Abstract
Aim:
In this study, a planned research work was conducted to investigate the nutrigenomic aspects of supplementation of Allium sativum (garlic) and Ocimum sanctum (holy basil) leaf powder on the growth performance and immune characteristics of broilers.
Materials and Methods:
A 6 weeks feeding trial was conducted with 280-day-old Ven Cobb broilers, distributed randomly into seven experimental groups. Each treatment had 4 replicates with 10 birds each. The birds of the control group (T1) were fed a basal diet formulated as per BIS standards. The broilers of treatment groups T2 and T3 were fed basal diet supplemented with the commercially available garlic powder (GP) at levels of 0.5% and 1.0% of the feed, respectively, while broilers in T4 and T5 were fed basal diet supplemented with commercial grade holy basil leaf powder (HBLP) at levels 0.5% and 1.0% of the feed, respectively. Birds in the T6 were fed with 0.5% GP and 0.5% HBLP, whereas T7 was fed with 1.0% GP and 1.0% HBLP. At the end of the feeding trial (6th week), blood samples were collected and analyzed for relative mRNA expression of toll-like receptors (TLR) 2, TLR 4 and TLR 7 using real-time polymerase chain reaction.
Results:
The mean body weight gain and feed conversion efficiency were improved (p<0.05) in broilers fed the GP and HBLP incorporated diets compared with the control group. The relative mRNA expression levels of TLR 2, TLR 4 and TLR 7 in the peripheral blood of the broilers were found to be increased (p<0.05) in the birds supplemented with graded levels of the GP and HBLP as compared to the untreated group.
Conclusion:
The present work concludes that the inclusion of GP and HBLP could enhance the production performance and immune status of birds by augmenting the T-cell mediated immune response and thereby protects them from disease without decreasing growth traits as a possible substitution to conventional antimicrobials.
Keywords: broilers, garlic, gene expression, holy basil, toll-like receptors
Introduction
Since time immemorial, traditional plants and their products, phytobiotics have been serving as an indispensable source of medicine in indigenous poultry production systems [1]. Today, around the globe, there is an increasing awareness about the emerging drug-resistant microbes, antimicrobial side effects and toxic residual effects of drugs in animal meat products which necessitate the search for newer feed alternatives or complementary medicines for the gut health maintenance and immunomodulation [2,3]. Therefore, the inherent utility of the indigenous herbal extracts/phytobiotics - such as Allium sativum (garlic), Ocimum sanctum (tulsi), Curcuma longa, Azadirachta indica, and Withania somnifera - are being searched out for the practical applications in poultry system for improving health and production indicating toward their immense therapeutic ability [4,5]. In the recent decade, there has been a vigorous emphasis on improving the growth and production performance in broiler industry which has adversely affected the immunological status of the broiler birds. This has thereby, altered the host defense mechanism by encountering the prevailing microbes such as bacteria, fungi, pathogenic viruses, endo and ecto-parasites, and several harmful toxins. Hence, researchers are now thinking toward the use of an array of antimicrobials of the herbal origin which have shown to possess multiple immunomodulatory actions such as phagocytosis, modulation of immunoglobulin and cytokine secretion, cellular co-receptor expression, class switching, lymphocyte expression, and histamine release [4].
Conventional herbal medicinal plants have been claimed to modulate the immune response, thereby augmenting non-specific immunity, essentially macrophages, granulocytes, natural killer cells and many complement functions [6,7]. Phytobiotics from herbs by acting as immunomodulators, serve as a potential alternative for the conventional chemotherapy in a variety of challenges by enhancing the natural defense mechanisms of the host. Several plant extracts, compounds, and formulations have also been patented including various polysaccharides, lectins, flavonoids, peptides, and tannins which are used in various in vitro models to assess their immune response [8]. The herbal preparations such as GP and HBLP have tremendous potential in improving the cell mediated immunity in poultry birds thereby benefiting the poultry sector immensely.
In the present experiment, we have investigated whether the A. sativum (garlic powder [GP]) and O. sanctum (holy basil leaf powder [HBLP]) incorporated diets, in compliance with maintaining growth and production performance could increase the relative mRNA expression of toll-like receptors (TLR 2, TLR 4 and TLR 7) thus enhance immunological status of the broilers by modulating their immune response.
Materials and Methods
Ethical approval
The animal experiment was conducted in accordance with guidelines approved by the Institutional Animal Ethics Committee, 12/CPCSEA Dated 8.4.2013 in the Department of Animal Nutrition, Lala Lajpat Rai University of Veterinary and Animal Sciences, Hisar.
Birds, experiment design, and management
A total of 280-day-old commercial broiler chicks (Ven Cobb) maintained for a period of 6-week in the Department of Animal nutrition, were randomly allotted to 1 of 7 treatments in a completely randomized design. Each treatment consisted of 4 replicate pens with 10 chicks in each. The birds of the control group (T1) were fed a basal diet formulated as per BIS standards [9] meeting the requirements of the growing phase. The ingredient and chemical composition of the basal diet as analyzed according to the standards laid down by Association of Official Analytical Chemists(AOAC) [10] and is presented in Table-1. Seven dietary treatments included the basal diet (T1) which acted as control group. The basal diets supplemented with 0.5% and 1.0% of the commercially available garlic powder (GP) in treatment groups T2 and T3, respectively. Whereas, the birds in the treatment groups T4 and T5 were fed at 0.5% and 1.0% of the commercial grade HBLP, respectively. Birds in the treatment group T6 was fed with 0.5% GP and 0.5% HBLP, whereas T7 treatment group was fed with 1.0% GP and 1.0% HBLP. The experimental design is presented in Table-2. The basal diets were formulated to meet the requirements for growing phase of the broilers as per the standards recommended by the Bureau of Indian Standards (BIS) (Table-1) [9]. All the herbal preparations used in the present experiment were purchased from the local market. Floor litter system was followed where the chicks were kept hygienically in separate pens. All the birds were reared adopting uniform management conditions. The chicks were brooded at 35°C during the 1st week and thereafter the temperature was reduced by 3°C every week until the temperature reached 25°C±1°C. The birds were vaccinated against prevailing diseases adopting a standard protocol. Individual body weight of chicks was recorded at 0 day age and thereafter weekly. Total feed consumed, growth rate, and feed conversion ratio (FCR) were calculated and presented in Table-2. At the end of the experiment, one bird from each replicate was slaughtered ethically by mechanical stunning followed by exsanguinities. Different carcass parameters - such as dressed weight (g), eviscerated weight (g), non-edible weight (g), and drawn weight (g) - were calculated and depicted in Table-3.
Table-1.
Ingredient (%) and chemical composition (% dry matter [DM] basis) of basal diet.
| Item | Starter diet | Finisher diet |
|---|---|---|
| Ingredient composition (%) | ||
| Maize | 53 | 57 |
| Soybean meal | 19 | 16 |
| Rice police | 3 | 4 |
| Ground nut cake | 12 | 11 |
| Fish meal | 7 | 5 |
| Soybean oil | 4 | 5 |
| Mineral mixture | 2.0 | 2.0 |
| *Feed additives | 0.29 | 0.29 |
| Chemical composition (% DM basis) | ||
| Crude protein | 22.04 | 20.08 |
| Crude fiber | 3.61 | 3.32 |
| Ether extract | 8.38 | 8.98 |
| Total ash | 6.18 | 5.86 |
| **Metabolizable energy (kcal/kg) | 3056 | 3163 |
Feed additives include Vitamin mixture-I - 10 g, vitamin, amino acid and Ca mixture-II - 20 g, coccidiostat (Dinitro-0-yoluamide) - 50 g, choline chloride - 50 g, Lysine - 50 g, DL- methionine - 80 g and chlortetracycline - 33.5 g/100 kg;
Calculated values - BIS (2007)
Table-2.
Experimental design.
| Treatment groups | Particulars | Number of replicates | Number of birds/replicate | Total |
|---|---|---|---|---|
| T1 | Control-standard broiler diet as per BIS (2007) specifications | 4 | 10 | 40 |
| T2 | Control+5 g garlic powder/kg of diet | 4 | 10 | 40 |
| T3 | Control+10 g garlic powder/kg of diet | 4 | 10 | 40 |
| T4 | Control+5 g holy basil leaf powder/kg of diet | 4 | 10 | 40 |
| T5 | Control+10 g holy basil leaf powder/kg of diet | 4 | 10 | 40 |
| T6 | Control+5 g garlic powder/kg of diet+5 g holy basil powder/kg of diet | 4 | 10 | 40 |
| T7 | Control+10 g garlic powder/kg of diet+10 g holy basil powder/kg of diet | 4 | 10 | 40 |
| Total | 280 |
Table-3.
Performance parameters viz. feed intake (g/bird), body weight gain (g/bird) and FCR in broilers under different dietary treatments during overall period of 6 weeks of experiment.
| Treatments | Feed intake (g/bird) | Body weight gain (g/bird) | FCR |
|---|---|---|---|
| T1 | 3771.75±4.23 | 1801.75a±1.79 | 2.09c±0.01 |
| T2 | 3713.25±11.94 | 1823.00ab±21.17 | 2.03bc±0.02 |
| T3 | 3687.25±79.93 | 1873.50b±9.13 | 1.96b±0.03 |
| T4 | 3645.75±61.90 | 1818.50ab±11.92 | 2.00bc±0.03 |
| T5 | 3634.25±17.80 | 1863.25b±8.41 | 1.95b±0.01 |
| T6 | 3627.50±6.64 | 2129.25c±22.83 | 1.70a±0.01 |
| T7 | 3640.25±4.23 | 2094.75c±32.93 | 1.73a±0.04 |
Mean values bearing different superscripts in a column differ significantly (p<0.05); T1: Basal diet, T2: 0.5% garlic powder, T3: 1% garlic powder, T4: 0.5% tulsi leaf powder, T5: 1% tulsi leaf powder, T6: Mixture of garlic powder and tulsi leaf powder at 0.5% each, T7: Mixture of garlic powder and tulsi leaf powder at 1% each, FCR=Feed conversion ratio
Blood collection and analysis
At the end of the feeding trial (6th week), blood samples were collected from one broiler per replicate, making four samples per treatment and thus a total of 28 samples were analyzed. About 2 ml of blood was collected from each bird via brachial wing vein puncture using sterilized syringes and 5 ml scalp vein needle set into vacutainer containing ethylene diamine tetraacetic acid (EDTA) for TLR mRNA expression. Plasma was prepared by centrifuging the blood at 3000 rpm for 10 min. The plasma was then transferred into a microcentrifuge tube using a Pasteur pipette and stored at −20°C until further analysis.
Reverse transcription (cDNA synthesis); RNA extraction and preparation of cDNA
Total RNA was isolated from blood samples by using TRIzol® as per the manufacturer’s instruction. In brief, 1 ml of TRIzol® reagent, 200 µl of chloroform was added to 600 ul of blood followed by centrifugation for phase separation and precipitation with isopropanol. Total RNA extracted was dissolved in 30 µL NFW and quantified using Qubit® 2.0 fluorometer (Invitrogen). Reverse transcription was carried out with total reaction volume of 20 μL using cDNA synthesis kit (Fermentas). Briefly, NFW (7.00 μL), 5X RT buffer (4 μL), 10 mM dNTPs (Fermentas) (2 μL), total RNA (5 μL), RT 200 IU/μL (1 μL), Random hexamer (1 μL). The polymerase chain reaction (RT-PCR) cyclic conditions were as initial incubation at 25°C for 5 min, reverse transcription at 42°C for 1 h, and deactivation at 70°C for 5 min in thermal cycler (Applied Biosystem). The cDNA was stored at −20°C till further use.
Real time PCR
For the analysis of temporal expression profile of different genes, real-time PCR was carried out using Step I plus real-time PCR system. For the real-time PCR reaction, SYBR Green dye based PCR mastermix (Affymetrix) was used, and all the instructions were followed as per the manufacturer. The reaction for the target gene, TLRs (TLR 2, TLR 4, and TLR 7), and the endogenous control, β-actin gene was carried out in triplicate along with non-template control as a negative control for each sample. The reaction mixture used to carry out the real-time PCR reaction for TLRs 2, 4 and 7; and β-actin gene contains 2X SYBR green PCR mastermix (Affymetrix, 12.5 μL), primers (forward and reverse 0.3 M each), NFW (variable), and template (2 μL). The cyclic conditions used for amplification were according to the instructions of the manufacturer. Amplification was done with denaturation for 15 min at 95°C, followed by 40 cycles of denaturation for 5 s at 95°C, and annealing/elongation for 30 s at 60°C, and a final melting curve analysis. The set of primers used for the real-time PCR is as shown in Table-4.
Table-4.
Oligonucleotide sequences of sense and antisense primers for real-time PCR products determined.
| Gene1 | Primer | Primer sequence2 | Accession No. | Product size |
|---|---|---|---|---|
| β-Actin | Sense | 5′-GAGAAATTGTGCGTGACATCA-3′ | L08165 | 152 |
| Antisense | 5′-CCTGAACCTCTCATTGCCA-3′ | |||
| TLR 2 | Sense | 5′-CATTCACCATGAGGCAGGGATAG-3′ | AB046533 | 157 |
| Antisense | 5′-GGTGCAGATCAAGGACACTAGGA-3′ | |||
| TLR 4 | Sense | 5′-TTCAGAACGGACTCTTGAGTGG-3′ | AY064697 | 131 |
| Antisense | 5′-CAACCGAATAGTGGTGACGTTG-3′ | |||
| TLR 7 | Sense | 5′-TTGCTGCTGTTGTCTTGAGTGAG-3′ | AJ627563 | 182 |
| Antisense | 5′-AACAACAGTGCATTTGACGTCCT-3′ |
Relative quantification by comparative CT method (ΔΔCT method)
The average CT (Threshold cycle) value obtained for the TLRs 2, 4 and 7 (target) gene was normalized to β-actin (endogenous control). The data obtained were subjected to comparative CT method [11] for the analysis of the expression levels of targeted TLR gene and an endogenous control. The sample at 26 h of incubation was selected as calibrator.
Sequencing of product
Amplicons were sequenced using the Big Dye Terminator Cycle Sequencing Kit (Applied Biosystems, Carlsbad, CA, USA) on an automatic ABI 3130 xl Genetic Analyzer (Applied Biosystems, Carlsbad, CA, USA). The sequence obtained shows 100% nucleotide identity with the TLR sequence of chicken available in the global database.
Statistical analysis
Data were analyzed by one-way ANOVA as a completely randomized design using the General Linear Model (GLM) procedure of SAS Institute. Individual cage was used as an experimental unit for analyzing the performance data, whereas each bird selected was used as the statistical unit for analyzing the data of gene expression. Differences among means were tested by the least significant difference method, and p<0.05 was considered to be statistically significant group.
Results
Growth performance and FCR
Data pertaining to the growth performance as depicted in Table-3 revealed that significantly (p<0.05) highest mean body weight gain was observed in the treatment groups T6 and T7, where birds were supplemented a combination of GP and HBLP at 0.5 g and 1.0 g/kg of the diet, respectively. It was then followed by treatment groups T3 and T5 in which hens were fed ration supplemented with GP and HBLP at higher levels of inclusion of 1.0 g/kg of feed, respectively. While, the control group T1 recorded lowest mean body weight gain among all the treatments at all age groups. No significant difference in body weight gain was observed among the dietary treatments T1, T2, and T4. However, no significant (p<0.05) difference in feed intake (g/bird) was observed between dietary treatment groups T2, T3, T4, T5, T6 and T7 as compared to the control group T1 over the entire period of the experiment (Table-3).
The current research work on the FCR as shown in Table-3 unveiled that FCR was found to be significantly (p<0.05) improved in T6 and T7 as compared to control group T1 due to dietary supplementation of GP and HBLP in combination at 0.5% and 1.0% of the feed, respectively. Similarly, significantly improved FCR was observed in T3 and T5 treatment groups where GP and HBLP were included in the diet of hens at 1.0% of the ration, respectively. However, no significant difference was observed among supplemental groups T2 and T4 compared to T1 (control).
The study results as depicted in Table-5 revealed that overall with respect to the whole period of experiment there was significant (p<0.05) increase in the carcass parameters, viz., dressed weight (g), eviscerated weight (g) and drawn weight (g) in treatment groups T3, T6 and T7, in which broilers were supplemented with GP either alone or in combination with HBLP. While no significant difference was observed on the non-edible weight (g) due dietary supplementation of either of the herbal products (GP and HBLP).
Table-5.
Carcass parameters viz. dressed weight (g), eviscerated weight (g), non-edible weight (g) and drawn weight (g) in broilers under different treatments.
| Treatments | Dressed weight (g) | Eviscerated weight (g) | Non-edible weight (g) | Drawn weight (g) |
|---|---|---|---|---|
| T1 | 1284.85a±7.84 | 1071.59a±5.41 | 503.75±2.12 | 1108.07a±6.43 |
| T2 | 1326.42ab±19.97 | 1093.38ab±21.25 | 452.97±1.59 | 1189.50ab±18.49 |
| T3 | 1395.67b±8.39 | 1170.62b±7.99 | 519.62±2.79 | 1264.05b±10.39 |
| T4 | 1301.25ab±13.65 | 1095.64ab±12.96 | 510.23±1.73 | 1187.84ab±9.49 |
| T5 | 1366.23b±10.24 | 1159.87ab±9.37 | 484.89±0.94 | 1254.89b±14.83 |
| T6 | 1613.48c±20.98 | 1367.78c±23.74 | 521.64±2.46 | 1478.33c±18.99 |
| T7 | 1573.85c±35.24 | 1335.40c±31.48 | 516.21±1.94 | 1443.91c±25.31 |
Mean values bearing different superscripts in a column differ significantly (p<0.05); T1: Basal diet, T2: 0.5% garlic powder, T3: 1% garlic powder, T4: 0.5% tulsi leaf powder, T5: 1% tulsi leaf powder, T6: Mixture of garlic powder and tulsi leaf powder at 0.5% each, T7: Mixture of garlic powder and tulsi leaf powder at 1% each
TLR mRNA gene expression of broilers
The differential expression level of TLRs, viz. TLR 2, TLR 4 and TLR 7 gene transcripts in the of Ven Cobb commercial broiler strains was studied by relative quantification method. The level of target mRNA in different treatment groups was determined by comparative Ct method (ΔΔCt method). The nutrigenomic expression analysis as presented in Table-6 and also depicted in Figures-1 and 2 revealed that relative mRNA expression of TLR 2 of broilers was found to be (p<0.05) enhanced in the treatment groups T4 and T5 supplemented with 0.5% and 1% of the HBLP fed individually or in combination with similar levels of garlic powder in treatment groups T6 and T7, respectively. While, as presented in Table-6 and shown in Figures-1 and 2 at the end of the 6 weeks of experimental protocol broilers had (p<0.05) higher relative mRNA expression of TLR 4 in the plasma of broilers fed diet supplemented with 0.5% and 1% of the HBLP in the treatment groups T4 and T5, respectively. Furthermore, the relative TLR 4 mRNA expression was found to be significantly higher in the treatment groups T6 and T7 where the birds were fed a combination of GP and HBLP at 0.5% and 1% in their diet, respectively. While no significant effect was observed in the relative mRNA expression of TLR 4 in the birds supplemented with 0.5% and 1% of the garlic powder in the treatment groups T2 and T3, respectively as compared to that of birds fed maize based basal diet in control T1 and other dietary treatments. However, the data pertaining to the relative mRNA levels of TLR 7 (Table-6) and shown in Figures-1 and 2 in the plasma of birds revealed slightly different pattern of expression and it was found that significantly highest levels of expression was observed in treatment group T3 supplemented at 1% of the garlic powder, followed by the treatment groups T6 and T7 in which the broilers were fed a combination of GP and HBLP at 0.5% and 1% of their ration, respectively. While no significant differences were observed in the experimental groups T2, T4 and T5 as compared to the control group and rest of the supplemental groups. However, treatment groups T6 and T7 did not show any significant (p<0.05) differences among themselves. In nutshell, experimental treatments containing GP and HBLP either alone or in their combination in the broiler’s diet have potent immune modulating activity by showing stimulatory effect on relative mRNA expression of TLR 2, TLR 4 and TLR 7 of the commercial broilers.
Table-6.
Relative quantitation expression analysis of the toll-like receptors (TLR 2, TLR 4 and TLR 7) with reference to the endogenous reference gene B actin.
| Sample name | Target Name | Cт | Cт Mean | Cт SD | ΔCт mean | RQ |
|---|---|---|---|---|---|---|
| T1 | TLR 2 | 16.567 | 16.544c | 0.0297 | −2.801 | 1.000 |
| T2 | 16.455 | 16.387b | 0.0591 | −2.457 | 0.787 | |
| T3 | 17.838 | 17.759e | 0.0695 | −2.552 | 0.841 | |
| T4 | 16.409 | 16.476bc | 0.1109 | −2.728 | 0.950 | |
| T5 | 15.560 | 15.408a | 0.1409 | −3.330 | 1.443 | |
| T6 | 17.029 | 16.958d | 0.0723 | −2.396 | 0.755 | |
| T7 | 17.125 | 17.097cd | 0.0248 | −2.320 | 0.716 | |
| T1 | TLR 4 | 18.563 | 18.5541e | 0.0201 | −0.791 | 1.000 |
| T2 | 17.995 | 18.009b | 0.0261 | −0.834 | 1.030 | |
| T3 | 20.110 | 20.104f | 0.1091 | −0.206 | 0.666 | |
| T4 | 18.336 | 18.329d | 0.0199 | −0.875 | 1.060 | |
| T5 | 16.827 | 16.846a | 0.0811 | −1.893 | 2.146 | |
| T6 | 18.587 | 18.630e | 0.0420 | −0.724 | 0.954 | |
| T7 | 18.201 | 18.217c | 0.0631 | −1.200 | 1.327 | |
| T1 | TLR 7 | 28.603 | 28.811cd | 0.1825 | 9.4658 | 1.000 |
| T2 | 28.506 | 28.727bcd | 0.2148 | 9.8828 | 0.749 | |
| T3 | 27.854 | 28.158a | 0.2643 | 7.8472 | 3.070 | |
| T4 | 28.352 | 28.454abcd | 0.0901 | 9.2500 | 1.161 | |
| T5 | 28.557 | 28.920d | 0.3323 | 10.180 | 0.609 | |
| T6 | 28.373 | 28.274ab | 0.0860 | 8.9186 | 1.461 | |
| T7 | 28.093 | 28.409abc | 0.4489 | 8.9919 | 1.388 |
Mean values bearing different superscripts in a column differ significantly (p<0.05). TLR=Toll-like receptors
Figure-1.
Amplification plot and melt curve for chicken toll-like receptors (TLR 2), TLR 4 and TLR 7. Panel A and B Amplification plot and melt curve for chicken TLRs 2. Panel C and D Amplification plot and melt curve for chicken TLRs 4. Panel E and F Amplification plot and melt curve for chicken TLRs 7.
Figure-2.
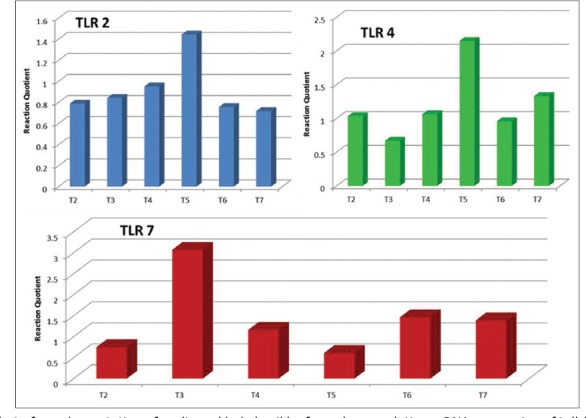
Effect of supplementation of garlic and holy basil leaf powder on relative mRNA expression of toll-like receptors 2 (TLR 2), TLR 4 and TLR 7 in the plasma of broilers. Significantly highest level of increase in the mRNA expression of TLR 2 and TLR 4 was observed in the treatment group T5. While, significantly highest increase in the mRNA expression of TLR 7 was observed in the treatment group T3 where broilers were fed garlic powder (GP) at 1% of the diet followed by the birds in the treatments groups T6 and T7 fed with increasing levels of 0.5% and 1% of the GP and holy basil leaf powder in combination, respectively.
Discussion
Beneficial effects of bioactive plant substances in animal nutrition may include the stimulation of appetite and feed intake, the improvement of endogenous digestive enzyme secretion, activation of immune responses and antibacterial, antiviral and antioxidant actions [12]. Thus, all the nutrients are directed toward growth promotion resulting in enhanced growth performance. The findings of the current work reported a significant (p<0.05) positive effect on average body weight gain by the supplementation of graded levels of the GP and HBLP either alone or in their combinations in commercial broilers at 2, 4 and 6 weeks of age. The improvement in weight gain of the birds using garlic in their rations may probably be due to the fact that allicin (an antibiotic substance found in garlic), inhibits growth of intestinal bacteria such as Staphylococcus aureus and Escherichia coli and inhibit aflatoxins producing fungi [13,14]. Resultantly, when the load of these bacteria in the intestine is low, birds may absorb more nutrients, leading to the improvement in weight gain of the birds using rations supplemented with A. sativum.
The basil plant possessing antioxidant properties results in increase in the digestive enzymes and decrease in bacterial activities and thus leading to muscle weight gain in broiler chicks [15]. Even the improvement in live body weight in broilers may be due to antibacterial effects related to garlic derivative propylpropane thiosulfonate (PTSO) that led to modulation of normal intestinal microflora by competitive exclusion and antagonism and thus improved nutrients digestibility in growing broilers [13,16].
The present investigation revealed that broilers supplemented with GP and HBLP at various levels and in their combinations led to utilization of their feed more efficiently than the birds fed ration without addition. The antibacterial properties of these herbal supplements resulted in better absorption of the nutrients present in the gut, finally leading to improved FCR. It can thus be concluded that there was significant positive effect on the average body weight and subsequent enhanced FCR due to supplementation of the diet with herbal products, GP and HBLP either individually or in combinations in the commercial broiler strains.
Medicinal herbs have shown to possess multiple immunomodulatory actions like phagocytosis, modulation of immunoglobulin and cytokine secretion, cellular co-receptor expression, class switching, lymphocyte expression, and histamine release [4]. In current work, it was observed that dietary inclusion of GP and HBLP either alone or in combination significantly increased the relative mRNA expression of TLR cell markers, which confirmed that these herbal feed additives could stimulate the T cell immune system in the plasma of broiler birds.
In the present investigation, we found that there was a significant increase in the relative mRNA expression of TLR 2 and TLR 4 in the plasma of the broilers fed diet supplemented with graded levels of the HBLP either alone or in combination with garlic powder. TLR 2 recognizes a variety of microbial components. These include lipoproteins/lipopeptides from various pathogens, peptidoglycan and lipoteichoic acid from Gram-positive bacteria [17]. TLR 4 is the principal receptor for lipopolysaccharide, which is a major component of outer membrane of gram-negative bacteria [18]. Several studies have shown that the essential oils and biologically active compounds in fresh leaves of O. sanctum are effective against bacteria such as E. coli, Shigella spp. Salmonella typhi, and Pseudomonas aeruginosa [19].
The antimicrobial action of essential oils in O. sanctum (Linn.) is attributed to monoterpene components which are mostly phenolic in nature. They exert membrane damaging effects to microbial strains and stimulate leakage of cellular potassium which is responsible for a lethal action related to cytoplasmic membrane damage [20]. Immunostimulant potential of ‘Tulsi’ is helpful in the treatment of immunosuppression. It shows its immunomodulatory effect by increase in interferon-γ, interleukin-4, T-helper cells, NK cells [21] thus reducing total bacterial count, increasing neutrophil and lymphocyte count and enhancing phagocytic activity and phagocytic index. Oil from ‘Tulsi’ seed can mediate GABAergic pathways and by this it can modulate both humoral and cell-mediated immunity [22]. Antimicrobial effects of basil essential oil could also be owed to the higher concentrations of linalool and eugenol [23]. Another study revealed that the ethanol and methanol extracts of O. sanctum had the ability to inhibit the growth of all test bacteria including E. coli and P. aeruginosa [24]. Herbs can influence selectively the microorganism by an antimicrobial activity thus favors better nutrient utilization and absorption or the stimulation of the immune system [25].
From the above reported studies and our result findings, it can be inferred that, supplementation of diet with 1% HBLP and GP improved performance, as holy basil leaf might have suppressed the growth of harmful organisms like Coliforms, thereby creating a conducive environment for the growth of the beneficial microbes like Lactobacillus, Bifidobacteria spp. and thereby, aid in digestion and give better performance. Similar mechanism of action would have occurred while supplementing GP and HBLP in combination at 0.5% and 1% dose level. Result findings related to the relative mRNA gene expression of TLR 2 and TLR 4 in the present study it can be inferred that HBLP given at 1% of feed showed better results as compared to the GP fed at 1% of the feed.
TLR 7 family is implicated in intracellular recognition of nucleic acids. The TLR 7 recognizes some antiviral compounds and single-stranded viral RNA. In this study, supplementation of diet with garlic powder either individually or in combination with HBLP significantly increased the relative mRNA expression of TLR 7 in the plasma of the broiler birds. Researchers are focusing on an extract of A. sativum called Ajoene, which appears to protect CD+ cells from attack by HIV early in the viral life cycle. Allicin present in the A. sativum can protect against plasmodium infection by enhancing the host innate as well as innate immunity [26]. Tulsi or holy basil is suggested to shorten the course of illness, clinical symptoms in patients suffering from viral hepatitis and also enhances survival of viral encephalitis patients. Based on the above discussion and our present research work, it can be concluded that GP and HBLP both individually or in combination possess potent antiviral properties reflected by the increased expression of TLR 7 in the plasma of the broilers.
Conclusions
Conventional medicinal herbs constitute an important aspect of applied biotechnological research and therefore as opposed to antimicrobial drugs and chemotherapeutic agents it can be employed for growth promotion and immunity booster in commercial broiler production systems. To summarize, the results of this study may lead us to conclude that the addition of GP and HBLP at higher level of 1% of the feed either alone or in combination in the diet of the broilers increased the relative mRNA expression of TLR 2, 4 and 7, although, among the two feed additives HBLP fed at 1% of diet was found to have better expression and production enhancing profile. Thus, the inclusion of the GP and HBLP could enhance the overall growth performance and immune status of birds by augmenting the T cell mediated immune response and thereby protects them from disease without decreasing performance traits.
Authors’ Contributions
This study is the part of M.V.Sc. research work of the first author NS, who carried out the research under the guidance of Professor NSM. SM, AK and SS helped during the trial. The article was drafted by NS. The revision was made by KB, RK and SM. All authors have read and approved the final version of the manuscript.
Acknowledgments
Authors are thankful for the help and support received from Department of Animal Nutrition and Animal Genetics for their help in carrying out our research. Also, we want to extend our gratitude toward Department of Animal Biotechnology, for the lab facility, guidance and financial assistance received under the RKVY project RKVY project no. 4029 C (g) AN-03 OA.
Competing Interests
The authors declare that they have no competing interests.
References
- 1.Gopi M, Karthik K, Manjunathachar H.V, Tamilmahan P, Kesavan M. Essential oils as a feed additive in poultry nutrition. Adv. Anim. Vet. Sci. 2014;2:1–7. [Google Scholar]
- 2.Chauhan R.S. Nutrition, immunity and livestock health. Indian Cow. Sci. Econ. J. 2010;7:2–13. [Google Scholar]
- 3.Chakraborty S, Pal S.K. Plants for cattle health: A review of ethnoveterinary herbs in veterinary health care. Ann. Ayurvedic Med. 2012;1:144–152. [Google Scholar]
- 4.Mahima R.A, Deb R, Latheef S.K, Samad H.A. Immunomodulatory and therapeutic potentials of herbal, traditional/indigenous and ethnovetrinary medicines. Pak. J. Biol. Sci. 2012;15:754–774. doi: 10.3923/pjbs.2012.754.774. [DOI] [PubMed] [Google Scholar]
- 5.Sridhar M, Suganthi R.U, Thammiaha V. Effect of dietary resveratrol in ameliorating aflatoxin B1-induced changes in broiler birds. J. Anim. Physiol. Anim. Nutr. 2014;99(6):1094–1104. doi: 10.1111/jpn.12260. [DOI] [PubMed] [Google Scholar]
- 6.Hashemi S.R, Davoodi H. Herbal plants as new immunostimulator in poultry industry: A review. Asian J. Anim. Vet. Adv. 2012;7:105–116. [Google Scholar]
- 7.Mirzaei-Aghsaghali A. Importance of medical herbs in animal feeding: A review. Ann. Biol. Res. 2012;3:918–923. [Google Scholar]
- 8.Cherng J.M, Chiang W, Chiang L.C. Immunomodulatory activities of common vegatables and spices of Umbelliferaeand its related coumarins and flavonoids. Food Chem. 2008;106:944–950. [Google Scholar]
- 9.Bureau of Indian Standards, BIS. (2007) Requirement for Chicken Feeds. IS: 1374-2007. Bureau of Indian Standards, New Delhi [Google Scholar]
- 10.AOAC. Official Methods of Analysis. 18th ed. Gaitherburg, Madison: Association of Official Analytical Chemists; 2007. [Google Scholar]
- 11.Livak K.J, Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C (T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 12.Toghyani M, Toghyani M, Gheisari A, Ghalamkari G, Eghbalsaied S. Evaluation of cinnamon and garlic as antibiotic growth promoter substitutions on performance, immune responses, serum biochemical and haematological parameters in broiler chicks. Livest. Sci. 2011;138:167–173. [Google Scholar]
- 13.Amagase H, Petesch B.L, Matsuura H, Kasuga S, Itakura Y. Intake of garlic and its bioactive components. J. Nutr. 2001;131:955S–962S. doi: 10.1093/jn/131.3.955S. [DOI] [PubMed] [Google Scholar]
- 14.Meraj I.C.A. Effect of Garlic and Neem Leaves Supplementation on the Performance of Broiler Chickens. Faisalabad, Pakistan: M.Sc. Thesis, Deptartement of Poultry Sciences, University of Agriculture; 1998. [Google Scholar]
- 15.Khan F.U, Durrani F.R, Sultan A, Khan R, Naz S. Effect of fenugreek (Trigonella foenum-graecum) seed extract on visceral organs of broiler chicks. ARPN. J. Agric. Biol. Sci. 2009;4:58–61. [Google Scholar]
- 16.Peinado M, Ruij R, Echavarri A, Aranda-Olmedo I, Rubio L. Garlic erivative PTS-O modulates intestinal microbiota composition and improves digestibility in growing broiler chickens. Anim. Feed Sci. Technol. 2013;181:87–92. [Google Scholar]
- 17.Takeda K, Akira S. Toll-like receptors in innate immunity. Int. Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 18.Kannaki T.R, Reddy M.R, Verma P.C, Shanmugam M. Chicken toll-like receptors and their role in immunity. World’s Poult. Sci. Assoc. 2010;66:727–738. [Google Scholar]
- 19.Prakash P, Gupta N. herapeutic uses of Ocimum sanctum Linn (tulsi) with a note on eugenol and its pharmacological actions: A short review. Indian J. Physiol. Pharmacol. 2005;49(2):125–131. [PubMed] [Google Scholar]
- 20.Mahamood K, Yaqoob U, Bajwa R. Antibacterial activity of essential oil of Ocimum sanctum (L) Mycopath. 2008;6:63–65. [Google Scholar]
- 21.Mondal S, Varma S, Bamola V.D, Naik S.N, Mirdha B.R. Double-blinded randomized controlled trial for immunomodulatory effects of tulsi (Ocimum sanctum Linn.) Leaf extract on healthy volunteers. J. Ethnopharmacol. 2011;136:452–456. doi: 10.1016/j.jep.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Mediratta P.K, Sharma K.K, Singh S. Evaluation of immunomodulatory potential of Ocimum sanctum seed oil and its possible mechanism of action. J. Ethanopharmacol. 2002;80:15–20. doi: 10.1016/s0378-8741(01)00373-7. [DOI] [PubMed] [Google Scholar]
- 23.Verma R.S, Bisht P.S, Padalia R.C, Saikia D, Chauhan A. Chemical composition and antibacterial activity of essential oil from two Ocimum spp. Grown in sub-tropical India during spring-summer cropping season. Asian J. Tradit. Med. 2011;6(5):211–217. [Google Scholar]
- 24.Pathmanathan M.K, Uthayarasa K, Jeyadevan J.P, Jeyaseelan E.C. In vitro antibacterial activity and phytochemical analysis of some selected medicinal plants. Int. J. Pharm. Biol. Arch. 2010;1(3):291–299. [Google Scholar]
- 25.Wenk C. Herbs and botanicals as feed additives in monogastric animals. Asian-Australas J. Anim. Sci. 2003;16(2):282–289. [Google Scholar]
- 26.Feng Y, Zhu X, Wang Q, Jiang Y, Shang H, Cui L, Cao Y. Allicin enhances proinflammatory immune responses and protects against acute murine malaria infection. Malar. J. 2012;11:268–296. doi: 10.1186/1475-2875-11-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sato K, Takahashi K, Tohno M, Miura Y, Kamada T, Ikegami S, Kitazawa H. Immunodulation in gut-associated lymphoid tissue of neonatal chicks by immunobiotic diets. Poult. Sci. 2009;88:2532–538. doi: 10.3382/ps.2009-00291. [DOI] [PubMed] [Google Scholar]
- 28.Bai S.P, Lu L, Luo X.G, Liu B. Kinetic of manganese absorption in ligated small intestinal segments of broilers. Poult. Sci. 2008;87:2596–2604. doi: 10.3382/ps.2008-00117. [DOI] [PubMed] [Google Scholar]


