrow grafts after low-dose total body irradiation: effects of high-dose corticosteroids and cyclosporine on engraftment. Blood 1995; 86: 4376–4381. [PubMed] [Google Scholar]
- Ustüner ET, Zdichavsky M, Ren X, Edelstein J, Maldonado C, Ray M et al. Long-term composite tissue allograft survival in a porcine model with cyclosporine/mycophenolate mofetil therapy. Transplantation 1998; 66: 1581–1587. [DOI] [PubMed] [Google Scholar]
- Zdichavsky M, Jones JW, Ustuner ET, Ren X, Edelstein J, Maldonado C et al. Scoring of skin rejection in a swine composite tissue allograft model. J Surg Res 1999; 85: 1–8. [DOI] [PubMed] [Google Scholar]
- Segel LD, Follette DM, Castellanos LM, Hayes R, Baker JM, Smolens IV. Steroid pretreatment improves graft recovery in a sheep orthotopic heart transplantation model. J Surg Res 1997; 16: 371–380. [PubMed] [Google Scholar]
- Schäcke H, Döcke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther 2002; 96: 23–43. [DOI] [PubMed] [Google Scholar]
- Schatz M, Patterson R, Zeitz S, O'Rourke J, Melam H. Corticosteroid therapy for the pregnant asthmatic patient. JAMA 1975; 233: 804–807. [PubMed] [Google Scholar]
- Sandborn WJ. Rational dosing of azathioprine and 6-mercaptopurine. Gut 2001; 48: 591–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong VW, Oellerich M. New developments in the immunosuppressive drug monitoring of cyclosporine, tacrolimus, and azathioprine. Clin Biochem 2001; 34: 9–16. [DOI] [PubMed] [Google Scholar]
- Weinshilboum RM, Sladek SL. Mercaptopurine pharmacogenetics: monogenic inheritance of erythrocyte thiopurine methyltransferase activity. Am J Hum Genet 1980; 32: 651–662. [PMC free article] [PubMed] [Google Scholar]
- Dervieux T, Médard Y, Baudouin V, Maisin A, Zhang D, Broly F et al. Thiopurine methyltransferase activity and its relationship to the occurrence of rejection episodes in paediatric renal transplant recipients treated with azathioprine. Br J Clin Pharmacol 1999; 48: 793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marbet U, Schmid I. Severe life-threatening diarrhea caused by azathioprine but not by 6-mercaptopurine. Digestion 2001; 63: 139–142. [DOI] [PubMed] [Google Scholar]
- Kahan BD. Efficacy of sirolimus compared with azathioprine for reduction of acute renal allograft rejection: a randomised multicentre study. The Rapamune US Study Group. Lancet 2000; 356: 194–202. [DOI] [PubMed] [Google Scholar]
- DeNeve W, Valeriote F, Edelstein M, Everett C, Bischoff M. In vivo DNA cross-linking by cyclophosphamide: comparison of human chronic lymphatic leukemia cells with mouse L1210 leukemia and normal bone marrow cells. Cancer Res 1989; 49: 3452–3456. [PubMed] [Google Scholar]
- Jolivet J, Cowan KH, Curt GA, Clendeninn NJ, Chabner BA. The pharmacology and clinical use of methotrexate. N Engl J Med 1983; 309: 1094–1104. [DOI] [PubMed] [Google Scholar]
- Connors TA, Cox PJ, Farmer PB, Foster AB, Jarman M. Some studies of the active intermediates formed in the microsomal metabolism of cyclophosphamide and isophosphamide. Biochem Pharmacol 1974; 23: 115–129. [DOI] [PubMed] [Google Scholar]
- Whirl-Carrillo M, McDonagh EM, Hebert JM, Gong L, Sangkuhl K, Thorn CF et al. Pharmacogenomics knowledge for personalized medicine. Clin Pharmacol Ther 2012; 92: 414–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce WR, Meeker BE, Valeriote FA. Comparison of the sensitivity of normal hematopoietic and transplanted lymphoma colony-forming cells to chemotherapeutic agents administered in vivo. J Natl Cancer Inst 1966; 37: 233–245. [PubMed] [Google Scholar]
- Hall JM, Ohno S, Pribnow JF. The effect of cyclophosphamide on an ocular immune response. I. Primary response. Clin Exp Immunol 1977; 30: 309–316. [PMC free article] [PubMed] [Google Scholar]
- Turk JL, Poulter LW. Selective depletion of lymphoid tissue by cyclophosphamide. Clin Exp Immunol 1972; 10: 285–296. [PMC free article] [PubMed] [Google Scholar]
- Stockman GD, Heim LR, South MA, Trentin JJ. Differential effects of cyclophosphamide on the B and T cell compartments of adult mice. J Immunol 1973; 110: 277–282. [PubMed] [Google Scholar]
- Stevenson HC, Fauci AS. Activation of human B lymphocytes. XII. Differential effects of in vitro cyclophosphamide on human lymphocyte subpopulations involved in B-cell activation. Immunology 1980; 39: 391–397. [PMC free article] [PubMed] [Google Scholar]
- Ahmed AR, Hombal SM. Cyclophosphamide (Cytoxan). A review on relevant pharmacology and clinical uses. J Am Acad Dermatol 1984; 11: 1115–1126. [DOI] [PubMed] [Google Scholar]
- Mullins GM, Colvin M. Intensive cyclophosphamide (NSC-26271) therapy for solid tumors. Cancer Chemother Rep 1975; 59: 411–419. [PubMed] [Google Scholar]
- Bergsagel DE, Robertson GL, Hasselback R. Effect of cyclophosphamide on advanced lung cancer and the hematological toxicity of large, intermittent intravenous doses. Can Med Assoc J 1968; 98: 532–538. [PMC free article] [PubMed] [Google Scholar]
- Fetting JH, Grochow LB, Folstein MF, Ettinger DS, Colvin M. The course of nausea and vomiting after high-dose cyclophosphamide. Cancer Treat Rep 1982; 66: 1487–1493. [PubMed] [Google Scholar]
- Bertino JR. Karnofsky memorial lecture. Ode to methotrexate. J Clin Oncol 1993; 11: 5–14. [DOI] [PubMed] [Google Scholar]
- Franklin TJ, Cook JM. The inhibition of nucleic acid synthesis by mycophenolic acid. Biochem J 1969; 113: 515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison AC, Eugui EM. Immunosuppressive and other effects of mycophenolic acid and an ester prodrug, mycophenolate mofetil. Immunol Rev 1993; 136: 5–28. [DOI] [PubMed] [Google Scholar]
- Hao L, Calcinaro F, Gill RG, Eugui EM, Allison AC, Lafferty KJ. Facilitation of specific tolerance induction in adult mice by RS-61443. Transplantation 1992; 53: 590–595. [DOI] [PubMed] [Google Scholar]
- Sollinger HW, Deierhoi MH, Belzer FO, Diethelm AG, Kauffman RS. RS-61443—a phase I clinical trial and pilot rescue study. Transplantation 1992; 53: 428–432. [DOI] [PubMed] [Google Scholar]
- Platz KP, Sollinger HW, Hullett DA, Eckhoff DE, Eugui EM, Allison AC. RS-61443—a new, potent immunosuppressive agent. Transplantation 1991; 51: 27–31. [DOI] [PubMed] [Google Scholar]
- Weir MR, Anderson L, Fink JC, Gabregiorgish K, Schweitzer EJ, Hoehn-Saric E et al. A novel approach to the treatment of chronic allograft nephropathy. Transplantation 1997; 64: 1706–1710. [DOI] [PubMed] [Google Scholar]
- Bestard O, Cruzado JM, Grinyó JM. Calcineurin-inhibitor-sparing immunosuppressive protocols. Transplant Proc 2005; 37: 3729–3732. [DOI] [PubMed] [Google Scholar]
- Hesselink DA, van Hest RM, Mathot RAA, Bonthuis F, Weimar W, de Bruin RWF et al. Cyclosporine interacts with mycophenolic acid by inhibiting the multidrug resistance-associated protein 2. Am J Transplant 2005; 5: 987–994. [DOI] [PubMed] [Google Scholar]
- van Gelder T, Klupp J, Barten MJ, Christians U, Morris RE. Comparison of the effects of tacrolimus and cyclosporine on the pharmacokinetics of mycophenolic acid. Ther Drug Monit 2001; 23: 119–128. [DOI] [PubMed] [Google Scholar]
- Goldblum R. Therapy of rheumatoid arthritis with mycophenolate mofetil. Clin Exp Rheumatol 1993; 11 (Suppl 8): S117–S119. [PubMed] [Google Scholar]
- Pillans P. Immunosuppressants—mechanisms of action and monitoring. Aust Prescr 2006; 29: 99–101. [Google Scholar]
- Calne RY, White DJ, Thiru S, Evans DB, McMaster P, Dunn DC et al. Cyclosporin A in patients receiving renal allografts from cadaver donors. Lancet 1978; 2: 1323–1327 (cited 23 February 2014). [DOI] [PubMed] [Google Scholar]
- Anjum T, Azam A, Irum W. Production of cyclosporine A by submerged fermentation from a local isolate of Penicillium fellutanum. Indian J Pharm Sci 2012; 74: 372–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begley DJ, Squires LK, Zloković BV, Mitrović DM, Hughes CC, Revest PA et al. Permeability of the blood–brain barrier to the immunosuppressive cyclic peptide cyclosporin A. J Neurochem 1990; 55: 1222–1230. [DOI] [PubMed] [Google Scholar]
- Tsuji A, Tamai I, Sakata A, Tenda Y, Terasaki T. Restricted transport of cyclosporin A across the blood–brain barrier by a multidrug transporter, P-glycoprotein. Biochem Pharmacol 1993; 46: 1096–1099. [DOI] [PubMed] [Google Scholar]
- Duncan N, Craddock C. Optimizing the use of cyclosporin in allogeneic stem cell transplantation. Bone Marrow Transplant 2006; 38: 169–174. [DOI] [PubMed] [Google Scholar]
- Storb R, Deeg HJ, Pepe M, Appelbaum F, Anasetti C, Beatty P et al. Methotrexate and cyclosporine versus cyclosporine alone for prophylaxis of graft-versus-host disease in patients given HLA-identical marrow grafts for leukemia: long-term follow-up of a controlled trial. Blood 1989; 73: 1729–1734. [PubMed] [Google Scholar]
- Halloran PF, Helms LM, Kung L, Noujaim J. The temporal profile of calcineurin inhibition by cyclosporine in vivo. Transplantation 1999; 68: 1356–1361. [DOI] [PubMed] [Google Scholar]
- Kiani A, Rao A, Aramburu J. Manipulating immune responses with immunosuppressive agents that target NFAT. Immunity 2000; 12: 359–372. [DOI] [PubMed] [Google Scholar]
- Takaori K, Nio Y, Inoue K, Tun T, Fukumoto M, Hashida T et al. A comparative study on immunosuppressive effects of cyclosporin A and FK 506 on peripheral blood lymphocytes in dogs. Biotherapy 1992; 4: 129–137. [DOI] [PubMed] [Google Scholar]
- Hunt J, Cheng A, Hoyles A, Jervis E, Morshead CM. Cyclosporin A has direct effects on adult neural precursor cells. J Neurosci 2010; 30: 2888–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichard L, Fabre I, Fabre G, Domergue J, Saint Aubert B, Mourad G et al. Cyclosporin A drug interactions. Screening for inducers and inhibitors of cytochrome P-450 (cyclosporin A oxidase) in primary cultures of human hepatocytes and in liver microsomes. Drug Metab Dispos 1990; 18: 595–606. [PubMed] [Google Scholar]
- Freeman DJ, Laupacis A, Keown PA, Stiller CR, Carruthers SG. Evaluation of cyclosporin–phenytoin interaction with observations on cyclosporin metabolites. Br J Clin Pharmacol 1984; 18: 887–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Qiu W, Liu Z, Zhu L, Liu Z, Tu J et al. Effects of genetic polymorphisms of CYP3A4, CYP3A5 and MDR1 on cyclosporine pharmacokinetics after renal transplantation. Clin Exp Pharmacol Physiol 2006; 33: 1093–1098. [DOI] [PubMed] [Google Scholar]
- Sangalli L, Bortolotti A, Jiritano L, Bonati M. Cyclosporine pharmacokinetics in rats and interspecies comparison in dogs, rabbits, rats, and humans. Drug Metab Dispos 1988; 16: 749–753. [PubMed] [Google Scholar]
- Kahan BD. Therapeutic drug monitoring of cyclosporine: 20 years of progress. Transplant Proc 2004; 36: 378S–391S. [DOI] [PubMed] [Google Scholar]
- Ruutu T, Niederwieser D, Gratwohl A, Apperley JF. A survey of the prophylaxis and treatment of acute GVHD in Europe: a report of the European Group for Blood and Marrow, Transplantation (EBMT). Chronic Leukaemia Working Party of the EBMT. Bone Marrow Transplant 1997; 19: 759–764. [DOI] [PubMed] [Google Scholar]
- Dunn CJ, Wagstaff AJ, Perry CM, Plosker GL, Goa KL. Cyclosporin: an updated review of the pharmacokinetic properties, clinical efficacy and tolerability of a microemulsion-based formulation (neoral)1 in organ transplantation. Drugs 2001; 61: 1957–2016. [DOI] [PubMed] [Google Scholar]
- Steffan J, Alexander D, Brovedani F, Fisch RD. Comparison of cyclosporine A with methylprednisolone for treatment of canine atopic dermatitis: a parallel, blinded, randomized controlled trial. Vet Dermatol 2003; 14: 11–22. [DOI] [PubMed] [Google Scholar]
- Pan H, Gazarian A, Buff S, Solla F, Gagnieu M, Leveneur O et al. Oral cyclosporine A in neonatal swines for transplantation studies. Immunopharmacol Immunotoxicol 2014; 100: 1–7. [DOI] [PubMed] [Google Scholar]
- Rose AH, Ilett KF, O'Donoghue HL, Hackett LP, Penhale WJ, Manning LS et al. Cyclosporin immunosuppression of sheep: pharmacokinetics and allograft survival. Vet Immunol Immunopathol 2001; 81: 23–36. [DOI] [PubMed] [Google Scholar]
- Bacigalupo A, Van Lint MT, Occhini D, Gualandi F, Lamparelli T, Sogno G et al. Increased risk of leukemia relapse with high-dose cyclosporine A after allogeneic marrow transplantation for acute leukemia. Blood 1991; 77: 1423–1428. [PubMed] [Google Scholar]
- Mengarelli A, Iori AP, Romano A, Cerretti R, Cerilli L, De Propris MS et al. One-year cyclosporine prophylaxis reduces the risk of developing extensive chronic graft-versus-host disease after allogeneic peripheral blood stem cell transplantation. Haematologica 2003; 88: 315–323. [PubMed] [Google Scholar]
- Whitworth JA, Mills EH, Coghlan JP, McDougall JG, Nelson MA, Spence CD et al. The haemodynamic effects of cyclosporin A in sheep. Clin Exp Pharmacol Physiol 1987; 14: 573–580. [DOI] [PubMed] [Google Scholar]
- Patton PR, Brunson ME, Pfaff WW, Howard RJ, Peterson JC, Ramos EL et al. A preliminary report of diltiazem and ketoconazole. Their cyclosporine-sparing effect and impact on transplant outcome. Transplantation 1994; 57: 889–892. [DOI] [PubMed] [Google Scholar]
- First MR, Schroeder TJ, Michael A, Hariharan S, Weiskittel P, Alexander JW. Cyclosporine-ketoconazole interaction. Long-term follow-up and preliminary results of a randomized trial. Transplantation 1993; 55: 1000–1004. [PubMed] [Google Scholar]
- Nash RA, Antin JH, Karanes C, Fay JW, Avalos BR, Yeager AM et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood 2000; 96: 2062–2068. [PubMed] [Google Scholar]
- Bolwell B, Sobecks R, Pohlman B, Andresen S, Rybicki L, Kuczkowski E et al. A prospective randomized trial comparing cyclosporine and short course methotrexate with cyclosporine and mycophenolate mofetil for GVHD prophylaxis in myeloablative allogeneic bone marrow transplantation. Bone Marrow Transplant 2004; 34: 621–625. [DOI] [PubMed] [Google Scholar]
- Greenberg ML, Uretsky BF, Reddy PS, Bernstein RL, Griffith BP, Hardesty RL et al. Long-term hemodynamic follow-up of cardiac transplant patients treated with cyclosporine and prednisone. Circulation 1985; 71: 487–494. [DOI] [PubMed] [Google Scholar]
- Palestine AG, Nussenblatt RB, Chan CC. Side effects of systemic cyclosporine in patients not undergoing transplantation. Am J Med 1984; 77: 652–656. [DOI] [PubMed] [Google Scholar]
- Kovalik M, Thoday KL, van den Broek AHM. The use of ciclosporin A in veterinary dermatology. Vet J 2012; 193: 317–325. [DOI] [PubMed] [Google Scholar]
- Murray BM, Paller MS, Ferris TF. Effect of cyclosporine administration on renal hemodynamics in conscious rats. Kidney Int 1985; 28: 767–774. [DOI] [PubMed] [Google Scholar]
- Ahn KO, Lim SW, Li C, Yang HJ, Ghee JY, Kim JY et al. Influence of angiotensin II on expression of toll-like receptor 2 and maturation of dendritic cells in chronic cyclosporine nephropathy. Transplantation 2007; 83: 938–947. [DOI] [PubMed] [Google Scholar]
- Kennedy MS, Yee GC, McGuire TR, Leonard TM, Crowley JJ, Deeg HJ. Correlation of serum cyclosporine concentration with renal dysfunction in marrow transplant recipients. Transplantation 1985; 40: 249–253. [DOI] [PubMed] [Google Scholar]
- Joss DV, Barrett AJ, Kendra JR, Lucas CF, Desai S. Hypertension and convulsions in children receiving cyclosporin A. Lancet 1982; 1: 906. [DOI] [PubMed] [Google Scholar]
- Cravedi P, Codreanu I, Satta A, Turturro M, Sghirlanzoni M, Remuzzi G et al. Cyclosporine prolongs delayed graft function in kidney transplantation: are rabbit anti-human thymocyte globulins the answer? Nephron Clin Pract 2005; 101: c65–c71. [DOI] [PubMed] [Google Scholar]
- Kawaguchi A, Sugimoto K, Fujimura A. Preventive effect of platelet-activating factor antagonist, Y-24180, against cyclosporine-induced acute nephrotoxicity. Life Sci 2001; 68: 1181–1190. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Momoi Y, Iwasaki T. Cyclosporine A inhibits the mRNA expressions of IL-2, IL-4 and IFN-gamma, but not TNF-alpha, in canine mononuclear cells. J Vet Med Sci 2007; 69: 887–892. [DOI] [PubMed] [Google Scholar]
- Citterio F. Evolution of the therapeutic drug monitoring of cyclosporine. Transplant Proc 2004; 36: 420S–425S. [DOI] [PubMed] [Google Scholar]
- Holt DW, Armstrong VW, Griesmacher A, Morris RG, Napoli KL, Shaw LM. International Federation of Clinical Chemistry/International Association of Therapeutic Drug Monitoring and Clinical Toxicology working group on immunosuppressive drug monitoring. Ther Drug Monit 2002; 24: 59–67. [DOI] [PubMed] [Google Scholar]
- Canadian Neoral Renal Transplantation Study Group. Absorption profiling of cyclosporine microemulsion (neoral) during the first 2 weeks after renal transplantation. Transplantation 2001; 72: 1024–1032. [DOI] [PubMed] [Google Scholar]
- Anglade E, Yatscoff R, Foster R, Grau U. Next-generation calcineurin inhibitors for ophthalmic indications. Expert Opin Investig Drugs 2007; 16: 1525–1540. [DOI] [PubMed] [Google Scholar]
- Benelli U, Lepri A, Del Tacca M, Nardi M. FK-506 delays corneal graft rejection in a model of corneal xenotransplantation. J Ocul Pharmacol Ther 1996; 12: 425–431. [DOI] [PubMed] [Google Scholar]
- Thomson AW, Bonham CA, Zeevi A. Mode of action of tacrolimus (FK506): molecular and cellular mechanisms. Ther Drug Monit 1995; 17: 584–591. [DOI] [PubMed] [Google Scholar]
- Scott LJ, McKeage K, Keam SJ, Plosker GL. Tacrolimus: a further update of its use in the management of organ transplantation. Drugs 2003; 63: 1247–1297. [DOI] [PubMed] [Google Scholar]
- Alomar A, Berth-Jones J, Bos JD, Giannetti A, Reitamo S, Ruzicka T et al. The role of topical calcineurin inhibitors in atopic dermatitis. Br J Dermatol. 2004; 151 (Suppl 70):3–27. [DOI] [PubMed] [Google Scholar]
- Schreiber SL, Crabtree GR. The mechanism of action of cyclosporin A and FK506. Immunol Today 1992; 13: 136–142. [DOI] [PubMed] [Google Scholar]
- Kinugasa F, Nagatomi I, Ishikawa H, Nakanishi T, Maeda M, Hirose J et al. Efficacy of oral treatment with tacrolimus in the renal transplant model in cynomolgus monkeys. J Pharmacol Sci 2008; 108: 529–534. [DOI] [PubMed] [Google Scholar]
- Wijnen RM, Ericzon BG, Tiebosch AT, Buurman WA, Groth CG, Kootstra G. Toxicology of FK506 in the cynomolgus monkey: a clinical, biochemical, and histopathological study. Transpl Int 1992; 5 (Suppl 1):S454–S458. [DOI] [PubMed] [Google Scholar]
- Griesemer A, Liang F, Hirakata A, Hirsh E, Lo D, Okumi M et al. Occurrence of specific humoral non-responsiveness to swine antigens following administration of GalT-KO bone marrow to baboons. Xenotransplantation 2010; 17: 300–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesemer AD, Lamattina JC, Okumi M, Etter JD, Shimizu A, Sachs DH et al. Linked suppression across an MHC-mismatched barrier in a miniature swine kidney transplantation model. J Immunol 2008; 181: 4027–4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi S, Xu D, Peng J, Vu MD, Wu J, Bekersky I et al. Effect of tacrolimus (FK506) and sirolimus (rapamycin) mono- and combination therapy in prolongation of renal allograft survival in the monkey. Transplantation 2000; 69: 1275–1283. [DOI] [PubMed] [Google Scholar]
- Zeiser R, Nguyen VH, Beilhack A, Buess M, Schulz S, Baker J et al. Inhibition of CD4+CD25+ regulatory T-cell function by calcineurin-dependent interleukin-2 production. Blood 2006; 108: 390–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albanell J, Dalmases A, Rovira A, Rojo F. mTOR signalling in human cancer. Clin Transl Oncol 2007; 9: 484–493. [DOI] [PubMed] [Google Scholar]
- Battaglia M, Stabilini A, Draghici E, Gregori S, Mocchetti C, Bonifacio E et al. Rapamycin and interleukin-10 treatment induces T regulatory type 1 cells that mediate antigen-specific transplantation tolerance. Diabetes 2006; 55: 40–49. [PubMed] [Google Scholar]
- Abraham RT, Gibbons JJ. The mammalian target of rapamycin signaling pathway: twists and turns in the road to cancer therapy. Clin Cancer Res 2007; 13: 3109–3114. [DOI] [PubMed] [Google Scholar]
- Lim J, Park M, Im K, Kim N, Park H, Lee S et al. IL-21 blockade modulates activated T- and B-cell homeostasis via BAFF pathway-mediated inhibition in a murine model of acute graft-versus-host disease. Exp Hematol 2014. [DOI] [PubMed]
- Hackstein H, Taner T, Logar AJ, Thomson AW. Rapamycin inhibits macropinocytosis and mannose receptor-mediated endocytosis by bone marrow-derived dendritic cells. Blood 2002; 100: 1084–1087. [DOI] [PubMed] [Google Scholar]
- Oliveira JG, Xavier P, Sampaio SM, Henriques C, Tavares I, Mendes AA et al. Compared to mycophenolate mofetil, rapamycin induces significant changes on growth factors and growth factor receptors in the early days post-kidney transplantation. Transplantation 2002; 73: 915–920. [DOI] [PubMed] [Google Scholar]
- Gallant-Haidner HL, Trepanier DJ, Freitag DG, Yatscoff RW. Pharmacokinetics and metabolism of sirolimus. Ther Drug Monit 2000; 22: 31–35. [DOI] [PubMed] [Google Scholar]
- Ko HT, Yin JL, Wyburn K, Wu H, Eris JM, Hambly BD et al. Sirolimus reduces vasculopathy but exacerbates proteinuria in association with inhibition of VEGF and VEGFR in a rat kidney model of chronic allograft dysfunction. Nephrol Dial Transplant 2013; 28: 327–336. [DOI] [PubMed] [Google Scholar]
- Salminen US, Maasilta PK, Taskinen EI, Harjula AL. Obliterative lesions in a heterotopic bronchial xenograft model—a preliminary study. Transplantation 2000; 70: 48–50. [PubMed] [Google Scholar]
- Hogan WJ, Little M, Zellmer E, Friedetzky A, Diaconescu R, Gisburne S et al. Postgrafting immunosuppression with sirolimus and cyclosporine facilitates stable mixed hematopoietic chimerism in dogs given sublethal total body irradiation before marrow transplantation from DLA-identical littermates. Biol Blood Marrow Transplant 2003; 9: 489–495. [DOI] [PubMed] [Google Scholar]
- Groth CG, Bäckman L, Morales JM, Calne R, Kreis H, Lang P et al. Sirolimus (rapamycin)-based therapy in human renal transplantation: similar efficacy and different toxicity compared with cyclosporine. Sirolimus European Renal Transplant Study Group. Transplantation 1999; 67: 1036–1042. [DOI] [PubMed] [Google Scholar]
- MacDonald AS. A worldwide, phase III, randomized, controlled, safety and efficacy study of a sirolimus/cyclosporine regimen for prevention of acute rejection in recipients of primary mismatched renal allografts. Transplantation 2001; 71: 271–280. [DOI] [PubMed] [Google Scholar]
- Schuurman HJ, Ringers J, Schuler W, Slingerland W, Jonker M. Oral efficacy of the macrolide immunosuppressant SDZ RAD and of cyclosporine microemulsion in cynomolgus monkey kidney allotransplantation. Transplantation 2000; 69: 737–742. [DOI] [PubMed] [Google Scholar]
- Pascual J. Everolimus in clinical practice—renal transplantation. Nephrol Dial Transplant 2006; 21 (Suppl 3):iii18–iii23. [DOI] [PubMed] [Google Scholar]
- Schuler W, Sedrani R, Cottens S, Häberlin B, Schulz M, Schuurman HJ et al. SDZ RAD, a new rapamycin derivative: pharmacological properties in vitro and in vivo. Transplantation 1997; 64: 36–42. [DOI] [PubMed] [Google Scholar]
- Crowe A, Bruelisauer A, Duerr L, Guntz P, Lemaire M. Absorption and intestinal metabolism of SDZ-RAD and rapamycin in rats. Drug Metab Dispos 1999; 27: 627–632. [PubMed] [Google Scholar]
- Formica RN, Lorber KM, Friedman AL, Bia MJ, Lakkis F, Smith JD et al. The evolving experience using everolimus in clinical transplantation. Transplant Proc 2004; 36: 495S–499S. [DOI] [PubMed] [Google Scholar]
- Bumbea V, Kamar N, Ribes D, Esposito L, Modesto A, Guitard J et al. Long-term results in renal transplant patients with allograft dysfunction after switching from calcineurin inhibitors to sirolimus. Nephrol Dial Transplant 2005; 20: 2517–2523. [DOI] [PubMed] [Google Scholar]
- Zhang C, Shan J, Lu J, Huang Y, Feng L, Long D et al. Rapamycin in combination with donor-specific CD4+CD25+Treg cells amplified in vitro might be realize the immune tolerance in clinical organ transplantation. Cell Immunol 2010; 264: 111–113. [DOI] [PubMed] [Google Scholar]
- Zeiser R, Leveson-Gower DB, Zambricki EA, Kambham N, Beilhack A, Loh J et al. Differential impact of mammalian target of rapamycin inhibition on CD4+CD25+Foxp3+ regulatory T cells compared with conventional CD4+ T cells. Blood 2008; 111: 453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DW, Azimzadeh A. Transplantation tolerance: one step towards the holy grail. Clin Immunol 2010; 136: 161. [DOI] [PubMed] [Google Scholar]
- Levings MK, Sangregorio R, Roncarolo MG. Human cd25(+)cd4(+) t regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J Exp Med 2001; 193: 1295–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffre O, Santolaria T, Calise D, Al Saati T, Hudrisier D, Romagnoli P et al. Prevention of acute and chronic allograft rejection with CD4+CD25+Foxp3+ regulatory T lymphocytes. Nat Med 2008; 14: 88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel V, Naujokat C, Sadeghi M, Weimer R, Renner F, Yildiz S et al. Observational support for an immunoregulatory role of CD3+CD4+CD25+IFN-gamma+ blood lymphocytes in kidney transplant recipients with good long-term graft outcome. Transpl Int 2008; 21: 646–660. [DOI] [PubMed] [Google Scholar]
- Fricke S, Rothe K, Hilger N, Ackermann M, Oelkrug C, Fricke C et al. Allogeneic bone marrow grafts with high levels of CD4(+) CD25(+) FoxP3(+) T cells can lead to engraftment failure. Cytometry A 2012; 81: 476–488. [DOI] [PubMed] [Google Scholar]
- Garden OA, Pinheiro D, Cunningham F. All creatures great and small: regulatory T cells in mice, humans, dogs and other domestic animal species. Int Immunopharmacol 2011; 11: 576–588. [DOI] [PubMed] [Google Scholar]
- Jeon E, Yoon B, Lim J, Oh H, Park H, Park M et al. Adoptive transfer of all-trans-retinal-induced regulatory T cells ameliorates experimental autoimmune arthritis in an interferon-gamma knockout model. Autoimmunity 2012; 45: 460–469. [DOI] [PubMed] [Google Scholar]
- Liu Y, Carlsson R, Comabella M, Wang J, Kosicki M, Carrion B et al. FoxA1 directs the lineage and immunosuppressive properties of a novel regulatory T cell population in EAE and MS. Nat Med 2014; 20: 272–282. [DOI] [PubMed] [Google Scholar]
- Colombo MP, Piconese S. Regulatory-T-cell inhibition versus depletion: the right choice in cancer immunotherapy. Nat Rev Cancer 2007; 7: 880–887. [DOI] [PubMed] [Google Scholar]
- Weiden PL, Storb R, Tsoi MS, Graham TC, Lerner KG, Thomas ED. Infusion of donor lymphocytes into stable canine radiation chimeras: implications for mechanism of transplantation tolerance. J Immunol 1976; 116: 1212–1219. [PubMed] [Google Scholar]
- Kumar S, Ponnazhagan S. Mobilization of bone marrow mesenchymal stem cells in vivo augments bone healing in a mouse model of segmental bone defect. Bone 2012; 50: 1012–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Lu W, Zhao Y, Rong P, Cao R, Gu W et al. Adipocytes derived from human bone marrow mesenchymal stem cells exert inhibitory effects on osteoblastogenesis. Curr Mol Med 2011; 11: 489–502. [DOI] [PubMed] [Google Scholar]
- Caplan AI. The mesengenic process. Clin Plast Surg 1994; 21: 429–435. [PubMed] [Google Scholar]
- Deans RJ, Moseley AB. Mesenchymal stem cells: biology and potential clinical uses. Exp Hematol 2000; 28: 875–884. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhao RC. Mesenchymal stem cells targeting the GVHD. Sci China C. Life Sci 2009; 52: 603–609. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringdén O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol 2003; 31: 890–896. [DOI] [PubMed] [Google Scholar]
- Meisel R, Zibert A, Laryea M, Göbel U, Däubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood 2004; 103: 4619–4621. [DOI] [PubMed] [Google Scholar]
- Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 2007; 25: 2739–2749. [DOI] [PubMed] [Google Scholar]
- Maccario R, Podestà M, Moretta A, Cometa A, Comoli P, Montagna D et al. Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica 2005; 90: 516–525. [PubMed] [Google Scholar]
- Krampera M, Cosmi L, Angeli R, Pasini A, Liotta F, Andreini A et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells 2006; 24: 386–398. [DOI] [PubMed] [Google Scholar]
- Jiang X, Zhang Y, Liu B, Zhang S, Wu Y, Yu X et al. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood 2005; 105: 4120–4126. [DOI] [PubMed] [Google Scholar]
- Zhu G, Zhou X, Lu H, Zhou J, Li A, Xu W et al. Human bone marrow mesenchymal stem cells express multiple hematopoietic growth factors. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2003; 11: 115–119. [PubMed] [Google Scholar]
- Ball LM, Bernardo ME, Roelofs H, Lankester A, Cometa A, Egeler RM et al. Cotransplantation of ex vivo expanded mesenchymal stem cells accelerates lymphocyte recovery and may reduce the risk of graft failure in haploidentical hematopoietic stem-cell transplantation. Blood 2007; 110: 2764–2767. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 2004; 363: 1439–1441. [DOI] [PubMed] [Google Scholar]
- Weng JY, Du X, Geng SX, Peng YW, Wang Z, Lu ZS et al. Mesenchymal stem cell as salvage treatment for refractory chronic GVHD. Bone Marrow Transplant 2010; 45: 1732–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma RR, Pollock K, Hubel A, McKenna D. Mesenchymal stem or stromal cells: a review of clinical applications and manufacturing practices. Transfusion 2014; 54: 1418–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boltze J, Arnold A, Walczak P, Jolkkonen J, Cui L, Wagner D. The Dark Side of the Force—constraints and complications of cell therapies for stroke. Front Neurol 2015; 6: 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev 2009; 18: 683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Kerkelä E, Bakreen A, Boltze J, Lukomska B, Jolkkonen J et al. Cerebral embolism caused by intra-arterial delivery of allogeneic bone-marrow mesenchymal stem cells in rats is related to cell dose and infusion velocity. Stem Cells Res 2015; 6: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl MG. Der immunsupprimierte Patient: Infektionsprophylaxe und -therapie. Uni-Med: Bremen, Germany. 2003. [Google Scholar]
- Appelbaum FR, Bacigalupo A, Soiffer R. Anti-T cell antibodies as part of the preparative regimen in hematopoietic cell transplantation—a debate. Biol Blood Marrow Transplant 2012; 18: S111–S115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohty M. Mechanisms of action of antithymocyte globulin: T-cell depletion and beyond. Leukemia 2007; 21: 1387–1394. [DOI] [PubMed] [Google Scholar]
- Godown J, Deal AM, Riley K, Bailliard F, Blatt J. Worsening bradycardia following antithymocyte globulin treatment of severe aplastic anemia. J Pediatr Pharmacol Ther 2011; 16: 218–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med 2006; 355: 1018–1028. [DOI] [PubMed] [Google Scholar]
- Weißmüller S, Kronhart S, Kreuz D, Schnierle B, Kalinke U, Kirberg J et al. TGN1412 induces lymphopenia and human cytokine release in a Humanized Mouse Model. PLoS One 2016; 11: e0149093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persky DO, Dornan D, Goldman BH, Braziel RM, Fisher RI, Leblanc M et al. Fc gamma receptor 3a genotype predicts overall survival in follicular lymphoma patients treated on SWOG trials with combined monoclonal antibody plus chemotherapy but not chemotherapy alone. Haematologica 2012; 97: 937–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneba M. Antikörpertherapie in der Hämatologie und Onkologie. Uni-Med: Bremen, Germany. 2001. [Google Scholar]
- Reff ME, Carner K, Chambers KS, Chinn PC, Leonard JE, Raab R et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood 1994; 83: 435–445. [PubMed] [Google Scholar]
- Li M, Xiao X, Zhang W, Liu L, Xi N, Wang Y. AFM analysis of the multiple types of molecular interactions involved in rituximab lymphoma therapy on patient tumor cells and NK cells. Cell Immunol 2014; 290: 233–244. [DOI] [PubMed] [Google Scholar]
- Impellizeri JA, Howell K, McKeever KP, Crow SE. The role of rituximab in the treatment of canine lymphoma: an ex vivo evaluation. Vet J 2006; 171: 556–558. [DOI] [PubMed] [Google Scholar]
- Fricke S, Hilger N, Fricke C, Schönfelder U, Behre G, Ruschpler P et al. Prevention of graft-versus-host-disease with preserved graft-versus-leukemia-effect by ex vivo and in vivo modulation of CD4(+) T-cells. Cell Mol Life Sci 2014; 71: 2135–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt F, Hilger N, Oelkrug C, Svanidze E, Ruschpler P, Eichler W et al. Flow cytometric analysis of the graft-versus-leukemia-effect after hematopoietic stem cell transplantation in mice. Cytometry A 2015; 87: 334–345. [DOI] [PubMed] [Google Scholar]
- Chao N. Control of GVHD: it's in our DNA!. Blood 2012; 119: 1102–1103. [DOI] [PubMed] [Google Scholar]
- Ford ML, Larsen CP. Translating costimulation blockade to the clinic: lessons learned from three pathways. Immunol Rev 2009; 229: 294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson AM, Vanguri VK, Sharpe AH. SnapShot: B7/CD28 costimulation. Cell 2009; 137: 974–4.e1. [DOI] [PubMed] [Google Scholar]
- Salomon B, Bluestone JA. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu Rev Immunol 2001; 19: 225–252. [DOI] [PubMed] [Google Scholar]
- Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol 2005; 23: 515–548. [DOI] [PubMed] [Google Scholar]
- Cerdan C, Martin Y, Courcoul M, Mawas C, Birg F, Olive D. CD28 costimulation up-regulates long-term IL-2R beta expression in human T cells through combined transcriptional and post-transcriptional regulation. J Immunol 1995; 154: 1007–1013. [PubMed] [Google Scholar]
- Freeman GJ, Boussiotis VA, Anumanthan A, Bernstein GM, Ke XY, Rennert PD et al. B7-1 and B7-2 do not deliver identical costimulatory signals, since B7-2 but not B7-1 preferentially costimulates the initial production of IL-4. Immunity 1995; 2: 523–532. [DOI] [PubMed] [Google Scholar]
- Cerdan C, Martin Y, Courcoul M, Brailly H, Mawas C, Birg F et al. Prolonged IL-2 receptor alpha/CD25 expression after T cell activation via the adhesion molecules CD2 and CD28. Demonstration of combined transcriptional and post-transcriptional regulation. J Immunol 1992; 149: 2255–2261. [PubMed] [Google Scholar]
- Fraser JD, Weiss A. Regulation of T-cell lymphokine gene transcription by the accessory molecule CD28. Mol Cell Biol 1992; 12: 4357–4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser JD, Irving BA, Crabtree GR, Weiss A. Regulation of interleukin-2 gene enhancer activity by the T cell accessory molecule CD28. Science 1991; 251: 313–316. [DOI] [PubMed] [Google Scholar]
- Thompson CB, Lindsten T, Ledbetter JA, Kunkel SL, Young HA, Emerson SG et al. CD28 activation pathway regulates the production of multiple T-cell-derived lymphokines/cytokines. Proc Natl Acad Sci USA 1989; 86: 1333–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstein T, June CH, Ledbetter JA, Stella G, Thompson CB. Regulation of lymphokine messenger RNA stability by a surface-mediated T cell activation pathway. Science 1989; 244: 339–343. [DOI] [PubMed] [Google Scholar]
- Freeman GJ, Gribben JG, Boussiotis VA, Ng JW, Restivo VA, Lombard LA et al. Cloning of B7-2: a CTLA-4 counter-receptor that costimulates human T cell proliferation. Science 1993; 262: 909–911. [DOI] [PubMed] [Google Scholar]
- Freeman GJ, Borriello F, Hodes RJ, Reiser H, Hathcock KS, Laszlo G et al. Uncovering of functional alternative CTLA-4 counter-receptor in B7-deficient mice. Science 1993; 262: 907–909. [DOI] [PubMed] [Google Scholar]
- Gimmi CD, Freeman GJ, Gribben JG, Sugita K, Freedman AS, Morimoto C et al. B-cell surface antigen B7 provides a costimulatory signal that induces T cells to proliferate and secrete interleukin 2. Proc Natl Acad Sci USA 1991; 88: 6575–6579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells AD, Walsh MC, Bluestone JA, Turka LA. Signaling through CD28 and CTLA-4 controls two distinct forms of T cell anergy. J Clin Invest 2001; 108: 895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson P, Henderson L, Chapman JR, Craig JC, Webster AC. Belatacept for kidney transplant recipients. Cochrane Database Syst Rev 2014; 11: CD010699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Townsend R, You X, Shen Y, Zhan P, Zhou Z et al. Pharmacokinetics, pharmacodynamics, and immunogenicity of belatacept in adult kidney transplant recipients. Clin Drug Invest 2014; 34: 117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilat N, Sayegh MH, Wekerle T. Costimulatory pathways in transplantation. Semin Immunol 2011; 23: 293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennberg L, Czech KA, Larsson LC, Mirza B, Bennet W, Song Z et al. Effects of immunosuppressive treatment on host responses against intracerebral porcine neural tissue xenografts in rats. Transplantation 2001; 71: 1797–1806. [DOI] [PubMed] [Google Scholar]
- Tillson M, Niemeyer GP, Welch JA, Brawner W, Swaim SF, Rynders P et al. Hematopoietic chimerism induces renal and skin allograft tolerance in DLA-identical dogs. Exp Hematol 2006; 34: 1759–1770. [DOI] [PubMed] [Google Scholar]
- Fuchs EJ. Transplantation tolerance: from theory to clinic. Immunol Rev 2014; 258: 64–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touzot M, Obada EN, Beaudreuil S, François H, Durrbach A. Complement modulation in solid-organ transplantation. Transplant Rev (Orlando) 2014; 28: 119–125. [DOI] [PubMed] [Google Scholar]
- Tiriveedhi V, Banan B, Deepti S, Nataraju A, Hachem R, Trulock E et al. Role of defensins in the pathogenesis of chronic lung allograft rejection. Hum Immunol 2014; 75: 370–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SR, Lee HJ, Cha SH, Jeong KJ, Lee Y, Jeon CY et al. Long-term survival and differentiation of human neural stem cells in nonhuman primate brain with no immunosuppression. Cell Transplant 2015; 24: 191–201. [DOI] [PubMed] [Google Scholar]
- Rogers SA, Tripathi P, Mohanakumar T, Liapis H, Chen F, Talcott MR et al. Engraftment of cells from porcine islets of Langerhans following transplantation of pig pancreatic primordia in non-immunosuppressed diabetic rhesus macaques. Organogenesis 2011; 7: 154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotti G, Palmisano A, Maggiore U, Buzio C. Vascular endothelium as a target of immune response in renal transplant rejection. Front Immunol 2014; 5: 505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma V, McNeill JH. To scale or not to scale: the principles of dose extrapolation. Br J Pharmacol 2009; 157: 907–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maczewski M, Mackiewicz U. Effect of metoprolol and ivabradine on left ventricular remodelling and Ca2+ handling in the post-infarction rat heart. Cardiovasc Res 2008; 79: 42–51. [DOI] [PubMed] [Google Scholar]
- West LJ, Pierce CM, Thomas WD. Lysergic acid diethylamide: its effects on a male Asiatic elephant. Science 1962; 138: 1100–1103. [DOI] [PubMed] [Google Scholar]
- Wagner D, Pösel C, Schulz I, Schicht G, Boltze J, Lange F et al. Allometric dose retranslation unveiled substantial immunological side effects of granulocyte colony-stimulating factor after stroke. Stroke 2014; 45: 623–626. [DOI] [PubMed] [Google Scholar]
- Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J 2008; 22: 659–661. [DOI] [PubMed] [Google Scholar]
- Saif MW, Choma A, Salamone SJ, Chu E. Pharmacokinetically guided dose adjustment of 5-fluorouracil: a rational approach to improving therapeutic outcomes. J Natl Cancer Inst 2009; 101: 1543–1552. [DOI] [PubMed] [Google Scholar]
- Baxter JD, Tomkins GM. Specific cytoplasmic glucocorticoid hormone receptors in hepatoma tissue culture cells. Proc Natl Acad Sci USA 1971; 68: 932–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Högger P. Was passiert im Körper mit den Glucocorticoiden? Pharmakokinetik und Pharmakodynamik. Pharm Unserer Zeit 2003; 32: 296–301. [DOI] [PubMed] [Google Scholar]
- Demuth DC, Müntener CR CliniPharm/CliniTox: Institut für Veterinärpharmakologie und -toxikologie, 2016. Available at: www.clinipharm.ch/ (last accessed 24 April 2016).
- Wishart Research Group. DrugBank Drug and Drug Target Database, 2016. Available at: http://www.drugbank.ca (last accessed 25 April 2016).
- Burgis E. Intensivkurs: Allgemeine und spezielle Pharmakologie: Zum GK2 und GK 3; mit 320 Tabellen. Urban und Fischer: München, Jena, Germany. 2001. [Google Scholar]
- Aytac HO, Iskit AB, Sayek I. Dexamethasone effects on vascular flow and organ injury in septic mice. J Surg Res 2014; 188: 496–502. [DOI] [PubMed] [Google Scholar]
- Hansen DK, LaBorde JB, Wall KS, Holson RR, Young JF. Pharmacokinetic considerations of dexamethasone-induced developmental toxicity in rats. Toxicol Sci 1999; 48: 230–239. [DOI] [PubMed] [Google Scholar]
- Samtani MN, Jusko WJ. Comparison of dexamethasone pharmacokinetics in female rats after intravenous and intramuscular administration. Biopharm Drug Dispos 2005; 26: 85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peets EA, Staub M, Symchowicz S. Plasma binding of betamethasone-3H, dexamethasone-3H, and cortisol-14C—a comparative study. Biochem Pharm 1969; 18: 1655–1663. [DOI] [PubMed] [Google Scholar]
- Feng Y, Zengmin T, Shuang L, Quanjn Z, Yaming W, Qiuxing H. Local effects of dexamethasone on immune reaction in neural transplantation. Transpl Immunol 2007; 18: 126–129. [DOI] [PubMed] [Google Scholar]
- Dietzel K, Estes K, Brewster M, Bodor N, Derendorf H. The use of 2-hydroxypropyl-β-cyclodextrin as a vehicle for intravenous administration of dexamethasone in dogs. Inter J of Pharm 1990; 59: 225–230. [Google Scholar]
- Plumb DC. Plumb's Veterinary Drug Handbook. PharmaVet; Distributed by Blackwell Publishers: Stockholm, WI, Ames, IA, USA. 2008. [Google Scholar]
- Löscher W, Ungemach FR, Kroker R (eds). Pharmakotherapie bei Haus- und Nutztieren. 6., aktualisierte Aufl. Berlin [u.a.]. Parey, Germany. 2003. [Google Scholar]
- Miyamoto H, Sunamori M, Yoshida T, Suzuki A. The effect of betamethasone on myocardial viability during cold cardioplegia in canine donor heart preservation. Surg Today 1994; 24: 449–455. [DOI] [PubMed] [Google Scholar]
- Wyns H, Meyer E, Watteyn A, Plessers E, de Baere S, de Backer P et al. Pharmacokinetics of dexamethasone after intravenous and intramuscular administration in pigs. Vet J 2013; 198: 286–288. [DOI] [PubMed] [Google Scholar]
- Plumb DC. Veterinary Drug Handbook. Iowa State Press: Ame, IA, USA. 2002. [Google Scholar]
- Samtani MN, Jusko WJ. Stability of dexamethasone sodium phosphate in rat plasma. Int J Pharm 2005; 301: 262–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey BM, Sieber M, Mettler D, Gänger H, Frey FJ. Marked interspecies differences between humans and pigs in cyclosporine and prednisolone disposition. Drug Metab Dispos 1988; 16: 285–289. [PubMed] [Google Scholar]
- Anon. Rote Liste, 2016. Available at: http://www.rote-liste.de/ (last accessed date 25 April 2016).
- Muhetaer G, Takeuchi H, Unezaki S, Kawachi S, Iwamoto H, Nakamura Y et al. Clinical significance of peripheral blood lymphocyte sensitivity to glucocorticoids for the differentiation of high-risk patients with decreased allograft function after glucocorticoid withdrawal in renal transplantation. Clin Ther 2014; 36: 1264–1272. [DOI] [PubMed] [Google Scholar]
- El Dareer SM, Struck RF, White VM, Mellett LB, Hill DL. Distribution and metabolism of prednisone in mice, dogs, and monkeys. Cancer Treat Rep 1977; 61: 1279–1289. [PubMed] [Google Scholar]
- al-Habet SM, Taraporewala IB, Lee HJ. Pharmacokinetics of prednisolone and its local anti-inflammatory steroid-21-oate ester derivatives. In vitro and in vivo comparative study. Drug Metab Dispos 1990; 18: 55–60. [PubMed] [Google Scholar]
- Nichols AI, D'Ambrosio R, Pyszczynski NA, Jusko WJ. Pharmacokinetics and pharmacodynamics of prednisolone in obese rats. J Pharmacol Exp Ther 1989; 250: 963–970. [PubMed] [Google Scholar]
- Paulus P, Holfeld J, Urbschat A, Mutlak H, Ockelmann PA, Tacke S et al. Prednisolone as preservation additive prevents from ischemia–reperfusion injury in a rat model of orthotopic lung transplantation. PLoS One 2013; 8: e73298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H, Wang L, Zhang X, Zhang G, Mai H, Han Y et al. Rapamycin, mycophenolate mofetil, methylprednisolone, and cytotoxic T-lymphocyte-associated antigen 4 immunoglobulin-based conditioning regimen to induce partial tolerance to hind limb allografts without cytoreductive conditioning. Transplant Proc 2008; 40: 1714–1721. [DOI] [PubMed] [Google Scholar]
- Chimalakonda AP, Mehvar R. Effects of methylprednisolone and its liver-targeted dextran prodrug on ischemia–reperfusion injury in a rat liver transplantation model. Pharm Res 2007; 24: 2231–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey FJ, Frey BM, Greither A, Benet LZ. Prednisolone clearance at steady state in dogs. J Pharmacol Exp Ther 1980; 215: 287–291. [PubMed] [Google Scholar]
- Tse FL, Welling PG. Prednisolone bioavailability in the dog. J Pharm Sci 1977; 66: 1751–1754. [DOI] [PubMed] [Google Scholar]
- Haishima A, Kawakami Y, Mizuno S, Kageyama T, Muto M, Suzuki T et al. Acute vascular and interstitial rejection following renal allograft transplantation in dogs. J Vet Med Sci 2002; 64: 1137–1140. [DOI] [PubMed] [Google Scholar]
- Inui K, Schäfers HJ, Aoki M, Wada H, Becker V, Ongsiek B et al. Effect of methylprednisolone and prostacyclin on bronchial perfusion in lung transplantation. Ann Thorac Surg 1993; 55: 464–469. [DOI] [PubMed] [Google Scholar]
- Salminen US, Uusitalo M, Ikonen T, Taskinen E, Morris RE, Harjula AL. Effect of immunosuppression on obliterative lesions in a heterotopic large-animal bronchial allograft model. Transplant Proc 1997; 29: 3155–3156. [DOI] [PubMed] [Google Scholar]
- Rossi G, Gatti S, Reggiani P, Galmarini D, Privitera G, Velio P et al. Small bowel transplantation under oral immunosuppression: experimental study in the pig. Transplant Proc 1997; 29: 1816–1818. [DOI] [PubMed] [Google Scholar]
- Alvinerie M, Tufenkji AE, Houin G, Toutain PL, Galtier P. Comparative incidence of experimental fascioliasis on corticosteroid pharmacokinetics in sheep. Comp Biochem Physiol C 1989; 94: 81–86. [DOI] [PubMed] [Google Scholar]
- Alvinerie M, Sutra JF, Galtier P, Toutain PL. Disposition of prednisolone in the course of subclinical fascioliasis in sheep. Xenobiotica 1993; 23: 483–493. [DOI] [PubMed] [Google Scholar]
- BC Transplant agency of the Provincial Health Services Authority (PHSA). Clinical Guidelines for Transplant Medications, 2015. Available at: http://www.transplant.bc.ca/health-professionals/pharmacy (last accessed 24 April 2016).
- Chan GL, Erdmann GR, Gruber SA, Matas AJ, Canafax DM. Azathioprine metabolism: pharmacokinetics of 6-mercaptopurine, 6-thiouric acid and 6-thioguanine nucleotides in renal transplant patients. J Clin Pharmacol 1990; 30: 358–363. [DOI] [PubMed] [Google Scholar]
- Anstey A, Lear JT. Azathioprine: clinical pharmacology and current indications in autoimmune disorders. BioDrugs 1998; 9: 33–47. [DOI] [PubMed] [Google Scholar]
- Eich DM, Nestler JE, Johnson DE, Dworkin GH, Ko D, Wechsler AS et al. Inhibition of accelerated coronary atherosclerosis with dehydroepiandrosterone in the heterotopic rabbit model of cardiac transplantation. Circulation 1993; 87: 261–269. [DOI] [PubMed] [Google Scholar]
- Goldberg VM, Powell A, Shaffer JW, Zika J, Bos GD, Heiple KG. Bone grafting: role of histocompatibility in transplantation. J Orthop Res 1985; 3: 389–404. [DOI] [PubMed] [Google Scholar]
- Sutherland DE, Steffes MW, Bauer GE, McManus D, Noe BD, Najarian JS. Isolation of human and porcine islets of Langerhans and islet transplantation in pigs. J Surg Res 1974; 16: 102–111. [DOI] [PubMed] [Google Scholar]
- Juma FD, Rogers HJ, Trounce JR, Bradbrook ID. Pharmacokinetics of intravenous cyclophosphamide in man, estimated by gas-liquid chromatography. Cancer Chemother Pharmacol 1978; 1: 229–231. [DOI] [PubMed] [Google Scholar]
- Riggs CE, Egorin MJ, Fuks JZ, Schnaper N, Duffey P, Colvin OM et al. Initial observations on the effects of delta 9-tetrahydrocannabinol on the plasma pharmacokinetics of cyclophosphamide and doxorubicin. J Clin Pharmacol 1981; 21: 90S–98S. [DOI] [PubMed] [Google Scholar]
- Sladek NE, Priest J, Doeden D, Mirocha CJ, Pathre S, Krivit W. PLasma half-life and urinary excretion of cyclophosphamide in children. Cancer Treat Rep 1980; 64: 1061–1066. [PubMed] [Google Scholar]
- Attema-de Jonge ME. Pharmacokinetically Guided Dosing of (High-Dose) Chemotherapeutic Agents. Utrecht University Repository: Utrecht, Netherlands. 2004. [Google Scholar]
- Storb R, Buckner CD, Dillingham LA, Thomas ED. Cyclophosphamide regimens in rhesus monkey with and without marrow infusion. Cancer Res 1970; 30: 2195–2203. [PubMed] [Google Scholar]
- Harris RN, Basseches PJ, Appel PL, Durski AM, Powis G. Carbon tetrachloride-induced increase in the antitumor activity of cyclophosphamide in mice: a pharmacokinetic study. Cancer Chemother Pharmacol 1984; 12: 167–172. [DOI] [PubMed] [Google Scholar]
- Many A, Schwartz RS. On the mechanism of immunological tolerance in cyclophosphamide-treated mice. Clin Exp Immunol 1970; 6: 87–99. [PMC free article] [PubMed] [Google Scholar]
- Hong PS, Srigritsanapol A, Chan KK. Pharmacokinetics of 4-hydroxycyclophosphamide and metabolites in the rat. Drug Metab Dispos 1991; 19: 1–7. [PubMed] [Google Scholar]
- Luo Q, Lu M, Luo L, Fu Y, Fu J, Huang Z. Immune tolerance induced by bone marrow cell transplantation combined with use of cyclophosphamide in diabetic rats with pancreatic transplantation. Sichuan Da Xue Xue Bao Yi Xue Ban 2011; 42: 106–108 124. [PubMed] [Google Scholar]
- Anthony LB, Long QC, Struck RF, Hande KR. The effect of cimetidine on cyclophosphamide metabolism in rabbits. Cancer Chemother Pharmacol 1990; 27: 125–130. [DOI] [PubMed] [Google Scholar]
- Emmerich I, Ottilie H, Hertzsch R, Bode C Vetidata: Institut für Pharmakologie, Pharmazie und Toxikologie der Veterinärmedizinischen Fakultät, 2016. Available at: www.vetidata.de (last accessed date 10 May 2016).
- Putnam CW, Halgrimson CG, Groth CG, Kashiwagi N, Porter KA, Starzl TE. Immunosuppression with cyclophosphamide in the dog. Clin Exp Immunol 1975; 22: 323–329. [PMC free article] [PubMed] [Google Scholar]
- Han J, Liu YL, Fan W, Chao J, Hou YQ, Yin YL et al. Dietary l-arginine supplementation alleviates immunosuppression induced by cyclophosphamide in weaned pigs. Amino Acids 2009; 37: 643–651. [DOI] [PubMed] [Google Scholar]
- Hofírek B, Drábek J. Cytostatic effect of cyclosposphamide on bone marrow in sheep. Acta Vet Brno 1980, 217–222.
- Isacoff WH, Morrison PF, Aroesty J, Willis KL, Block JB, Lincoln TL. Pharmacokinetics of high-dose methotrexate with citrovorum factor rescue. Cancer Treat Rep 1977; 61: 1665–1674. [PubMed] [Google Scholar]
- Burak KW, Urbanski SJ, Swain MG. Successful treatment of refractory type 1 autoimmune hepatitis with methotrexate. J Hepatol 1998; 29: 990–993. [DOI] [PubMed] [Google Scholar]
- Ashar JN, Mathur A, Sangwan VS. Immunosuppression for Mooren's ulcer: evaluation of the stepladder approach—topical, oral and intravenous immunosuppressive agents. Br J Ophthalmol 2013; 97: 1391–1394. [DOI] [PubMed] [Google Scholar]
- Guo P, Wang X, Liu L, Belinsky MG, Kruh GD, Gallo JM. Determination of methotrexate and its major metabolite 7-hydroxymethotrexate in mouse plasma and brain tissue by liquid chromatography-tandem mass spectrometry. J Pharm Biomed Anal 2007; 43: 1789–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonetti A, Chatelut E, Kim S. An extended-release formulation of methotrexate for subcutaneous administration. Cancer Chemother Pharmacol 1994; 33: 303–306. [DOI] [PubMed] [Google Scholar]
- Joseph A, Munroe K, Housman M, Garman R, Richards S. Immune tolerance induction to enzyme-replacement therapy by co-administration of short-term, low-dose methotrexate in a murine Pompe disease model. Clin Exp Immunol 2008; 152: 138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheufler E, Zetler G, Iven H. Pharmacokinetics and organ distribution of methotrexate in the rat. Pharmacology 1981; 23: 75–81. [DOI] [PubMed] [Google Scholar]
- Park JM, Moon CH, Lee MG. Pharmacokinetic changes of methotrexate after intravenous administration to streptozotocin-induced diabetes mellitus rats. Res Commun Mol Pathol Pharmacol 1996; 93: 343–352. [PubMed] [Google Scholar]
- Hashizume H, Rutkowski MD, Weinstein JN, DeLeo JA. Central administration of methotrexate reduces mechanical allodynia in an animal model of radiculopathy/sciatica. Pain 2000; 87: 159–169. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Nakagawa M, Masuda C, Ide M, Uehara R, Ichikawa Y et al. Short-term low dose methotrexate ameliorates abnormal bone metabolism and bone loss in adjuvant induced arthritis. J Rheumatol 1997; 24: 1890–1895. [PubMed] [Google Scholar]
- Ramu A, Fusner JE, Blaschke T, Glaubiger DL. Probenecid inhibition of methotrexate-cerebrospinal fluid pharmacokinetics in dogs. Cancer Treat Rep 1978; 62: 1465–1470. [PubMed] [Google Scholar]
- Storb R, Yu C, Wagner JL, Deeg HJ, Nash RA, Kiem HP et al. Stable mixed hematopoietic chimerism in DLA-identical littermate dogs given sublethal total body irradiation before and pharmacological immunosuppression after marrow transplantation. Blood 1997; 89: 3048–3054. [PubMed] [Google Scholar]
- Sandberg DI, Solano J, Petito CK, Mian A, Mou C, Koru-Sengul T et al. Safety and pharmacokinetic analysis of methotrexate administered directly into the fourth ventricle in a piglet model. J Neurooncol 2010; 100: 397–406. [DOI] [PubMed] [Google Scholar]
- Harker GJ, Stephens FO, Wass J, Zbroja RA, Chick BF, Grace J. A preliminary report on the suitability of sheep epidermal squamous cell carcinoma as a solid tumor model in the evaluation of intra-arterial methotrexate administration. J Surg Oncol 1986; 32: 65–72. [DOI] [PubMed] [Google Scholar]
- Bullingham RE, Nicholls AJ, Kamm BR. Clinical pharmacokinetics of mycophenolate mofetil. Clin Pharmacokinet 1998; 34: 429–455. [DOI] [PubMed] [Google Scholar]
- Aw MM, Dhawan A, Samyn M, Bargiota A, Mieli-Vergani G. Mycophenolate mofetil as rescue treatment for autoimmune liver disease in children: a 5-year follow-up. J Hepatol 2009; 51: 156–160. [DOI] [PubMed] [Google Scholar]
- Morris RE, Hoyt EG, Murphy MP, Eugui EM, Allison AC. Mycophenolic acid morpholinoethylester (RS-61443) is a new immunosuppressant that prevents and halts heart allograft rejection by selective inhibition of T- and B-cell purine synthesis. Transplant Proc 1990; 22: 1659–1662. [PubMed] [Google Scholar]
- Sugioka N, Chen SH, Hayashida K, Koyama H, Ohta T, Kishimoto H et al. Stability and pharmacokinetic studies of a new immunosuppressant, mycophenolate mofetil (RS-61443), in rats. Biopharm Drug Dispos 1995; 16: 591–601. [DOI] [PubMed] [Google Scholar]
- Allison AC, Almquist SJ, Muller CD, Eugui EM. In vitro immunosuppressive effects of mycophenolic acid and an ester pro-drug, RS-61443. Transplant Proc 1991; 23: 10–14. [PubMed] [Google Scholar]
- Echeverri GJ, McGrath K, Bottino R, Hara H, Dons EM, van der Windt DJ et al. Endoscopic gastric submucosal transplantation of islets (ENDO-STI): technique and initial results in diabetic pigs. Am J Transplant 2009; 9: 2485–2496. [DOI] [PubMed] [Google Scholar]
- Rainer WGG, Levay-Young BK, Nakhleh RE, Shearer JD, Dunning M, Nelson CM et al. Portal donor-specific blood transfusion and mycophenolate mofetil allow steroid avoidance and tacrolimus dose reduction with sustained levels of chimerism in a pig model of intestinal transplantation. Transplantation 2004; 77: 1500–1506. [DOI] [PubMed] [Google Scholar]
- Treska V, Molacek J, Kobr J, Racek J, Trefil L, Hes O. Ischemic training and immunosuppressive agents reduce the intensity of ischemic reperfusion injury after kidney transplantation. Exp Clin Transplant 2006; 4: 439–444. [PubMed] [Google Scholar]
- Wei L, Xue T, Yang H, Zhao G, Zhang G, Lu Z et al. Modified uterine allotransplantation and immunosuppression procedure in the sheep model. PLoS One 2013; 8: e81300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henny FC, Kleinbloesem CH, Moolenaar AJ, Paul LC, Breimer DD, van Es LA. Pharmacokinetics and nephrotoxicity of cyclosporine in renal transplant recipients. Transplantation 1985; 40: 261–265. [DOI] [PubMed] [Google Scholar]
- Xue L, Zhang W, Ding X, Zhang J, Bao J, Miao L. Population pharmacokinetics and individualized dosage prediction of cyclosporine in allogeneic hematopoietic stem cell transplant patients. Am J Med Sci 2014; 348: 448–454. [DOI] [PubMed] [Google Scholar]
- Anderson JE, Blaschke TF. Ketoconazole inhibits cyclosporine metabolism in vivo in mice. J Pharmacol Exp Ther 1986; 236: 671–674. [PubMed] [Google Scholar]
- Lagodzinski Z, Górski A, Wasik M. Effect of FK506 and cyclosporine on primary and secondary skin allograft survival in mice. Immunology 1990; 71: 148–150. [PMC free article] [PubMed] [Google Scholar]
- Wagner O, Schreier E, Heitz F, Maurer G. Tissue distribution, disposition, and metabolism of cyclosporine in rats. Drug Metab Dispos 1987; 15: 377–383. [PubMed] [Google Scholar]
- Bolling SF, Lin H, Annesley TM, Boyd JA, Gallagher KP, Levy RJ. Local cyclosporine immunotherapy of heart transplants in rats enhances survival. J Surg Res 1991; 10: 577–583. [PubMed] [Google Scholar]
- McDonald JW, Liu XZ, Qu Y, Liu S, Mickey SK, Turetsky D et al. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat Med 1999; 5: 1410–1412. [DOI] [PubMed] [Google Scholar]
- Wassef R, Cohen Z, Langer B. Pharmacokinetic profiles of cyclosporine in rats. Influence of route of administration and dosage. Transplantation 1985; 40: 489–493. [DOI] [PubMed] [Google Scholar]
- Archer TM, Boothe DM, Langston VC, Fellman CL, Lunsford KV, Mackin AJ. Oral cyclosporine treatment in dogs: a review of the literature. J Vet Intern Med 2014; 28: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaden SL, Riviere JE. Pharmacokinetics, inhibition of lymphoblast transformation, and toxicity of cyclosporine in clinically normal pigs. Am J Vet Res 1990; 51: 399–403. [PubMed] [Google Scholar]
- Rosengard BR, Ojikutu CA, Guzzetta PC, Smith CV, Sundt TM, Nakajima K et al. Induction of specific tolerance to class I-disparate renal allografts in miniature swine with cyclosporine. Transplantation 1992; 54: 490–497. [DOI] [PubMed] [Google Scholar]
- Bom-van Noorloos AA, Visser JJ, Drexhage HA, Meijer S, Hoitsma HF. Cyclosporin A in orthotopic porcine liver transplantation. Long-term survival after short-term treatment. Eur Surg Res 1984; 16: 329–335. [DOI] [PubMed] [Google Scholar]
- Charles BG, Filippich LJ, Pass MA. Pharmacokinetics and absolute bioavailability of cyclosporin following intravenous and abomasal administration to sheep. J Pharm Pharmacol 1993; 45: 821–824. [DOI] [PubMed] [Google Scholar]
- Jusko WJ, Piekoszewski W, Klintmalm GB, Shaefer MS, Hebert MF, Piergies AA et al. Pharmacokinetics of tacrolimus in liver transplant patients*. Clin Pharmacol Ther 1995; 57: 281–290. [DOI] [PubMed] [Google Scholar]
- Venkataramanan R, Swaminathan A, Prasad T, Jain A, Zuckerman S, Warty V et al. Clinical pharmacokinetics of tacrolimus. Clin Pharmacokinet 1995; 29: 404–430. [DOI] [PubMed] [Google Scholar]
- Venkataramanan R, Jain A, Cadoff E, Warty V, Iwasaki K, Nagase K et al. Pharmacokinetics of FK 506: preclinical and clinical studies. Transplant Proc 1990; 22: 52–56. [PMC free article] [PubMed] [Google Scholar]
- Regazzi MB, Rinaldi M, Molinaro M, Pellegrini C, Calvi M, Arbustini E et al. Clinical pharmacokinetics of tacrolimus in heart transplant recipients. Ther Drug Monit 1999; 21: 2–7. [DOI] [PubMed] [Google Scholar]
- Ogata H, Matsui T, Nakamura M, Iida M, Takazoe M, Suzuki Y et al. A randomised dose finding study of oral tacrolimus (FK506) therapy in refractory ulcerative colitis. Gut 2006; 55: 1255–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Ma A, Dun H, Hu Y, Zeng L, Bai J et al. Effects of ASKP1240 combined with tacrolimus or mycophenolate mofetil on renal allograft survival in cynomolgus monkeys. Transplantation 2014; 98: 267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Peng J, Luo H, Loubeau M, Wan X, Xu D et al. Compromised kidney graft rejection response in Vervet monkeys after withdrawal of immunosuppressants tacrolimus and sirolimus. Transplantation 2000; 69: 1555–1561. [DOI] [PubMed] [Google Scholar]
- Takada K, Usuda H, Oh-Hashi M, Yoshikawa H, Muranishi S, Tanaka H. Pharmacokinetics of FK-506, a novel immunosuppressant, after intravenous and oral administrations to rats. J Pharmacobiodyn 1991; 14: 34–42. [DOI] [PubMed] [Google Scholar]
- Cavalli RC, Tambara Filho R, Gomes Regina de PX, da Luz Veronez DA, Slongo J, Fraga RD. Analysis of the histology of the scar bladder and biochemical parameters of rats with a solitary kidney undergoing immunosuppression with tacrolimus. Acta Cir Bras 2014; 29: 508–514. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Jin MB, Shimamura T, Yamashita K, Taniguchi M, Nomura M et al. A new immunosuppressant, FTY720, in canine kidney transplantation: effect of single-drug, induction and combination treatments. Transpl Int 2004; 17: 574–584. [DOI] [PubMed] [Google Scholar]
- Madariaga ML, Michel SG, Tasaki M, Villani V, La Muraglia GM, Sihag S et al. Induction of cardiac allograft tolerance across a full MHC barrier in miniature swine by donor kidney cotransplantation. Am J Transplant 2013; 13: 2558–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DM, Zhu X, Shiba H, Irefin S, Trenti L, Cocieru A et al. Adenosine restores the hepatic artery buffer response and improves survival in a porcine model of small-for-size syndrome. Liver Transpl 2009; 15: 1448–1457. [DOI] [PubMed] [Google Scholar]
- MacDonald A, Scarola J, Burke JT, Zimmerman JJ. Clinical pharmacokinetics and therapeutic drug monitoring of sirolimus. Clin Ther 2000; 22: B101. [DOI] [PubMed] [Google Scholar]
- Brattström C, Säwe J, Tydén G, Herlenius G, Claesson K, Zimmerman J et al. Kinetics and dynamics of single oral doses of sirolimus in sixteen renal transplant recipients. Ther Drug Monit 1997; 19: 397–406. [DOI] [PubMed] [Google Scholar]
- MacDonald A, Scarola J, Burke JT, Zimmerman JJ. Clinical pharmacokinetics and therapeutic drug monitoring of sirolimus. Clin Ther 2000; 22 (Suppl B):B101–B121. [DOI] [PubMed] [Google Scholar]
- Supko JG, Malspeis L. Dose-dependent pharmacokinetics of rapamycin-28-N,N-dimethylglycinate in the mouse. Cancer Chemother Pharmacol 1994; 33: 325–330. [DOI] [PubMed] [Google Scholar]
- Baker H, Sidorowicz A, Sehgal SN, Vézina C. Rapamycin (AY-22,989), a new antifungal antibiotic. III. In vitro and in vivo evaluation. J Antibiot 1978; 31: 539–545. [DOI] [PubMed] [Google Scholar]
- Pilon CB, Petillon S, Naserian S, Martin GH, Badoual C, Lang P et al. Administration of low doses of IL-2 combined to rapamycin promotes allogeneic skin graft survival in mice. Am J Transplant 2014; 14: 2874–2882. [DOI] [PubMed] [Google Scholar]
- Carvalho SR, Watts AB, Peters JI, Liu S, Hengsawas S, Escotet-Espinoza MS et al. Characterization and pharmacokinetic analysis of crystalline versus amorphous rapamycin dry powder via pulmonary administration in rats. Eur J Pharm Biopharm 2014; 88: 136–147. [DOI] [PubMed] [Google Scholar]
- Paoloni MC, Mazcko C, Fox E, Fan T, Lana S, Kisseberth W et al. Rapamycin pharmacokinetic and pharmacodynamic relationships in osteosarcoma: a comparative oncology study in dogs. PLoS One 2010; 5: e11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson JC, Allstadt SD, Fan TM, Khanna C, Lunghofer PJ, Hansen RJ et al. Pharmacokinetics of orally administered low-dose rapamycin in healthy dogs. Am J Vet Res 2016; 77: 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Gao R, Jiang J, Cheng S, Yuan J, Wang C et al. Pharmacokinetics of rapamycin-eluting stents in miniswine coronary model. Chin Med J 2004; 117: 1459–1463. [PubMed] [Google Scholar]
- Chapman TM, Perry CM. Everolimus. Drugs 2004; 64: 861–872; discussion 873–874. [DOI] [PubMed] [Google Scholar]
- van Damme-Lombaerts R, Webb NAY, Hoyer PF, Mahan J, Lemire J, Ettenger R et al. Single-dose pharmacokinetics and tolerability of everolimus in stable pediatric renal transplant patients. Pediatr Transplant 2002; 6: 147–152. [DOI] [PubMed] [Google Scholar]
- Majewski M, Korecka M, Joergensen J, Fields L, Kossev P, Schuler W et al. Immunosuppressive TOR kinase inhibitor everolimus (RAD) suppresses growth of cells derived from posttransplant lymphoproliferative disorder at allograft-protecting doses. Transplantation 2003; 75: 1710–1717. [DOI] [PubMed] [Google Scholar]
- O'Reilly T, McSheehy PMJ, Kawai R, Kretz O, McMahon L, Brueggen J et al. Comparative pharmacokinetics of RAD001 (everolimus) in normal and tumor-bearing rodents. Cancer Chemother Pharmacol 2010; 65: 625–639. [DOI] [PubMed] [Google Scholar]
- Maasilta PK, Vainikka, Tiina LS, Alho HS, Salminen U. Immune cells in a heterotopic lamb-to-pig bronchial xenograft model. Transpl Int 2005; 18: 1100–1108. [DOI] [PubMed] [Google Scholar]
- Yu X, Huang C, Song B, Xiao Y, Fang M, Feng J et al. CD4+CD25+ regulatory T cells-derived exosomes prolonged kidney allograft survival in a rat model. Cell Immunol 2013; 285: 62–68. [DOI] [PubMed] [Google Scholar]
- Mezrich JD, Kesselheim JA, Johnston DR, Yamada K, Sachs DH, Madsen JC. The role of regulatory cells in miniature swine rendered tolerant to cardiac allografts by donor kidney cotransplantation. Am J Transplant 2003; 3: 1107–1115. [DOI] [PubMed] [Google Scholar]
- Fukuda Y, Horie N, Satoh K, Yamaguchi S, Morofuji Y, Hiu T et al. Intra-arterial transplantation of low-dose stem cells provides functional recovery without adverse effects after stroke. Cell Mol Neurobiol 2014; 35: 399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielcarek M, Storb R, Georges GE, Golubev L, Nikitine A, Hwang B et al. Mesenchymal stromal cells fail to prevent acute graft-versus-host disease and graft rejection after dog leukocyte antigen-haploidentical bone marrow transplantation. Biol Blood Marrow Transplant 2011; 17: 214–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jui H, Lin C, Hsu W, Liu Y, Hsu R, Chiang B et al. Autologous mesenchymal stem cells prevent transplant arteriosclerosis by enhancing local expression of interleukin-10, interferon-γ, and indoleamine 2,3-dioxygenase. Cell Transplant 2012; 21: 971–984. [DOI] [PubMed] [Google Scholar]
- Jellema RK, Wolfs TGAM, Lima Passos V, Zwanenburg A, Ophelders DRMG, Kuypers E et al. Mesenchymal stem cells induce T-cell tolerance and protect the preterm brain after global hypoxia-ischemia. PLoS One 2013; 8: e73031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ternant D, Büchler M, Bénéton M, Alván G, Ohresser M, Touchard G et al. Interindividual variability in the concentration-effect relationship of antilymphocyte globulins—a possible influence of FcgammaRIIIa genetic polymorphism. Br J Clin Pharmacol 2008; 65: 60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringdén O, Quadracci LJ, Groth CG. The effects of anti-lymphocyte globulin on lymphocyte subpopulations in recipients of kidney allografts. Scand J Urol Nephrol Suppl 1977; 42: 86–89. [PubMed] [Google Scholar]
- Wolschke C, Zabelina T, Ayuk F, Alchalby H, Berger J, Klyuchnikov E et al. Effective prevention of GVHD using in vivo T-cell depletion with anti-lymphocyte globulin in HLA-identical or -mismatched sibling peripheral blood stem cell transplantation. Bone Marrow Transplant 2014; 49: 126–130. [DOI] [PubMed] [Google Scholar]
- Balner H, Yron I, Dersjant H, Zaalberg O, Betel I. ALG treatment of Rhesus monkeys. Attempts to reduce complications by the induction of tolerance. Transplantation 1970; 10: 416–424. [PubMed] [Google Scholar]
- Gotoh M, Fukuzaki T, Monden M, Dono K, Kanai T, Yagita H et al. A potential immunosuppressive effect of anti-lymphocyte function-associated antigen-1 monoclonal antibody on islet transplantation. Transplantation 1994; 57: 123–126. [PubMed] [Google Scholar]
- Sun S, Greenstein SM, Schechner RS, Tellis VA. Prolonged small bowel allograft survival following repeated intrathymic injections of donor splenocytes and peripheral anti-lymphocyte treatment. Transplant Proc 1999; 31: 588–589. [DOI] [PubMed] [Google Scholar]
- Hartner WC, Markees TG, De Fazio SR, Shaffer D, Van Der Werf WJ, Gilchrist B et al. Effect of early administration of donor bone marrow cells on renal allograft survival in dogs treated with antilymphocyte serum and cyclosporine. Transplantation 1995; 59: 131–134. [DOI] [PubMed] [Google Scholar]
- Bunn D, Lea CK, Bevan DJ, Higgins RM, Hendry BM. The pharmacokinetics of anti-thymocyte globulin (ATG) following intravenous infusion in man. Clin Nephrol 1996; 45: 29–32. [PubMed] [Google Scholar]
- Bieber CP, Lydick E, Griepp RB, David LA, Oyer PE, Stinson EB. Radioimmune assay of heterologous serum gamma-globulin in patients receiving rabbit antihuman thymocyte globulin. Transplantation 1975; 20: 393–398. [DOI] [PubMed] [Google Scholar]
- Yang J, Wang J, Men T, Zhang X, Li X, Shen B et al. Comparison of clinical outcome of low-dose and high-dose rabbit antithymocyte globulin induction therapy in renal transplantation: a single-center experience. Ann Transplant 2014; 19: 277–282. [DOI] [PubMed] [Google Scholar]
- D'Addio F, Boenisch O, Magee CN, Yeung MY, Yuan X, Mfarrej B et al. Prolonged, low-dose anti-thymocyte globulin, combined with CTLA4-Ig, promotes engraftment in a stringent transplant model. PLoS One 2013; 8: e53797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez-Ortiz R, Bestard O, Llaudó I, Franquesa M, Cerezo G, Torras J et al. Induction of suppressive allogeneic regulatory T cells via rabbit antithymocyte polyclonal globulin during homeostatic proliferation in rat kidney transplantation. Transpl Int 2014; 28: 108–119. [DOI] [PubMed] [Google Scholar]
- Wang Z, Kuhr CS, Allen JM, Blankinship M, Gregorevic P, Chamberlain JS et al. Sustained AAV-mediated dystrophin expression in a canine model of Duchenne muscular dystrophy with a brief course of immunosuppression. Mol Ther 2007; 15: 1160–1166. [DOI] [PubMed] [Google Scholar]
- Liu X, Hering BJ, Mellert J, Brandhorst D, Brandhorst H, Federlin K et al. Prevention of primary nonfunction after porcine islet allotransplantation. Transplant Proc 1997; 29: 2071–2072. [DOI] [PubMed] [Google Scholar]
- Scheidhauer K, Wolf I, Baumgartl H, Schilling C, von, Schmidt B, Reidel G et al. Biodistribution and kinetics of (131)I-labelled anti-CD20 MAB IDEC-C2B8 (rituximab) in relapsed non-Hodgkin's lymphoma. Eur J Nucl Med Mol Imaging 2002; 29: 1276–1282. [DOI] [PubMed] [Google Scholar]
- Tobinai K, Kobayashi Y, Narabayashi M, Ogura M, Kagami Y, Morishima Y et al. Feasibility and pharmacokinetic study of a chimeric anti-CD20 monoclonal antibody (IDEC-C2B8, rituximab) in relapsed B-cell lymphoma. The IDEC-C2B8 Study Group. Ann Oncol 1998; 9: 527–534. [DOI] [PubMed] [Google Scholar]
- Mao C, Brovarney MR, Dabbagh K, Birnböck HF, Richter WF, Del Nagro CJ. Subcutaneous versus intravenous administration of rituximab: pharmacokinetics, CD20 target coverage and B-cell depletion in cynomolgus monkeys. PLoS One 2013; 8: e80533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor CGA, Davies WR, Oi K, Teotia SS, Schirmer JM, Risdahl JM et al. Cardiac xenotransplantation: recent preclinical progress with 3-month median survival. J Thorac Cardiovasc Surg 2005; 130: 844–851. [DOI] [PubMed] [Google Scholar]
- Kagan L, Zhao J, Mager DE. Interspecies pharmacokinetic modeling of subcutaneous absorption of rituximab in mice and rats. Pharm Res 2014; 31: 3265–3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincenti F, Blancho G, Durrbach A, Friend P, Grinyo J, Halloran PF et al. Five-year safety and efficacy of belatacept in renal transplantation. J Am Soc Nephrol 2010; 21: 1587–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth RN, Nam AJ, Stanwix MG, Kukuruga D, Drachenberg CI, Bluebond-Langner R et al. Prolonged survival of composite facial allografts in non-human primates associated with posttransplant lymphoproliferative disorder. Transplantation 2009; 88: 1242–1250. [DOI] [PubMed] [Google Scholar]
- Hering BJ, Wijkstrom M, Graham ML, Hårdstedt M, Aasheim TC, Jie T et al. Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nat Med 2006; 12: 301–303. [DOI] [PubMed] [Google Scholar]
- Sharabi Y, Aksentijevich I, Sundt TM, Sachs DH, Sykes M. Specific tolerance induction across a xenogeneic barrier: production of mixed rat/mouse lymphohematopoietic chimeras using a nonlethal preparative regimen. J Exp Med 1990; 172: 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian S, Demetris AJ, Murase N, Rao AS, Fung JJ, Starzl TE. Murine liver allograft transplantation: tolerance and donor cell chimerism. Hepatology 1994; 19: 916–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayumi H, Himeno K, Shin T, Nomoto K. Drug-induced tolerance to allografts in mice. VI. Tolerance induction in H-2-haplotype-identical strain combinations in mice. Transplantation 1985; 40: 188–194. [DOI] [PubMed] [Google Scholar]
- Ben Nasr M, Vergani A, Avruch J, Liu L, Kefaloyianni E, D'Addio F et al. Co-transplantation of autologous MSCs delays islet allograft rejection and generates a local immunoprivileged site. Acta Diabetol 2015; 52: 917–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly S, Bliss TM, Shah AK, Sun GH, Ma M, Foo WC et al. Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proc Natl Acad Sci USA 2004; 101: 11839–11844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamura N, Nakahara K, Kino T, Goto T, Aoki H, Yamaguchi I et al. Prolongation of skin allograft survival in rats by a novel immunosuppressive agent, FK506. Transplantation 1988; 45: 206–209. [DOI] [PubMed] [Google Scholar]
- Niemeyer GP, Boudreaux MK, Goodman-Martin SA, Monroe CM, Wilcox DA, Lothrop CD. Correction of a large animal model of type I Glanzmann's thrombasthenia by nonmyeloablative bone marrow transplantation. Exp Hematol 2003; 31: 1357–1362. [DOI] [PubMed] [Google Scholar]
- Lanza RP, Ecker DM, Kühtreiber WM, Marsh JP, Ringeling J, Chick WL. Transplantation of islets using microencapsulation: studies in diabetic rodents and dogs. J Mol Med 1999; 77: 206–210. [DOI] [PubMed] [Google Scholar]
- Fuchimoto Y, Huang CA, Yamada K, Shimizu A, Kitamura H, Colvin RB et al. Mixed chimerism and tolerance without whole body irradiation in a large animal model. J Clin Invest 2000; 105: 1779–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanjani ED, Almeida-Porada G, Flake AW. The human/sheep xenograft model: a large animal model of human hematopoiesis. Int J Hematol 1996; 63: 179–192. [DOI] [PubMed] [Google Scholar]
- Wheeler DL, Lane JM, Seim HB, Puttlitz CM, Itescu S, Turner AS. Allogeneic mesenchymal progenitor cells for posterolateral lumbar spine fusion in sheep. Spine J 2014; 14: 435–444. [DOI] [PubMed] [Google Scholar]
- Mohiuddin MM, Singh AK, Corcoran PC, Hoyt RF, Thomas ML, Lewis BGT et al. One-year heterotopic cardiac xenograft survival in a pig to baboon model. Am J Transplant 2014; 14: 488–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Schuetz C, Elias N, Veillette GR, Wamala I, Varma M et al. Up to 9-day survival and control of thrombocytopenia following alpha1,3-galactosyl transferase knockout swine liver xenotransplantation in baboons. Xenotransplantation 2012; 19: 256–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higginbotham L, Mathews D, Breeden CA, Song M, Farris AB, Larsen CP et al. Pre-transplant antibody screening and anti-CD154 costimulation blockade promote long-term xenograft survival in a pig-to-primate kidney transplant model. Xenotransplantation 2015; 22: 221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen CP, Pearson TC, Adams AB, Tso P, Shirasugi N, Strobert E et al. Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. Am J Transplant 2005; 5: 443–453. [DOI] [PubMed] [Google Scholar]
- Iwase H, Liu H, Wijkstrom M, Zhou H, Singh J, Hara H et al. Pig kidney graft survival in a baboon for 136 days: longest life-supporting organ graft survival to date. Xenotransplantation 2015; 22: 302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Sogawa H, Boskovic S, Abrahamian G, Smith R, Wee S et al. CD154 blockade for induction of mixed chimerism and prolonged renal allograft survival in nonhuman primates. Am J Transplant 2004; 4: 1391–1398. [DOI] [PubMed] [Google Scholar]
- Wu K, Xiang F, Yuan J, Zeng Z, Zhou H, Chang S et al. A combination of donor specific transfusion and rapamycin prolongs cardiac allograft survival in mice. Transplant Proc 2008; 40: 3699–3701. [DOI] [PubMed] [Google Scholar]
- Gorczynski RM, Bransom J, Cattral M, Huang X, Lei J, Xiaorong L et al. Synergy in induction of increased renal allograft survival after portal vein infusion of dendritic cells transduced to express TGFbeta and IL-10, along with administration of CHO cells expressing the regulatory molecule OX-2. Clin Immunol 2000; 95: 182–189. [DOI] [PubMed] [Google Scholar]
- Sriwatanawongsa V, Davies HS, Calne RY. The essential roles of parenchymal tissues and passenger leukocytes in the tolerance induced by liver grafting in rats. Nat Med 1995; 1: 428–432. [DOI] [PubMed] [Google Scholar]
- Chen G, Li J, Chen L, Lai X, Qiu J. ECDI-fixed allogeneic splenocytes combined with α1-antitrypsin prolong survival of rat renal allografts. Int Immunopharmacol 2015; 26: 43–49. [DOI] [PubMed] [Google Scholar]
- Strober S, Modry DL, Hoppe RT, Pennock JL, Bieber CP, Holm BI et al. Induction of specific unresponsiveness to heart allografts in mongrel dogs treated with total lymphoid irradiation and antithymocyte globulin. J Immunol 1984; 132: 1013–1018. [PubMed] [Google Scholar]
- Todo S, Kam I, Lynch S, Starzl TE. Animal research in liver transplantation with special reference to the dog. Semin Liver Dis 1985; 5: 309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todo S, Porter KA, Kam I, Lynch S, Venkataramanan R, DeWolf A et al. Canine liver transplantation under Nva2-cyclosporine versus cyclosporine. Transplantation 1986; 41: 296–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory CR, Kyles AE, Bernsteen L, Mehl M. Results of clinical renal transplantation in 15 dogs using triple drug immunosuppressive therapy. Vet Surg 2006; 35: 105–112. [DOI] [PubMed] [Google Scholar]
- Katayama M, Igarashi H, Fukai K, Tani K, Momota Y, Kamishina H et al. Fluconazole decreases cyclosporine dosage in renal transplanted dogs. Res Vet Sci 2010; 89: 124–125. [DOI] [PubMed] [Google Scholar]
- Mezrich JD, Yamada K, Lee RS, Mawulawde K, Benjamin LC, Schwarze ML et al. Induction of tolerance to heart transplants by simultaneous cotransplantation of donor kidneys may depend on a radiation-sensitive renal-cell population. Transplantation 2003; 76: 625–631. [DOI] [PubMed] [Google Scholar]
- Tonsho M, Michel S, Ahmed Z, Alessandrini A, Madsen JC. Heart transplantation: challenges facing the field. Cold Spring Harb Perspect Med 2014; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez ER, Ramirez Nessetti DK, Nessetti MBR, Khatamee M, Wolfson MR, Shaffer TH et al. Pregnancy and outcome of uterine allotransplantation and assisted reproduction in sheep. J Min Invas Gynecol 2011; 18: 238–245. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.