Abstract
Background & Aims
Granulocyte macrophage colony-stimulating factor (GM-CSF) treatment induces clinical response in patients with active Crohn’s disease. To explore whether monocytes mediate GM-CSF effects in vivo, we used a mouse model of chronic colitis induced by dextran sulfate sodium (DSS).
Methods
Murine bone marrow-derived monocytes were activated with GM-CSF in vitro, and gene expression, phenotype, and function of GM-CSF-activated monocytes (GMaM) were analyzed. Therapeutic effects of GMaM were assessed in a model of chronic colitis induced by repeated cycles of DSS. Monocytes were administered intravenously and their immunomodulatory functions were evaluated in vivo by clinical monitoring, histology, endoscopy, immunohistochemistry, and expression of inflammatory markers in the colon. The distribution of injected monocytes in the intestine was measured by in vivo imaging.
Results
GMaM expressed significantly higher levels of anti-inflammatory molecules. Production of reactive oxygen species was also increased while phagocytosis and adherence were decreased. GMaM up-regulated CD39 and CD73, which allows the conversion of adenosine triphosphate into adenosine and coincided with the induction of Foxp3+ (forkhead-box-protein P3 positive) regulatory T cells (Treg) in cocultures of GMaM and naive T cells. In chronic DSS-induced colitis, adoptive transfer of GMaM led to significant clinical improvement, as demonstrated by reduced weight loss, inflammatory infiltration, ulceration, and colon shrinkage. As GMaM migrated faster and persisted longer in the inflamed intestine compared with control monocytes, their presence induced Treg generation in vivo.
Conclusions
GM-CSF leads to specific monocyte activation that modulates experimental colitis via mechanisms that include the induction of Treg. We demonstrate a possible mechanism of Treg induction through CD39 and CD73 expression on monocytes.
Keywords: Adaptive Immunity, Dextran Sulfate Sodium, Experimental Colitis, Immune Response, Innate Immunity, GM-CSF, Monocyte, T Cell
Abbreviations used in this paper: ALDH, aldehyde dehydrogenase; Arg1, arginase 1; ATP, adenosine triphosphate; CD, Crohn’s disease; CD39, E-NTPDase; CD73, ecto-5′-nucleotidase; CFSE, carboxyfluorescein succinimidyl ester; DC, dendritic cells; DSS, dextran sulfate sodium; FCS, fetal calf serum; Foxp3, forkhead-box-protein P3; GM-CSF, granulocyte macrophage colony-stimulating factor; GMaM, granulocyte-macrophage colony-stimulating factor–activated monocytes; IBD, inflammatory bowel disease; IL, interleukin; IL-1Ra, IL-1 receptor antagonist; LPS, lipopolysaccharide; MACS, magnetic-activated cell sorting; MEICS, murine endoscopic index of colitis severity; NO, nitric oxide; OD, optical density; PBS, phosphate-buffered saline; PCR, polymerase chain reaction; qRT-PCR, quantitative reverse-transcription polymerase chain reaction; RA, retinoic acid; ROS, reactive oxygen species; TNFα, tumor necrosis factor α; Treg, regulatory T cells; WT, wild type
Summary.
Monocytes can mediate the clinical response of granulocyte macrophage colony-stimulating factor (GM-CSF) in colitis. GM-CSF-activated monocytes express adenosine triphosphate–converting enzymes (CD39/CD73) to generate adenosine which possibly induces regulatory T cells. This mechanism of monocytes may control intestinal inflammation.
Crohn’s disease (CD) is a chronic inflammatory bowel disease (IBD) that may affect the whole gastrointestinal tract. It was initially hypothesized that the inflammatory pathology in CD was exclusively a result of dysregulated adaptive immune responses (eg, T cells).1 However, more recent data implicate roles for innate as well as adaptive immunity in CD pathogenesis,2, 3 supported by the discovery of genetic defects associated with innate immune functions (eg, mutations in nucleotide-binding oligomerization domain-containing protein 2) in CD patients.4 Further support comes from the observation that monocyte/macrophages of some CD patients fail to secrete proinflammatory cytokines and chemokines.5, 6 Therefore, innate immune cells and particularly blood monocytes, the source of macrophages and dendritic cells (DCs) in inflamed intestinal mucosa,7 play key roles in CD pathogenesis.
Monocytes and their derivatives are crucial for most phases of immune reactions, ranging from the progression to resolution of inflammatory lesions. This functional diversity reflects the phenotypic heterogeneity of monocytes and macrophages, encompassing classic proinflammatory monocyte/macrophages (M1-polarized) to alternatively activated macrophages (M2-polarized) generated by exposure to interleukin 4 (IL-4)/IL-13. In addition, it is known that regulatory monocytes and macrophages that produce IL-10 or transforming growth factor-β are generated in response to glucocorticoids or interferon-γ alone.8, 9 Advances in our understanding of how CD can result from an altered innate immune function have led to new therapeutic approaches that enhance innate immunity and host defense.
Stimulators of innate immunity have been tested, including granulocyte macrophage colony-stimulating factor (GM-CSF), a classic growth factor for innate immune cells of the myeloid lineage.10 GM-CSF therapy has been observed to provide partial protection against intestinal inflammation in a mouse dextran sulfate sodium (DSS) colitis model,11, 12 and GM-CSF–deficient mice show increased susceptibility to DSS-induced colitis.13 Mechanisms by which GM-CSF exerts beneficial effects in CD have not been fully elucidated, but they likely involve key mediators of innate and adaptive immunity, possibly including forkhead-box-protein P3 positive (Foxp3+) regulatory T cells (Treg) recruitment via CD11b+CD103+ DCs.14, 15
Interestingly, GM-CSF injection into mice with DSS-induced colitis results in an accumulation of splenic CD11b+ cells that promote wound closure and epithelial cell proliferation in vitro. Furthermore, transfer of in vivo GM-CSF-expanded splenic CD11b+ cells into DSS-treated mice significantly reduces disease severity.11 It remains to be shown however, how the expansion of GM-CSF-activated monocytes (GMaM) leads to beneficial effects in IBD.
Because of the central role of different monocyte subtypes and their derivative cells in the immune system, we hypothesized that the effects of GM-CSF on mucosal inflammation could be mediated via monocytes. Therefore, we activated bone marrow-derived monocytes with GM-CSF, characterized their phenotype, and examined their functional properties in vitro. The therapeutic potential of the GMaM was assessed in a DSS-induced model of chronic colitis and verified in the T-cell transfer colitis model. We show that GMaM are both gut homing and strong inhibitors of DSS-induced colitis, most likely by inducing Treg that are generated in high numbers by GMaM in vitro when cocultured with naive T cells. Furthermore, GM-CSF mediates up-regulation of E-NTPDase (CD39) and ecto-5′-nucleotidase (CD73) on GMaM, which is associated with enhanced conversion of adenosine triphosphate (ATP) to adenosine and is likely used for the observed induction of Treg. Our findings suggest that GMaM use CD39 and CD73 to differentiate Treg from naive T cells, which combined with other unique immunomodulatory features of GMaM can lead to amelioration of excessive intestinal inflammation.
Materials and Methods
Mice
The B6.129P(Cg)-Ptprca Cx3cr1tm1Litt/LittJ, Rag1−/−, CD45.1 (C57/BL6-Ly 5.1), and corresponding wild-type (WT) C57BL/6J mice were obtained from the Jackson Laboratory (Bar Harbor, ME). The CD39−/− mice were kindly provided by Prof. Kettenmann from the Max-Delbrueck-Center, Berlin, Germany. All mice were housed and bred under specific pathogen-free conditions and were used between 8 and 14 weeks of age. All animal studies were performed in accordance to approved protocols of the animal welfare committee of the North Rhine-Westphalia State Agency for Nature, Environment, and Consumer Protection, Recklinghausen, Germany (LANUV NRW Reference No. 87-51.04.2010.A113).
Isolation of Bone Marrow-Derived Monocytes and In Vitro GM-CSF Treatment
Bone marrow-derived monocytes and GMaM were generated in vitro from freshly isolated bone marrow precursor cells. Briefly, femurs and tibias were dissected from mice, tissue cleaned off, then flushed with cold phosphate-buffered saline (PBS)/1% fetal calf serum (FCS). Erythrocytes were removed by incubation in tris ammonium chloride buffer, and the cells further separated on a Ficoll gradient (Biochrom, Berlin, Germany). The interphase was collected, and the remaining T cells (CD90+), B cells (CD19+), and DCs (CD11c+) were removed using magnetic beads coupled to anti-CD90, anti-CD19, and anti-CD11c antibodies using magnetic-activated cell sorting (MACS) technology according to the manufacturer’s instructions (Miltenyi Biotech, Bergisch Gladbach, Germany). The remaining cells were cultured for 2 days in 20% L929 cell supernatant (containing M-CSF) conditioned Dulbecco’s modified Eagle medium containing 2 mM glutamine, 0.1 mM nonessential amino acids (all Invitrogen, Karlsruhe, Germany), penicillin (100 U/mL), streptomycin (100 μg/mL), and 10% heat-inactivated FCS (all Biochrom) including additional supplementation with 150 U/mL recombinant murine GM-CSF (ImmunoTools, Friesoythe, Germany) for induction of GMaM.
Induction of Colitis and Treatment
Mice were fed with 2% (wt/vol) DSS (molecular weight 36–50 kDa; MP Biomedicals, Santa Ana, CA) dissolved in UV-sterilized tap water ad libitum for 3 days, followed by 4 days of untreated tap water alone. The treatment cycle was repeated twice subsequently (three cycles in total). Non-DSS-treated control animals received tap water alone throughout the experiment. GMaM or control monocytes (2 × 106 cells) were administered intravenously 1 day before starting the third DSS treatment cycle.
For induction of transfer colitis, syngeneic CD4+ T cells were prepared from spleens of C57BL/6 mice, and CD25+ cells were removed using MACS technology. We adoptively transferred 1 × 106 CD4+CD25− T-cells into Rag1−/− mice (on C57BL/6 background) intravenously. The weight of the animals was monitored frequently (at least every 2 days) until they lost body weight on consecutive days and colitis had been established. All animals that fulfilled the criterion of fully developed colitis by days 19–22 were intravenously injected with 2 × 106 monocytes per mouse (GMaM or control monocytes), and their weight was monitored for additional 10 days.
In Vivo Induction of Regulatory T Cells by GMaM
We injected Rag1−/− mice (on C57BL/6 background) with 2 × 106 CD4+ T cells (intravenous) to reconstitute the T-cell pool; to some mice, 2 × 106 GMaM or control monocytes (WT or CD39−/−) were simultaneously injected (intravenous). After 7 days, the spleens were removed, a single-cell suspension was prepared, and the cells were counted. Afterward, the presence of Foxp3+ CD4+ T cells was evaluated by flow cytometry as described in the flow cytometry section. Finally, the total Treg cell number was calculated based on number of total splenocytes.
Assessment of Colon Inflammation and Colonoscopy
Mice were weighed daily and were visually inspected for hunched posture, diarrhea, and rectal bleeding. On day 20, mice from each group were anesthetized with isoflurane (100% v/v, 1.5 vol. %, 1.5 L/min) (Florene; Abbott, Wiesbaden, Germany) and were given an enema (Freka-Clyss; Fresenius Kabi, Sèvres, France).
High-resolution colonoscopy was performed using a veterinary endoscopy workstation (Coloview; Karl Storz, Tuttlingen, Germany) to assess colitis. Under visual control, the rigid miniature endoscope (1.9-mm outer diameter) was inserted according to anatomic conditions. The modified murine endoscopic index of colitis severity (MEICS), which evaluates thickening of the colon, changing vascularity, and the presence of fibrin, granular mucosal surfaces, and also stool consistency (0–3 points each, maximum of 15 points), was used to investigate colonic inflammation.16
To measure colon length, the colon was excised between the ileocecal junction and proximal rectum and was measured with a ruler without stretching the organ. Subsequently, the colon was rinsed with ice-cold PBS and cut open longitudinally. A specimen of the distal colon was taken and immediately frozen in liquid nitrogen. The remaining colon was embedded for cryosectioning in a “swiss roll” configuration.17
Quantitative Real-Time Polymerase Chain Reaction
Gene expression analysis in mouse monocytes after treatment with GM-CSF for 48 hours was performed by quantitative real-time reverse-transcription polymerase chain reaction (qRT-PCR) as described previously elsewhere.18 The total RNA was isolated from distal colon tissue using a Precellys24 (Bertin Technologies, Montigny-le-Bretonneux, France) and a NucleoSpin RNA II Kit (Macherey-Nagel, Düren, Germany) according to the manufacturer’s instructions. We performed PCR amplifications on a CFX384 Touch real-time PCR detection system (Bio-Rad Laboratories, Munich, Germany). Relative gene expression was normalized to the endogenous housekeeping control gene ribosomal protein L13a, and the relative expression of respective genes was calculated by the comparative threshold cycle method. The primers used for qRT-PCR analysis are given in Supplementary Table 1.
Flow Cytometry
A FACSCanto Flow Cytometer (BD Biosciences, Heidelberg, Germany) was used for flow cytometry measurements, and the analysis was performed using FlowJo software (version 9.7; TreeStar, Ashland, OR). Antibody staining of cells was routinely performed with 1 μg/mL of each antibody. For detection of cell-surface molecules, flow cytometry was performed as described elswhere.19 For coculture experiments, intracellular staining of Foxp3 expression was performed using the Foxp3/Transcription Factor Staining Buffer Set (eBioscience, San Diego, CA) according to the manufacturer’s instructions. The monoclonal antibodies used for surface and intracellular staining are given in Supplementary Table 2. The expression of CX3CR1 on monocytes was evaluated using reporter mice that express a green fluorescent protein under transcriptional control of the CX3CR1 promoter (B6.129P(Cg)-Ptprca Cx3cr1tm1Litt/LittJ).
Phagocytosis, Adhesion, and Oxidative Burst
For evaluation of phagocytic capacity, WT or CD39−/− GMaM and control monocytes were incubated with carboxylate-modified polystyrene fluorescent yellow-green beads (Sigma-Aldrich, Taufkirchen, Germany) for 4 hours at a 1:10 ratio of monocytes to beads. Phagocytosis rates were determined by flow cytometry.
For determination of cell adhesion, either control monocytes or GMaM (2 × 105) were seeded in triplicates into 96-well flat-bottom plastic tissue culture plates and incubated at 37°C and 5% CO2 for 4 hours. Nonadhering cells were removed by washing twice. Remaining adherent cells were fixed with 2% glutaraldehyde (Sigma-Aldrich) for 10 minutes, washed twice with distilled H2O, and stained with 0.5% crystal violet (Merck Millipore, Darmstadt, Germany) in 2% ethanol (pH 6.0) for 15 minutes at room temperature. The cells were washed three times and lysed by adding 10% acetic acid, and the optical density (OD) at 560 nm was determined using an Asys Expert 96 Microplate Enzyme-Linked Immunosorbent Assay reader (Anthos Mikrosysteme, Krefeld, Germany).
For the determination of reactive oxygen species (ROS), monocytes were stimulated for 4 hours at 37°C with Escherichia coli 055:B5–derived lipopolysaccharide (LPS) (10 ng/mL; Sigma-Aldrich) in the presence of 15 μM dihydrorhodamine 123 (Merck, Darmstadt, Germany) for the final 15 minutes. Stimulated monocytes were then analyzed by flow cytometry.
Analysis of Cytokine Levels
Cytokine secretion of WT or CD39−/− GMaM and control monocytes after 16 hours of LPS treatment (100 ng/mL; Sigma-Aldrich) was measured from cell culture supernatants. Murine tumor necrosis factor α (TNFα), IL-1β, IL-6, and IL-10 were measured by using a bead-based multiplex assay (mouse TH1/TH2 10plex FlowCytomix; eBioscience) according to the manufacturer’s instructions.
Aldehyde Dehydrogenase, Arginase Activity, and Nitrite Assay
To indirectly calculate the production of the vitamin A metabolite, retinoic acid (RA), in GMaM and control monocytes, aldehyde dehydrogenase (ALDH) activity was measured with a commercially available ALDEFLUOR detection kit (StemCell Technologies, Vancouver, BC, Canada) according to the manufacturer’s protocol. Dead cells were excluded by counterstaining with 5 μL of 7-aminoactinomycin D (Biolegend, San Diego, CA).
Arginase activity was evaluated by measuring the conversion of arginine to ornithine and detectable urea in cell lysates of GMaM and control monocytes using an arginase assay kit (Abnova, Walnut, CA). In brief, we lysed 1 × 106 cells in 100 μL of 10 mM Tris-HCl (pH 7.4) containing proteinase inhibitors and 0.4% Triton X-100. Lysates were centrifuged for 10 minutes at 4°C at 14,000g, and 40 μL of supernatants mixed with 10 μL 5X substrate buffer were transferred in a 96-well flat-bottom plate and incubated for 2 hours at 37°C. The reaction was stopped by adding 200 μL of urea reagent (kit component) to all wells. The plate was incubated at room temperature for 1 hour, and urea production was determined by measuring the OD at 430 nm.
To measure basal nitric oxide (NO) production in supernatants of WT or CD39−/− GMaM and control monocytes after 48 hours of culture, we used the Griess reagent system (Promega, Fitchburg, WI) according to the manufacturer’s protocol. We read the OD at 550 nm with a spectrophotometer. The sample nitrite concentration was calculated from a nitrite standard reference curve.
Coculture and Inhibitors
Naïve T cells were isolated from C57Bl/6J splenocytes using the pan T cell kit II (Miltenyi Biotec) according to the manufacturer’s protocol and were cocultured with respective monocytes at 37°C and 5% CO2 in RPMI 1640 supplemented with 10% FCS, penicillin (100 U/mL), streptomycin (100 μg/mL), 15 mM HEPES (pH 7.4), 2 mM l-glutamine, and 1% nonessential amino acids. We applied 1 × 105 splenic T cells to each well (96-well plate), and 2 × 104 GMaM or control monocytes were added (ratio of 5:1 for T cells/monocytes, triplicates for each condition) and incubated for 5 days without further T-cell stimulation. For detecting cell proliferation, we labeled T cells with carboxyfluorescein succinimidyl ester (CFSE) (eBioscience). The CFSE was diluted to a working concentration of 500 nM in PBS, with a 100-μL CFSE working solution used for 1 × 106 cells. The T cells were stained for 4 minutes at room temperature and subsequently were washed twice with PBS/1% FCS. The staining efficiency of CFSE was routinely controlled by flow cytometry.
To inhibit CD39 and/or CD73, 200 μM ARL 67156 trisodium salt and/or α,β-methyleneosine 5′-diphosphate sodium salt (Merck Millipore), respectively, were used. For evaluation of CD39 blocking efficacy, 10μM adenosine (Sigma-Aldrich) was added. In addition, 50 μM or 100 μM Nω-hydroxy-nor-l-arginine (Sigma-Aldrich) was used to inhibit arginase activity. To block IL-10-mediated effects, 5 μg/mL anti-IL-10 (clone: JES5–16E3) or 5 μg/mL anti-IL-10 receptor (clone: 1B1.3a) antibodies (both Biolegend) were used.
Immunohistochemistry
Cryosections (5 μm) were prepared from Cryo-OCT (Tissue-Tek; Fisher Scientific, Schwerte, Germany) embedded colon tissue. After the slides had been fixed for 10 minutes, we stained them with H&E. The sections were independently evaluated by two blinded investigators using a scoring system to grade inflammation and cell infiltration from 0 to 3 (0 = none; 1 = mild; 2 = moderate; 3 = severe) and the occurrence of ulcerations from 0 to 3 (0 = none; 1 = ulcers spanning up to two crypts; 2 = ulcers spanning 3 to 10 crypts; 3 = ulcers spanning more than 10 crypts). The grading was performed for the proximal and the distal part of the colon separately, with a combined maximal grading score of 12. For the staining of infiltrating phagocytes, a monoclonal antibody raised against CD11b (clone: M1/70; BD Biosciences) was used, and the presence of regulatory T cells was evaluated using a monoclonal antibody against Foxp3 (clone: MF-14; Biolegend). Secondary antibody binding and subsequent peroxidase reaction was performed as described previously.20
In Vivo Cell Tracking
For in vivo cell tracking, monocytes were stained with a commercially available lipophilic tracer 1,1-dioctadecyl-3,3,3,3-tetramethylindotricarbocyanine iodide (Life Technologies, Darmstadt, Germany) with an emission maximum of 782 nm as described elsewhere.21 In vivo distribution of labeled cells across the intestine was studied 48 hours and 96 hours after intravenous injection using a planar small animal fluorescence-mediated tomography system (FMT 2500; VisEn Medical, Bedford, MA) as described elsewhere.21 Ex vivo optical imaging of the dissected intestine placed on a petri dish 96 hours after intravenous injection was performed using a whole-body multichannel small animal fluorescence reflectance imager (in vivo FX Pro; Bruker, Billerica, MA). The monocyte migration toward Peyer’s patches was statistically analyzed by first selecting the regions of interest, which were Peyer’s patches observed in white-light images. These images were then overlaid with corresponding fluorescence images, and the mean fluorescent intensities evaluated from these regions of interest. Mice that received unlabeled monocytes served as the control for autofluorescence discrimination.
Monocyte Migration Into Inflamed Colonic Lamina Propria
Migratory capacity of WT or CD39−/− control monocytes and GMaM was assessed in CD45.1 (on C57BL/6 background) mice with chronic DSS-induced colitis. One day before the start of the third DSS-treatment cycle, mice received either CD45.2 WT control monocytes, WT GMaM, CD39−/− control monocytes, or CD39−/− GMaM (2 × 106 each). Two days after injection, lamina propria mononuclear cells from the colon were isolated using the Lamina Propria Dissociation Kit and a GentleMACS Octo Dissociator (both Miltenyi Biotech) according to the manufacturer’s protocol. Infiltrating CD45.2-positive cells were stained and measured by flow cytometry as described earlier.
Statistics
Data are expressed as mean ± standard error of the mean (SEM) and were tested for statistical significance using the Student t-test and one-way or two-way analysis of variance (ANOVA) with Bonferroni correction as indicated. P < .05 was considered statistically significant. All calculations were performed using Prism version 6 for Macintosh (GraphPad Software, San Diego, CA).
Results
GM-CSF-Activated Monocytes Are Phenotypically Distinct Monocytes
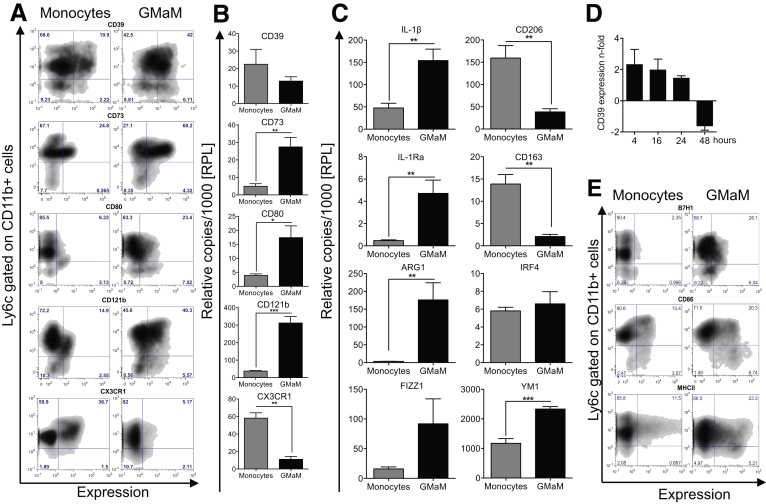
Activated monocytes were generated from bone marrow precursor cells of C57Bl/6 mice by GM-CSF treatment for 48 hours. GMaM were analyzed by flow cytometry for several surface molecules known to be expressed on monocyte subsets and implicated for further development into specialized macrophages ranging from M1-polarized to M2-polarized. We found E-NTPDase (CD39), ecto-5′-nucleotidase (CD73), and the IL-1 decoy receptor (CD121b) highly up-regulated after GM-CSF activation. The fractalkine receptor (CX3CR1) that is expressed by alternatively activated monocytes (rolling monocytes) that can give rise to M2-polarized macrophages22 was down-regulated on Ly6c+CD11b+ monocytes (Figure 1A). The costimulatory molecules CD80 (Figure 1A), CD86, MHCII, and B7-H1 (see Figure 1E) were highly up-regulated on GMaM. Expression of cell surface markers was confirmed by mRNA expression analysis, except for CD39 mRNA, which was not differentially expressed after 48 hours of GM-CSF activation compared with untreated monocytes (see Figure 1B) but earlier after 4, 16, and 24 hours (see Figure 1D).
Figure 1.
Granulocyte macrophage colony-stimulating factor (GM-CSF)-activated monocytes have a distinct phenotype. Bone marrow-derived control monocytes and GM-CSF-activated monocytes (GMaM) were generated in vitro for 48 hours. (A) Resulting cells were stained for cell-surface molecules and analyzed by flow cytometry. Expression is shown as representative dot plots gated on CD11b+ cells, showing Ly6c expression (y axis) versus respective cell surface molecules (x axis). (B) Expression of cell surface molecules was validated using quantitative reverse-transcription polymerase chain reaction (qRT-PCR) (n = 5–6). (C) The mRNA levels of several monocyte subset defining molecules were investigated by qRT-PCR (n = 5–6). (D) Time-dependent expression of CD39 mRNA in GMaM using qRT-PCR shown as n-fold expression compared with control monocytes (n = 3). (E) Representative dot plots of costimulatory molecules gated on CD11b+ cells show Ly6c expression (y axis) versus respective cell surface molecules (x axis). (B–D) Graphs show mean values (± standard error of the mean), and statistical significance was determined by unpaired Student t test. *P < .05; **P < .01; ***P < .001.
We performed additional qRT-PCR experiments to further investigate effects of GM-CSF on activation and polarization in monocytes. We found increased expression of arginase 1 (Arg1), IL-1β, and also the IL-1β counterregulating IL-1 receptor antagonist (IL-1Ra) in GMaM (see Figure 1C). Interestingly, M2 polarization marker genes were discordantly expressed. For example, found in inflammatory zone 1 (FIZZ1) and chitinase-3-like-3 (YM1) were increased, whereas the mannose receptor (CD206) and scavenger receptor CD163 were decreased. The transcription factor Interferon regulatory factor 4 (IRF4), previously implicated in M2 polarization,23 was not differentially expressed (see Figure 1C). Although GMaM share markers of both classic and nonclassic monocytes and their derivative macrophages, their phenotype does not completely match either of these monocyte subsets described in the literature. Therefore, we consider GMaM to be monocytes in a unique and distinct state of activation.
GM-CSF Treatment Modulates Monocyte Functions
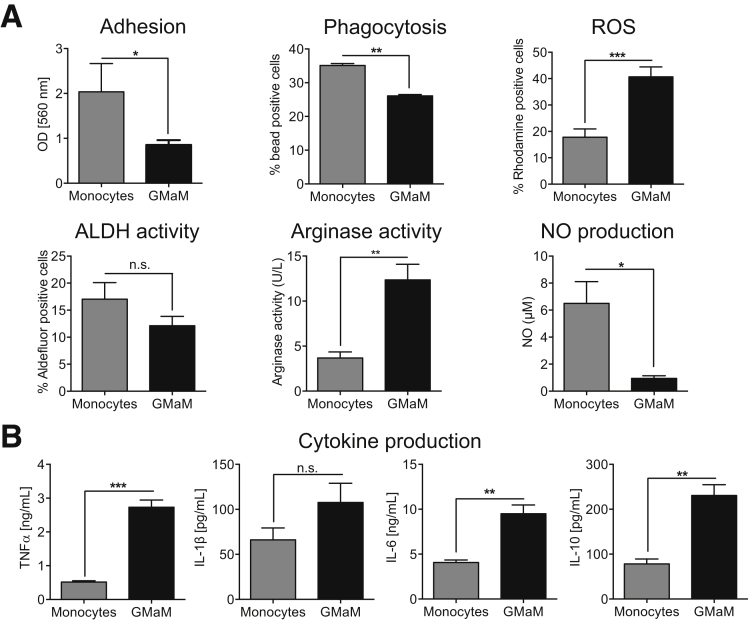
Monocytes play key roles in fighting foreign particles via phagocytosis or production of ROS but also in activation and regulation of cells of the adaptive immune system, either by secretion of cytokines or by cell contact–mediated mechanisms. We therefore investigated whether GM-CSF might modify the activity of monocytes in either of these capacities. Adherence of GMaM to plastic culture plates and their phagocytic activities were significantly reduced compared with untreated monocytes (Figure 2A). Importantly, ROS formation and thus the capacity to kill pathogens was significantly enhanced in GMaM (see Figure 2A). Conversely, RA production, as determined by ALDH activity, was slightly diminished in GMaM (see Figure 2A). We found Arg1 activity significantly increased in GMaM; because NO synthase and Arg1 compete for the same substrate (l-arginine), NO production was also strongly reduced (see Figure 2A).
Figure 2.
Granulocyte macrophage colony-stimulating factor (GM-CSF) treatment modulates monocyte functions. (A) Upper left/middle panel: Potential of granulocyte-macrophage colony-stimulating factor–activated monocytes (GMaM) and control monocytes to adhere to plastic surfaces and phagocytosis of latex beads (n = 5). Upper right panel: Reactive oxygen species (ROS) production of GMaM and control monocytes as rhodamine positive cells (n = 11). Lower left panel: To indirectly estimate the production of retinoic acid (RA), aldehyde dehydrogenase (ALDH) activity measured using Aldefluor and displayed as the percentage of Aldefluor-positive cells (n = 4). Lower middle panel: Nitrite oxide (NO) production in cell culture supernatants of GMaM compared with control monocytes (n = 3). Lower right panel: Arginase activity evaluated by measuring the conversion of arginine to ornithine and detectable urea in cell lysates (n = 3). (B) Cytokine secretion of GMaM and control monocytes after lipopolysaccharide treatment measured in cell culture supernatants (n = 3). Statistical significance was determined by unpaired Student t test. *P < .05; **P < .01; ***P < .001; n.s., not statistically significant.
Next, we analyzed the influence of GM-CSF (treatment for 48 hours) on cytokine production in response to LPS stimulation for 16 hours. We observed an increased release of proinflammatory IL-1β, TNFα, and IL-6 from GMaM compared with control monocytes after LPS stimulation (see Figure 2B). Interestingly, we also observed an increased release of anti-inflammatory IL-10 (see Figure 2B).
GMaM Have a Therapeutic Effect in DSS-Induced Colitis
After we had evaluated the specific activation pattern of GMaM in vitro, because monocytes represent the exclusive source of intestinal macrophages we tested the in vivo therapeutic potential of GMaM in a mouse colitis model. We chose the chronic DSS-induced colitis model because it mimics many of the pathologic features of CD (eg, epithelial degradation and bacterial invasion) and thereby allows testing for GMaM-mediated effects that may alter intestinal macrophage function. Colitis was induced by repetitive administration of DSS in drinking water, with disease severity primarily assessed by monitoring body weight loss.
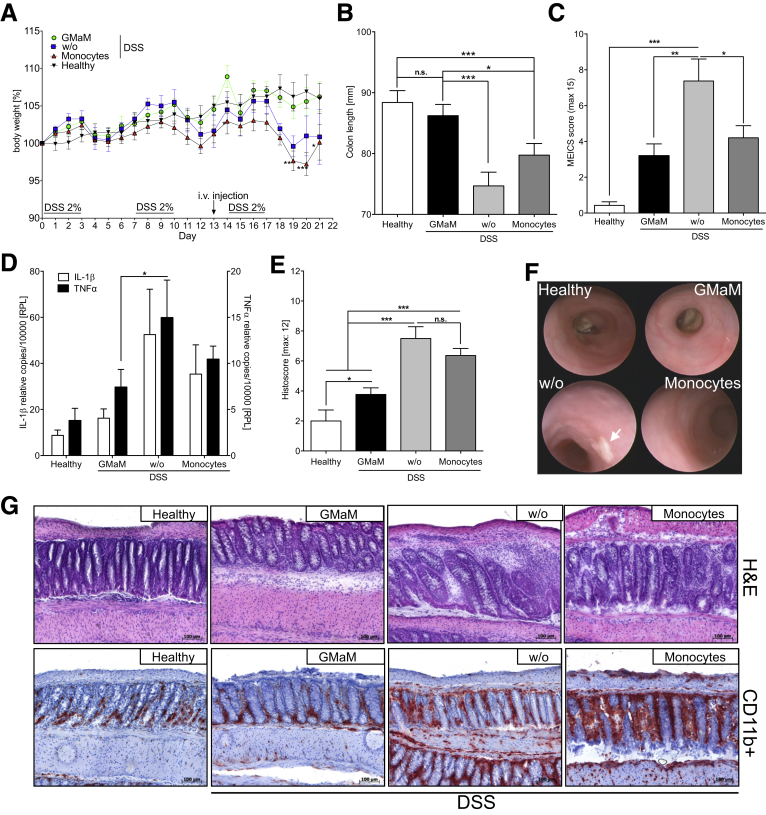
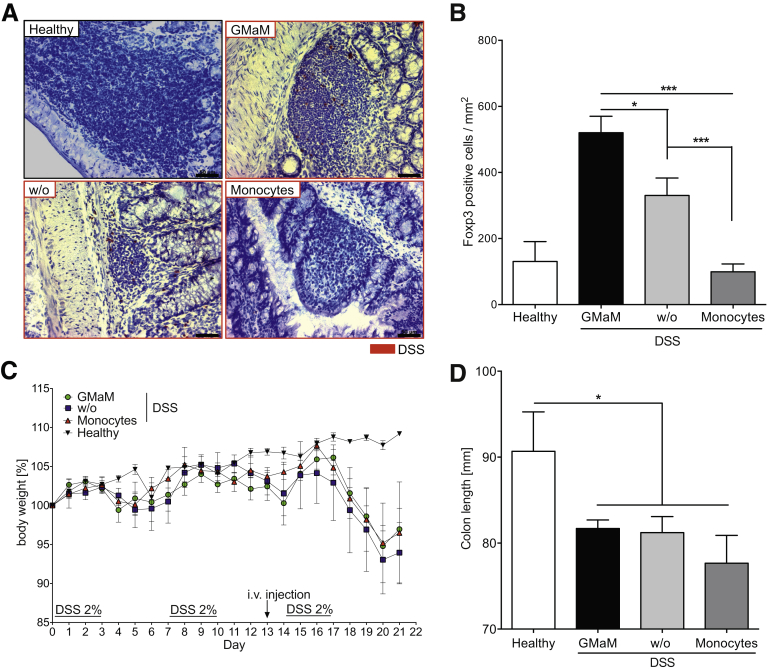
We observed significant weight loss in untreated mice and mice treated with control monocytes (Figure 3A). In striking contrast, GMaM-treated mice showed no weight loss after DSS exposure (see Figure 3A, filled circle) and generally displayed better disease outcomes when taking behavior, hunched posture, diarrhea, and rectal bleeding into account (data not shown). This observation was supported by the findings of significantly less colon shrinkage (see Figure 3B, black bar) and significantly lower MEICS scores (see Figure 3C, black bar) as assessed by colonoscopy (see Figure 3F, example pictures). The GMaM-treated mice also showed significantly lower IL-1β and TNFα mRNA levels in the distal colon after DSS exposure than the mice receiving control monocytes (see Figure 3D). Furthermore, GMaM treatment led to significantly reduced intestinal inflammation as indicated by reduced prevalence of CD11b+ phagocytes (eg, neutrophils), moderate colonic wall thickening, less goblet cell loss (see Figure 3G), and lower histopathology score values compared with controls (see Figure 3E). Together, these results demonstrate a clear therapeutic benefit of GMaM delivery in an in vivo colitis model.
Figure 3.
Granulocyte-macrophage colony-stimulating factor–activated monocytes (GMaM) have a therapeutic effect in dextran sulfate sodium (DSS)-induced colitis in wild-type C57Bl/6 mice. (A) Chronic colitis induced by repeated oral administration of DSS in C57Bl/6 mice. One day before the start of the third treatment cycle, mice received either control monocytes, GMaM, or phosphate-buffered saline as vehicle. Body weight was monitored daily and is shown as the percentage of weight change of three independent experiments with C57Bl/6 mice (n = 7–15 per treatment group). (B) On day 21, colons were removed, and the colon lengths were measured for each treatment group. Graphs show the mean ± standard error of the mean (SEM). (C) Murine endoscopic index of colitis severity (MEICS) scores (mean ± SEM) based on high-resolution colonoscopy. (D) Proinflammatory cytokines (interleukin 1β = white bars, tumor necrosis factor α = black bars) were measured in DSS-treated and healthy control C57Bl/6 mice by quantitative reverse-transcription polymerase chain reaction in distal colon biopsies taken at the end of the experiment. Mean ± SEM for three independent experiments (n = 11 per treatment group). (E) Colon inflammation scores (mean ± SEM) were graded by two blinded investigators and performed for the proximal and the distal part of the colon separately (n = 11). (F) Representative pictures of chronic colitis in C57Bl/6 mice on day 20 for two independent experiments are shown (arrow shows fibrin plaque; n = 11 per treatment group). (G) Representative microscopic colon images of mice with colitis and healthy control mice are shown (H&E and CD11b staining; scale bar: 100 μm). Statistical significance was determined by unpaired Student t test except for body weight change where two-way analysis of variance with Bonferroni correction was used. *P < .05; **P < .01; ***P < .001.
GMaM Infiltrate the Intestine in Higher Numbers Compared With Control Monocytes
To address whether the beneficial effects of GMaM treatment are a direct effect of the transferred monocytes, we investigated the in vivo migratory potential of GMaM within the context of chronic DSS-induced colitis (Figure 4). We specifically aimed to show that GMaM migrate to the site of inflammation, thus supporting a direct mechanistic role for their amelioration of colitis severity.
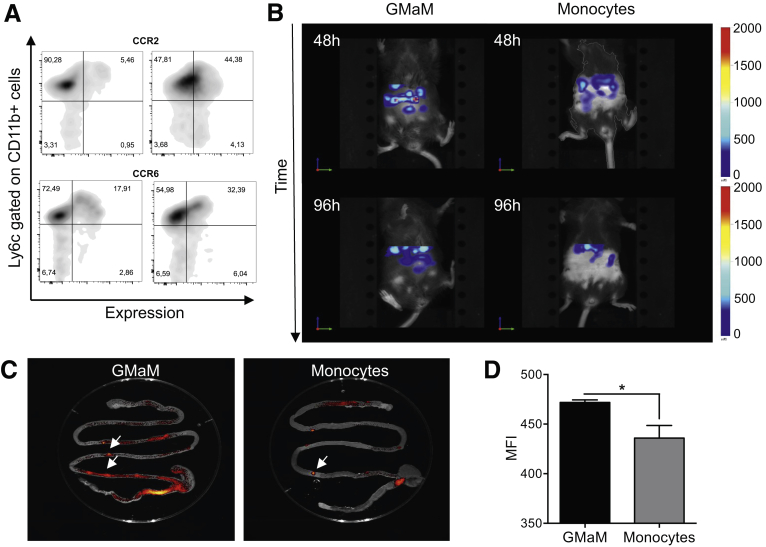
Figure 4.
Granulocyte-macrophage colony-stimulating factor–activated monocytes (GMaM) infiltrate the intestine at higher numbers and persist longer. (A) GMaM and control monocytes were stained for cell surface expression of gut homing molecules and analyzed by flow cytometry. Expression is shown as representative dot plots gated on CD11b+ cells showing Ly6c expression (y axis) versus respective cell surface molecules (x axis). (B) GMaM and control monocytes were labeled with 1,1-dioctadecyl-3,3,3,3-tetramethylindotricarbocyanine iodide and injected intravenously at day 13 of chronic dextran sulfate sodium–induced colitis. Monocyte infiltration in the intestine was visualized after 48 hours and 96 hours, and representative pictures are shown (n = 3). (C) After 96 hours, the intestine was removed and monocyte infiltration especially into Peyer’s patches, visualized by fluorescence reflectance imaging, was evaluated (arrow shows Peyer’s patches). (D) Mean fluorescence intensity (mean ± SEM) of Peyer’s patches is shown (n = 5–7). Statistical significance was determined by unpaired Student t-test. *P < .05.
In performing flow cytometry–based staining of gut homing molecules on monocytes (eg, α4β7, CCR7, CCR9, CD103), we found CCR2, necessary for migration toward inflammatory sites,24 and CCR6, used for the migration toward lymph follicles such as Peyer’s patches,25 to be up-regulated on GMaM (Figure 4A). CCR7, CCR9, CD103, and α4β7, however, were not regulated on GMaM compared with the control monocytes.
When GMaM or control monocytes were labeled with a near infrared fluorescent dye and injected intravenously 1 day before the last DSS treatment cycle, the GMaM were significantly enriched in the intestine relative to the control monocytes at 48 hours after injection. This effect was more pronounced after 96 hours when, by contrast, the control monocytes were mostly absent (Figure 4B and Supplementary Video 1, GMaM 48 hours after injection and Supplementary Video 2, monocytes 48 hours after injection). To allow a more detailed view of the GMaM infiltration sites, we removed the intestine after 96 hours to study the monocyte infiltration by fluorescence reflectance imager technology (see Figure 4C). We observed significantly more infiltration of GMaM into Peyer’s patches (arrows) than untreated control monocytes (see Figure 4C and D).
Thus, we show that GMaM not only migrate faster to the inflamed tissue compared with control monocytes but persist within the tissue for significantly longer periods. These results reveal preferential homing of GMaM to sites of intestinal inflammation, which coincides with expression of CCR2 and CCR6 and directly correlates with improved disease outcome in the DSS colitis model.
Foxp3+ T-Cell Induction by GMaM Is Required for Therapeutic Effectiveness in DSS-induced Colitis
From the in vivo cell tracking studies it was apparent that GMaM directly migrate toward secondary lymphatic organs such as Peyer’s patches. Therefore, we asked whether GMaM might exert their protective influence via interactions with other immune cell types. Accordingly, we performed immunostaining for a range of immune markers in colon cryosections of DSS-treated mice injected with either GMaM or control monocytes. Interestingly, compared with all other groups, we found that Foxp3-expressing Treg were significantly enriched in the lymph follicles of mice that received GMaM (Figure 5A and B). In addition, an increased presence of Foxp3+ cells (red staining) was only found in the lymph follicles, not in the lamina propria (see Figure 5A).
Figure 5.
Granulocyte-macrophage colony-stimulating factor–activated monocytes (GMaM) induce Foxp3+T cells in dextran sulfate sodium (DSS)-induced colitis, and T cells mediate the beneficial effect of GMaM. (A) Regulatory T cells (Treg) were stained in wild-type (WT) mice treated with DSS (red frames) and control WT mice using a monoclonal antibody raised against Foxp3. Examples of colonic lymph follicles harboring Treg are shown for each treatment group (scale bar: 50 μm). (B) The number of Foxp3+ cells in lymph follicles was quantified and is displayed as mean ± standard error of the mean (SEM) of positive cells per mm2 (n = 8–12). (C) Chronic colitis was induced by repeated oral administration of DSS in Rag1−/− mice. Untreated Rag1−/− mice that received only plain water served as controls. One day before the start of the third treatment cycle, mice received control monocytes, GMaM, or as a vehicle control phosphate-buffered saline alone, intravenously by the tail vein. Body weight of Rag1−/− mice was monitored and is shown as the percentage of weight change of two independent experiments (n = 6 per treatment group). (D) Colons were removed, and the colon lengths were measured for each treatment group. Graphs show the mean ± SEM. Statistical significance was determined by unpaired student’s t-test. *P < .05; ***P < .001.
We tested the requirement for Treg in GMaM-mediated protection against colitis in Rag1−/− homozygous null mice, which lack all mature adaptive immune cells including functional T-cell subsets. GMaM injection was not protective against DSS-induced colitis in Rag1−/− mice as evidenced by their increased body weight loss and colon shrinkage (see Figure 5C and D). In comparison with our earlier observations in WT mice injected with GMaM (see Figure 3A), our results show that adaptive immunity is highly required for GMaM-mediated protection against colitis and provide evidence to support a mechanistic role for Foxp3-expressing Tregs.
GMaM Showed Beneficial Effects in T-Cell Transfer Colitis and Led to an Increased Number of Splenic Regulatory T Cells In Vivo
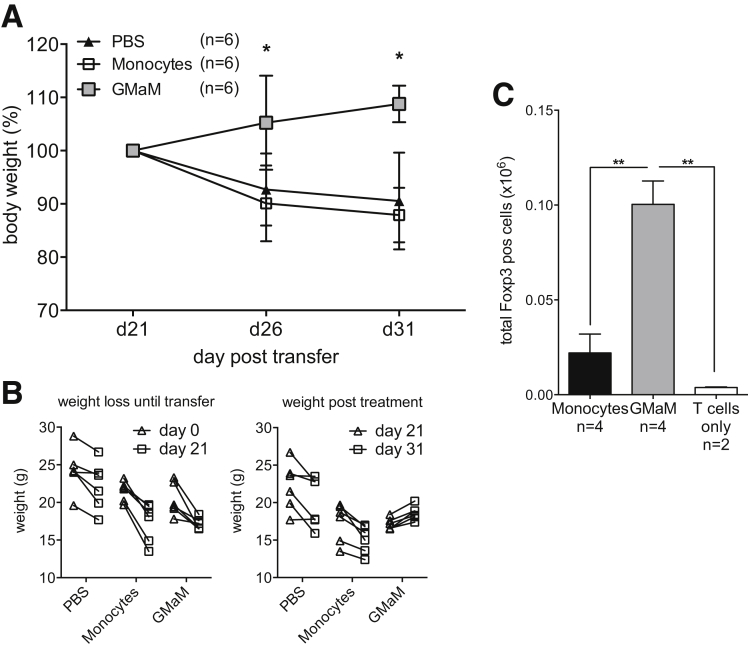
To further investigate the crosstalk between innate and adaptive immune mechanisms we examined a T-cell–dependent model of chronic colitis. Here, T cells that were depleted for regulatory cells were injected in Rag1−/− mice that subsequently develop colitis. Again by injecting GMaM into Rag1−/− mice that developed colitis symptoms (eg, weight loss) we could show a therapeutic effect of GMaM (Figure 6A and B).
Figure 6.
Granulocyte-macrophage colony-stimulating factor–activated monocytes (GMaM) showed beneficial effects in T-cell transfer colitis and led to increasing numbers of splenic regulatory T cells (Treg) in vivo. (A) T-cell transfer colitis was induced by intravenous injection of T cells in Rag1−/− mice. Monocyte transfer (intravenous) was performed when mice had developed colitis symptoms between days 19 and 22. Body weight was monitored and is shown as the percentage of weight change (mean ± standard error of the mean [SEM]) of two independent experiments with Rag1−/− mice (n = 6 per treatment group). (B) Weight of individual Rag1−/− mice of different groups was measured before (days 0 to 21) and after (days 21 to 31) monocyte transfer. (C) Rag1−/− mice were simultaneously injected with CD4+ T cells and GMaM or control monocytes. After 7 days, the spleens were removed, and the presence of Foxp3+ CD4+ T cells was evaluated by flow cytometry. Shown is the mean ± SEM of total Treg cells based on the number of splenocytes. Statistical significance was determined by (A) two-way or (C) one-way analysis of variance with Bonferroni correction. *P < .05; **P < .01.
Unfortunately, it was not possible to detect Foxp3+ Treg in histology sections of the intestine (data not shown). Therefore, we performed an additional set of experiments where we injected CD4+ T cells together with control monocytes or GMaM intravenously into Rag1−/− mice. After 1 week we isolated the spleen of these mice. Here we could show in vivo an increased total cell number of Foxp3+ CD4+ T cells in splenocytes of Rag1−/− mice, which together with CD4+ T cells received GMaM (see Figure 6C).
GMaM Induce Proliferation and Differentiation of Foxp3+ CD4+ T Cells via Adenosine/CD39 but Not Arginase In Vitro
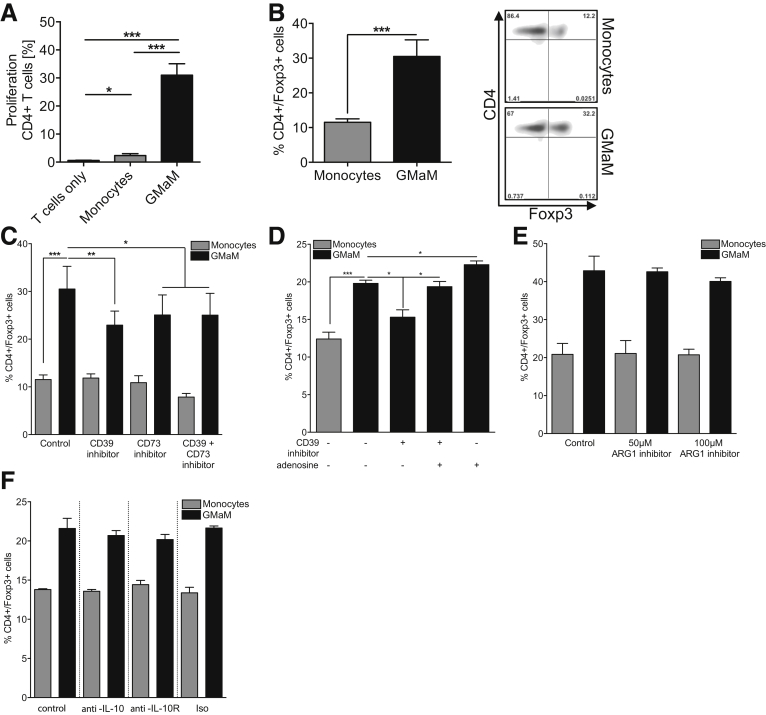
The in vivo experiments found circumstantial evidence to support a role for Treg in GMaM-induced protection against colitis. To formally substantiate this, we investigated whether GMaM can directly promote activation and differentiation of Treg from a naive T-cell pool in vitro. We performed coculture experiments using a 5:1 ratio of naive splenic T cells to monocytes. We found significantly higher proliferation of CD4+ and CD8+ T cells (Figure 7A and data not shown) coincubated with GMaM compared with the untreated monocytes. Additionally we demonstrated that GMaM could induce Foxp3 expression in Treg in vitro (see Figure 6B).
Figure 7.
Coculture of Granulocyte-macrophage colony-stimulating factor–activated monocytes (GMaM) with naive T cells led to proliferation and induction of Foxp3+CD4+T cells via adenosine/CD39 but not by arginase. (A) Naive carboxyfluorescein succinimidyl ester (CFSE)-labeled T cells were cocultured with respective monocytes (ratio 5:1, triplicates for each condition) or left alone as control. Cocultures were incubated for 5 days without further T-cell stimulation. Proliferation was evaluated as a decrease in CFSE fluorescence and quantified by analyzing the percentage of proliferating CD4+ T cells (n = 5). (B) T cells were cocultured with GMaM or control monocytes at a ratio of 5:1 (T cells/monocytes). Cells were stained for CD4 and Foxp3 expression and analyzed by flow cytometry. Dot plots (right panel) are representative for graph (left panel) that includes mean (± standard error of the mean) of five independent experiments. (C) To inhibit the function of CD39 and/or CD73, either CD39 inhibitor and/or CD73 inhibitor were added to the cocultures, respectively (n = 6). (D) To further evaluate the role of adenosine in regulatory T-cell induction, we added 10 μM adenosine to some cocultures (n = 4). (E) Arginase activity during cocultures was blocked using 50 μM or 100 μM ARG1 inhibitor (n = 4). (F) To inhibit the function of interleukin 10 (IL-10), either anti-IL-10 or anti-IL-10 receptor antibodies were used. (C–F) Cells were stained for CD4 and Foxp3 expression, and the percentages of double-positive T cells are shown. Statistical significance was determined by one-way analysis of variance with Bonferroni correction. *P < .05; **P < .01; ***P < .001.
Several self-molecules and mediators have been reported to induce Treg differentiation,26, 27 including adenosine and arginase.28, 29, 30 Interestingly, in our earlier phenotypic characterization of GMaM we observed increased expression of CD39 and CD73 (see Figure 1A and B), both of which play roles in anti-inflammatory adenosine biosynthesis.31 Similarly, we also observed increased expression and activity of Arg1 (see Figures 1C and 2A). Taking these observations into account, we examined whether either or both of these pathways were required for GMaM-dependent induction of Treg.
Pharmacologic inhibition of either CD39 or CD73 significantly reduced GMaM-dependent induction of Foxp3+ Treg from naive T cells compared with the control cultures (see Figure 7C). This inhibitory effect was completely abolished by resubstituting adenosine in CD39 inhibition experiments. Furthermore, addition of adenosine alone (ie, incubation without the CD39 inhibitor) even increased GMaM-mediated Treg induction (see Figure 7D). In contrast, inhibition of arginase activity and blocking IL-10 or IL-10 receptor had no effect on the ability of GMaM to induce a Treg phenotype under the conditions tested (see Figure 7E and F). Collectively these results identify GMaM as potent inducers of Treg via activation of anti-inflammatory adenosine biosynthesis.
CD39 Is Responsible for Selected GMaM Functions
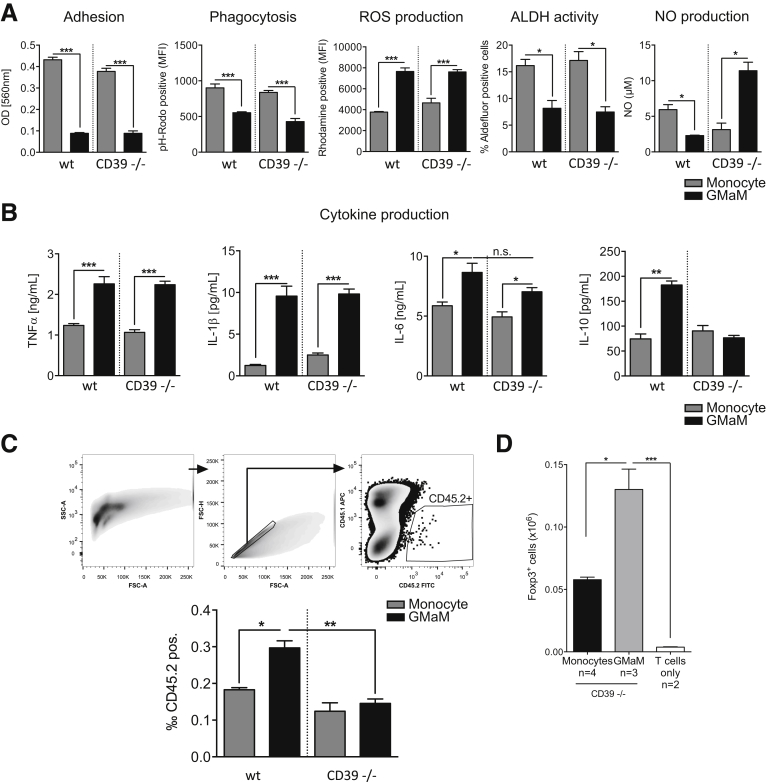
As we had found CD39 to be necessary for GMaM-mediated Treg induction, we were also interested in the role of CD39 in other previously observed GMaM functions. We repeated some functional assays with CD39−/− GMaM and could show that CD39 only played a role in NO production. All other monocyte functions (eg, adhesion, phagocytosis, ROS and RA production) were comparable between WT and CD39−/− GMaM (Figure 8A).
Figure 8.
Functional characterization of CD39-deficient granulocyte-macrophage colony-stimulating factor–activated monocytes (GMaM) in vitro and their migration in vivo. Bone marrow-derived control monocytes and GMaM were generated from CD39−/− and corresponding wild-type (WT) mice in vitro. (A) The influence of CD39 on GMaM functions was evaluated. The ability to adhere to plastic surfaces and phagocytosis of pHrodo particles is shown (n = 3). Reactive oxygen species (ROS) production is presented as mean fluorescent intensities of rhodamine positive cells (n = 3). To indirectly measure the production of retinoic acid (RA), aldehyde dehydrogenase (ALDH) activity was measured using Aldefluor and displayed as the percentage of Aldefluor-positive cells (n = 3). Nitric oxide (NO) production was determined in cell culture supernatants (n = 3). (B) Cytokine secretion after lipopolysaccharide (LPS) treatment was measured in cell culture supernatants using a bead-based multiplex assay (n = 6). (C) Chronic colitis was induced using DSS in CD45.1 mice. One day before starting the third dextran sulfate sodium (DSS) treatment cycle, the mice received CD45.2 control monocytes, GMaM, CD39−/− control monocytes, or CD39−/− GMaM (2 × 106/mouse). Two days after injection, lamina propria mononuclear cells from the colon were isolated, and infiltrating CD45.2 positive cells were stained and measured by flow cytometry. Shown is the gating strategy and respective bar graph (mean ± standard error of the mean [SEM]; n = 3–4). (D) Rag1−/− mice were simultaneously injected with naive WT CD4+ T cells (2 × 106/mouse) and CD39−/− GMaM or CD39−/− control monocytes (2 × 106/mouse). Spleens were removed after 7 days, and the presence of Foxp3+ CD4+ T cells was evaluated by flow cytometry. Shown is the mean ± SEM of total regulatory T-cells based on the number of total splenocytes. Statistical significance was determined by one-way analysis of variance with Bonferroni correction. *P < .05; **P < .01; ***P < .001; n.s., not significant.
With regard to LPS-induced cytokine production CD39−/− GMaM failed to produce IL-10, whereas an observed GM-CSF–mediated increased release of IL-1β, TNFα, and IL-6 was also seen in CD39−/− GMaM (see Figure 8B). One striking feature of GMaM was the higher migratory potential toward sites of inflammation in the intestine.
Taking into account that CD39 has been described to be involved in migration of monocytes32 we performed in vivo migration experiments with CD39−/− GMaM. To be able to distinguish between donor and recipient cells, we used CD45.2-expressing GMaM (WT and CD39−/−) and transferred them into CD45.1 recipient mice that were induced to develop colitis using DSS. Chronic colitis was induced according to the standard DSS-treatment regime (three cycles DSS dissolved in tap water ad libitum for 3 days, followed by 4 days of untreated tap water alone) in CD45.1 mice. The CD45.2 expressing CD39−/− or WT monocytes were injected into CD45.1 recipients; 2 days later colonic lamina propria mononuclear cells were isolated, and the amount of infiltrating CD45.2 expressing cells were analyzed. We could show that more WT GMaM migrated toward the colonic lamina propria compared with the WT control monocytes. Interestingly, CD39−/− GMaM did not show an augmented migration toward the inflamed colon compared to WT and CD39−/− control monocytes (see Figure 8C). Hence, the GMaM of CD39−/− origin did migrate less efficiently to the colon compared with the GMaM from WT origin. This suggests and confirms the role of CD39 in migration of monocytes.
Finally, we asked whether a direct in vivo effect of CD39 on GMaM is responsible for Treg cell induction. To this end, we injected CD39−/− GMaM and WT responder T cells in parallel into Rag1−/− mice as described earlier (see Figure 6C). However, in this setting we did not observe a differential effect of CD39 as CD39−/− GMaM were able to induce Treg cells in the spleen of the recipient mice (see Figure 8D).
Discussion
Based on observations and reports in CD that showed defects associated with innate immunity, it was hypothesized that this arm of the immune system is central for the development of CD. Therefore, clinical studies were performed using colony-stimulating factors (eg, GM-CSF) to modulate innate immune cell functions. GM-CSF therapy has been shown to ameliorate CD in some patient groups and provides partial protection against intestinal inflammation in a mouse DSS colitis model (reviewed in Däbritz2). Because GM-CSF acts primarily on monocyte/macrophages, here we have focused on the mechanisms by which GMaM might exert their presumed therapeutic action.
Phenotypically, GMaM did not fit into current monocyte subtype definitions dividing monocytes according to surface markers and functions in classic/inflammatory, intermediate, and nonclassic monocytes.33 Markers for inflammatory monocytes, such as costimulatory CD80 and proinflammatory IL-1β, are up-regulated on GMaM but also anti-inflammatory molecules like CD121b, IL-1Ra, and Arg1. Furthermore, CX3CR1, CD163, and CD206 that are described to be hallmarks of nonclassic monocytes are down-regulated in GMaM. Because nonclassic monocytes are known to give rise to M2-polarized macrophages,34, 35 it was noticeable that the M2 markers, FIZZ1 and YM1, were both up-regulated. In summary, despite their phenotypical diversity, GMaM mainly seem to represent monocytes in a developmental stage passaging toward M2-polarized macrophages.
The distinct phenotype of GMaM likely reflects their diverse functional properties. GMaM showed diminished adhesion and phagocytosis, and increased production of ROS needed for the killing of pathogens; but it also recently has been described to play a key role in the development of M2-polarized macrophages.36 In addition, inflammatory NO production was significantly reduced while anti-inflammatory arginase activity increased; thus, these cells seemed to have a more anti-inflammatory status.
Because GMaM displayed an anti-inflammatory phenotype, we asked whether these cells might confer therapeutic benefits in vivo. DSS-induced colitis has been previously used to evaluate GM-CSF effects in the mouse, and it provides a robust model through which mechanisms of mucosal innate immunity and intestinal barrier function can be dissected.9, 11, 13, 37 We established a new protocol that more closely resembles a chronic form of DSS-induced colitis with typical clinical symptoms and moderate weight loss but manifested barrier damage. The adoptive transfer of GMaM into mice with established chronic DSS-induced colitis displayed a beneficial effect on specific clinical parameters, including body weight, colon length, and MEICS scores as assessed by small animal endoscopy.
GMaM treatment of mice with DSS-induced colitis resulted in a reduced number of inflammatory cellular infiltrates, and related production of proinflammatory cytokines such as IL-1β and TNFα in the colon was dramatically decreased. In addition, the significant up-regulation of the decoy receptor for IL-1 (CD121b) and IL-1Ra on GMaM further supports the beneficial effect of GMaM by sequestering colitis-associated IL-1β in the intestine of mice with DSS-induced colitis.38 Moreover, GMaM displayed highly selective gut homing activity, particularly targeting lymphoid follicles (ie, Peyer’s patches), which is also reflected by the increased expression of CCR2 and CCR6. Furthermore, up-regulated CD39 might support migration of GMaM as we found less migration toward inflamed colonic lamina propria when we used CD39−/− GMaM for in vivo migration studies.
In situ staining revealed an increased prevalence of Foxp3+ Treg, potentially indicative of an indirect immunomodulatory role of GMaM. In support of this notion, Brem-Exner et al9 showed that interferon-γ–induced macrophages could induce Foxp3+ Treg in vitro. Moreover, we showed that naive T cells when cocultured with GMaM were induced to proliferate and acquired a Foxp3+ Treg phenotype in vitro.
Assessment of GMaM function in Rag1−/− mice provided key evidence that functional T cell subsets and most likely Treg are required for the immunomodulatory effects of GMaM. More strikingly, the addition of T cells together with GMaM into Rag1−/− mice provided direct evidence of GMaM’s capability to differentiate Foxp3+ Tregs also in vivo. Therefore, GMaM can induce protective Treg within the context of experimental colitis via direct interactions within intestinal lymphoid follicles and Peyer’s patches.
We have also examined possible mechanisms by which GMaM may program naive T cells to a Treg phenotype. Recent studies have shown that IL-10 produced by CD103+ DCs or CX3CR1+ intestinal macrophages39, 40 is required for intestinal Treg induction and thus maintenance of gut homeostasis.41 Indeed, in our study GMaM were shown to release high levels of IL-10. However, IL-10 and IL-10 receptor blockade studies showed that IL-10 is not required for GMaM-dependent induction of Treg under the in vitro conditions tested. In addition, CD39−/− GMaM failed to produce higher amounts of IL-10. Nevertheless elevated numbers of splenic Treg were found in vivo when CD39−/− GMaM and WT T cells were simultaneously injected into Rag1−/− mice. GMaM also produced high levels of proinflammatory IL-6 and TNFα; the latter has been reported to drive expansion of Treg.42 Again, by blocking with specific antibodies we could rule out a role for TNFα in GMaM-mediated Treg induction (data not shown).
Other molecules involved in Treg induction by antigen-presenting cells are RA43 and arginase.30 We detected a significant down-regulation of the RA-converting enzyme ALDH activity, necessary for RA production, in GMaM. Therefore, it is very unlikely that GMaM reprogram naive T cells into Treg by this mechanism. Another major inducer of Treg is arginase, and we detected a significant up-regulation of Arg1 mRNA expression as well as increased Arg1 activity in GMaM. Surprisingly, Treg did develop in cocultures with GMaM even in the presence of Arg1 inhibition. As we also found up-regulation of ectonucleotidases on GMaM, we consequently investigated whether these molecular pathways could be involved in the observed Treg induction.
It is known that the proinflammatory danger signal ATP can be processed by monocyte expressed E-NTPDase (CD39), which catalyzes ATP and adenosine diphosphate to adenosine monophosphate.44 In a further step, ecto-5′-nucleotidase (CD73) rapidly dephosphorylates adenosine monophosphate into anti-inflammatory adenosine.45 Adenosine has been described to be of importance for regulatory mechanisms in the immune system and in induction of Treg.28 Because this pathway promotes an anti-inflammatory milieu, its potential has already been recognized and tested in the context of IBD. CD39-deficient mice consistently developed more severe DSS-induced colitis, and CD39 somatic mutations have been found to be associated with IBD.46
It has been described that CD39 and CD73 are jointly up-regulated on M2-polarized macrophages47 and that Treg express high numbers of these enzymes.48 We therefore hypothesized that this could provide at least one possible mechanism by which GMaM exert their anti-inflammatory function directly in the intestine by sequestering ATP, or by interaction with cells of the adaptive immune system by providing adenosine. Until now, it was thought that Treg form an autocrine loop using self-generated adenosine that binds to Treg-expressed adenosine receptors (eg, A2AR) resulting in Treg expansion and higher Foxp3 expression.29, 49 However, it remains unproven as to whether these activities degrading ATP to adenosine could be provided by a second cell type that is surrounding the cell, in our case the monocyte, with an adenosine-rich “purinergic halo,” thereby modifying cells in close proximity. We demonstrate that CD39 and CD73 have a decisive impact on GMaM-induced Treg differentiation in vitro, as Treg induction from naive T cells was indeed dependent on CD39 and CD73 activity as well as on adenosine provided by monocytes. However, because Treg induction by GMaM is not completely blocked by inhibition of CD39 and CD73, an as-yet-unidentified mechanism could also contribute to Treg differentiation. This idea is supported by the finding that CD39−/− GMaM, when added together with WT T cells into Rag1−/− mice also showed the capability to differentiate Foxp3+ Tregs in vivo. Nevertheless, in this setting it is possible that other host cells such as monocytes/macrophages or CD39-expressing epithelial cells could compensate for the lack of CD39 on CD39−/− GMaM. However, it remains to be shown and needs future study, for example, by using CD39−/− and CD73−/− as well as double-knockout T cells and/or monocytes to dissect the in vivo role of CD39 on Treg cell induction during inflammation.
Taken together, we have shown that GM-CSF induces a specifically activated monocyte subset in mice that is characterized by less pronounced amplification of classic innate immune mechanisms (important for their role in host defense) and more induction of M2-polarized macrophage-related immunomodulatory functions (important for their cross-talk with adaptive immunity). The resulting monocyte phenotype, which we have termed GMaM, has a protective and therapeutic role in chronic DSS-induced colitis in mice. The beneficial effects of GMaM are at least in part attributable to their potency to modulate the T-cell compartment. As a novel pathway involved in the interaction of innate and adaptive immunity, we propose that GMaM may act through CD39 and CD73 to convert ATP to adenosine to induce regulatory T cells from naive T cells.
Acknowledgments
The authors thank Michel Eisenblätter for advice and assistance with in vivo imaging. They also thank Melanie Saers, Susanne Schleifenbaum, Claudia Terwesten-Solé, Eva Nattkemper, and Andrea Stadtbäumer for excellent technical work. For providing CD39−/− mice, the authors thank Marina Matyash, PhD, and Prof. Helmut Kettenmann. The authors thank Faekah Gohar, MD, for carefully reading the manuscript.
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding This study was funded by grants of the Broad Medical Research Program of the Eli and Edythe Broad Foundation (BMRP IBD0201 to D.F. and J.D.); the German Research Foundation (DFG DA1161/4-1 to J.D. and D.F.; DFG SU195/3-2 to G.V.; DFG SF1009B08 to M.B.); and the Innovative Medical Research Program of the University of Münster (IMF DÄ120904 and DÄ3Ü21003 to J.D. and D.F.). J.D. is also supported by a research fellowship awarded by the German Research Foundation (DFG DA1161/5–1).
Supplementary Material
Supplementary Table 1.
Primer Sequences for Quantitative Reverse Transcription Polymerase Chain Reaction
| Gene | Forward Primer Sequence (5′-3′) | Reverse Primer Sequence (5′-3′) |
|---|---|---|
| Arg1 | CTC CAA GCC AAA GTC CTT AGA G | GGA GCT GTC ATT AGG GAC ATC A |
| CD121b | GCA TCC CAC TGT GAG CAA ATG | GCA AGT AGG AGA CAT GAG GCA GAG |
| CD163 | GTC TCT GAG GCT GAC CAA CGA A | CAC AGT GGT TGG AGA CAT ATT GCT |
| CD206 | AGA CGA AAT CCC TGC TAC TGA A | TAG AAA GGA ATC CAC GCA GTC T |
| CD39 | AAG GTG AAG AGA TTT TGC TCC AA | TTT GTT CTG GGT CAG TCC CAC |
| CD73 | GGA CAT TTG ACC TCG TCC AAT | GGG CAC TCG ACA CTT GGT G |
| CD80 | AAA TAT GGA GAT GCT CAC GTG TCA G | CTG TTA TTA CTG CGC CGA ATC C |
| CX3CR1 | TCA TCA GCA TCG ACC GGT ACC | TGA CAC CGT GCT GCA CTG TC |
| FIZZ1 | CCT GGA ACC TTT CCT GAG ATT CTG | GAT GCA GAT GAG AAG GGA ACA AGT |
| IL-1Ra | TTT AGC TCA CCC ATG GCT TCA G | CAG CAA TGA GCT GGT TGT TTC TC |
| IL-1β | TGT CTT GGC CGA GGA CTA AGG | TGG GCT GGA CTG TTT CTA ATG C |
| IRF4 | CCG ACA GTG GTT GAT CGA CC | CCT CAC GAT TGT AGT CCT GCT T |
| RPL | TGG TCC CTG CTG CTC TCA AG | GGC CTT TTC CTT CCG TTT CTC |
| TNFα | AGA AAC ACA AGA TGC TGG GAC AGT | CCT TTGCAG AAC TCA GGAATG G |
| YM1 | GGA GTA GAG ACC ATG GCA CTG AAC | GAC TTG CGT GAC TAT GAA GCA TTG |
Supplementary Table 2.
Antibodies for Flow Cytometry
| Molecule | Clone | Manufacturer |
|---|---|---|
| B7H1 | 29E.2A3 | Biolegend (San Diego, CA) |
| CCR2 | 475301 | R&D Systems (Abington, UK) |
| CCR6 | 29–2L17 | Biolegend |
| CD11b | M1/70 | Biolegend |
| CD121b | 4E2 | BD Bioscience (Heidelberg, Germany) |
| CD39 | 24DMS1 | eBioscience (San Diego, CA) |
| CD4 | RM4–5 | Biolegend |
| CD45.1 | A20 | Biolegend |
| CD45.2 | 104 | Biolegend |
| CD73 | TY/11.8 | Biolegend |
| CD80 | 16–10A1 | Biolegend |
| CD86 | IT2.2 | Biolegend |
| Foxp3 | FJK-16s | eBioscience |
| Ly6c | HK1.4 | Biolegend |
| MHCII (I-A/I-E) | M5/114.15.2 | eBioscience |
Granulocyte-macrophage colony-stimulating factor–activated monocytes 48 hours after injection.
Monocytes 48 hours after injection.
References
- 1.Di Sabatino A., Biancheri P., Rovedatti L. New pathogenic paradigms in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:368–371. doi: 10.1002/ibd.21735. [DOI] [PubMed] [Google Scholar]
- 2.Dabritz J. Granulocyte macrophage colony-stimulating factor and the intestinal innate immune cell homeostasis in Crohn’s disease. Am J Physiol Gastrointest Liver Physiol. 2014;306:G455–G465. doi: 10.1152/ajpgi.00409.2013. [DOI] [PubMed] [Google Scholar]
- 3.Glocker E., Grimbacher B. Inflammatory bowel disease: is it a primary immunodeficiency? Cell Mol Life Sci. 2012;69:41–48. doi: 10.1007/s00018-011-0837-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaser A., Zeissig S., Blumberg R.S. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith A.M., Rahman F.Z., Hayee B. Disordered macrophage cytokine secretion underlies impaired acute inflammation and bacterial clearance in Crohn’s disease. J Exp Med. 2009;206:1883–1897. doi: 10.1084/jem.20091233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marks D.J., Harbord M.W., MacAllister R. Defective acute inflammation in Crohn’s disease: a clinical investigation. Lancet. 2006;367:668–678. doi: 10.1016/S0140-6736(06)68265-2. [DOI] [PubMed] [Google Scholar]
- 7.Zhou L., Braat H., Faber K.N. Monocytes and their pathophysiological role in Crohn’s disease. Cell Mol Life Sci. 2009;66:192–202. doi: 10.1007/s00018-008-8308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varga G., Ehrchen J., Tsianakas A. Glucocorticoids induce an activated, anti-inflammatory monocyte subset in mice that resembles myeloid-derived suppressor cells. J Leukoc Biol. 2008;84:644–650. doi: 10.1189/jlb.1107768. [DOI] [PubMed] [Google Scholar]
- 9.Brem-Exner B.G., Sattler C., Hutchinson J.A. Macrophages driven to a novel state of activation have anti-inflammatory properties in mice. J Immunol. 2008;180:335–349. doi: 10.4049/jimmunol.180.1.335. [DOI] [PubMed] [Google Scholar]
- 10.Hamilton J.A. Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immunol. 2008;8:533–544. doi: 10.1038/nri2356. [DOI] [PubMed] [Google Scholar]
- 11.Bernasconi E., Favre L., Maillard M.H. Granulocyte-macrophage colony-stimulating factor elicits bone marrow-derived cells that promote efficient colonic mucosal healing. Inflamm Bowel Dis. 2010;16:428–441. doi: 10.1002/ibd.21072. [DOI] [PubMed] [Google Scholar]
- 12.Sainathan S.K., Hanna E.M., Gong Q. Granulocyte macrophage colony-stimulating factor ameliorates DSS-induced experimental colitis. Inflamm Bowel Dis. 2008;14:88–99. doi: 10.1002/ibd.20279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu Y., Hunt N.H., Bao S. The role of granulocyte macrophage-colony-stimulating factor in acute intestinal inflammation. Cell Res. 2008;18:1220–1229. doi: 10.1038/cr.2008.310. [DOI] [PubMed] [Google Scholar]
- 14.Farache J., Zigmond E., Shakhar G., Jung S. Contributions of dendritic cells and macrophages to intestinal homeostasis and immune defense. Immunol Cell Biol. 2013;91:232–239. doi: 10.1038/icb.2012.79. [DOI] [PubMed] [Google Scholar]
- 15.Bogunovic M., Ginhoux F., Helft J. Origin of the lamina propria dendritic cell network. Immunity. 2009;31:513–525. doi: 10.1016/j.immuni.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Becker C., Fantini M.C., Wirtz S. In vivo imaging of colitis and colon cancer development in mice using high resolution chromoendoscopy. Gut. 2005;54:950–954. doi: 10.1136/gut.2004.061283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melgar S., Karlsson L., Rehnstrom E. Validation of murine dextran sulfate sodium-induced colitis using four therapeutic agents for human inflammatory bowel disease. Int Immunopharmacol. 2008;8:836–844. doi: 10.1016/j.intimp.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 18.Dabritz J., Friedrichs F., Weinhage T. The functional -374T/A polymorphism of the receptor for advanced glycation end products may modulate Crohn’s disease. Am J Physiol Gastrointest Liver Physiol. 2011;300:G823–G832. doi: 10.1152/ajpgi.00115.2010. [DOI] [PubMed] [Google Scholar]
- 19.Schwarzmaier D., Foell D., Weinhage T. Peripheral monocyte functions and activation in patients with quiescent Crohn’s disease. PLoS One. 2013;8:e62761. doi: 10.1371/journal.pone.0062761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sunderkotter C., Beil W., Roth J. Cellular events associated with inflammatory angiogenesis in the mouse cornea. Am J Pathol. 1991;138:931–939. [PMC free article] [PubMed] [Google Scholar]
- 21.Eisenblätter M., Ehrchen J., Varga G. In vivo optical imaging of cellular inflammatory response in granuloma formation using fluorescence-labeled macrophages. J Nucl Med. 2009;50:1676–1682. doi: 10.2967/jnumed.108.060707. [DOI] [PubMed] [Google Scholar]
- 22.Geissmann F., Manz M.G., Jung S. Development of Monocytes, Macrophages, and Dendritic Cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Satoh T., Takeuchi O., Vandenbon A. The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat Immunol. 2010;11:936–944. doi: 10.1038/ni.1920. [DOI] [PubMed] [Google Scholar]
- 24.Serbina N.V., Jia T., Hohl T.M. Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol. 2008;26:421–452. doi: 10.1146/annurev.immunol.26.021607.090326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mowat A.M., Agace W.W. Regional specialization within the intestinal immune system. Nat Rev Immunol. 2014;14:667–685. doi: 10.1038/nri3738. [DOI] [PubMed] [Google Scholar]
- 26.Barnes M.J., Griseri T., Johnson A.M.F. CTLA-4 promotes Foxp3 induction and regulatory T cell accumulation in the intestinal lamina propria. Mucosal Immunol. 2013;6:324–334. doi: 10.1038/mi.2012.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peterson R.A. Regulatory T-cells: diverse phenotypes integral to immune homeostasis and suppression. Toxicol Pathol. 2012;40:186–204. doi: 10.1177/0192623311430693. [DOI] [PubMed] [Google Scholar]
- 28.Ohta A., Kini R., Ohta A. The development and immunosuppressive functions of CD4(+) CD25(+) FoxP3(+) regulatory T cells are under influence of the adenosine-A2A adenosine receptor pathway. Front Immunol. 2012;3:190. doi: 10.3389/fimmu.2012.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ehrentraut H., Westrich J.A., Eltzschig H.K. Adora2b adenosine receptor engagement enhances regulatory T cell abundance during endotoxin-induced pulmonary inflammation. PLoS One. 2012;7:e32416. doi: 10.1371/journal.pone.0032416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang J., Thangamani S., Kim M.H. Retinoic acid promotes the development of Arg1-expressing dendritic cells for the regulation of T-cell differentiation. Eur J Immunol. 2013;43:967–978. doi: 10.1002/eji.201242772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Antonioli L., Pacher P., Vizi E.S., Hasko G. CD39 and CD73 in immunity and inflammation. Trends Mol Med. 2013;19:355–367. doi: 10.1016/j.molmed.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goepfert C., Sundberg C., Sevigny J. Disordered cellular migration and angiogenesis in cd39-null mice. Circulation. 2001;104:3109–3115. doi: 10.1161/hc5001.100663. [DOI] [PubMed] [Google Scholar]
- 33.Ziegler-Heitbrock L., Ancuta P. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116:e74–e80. doi: 10.1182/blood-2010-02-258558. [DOI] [PubMed] [Google Scholar]
- 34.Martinez F.O., Gordon S., Locati M. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177:7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 35.Auffray C., Sieweke M.H., Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y., Choksi S., Chen K. ROS play a critical role in the differentiation of alternatively activated macrophages and the occurrence of tumor-associated macrophages. Cell Res. 2013;23:898–914. doi: 10.1038/cr.2013.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perse M., Cerar A. Dextran sodium sulphate colitis mouse model: traps and tricks. J Biomed Biotechnol. 2012;2012:718617. doi: 10.1155/2012/718617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bauer C., Duewell P., Mayer C. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut. 2010;59:1192–1199. doi: 10.1136/gut.2009.197822. [DOI] [PubMed] [Google Scholar]
- 39.Bain C.C., Mowat A.M. Intestinal macrophages—specialised adaptation to a unique environment. Eur J Immunol. 2011;41:2494–2498. doi: 10.1002/eji.201141714. [DOI] [PubMed] [Google Scholar]
- 40.Kayama H., Ueda Y., Sawa Y. Intestinal CX3C chemokine receptor 1(high) (CX3CR1(high)) myeloid cells prevent T-cell-dependent colitis. Proc Natl Acad Sci USA. 2012;109:5010–5015. doi: 10.1073/pnas.1114931109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murai M., Turovskaya O., Kim G. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat Immunol. 2009;10:1178–1184. doi: 10.1038/ni.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bilate A.M., Lafaille J.J. Can TNF-alpha boost regulatory T cells? J Clin Invest. 2010;120:4190–4192. doi: 10.1172/JCI45262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soroosh P., Doherty T.A., Duan W. Lung-resident tissue macrophages generate Foxp3(+) regulatory T cells and promote airway tolerance. J Exp Med. 2013;210:775–788. doi: 10.1084/jem.20121849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pulte E.D., Broekman M.J., Olson K.E. CD39/NTPDase-1 activity and expression in normal leukocytes. Thromb Res. 2007;121:309–317. doi: 10.1016/j.thromres.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Szabo C., Pacher P. The outsiders: emerging roles of ectonucleotidases in inflammation. Sci Transl Med. 2012;4:146ps14. doi: 10.1126/scitranslmed.3004378. [DOI] [PubMed] [Google Scholar]
- 46.Friedman D.J., Künzli B.M., A-Rahim Y.I. From the cover: CD39 deletion exacerbates experimental murine colitis and human polymorphisms increase susceptibility to inflammatory bowel disease. Proc Natl Acad Sci USA. 2009;106:16788–16793. doi: 10.1073/pnas.0902869106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zanin R.F., Braganhol E., Bergamin L.S. Differential macrophage activation alters the expression profile of NTPDase and ecto-5′-nucleotidase. PLoS One. 2012;7:e31205. doi: 10.1371/journal.pone.0031205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deaglio S., Dwyer K.M., Gao W. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bynoe M.S., Viret C. Foxp3+CD4+ T cell-mediated immunosuppression involves extracellular nucleotide catabolism. Trends Immunol. 2008;29:99–102. doi: 10.1016/j.it.2007.12.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Granulocyte-macrophage colony-stimulating factor–activated monocytes 48 hours after injection.
Monocytes 48 hours after injection.