Abstract
Hepatic steatosis and steatohepatitis are common histologic findings that can be caused by multiple etiologies. The three most frequent causes for steatosis/steatohepatitis are alcohol (alcoholic steatohepatitis, ASH), obesity/metabolic syndrome (nonalcoholic steatohepatitis, NASH), and environmental toxicants (toxicant-associated steatohepatitis, TASH). Hepatic steatosis is an early occurrence in all three forms of liver disease, and they often share common pathways to disease progression/severity. Disease progression is a result of both direct effects on the liver as well as indirect alterations in other organs/tissues such as intestine, adipose tissue, and the immune system. Although the three liver diseases (ASH, NASH, and TASH) share many common pathogenic mechanisms, they also exhibit distinct differences. Both shared and divergent mechanisms can be potential therapeutic targets. This review provides an overview of selected important mechanistic similarities and differences in ASH, NASH, and TASH.
Keywords: Alcoholic Steatohepatitis, Mechanisms, Nonalcoholic Steatohepatitis, Toxicant-Associated Steatohepatitis
Abbreviations used in this paper: ALD, alcoholic liver disease; ALT, alanine aminotransferase; ASH, alcoholic steatohepatitis; AST, aspartate transaminase; BMI, body mass index; CYP2E1, cytochrome P450 isoform 2E1; ECM, extracellular matrix; ER, endoplasmic reticulum; HCC, hepatocellular carcinoma; HDAC, histone deacetylase; HSC, hepatic stellate cell; IL, interleukin; LA, linoleic acid; LPS, lipopolysaccharide; miR, microRNA; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; NK, natural killer; NKT, natural killer T; OXLAM, oxidized linoleic acid metabolite; PAI-1, plasminogen activator inhibitor-1; PCB153, 2,2′,4,4′,5,5′-hexachlorobiphenyl; PPAR, peroxisome proliferator-activated receptor; RNS, reactive nitrogen species; SNP, single-nucleotide polymorphism; TASH, toxicant-associated steatohepatitis; TAFLD, toxicant-associated fatty liver disease; TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin; TH, helper T cell; TLR, Toll-like receptor; TNF, tumor necrosis factor; VA, U.S. Department of Veterans Affairs/Veterans Administration
Summary.
This article reviews selected important mechanistic similarities and differences in alcoholic steatohepatitis, nonalcoholic steatohepatitis, and toxicant-associated steatohepatitis.
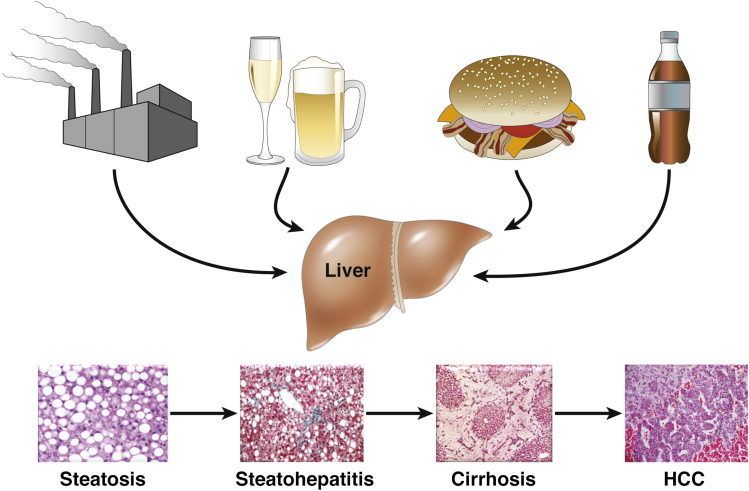
Hepatic steatosis and steatohepatitis are common histologic findings that can be caused by multiple etiologies (Figure 1). The three most frequent causes for steatosis/steatohepatitis are alcohol, obesity/metabolic syndrome, and environmental toxicants, as reviewed herein.
Figure 1.
Multiple etiologic factors and metabolic pathways lead to the same histologic liver abnormalities.
Alcohol remains one of the most common causes of both acute and chronic liver disease in the United States.1 In Western countries, up to 50% of cases of end-stage liver disease have alcohol as a major etiologic factor.2 Excessive alcohol consumption is the third leading preventable cause of death in the United States. Alcohol-related deaths, excluding accidents/homicides, accounted for 22,073 deaths in the United States in 2006, with 13,000 of those specifically attributed to alcoholic liver disease (ALD).3 Cirrhosis from any cause represents the 12th leading cause of death in the United States, and 45.9% of all cirrhosis deaths are attributed to alcohol.4 As shown by early studies involving controlled drinking with subsequent liver biopsies in volunteers, almost everyone who drinks heavily for 12 weeks will develop fatty liver.5, 6 This usually resolves with abstinence, but a subset of people who continue to drink heavily will develop alcoholic hepatitis, which may progress to cirrhosis or even hepatocellular carcinoma (HCC).
The progression of ALD is somewhat similar to nonalcoholic fatty liver disease (NAFLD) and toxicant-associated fatty liver disease (TAFLD) in that it generally occurs over several years. Importantly, studies from the Veterans Administration (VA) have shown that patients with cirrhosis and superimposed alcoholic hepatitis had >60% mortality over a 4-year period, with most of those deaths occurring in the first few months.7 Thus, the prognosis for this aggressive stage of ALD is worse than for many common types of cancer, such as breast, prostate, and colon.
We have known that hepatic steatosis is associated with obesity since at least the 1950s. However, it was not until 1980, when Ludwig et al8 coined the term “nonalcoholic steatohepatitis—NASH” to describe this previously unnamed condition that often occurred in cirrhotic patients, that its clinical importance became recognized. NAFLD encompasses a pathologic spectrum of liver disease that ranges from steatosis to steatohepatitis, cirrhosis, and hepatocellular carcinoma. NAFLD is by far the most common cause of liver disease and abnormal enzymes in children and adults in the United States, with about one-third of adults thought to have NAFLD. The U.S. unselected prevalence of NASH is estimated to be 2% to 5%. Dietary factors, including high-fat and high-fructose diets, have been associated with the development of NASH.
Over 60 million unique chemicals were registered with the Chemical Abstracts Service Registry as of May 2011. With the rapid pace of new chemical discovery and commercialization, it is impossible to fully define the potential impact of these substances on the liver. However, the problem appears significant: 33% of the 677 most common workplace chemicals reported in the National Institute of Occupational Safety and Health Pocket Guide are associated with hepatotoxicity.9 We first coined the term toxicant-associated steatohepatitis (TASH) in 2010, related to a cohort of patients with high vinyl chloride exposure who had classic steatohepatitis on liver biopsy but were not obese and did not drink alcohol.10 Many classes of industrial chemicals have been associated with steatosis or steatohepatitis. These include (but are not limited to): solvents and other halogenated hydrocarbons, volatile organic mixtures, persistent organic pollutants, pesticides, and some nitro-organic compounds.11 Recently enacted federal legislation (the Janey Ensminger Act of 2012) mandates medical coverage through the Department of Veteran’s Affairs for hepatic steatosis in military personnel who were exposed to solvents in the drinking water at Marine Corps Base Camp Lejeune.12 In addition to exposure level, an individual’s susceptibility to chemical-induced liver disease is determined by polymorphisms in the genes of xenobiotic metabolism, concomitant use of alcohol or prescription medications, nutritional factors, and obesity—as many organic chemicals are lipid soluble.
This article reviews the mechanisms for the development of steatohepatitis, highlighting mechanistic differences as well as many common pathways between the three major etiologies (Table 1). For example, alcohol and the environmental toxicant vinyl chloride are both metabolized through cytochrome P450 2E1 (CYP2E1) to form toxic aldehyde intermediates. Moreover, fructose is also metabolized to an aldehyde. Thus, three divergent forms of steatohepatitis can have an aldehyde as an intermediate. On the other hand, dietary unsaturated fat may play a protective role in NASH, but n-6 unsaturated fat appears to augment ALD. There can also be major interactions between types of steatohepatitis. For example, high-fat feeding and subsequent NASH markedly reduces glutathione S-transferases, which play a protective role against a variety of environmental toxicants and alcohol. This review evaluates selected mechanistic similarities and differences in alcoholic steatohepatitis (ASH), NASH, and TASH.
Table 1.
Clinical/Biochemical Characteristics
| Liver Disease | Cell Death | Proinflammatory Cytokines | Adiponectin | Oxidative Stress | Nutrition/BMI | Dietary “Cofactor” | Mechanism-Targeted Treatments |
|---|---|---|---|---|---|---|---|
| ASH | Necrotic/apoptotic ↑ AST |
↑↑↑ | ↑↑ | ↑ | BMI normal, malnutrition | n-6 unsaturated fat | Lifestyle modification Anticytokines (e.g., anakinra) Probiotics Anti-LPS |
| NASH | Apoptotic/necrotic ↑ ALT |
↑ | ↓ | ↑↑ | BMI ↑, overnutrition | Saturated fat, fructose | Lifestyle modification Vitamin E Antioxidants Probiotics |
| TASH | Necrotic/apoptotic Normal AST/ALT |
↑↑ | ↓ | ↑ | BMI normal, normal nutrition | Unclear, possibly high-fat diet | Lifestyle modification Aldehyde/toxin scavengers Antioxidants |
ALT, alanine aminotransferase; ASH, alcoholic steatohepatitis; AST, aspartate transaminase; BMI, body mass index; LPS, lipopolysaccharide; NASH, nonalcoholic steatohepatitis; TASH, toxicant-associated steatohepatitis.
Mechanisms of Liver Disease
Nutritional Abnormalities
Moderate/severe alcoholic hepatitis is usually associated with malnutrition. In large VA cooperative studies, virtually every patient with alcoholic hepatitis had some degree of malnutrition.13 Almost 50% of patients’ energy intake came from alcohol. Although their calorie intake was frequently adequate, their intake of protein and critical micronutrients was often deficient. A classic example of micronutrient deficiency is zinc deficiency.14, 15 Alcoholics regularly have decreased dietary intake of zinc as well as poor absorption and increased excretion. Moreover, oxidative stress causes zinc to be released from critical zinc-finger proteins. The cumulative negative impact on critical zinc-finger proteins can lead to liver injury, altered fat metabolism, and impaired liver regeneration as well as produce classic clinical manifestations of zinc deficiency in humans such as night blindness or skin lesions.
In the VA cooperative studies, the severity of liver disease correlated with malnutrition. Patients were given a balanced 2500-kcal hospital diet which was carefully monitored by a dietitian. Voluntary oral food intake correlated in a stepwise fashion with 6-month mortality data. Thus, patients who voluntarily consumed more than 3000 kcal/day had virtually no mortality, whereas those consuming less than 1000 kcal/day had greater than 80% 6-month mortality. Thus, global malnutrition and specific micronutrient depletion (eg, zinc) appear to play a role in alcoholic hepatitis.
The type of dietary fat consumed also appears to play an important role in the pathogenesis of ALD. Several studies have shown that dietary saturated fat protects against alcohol-induced liver disease in rodents, whereas dietary unsaturated fat enriched in linoleic acid (LA) promotes alcohol-induced liver damage.16 The mechanism(s) by which the combination of LA and alcohol promotes liver injury are not fully understood. LA is the most abundant polyunsaturated fatty acid in human diets and in human plasma and membrane lipids. Dietary intake of LA has more than tripled over the past century. LA can be enzymatically converted to bioactive oxidation products—oxidized LA metabolites (OXLAMs)—primarily via the actions of 12/15-lipoxygenase or nonenzymatically via free-radical-mediated oxidation response to oxidative stress. OXLAMs (either alone or in conjunction with ethanol) can induce increased gut permeability and hepatic mitochondrial dysfunction in experimental ALD. OXLAMs are also postulated to play an etiologic role in NASH.
In contrast to ASH, NASH is commonly associated with overnutrition and obesity. This is especially true in the United States. However, it is important to note that in most well-performed studies, especially pediatric studies, the amount of total calories consumed is similar in obese patients with or without NAFLD/NASH. Thus, total caloric intake does not seem to be the discriminating factor in the development of fatty liver. Individual dietary components have been postulated to play a role. High-carbohydrate diets, especially those high in sugared drinks including fructose, have been implicated.17 Moreover, endogenous production of fructose from high glucose intake has also been implicated in experimental NAFLD.18 Similarly, high-fat diets likely play a role in NAFLD. However, diets high in saturated fats have generally been implicated in NAFLD (compared to unsaturated fats with ASH). These saturated fats are thought to cause hepatic lipotoxicity. Moreover, certain fatty acids may activate Toll-like receptors and induce inflammation/injury. There is increased visceral adiposity in human and experimental NAFLD, and the adipocytes are enlarged and inflamed. On the other hand, adipocytes from ALD patients are actually smaller than normal but still inflamed.
Although the vinyl chloride-exposed patients originally described with TASH were not overweight,10 other chemicals are obesogens that disrupt endocrine signaling to cause steatosis.19, 20 Likewise, chemicals may modify the hepatic response to diet-induced obesity and mediate the transition from steatosis to steatohepatitis. This has recently been demonstrated for important food contaminants including polychlorinated biphenyls,21 arsenic,22 perfluorooctanoic acid,23 and water disinfection byproducts.24, 25 High-fat diet and chemical coexposures can impact hepatic inflammation,22, 23, 24, 25 oxidative stress,23, 24, 25, 26 fibrosis,22, 24 and aryl hydrocarbon receptor and/or nuclear receptor signaling,21, 23, 27 Not only may ethanol worsen TASH,28 solvents may also increase ethanol drinking behavior.29, 30 Thus, nutritional status modulates environmental liver disease, and environmental chemicals, in turn, influence the development and severity of both ALD and NAFLD. Dietary supplementation with oligofructose has been shown to improve steatohepatitis associated with arsenic and high-fat diet coexposures,31 indicating a potential therapeutic role for nutrition in TASH.
Intestinal Barrier Dysfunction/Microbiota
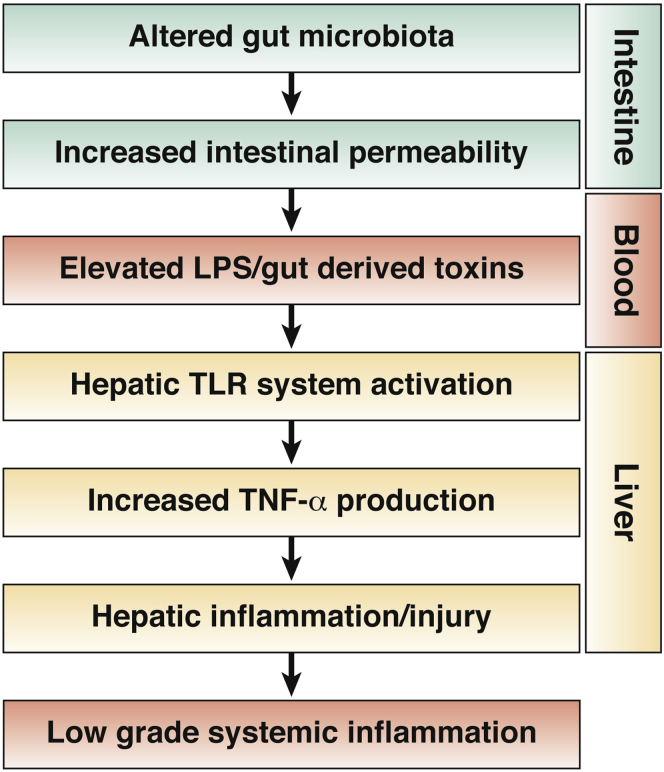
Alcohol, and specifically acetaldehyde, disrupts tight junction proteins and increases gut permeability both in vitro and in vivo; increased endotoxin levels are regularly observed in rodent models of ALD. Elevated endotoxin levels in ALD may originate from 1) Gram-negative bacterial overgrowth in the intestine, 2) increased intestinal permeability, and/or 3) impaired hepatic clearance of endotoxin.32 Endotoxin then stimulates the production of tumor necrosis factor (TNF) and other proinflammatory cytokines through Toll-like receptor 4 (TLR4) signaling, which plays a critical role in the development and progression of ALD (Figure 2). Other bacteria-derived toxins, such as peptidoglycan and flagellin, may also impact TLR signaling and proinflammatory cytokine production.32 Indeed, injected peptidoglycan increases liver injury/inflammation in alcohol-fed compared with control-fed mice, and ethanol feeding increases peptidoglycan levels.32, 33 Moreover, chronic alcohol feeding increases hepatic TLRs and thus sensitizes hepatocytes to inflammation/injury induced by translocation of gut-derived bacteria/toxins. Endotoxin not only plays a role in the fatty liver and liver injury of experimental ALD, but it also appears to play a role in hepatic fibrosis. In vitro assays as well as in vivo mixed chimerism studies show that endotoxin primes stellate cells for transforming growth factor–stimulated collagen production.34 Thus, lipopolysaccharide (LPS) also plays a role in fibrosis induction and progression.
Figure 2.
Alteration in gut-barrier function can lead to translocation of gut-derived products/toxins, which translocate and activate Toll-like receptors with subsequent production of inflammatory mediators, liver injury, and low-grade systemic inflammation.
Alterations in the gut microbiome likely play a major role in the development/progression of gut barrier dysfunction, endotoxemia, and liver injury/fibrosis of ALD.35 We have shown that ethanol consumption can cause a time-dependent decline in the abundance of both Bacteriodetes and Firmicutes, which was accompanied by a proportional increase in Actinobacteria and Proteobacteria;36 notably, the latter phylum encompasses pathogenic Gram-negative species such as Escherichia, Salmonella, Vibrio, and Helicobacter. These results strongly suggest that the increase in plasma endotoxin levels and hepatic inflammation are consequences of the expansion of the Gram-negative bacteria from the Proteobacteria phylum, which occurs in response to chronic ethanol consumption. Importantly, gut microbiome changes are important in the pathogenesis of human ALD (as well as human NAFLD/TAFLD), and probiotic therapy has improved liver enzymes in clinical trials in human ASH/NASH.37, 38, 39
The stability of the normal intestinal microbiome is influenced by several factors in the luminal environment, including gastric acidity, gut motility, bile salts, immunologic defense factors, colonic pH, and the competition between microorganisms for nutrients and intestinal binding sites. An altered luminal environment may lead to modifications in the microbial composition by supporting the growth of specific genera. Thus, a major increase of Alcaligenes (an alkaline-tolerant genus) correlates with an increase in fecal pH and a decrease in fecal short-chain fatty acids. Further, some short-chain fatty acids (eg, butyrate) have important signaling functions and epigenetic consequences, and they are a critical energy source for the intestine.40 Increased luminal pH, leading to pathogenic alterations, has been implicated in diverse disease states ranging from infantile diarrhea to liver cirrhosis.
Substantial data from experimental animal studies support the concept that the role of gut bacteria in NAFLD/NASH is multifactorial and includes regulation of energy homeostasis,41 modulation of choline42 and bile acid metabolism,43 and/or the ability to generate bacteria-derived toxins such as LPS.44 Small intestine bacterial overgrowth has also been linked to NASH pathogenesis.45 Elevated representation of Escherichia, alcohol-producing bacteria, was observed in parallel with increased blood alcohol concentration in NASH patients, suggesting a novel mechanism for the pathogenesis of NASH: gut microbiota enriched in alcohol-producing bacteria (eg, E. coli) constantly produce more alcohol, which in turn is known to play an important role in the disruption of intestinal tight junctions, hepatic oxidative stress, and liver inflammation.46
The gut bacteria may facilitate progression from the simple steatosis to NASH. Dysbiosis associated with the loss of NLRP3 (NOD-like receptor family, pyrin domain-containing 3), and NLRP6 inflammasomes resulted in increased influx of LPS and bacterial DNA to the liver; these bacterial products stimulate TLR4 and TLR9, respectively, leading to enhanced hepatic TNFα expression which drives NASH progression.47 The gut microbiota may also contribute to hepatic fibrosis via stimulation of TLR9-dependent profibrotic pathways in hepatic Kupffer cells.48 An exciting advance in the field has been the recent observation that gut microbiota transplantation from donor mice with NAFLD replicated the phenotype in wild-type recipients, demonstrating that NAFLD is a potentially transmissible process.49
Environmental toxicants can also alter the gut microbiome and its metabolic activity. Exposure to the environmental toxicant arsenic affects large populations both worldwide and in the United States, especially through contamination of drinking water. Recent studies by Lu et al50 showed that arsenic exposure produces major alterations in the gut microbiome, and arsenic-treated mice were clearly delineated from control mice on metabolic profiles by principal component analysis. Moreover, clear-cut interactions between the high-fat diet and arsenic-induced steatohepatitis as well as altered fecal metabolites/microbiome have also been reported.22, 31, 51 Likewise, polychlorinated biphenyl exposures have been associated with both intestinal dysbiosis52 and increased gut permeability.53
Immune Alterations and Inflammatory Mediators
The immune system, including both innate—mediated by neutrophils, macrophages, natural killer (NK) and natural killer T (NKT) cells—and adaptive—mediated by T and B cells—immune responses, is an important pathogenic component of fatty liver diseases.54 Macrophages and liver Kupffer cells are major producers of proinflammatory and anti-inflammatory cytokines (eg, TNFα, interleukin 8 [IL-8], and IL-10) and play a critical role, particularly in early fatty liver disease.55 Moreover, Kupffer cells can activate the adaptive immune cells (T cells) and hepatic stellate cells (HSCs) via IL-6 and transforming growth factor-β. Kupffer cell depletion was shown to reduce hepatic damage and inflammation in alcohol-induced56 and choline-deficient diet-induced steatohepatitis.57
Neutrophils are an initial inflammatory response to injury, and their infiltration into the liver in response to chemoattractant cytokines is a pathologic hallmark of fatty liver and most prominently ALD.58, 59 The contribution of neutrophils in NASH development is also becoming evident in mouse models60, 61 and human NASH,62 with the occurrence of both apoptosis and necrosis shown in humans.63
Hepatic inflammation along with steatosis and hepatotoxicity is observed in TAFLD, but the data are scarce. Dioxin (2,3,7,8-tetrachlorodibenzo-p-dioxin [TCDD]) administration in immature, ovariectomized C57B/l/6 mice either alone or in combination with another environmental pollutant, PCB153 (2,2′,4,4′,5,5′-hexachlorobiphenyl), resulted in hepatic histologic changes that included lipid accumulation and inflammatory cell infiltration.64 Subchronic exposure of mice to the pesticide malathion for 28 days resulted in hepatic steatosis and inflammation with neutrophil activation.65 Further studies after exposures to various toxins will better establish the role of immune alterations in TAFLD.
The adaptive immune system is considered important in fatty liver, particularly in the advanced stages with a more progressive phenotype and significant fibrosis. Alcoholic hepatitis patients have an increased number of T cells in the liver and increased circulating antibodies against lipid peroxidation adducts, suggesting that adaptive immune activation may contribute to the ALD pathogenesis.66, 67, 68, 69 Adaptive immune responses also contribute to hepatic inflammation in NAFLD.70
Studies in humans and animals suggest that NKT cells may be significant contributors to inflammation, cell death, and fibrosis in NAFLD.69, 71, 72 Moreover, evidence demonstrates a significant decrease of CD4+ regulatory T cells in a high fat diet mouse model of fatty liver.73, 74 Recently, another subset of helper T cells, TH17 cells, that secrete IL-17 and induce inflammation via neutrophils has been identified and is thought to play a role in fatty liver. TH17 responses are involved in human ALD75 and may be important for progression from simple steatosis to steatohepatitis in NAFLD.76 Gadd et al77 recently reported that in all stages of NAFLD the portal tracts were enriched by CD68+ macrophages and CD8+ lymphocytes. Further, Miyagi et al78 demonstrated a protective role of invariant NKT cells in the progression from inflammation to fibrosis, without alteration of steatosis, in a high-fat mouse model.
Cross-talk between various immune cells as well as interactions with other liver cells may also be critical determinants in fatty liver.79 T cells are important activators of HSCs; while activated, HSCs can act as antigen-presenting cells to stimulate T cells (including NKT cells and CD4+ and CD8+ T cells).80 Some studies have suggested that regulatory T cells may promote oncogenesis and tumor progression leading to HCC because they mediate immunosuppressive effects and inhibit NK and CD8+ T cells.81 In addition, hepatocytes are major producers of immunomodulatory cytokines such as IL-8,82 and they are targets for cytokine toxicity. Thus, many liver cells and peripheral infiltrating immune cells are involved inflammation and damage in fatty liver.
Fat-derived products (such as acrolein, leukotrienes, and OXLAMs), and advanced glycation end products can also cause liver inflammation and injury.83, 84 Factors that activate the inflammasome, such as uric acid, can induce production of IL-1 and IL-18. The release of damage-associated molecular patterns from dying cells, is also believed to trigger sterile inflammation following tissue injury.85 All these inflammatory/fibrotic mediators have been shown to be increased in fatty liver and are potential targets for therapeutic intervention.
Oxidative Stress and Lipid Peroxidation
Oxidative stress is an imbalance between pro-oxidants and antioxidants. Reactive oxygen species and reactive nitrogen species (RNS) are products of normal metabolism and can be beneficial to the host (eg, by contributing to bacterial killing).86 Overproduction of reactive oxygen species and RNS, inadequate antioxidant defenses (eg, low levels of vitamins, selenium, or mitochondrial glutathione), or both can lead to liver injury. Oxidative stress in ASH, NASH, or TASH is usually documented by detection of one of several indirect markers: 1) protein oxidation (eg, protein thiol or carbonyl products), 2) lipid oxidation (eg, isoprostanes or malondialdehyde), 3) DNA oxidation (eg, oxodeoxyguanosine), or 4) depletion or induction of antioxidant defenses (eg, vitamin E, glutathione, or thioredoxin).87
The stimulus for oxidative stress in the liver comes from multiple sources. In hepatocytes, CYP2E1 activity increases after alcohol consumption—in part because of stabilization of messenger RNA (mRNA). Similarly, CYP2E1 activity is increased in NAFLD. The CYP2E1 system leaks electrons to initiate oxidative stress.86 CYP2E1 is localized in the hepatic lobule in areas of alcohol-induced liver injury. Moreover, overexpression of CYP2E1 in mice and in HepG2 cells (a human hepatoma cell line) in vitro leads to enhanced alcohol hepatotoxicity. Nonparenchymal cells and infiltrating inflammatory cells (eg, polymorphonuclear neutrophils) are another major source of pro-oxidants that are used for normal cellular processes such as killing invading organisms. Infiltrating neutrophils use enzyme systems such as myeloperoxidase to generate hypochlorous acid (HClO−, a halide species that causes oxidative stress) and RNS.
Hepatic steatosis in TAFLD caused by exposure to methyl mercury is associated with increased lipid peroxidation products in rat livers.88 Inhibition of pyruvate dehydrogenase in the mitochondria, with resultant mitochondrial uncoupling and an increase in hydrogen peroxide production,89 has been shown with elevated free radicals and lipid peroxidation products in multiple studies of arsenic-induced TAFLD.90 Carbon tetrachloride is another well-studied hepatotoxicant that induces hepatic steatosis and injury after cleavage of CCl4 by CYP2E1, which generates the trichloromethyl radical and leads to lipid peroxidation and membrane damage.91 We recently demonstrated that PCB153 exposure causes TASH with hepatic antioxidant depletion.26
Oxidative stress can mediate liver injury through at least two major pathways: direct cell injury and cell signaling. Direct cell injury is indicated by markers such as lipid peroxidation and DNA damage. An even greater role is played by signaling pathways; for example, activation of transcription factors such as nuclear factor κB plays a critical role in the production of proinflammatory cytokines such as TNF.
Of all the mechanisms related to ASH, NASH, and TASH, oxidative stress has probably been the most widely studied. Antioxidant therapy offers potential as a clinical intervention for steatohepatitis. Importantly, there is no therapy approved by the U.S. Food and Drug Administration for any form of steatohepatitis. Moreover, vitamin E therapy (800 IU per day) is possibly the only widely accepted therapy for any form of steatohepatitis (in this case, NASH; see Table 1).92 Unfortunately, therapy is beyond the scope of this article, but each of the listed mechanisms for steatohepatitis represents a potential therapeutic target.
Endoplasmic Reticulum Stress
Endoplasmic reticulum (ER) stress, or the unfolded protein response pathway, is activated by conditions of protein overload or increased unfolded proteins. Once triggered, this signaling pathway results in adaptation and recovery of homeostasis; however, severe or prolonged ER stress can ultimately result in cell death.
Increasing evidence has demonstrates that ER stress is a common feature of many liver diseases, including ASH and NASH.93, 94 Alcohol-induced ER stress is seen in experimental alcohol-feeding models in mice, micropigs, rats, and zebrafish.95, 96, 97, 98 ER stress has been also been reported in human patients with ALD,99, 100 with up-regulation of multiple ER stress markers, which correlated with dysregulated lipid metabolism and impaired insulin signaling. This suggests that ER stress is integral to ALD pathogenesis in human alcoholics. The induction of hepatic ER stress has been described in several genetic and diet-induced murine models of obesity, insulin resistance, and NAFLD, and in the livers of patients with NAFLD.101, 102, 103, 104
Some upstream mechanisms that are demonstrated to cause ER stress in NAFLD include 1) hepatic steatosis/excess fatty acids, 2) oxidative stress and deficient nuclear factor erythroid 2-related factor 2 (Nrf2), 3) impaired hepatic autophagy in NAFLD patients and murine models of NAFLD, and 4) down-regulation of adiponectin.105, 106, 107 Several alcohol-induced factors are also known to cause ER stress in ASH, including acetaldehyde and toxic lipid-derived aldehydes and metabolites, oxidative stress, dysregulated methionine metabolism, aberrant epigenetic modifications, altered interferon regulatory factor 3 (IRF3)/Sting signaling, and disruption of calcium homeostasis.108, 109
Overall, hepatic ER stress occurs in both ASH and NASH in many species including humans, and is now accepted as an important mechanism in disease pathogenesis and progression. However, because ER stress appears to be both a cause and a consequence of other accompanying alterations, the question of association versus causality remains. The association of ER stress and TAFLD is highly likely but less well investigated. Thus, the exact role of ER stress in the pathogenesis of ALD, NAFLD, and TAFLD warrants further investigation to facilitate the development of therapies.
Fibrin/Extracellular Matrix
Fibrosis results from an imbalance between production and resorption of extracellular matrix (ECM) caused by a complex interplay between activation/transdifferentiation of HSCs, profibrogenic growth factors and cytokines, and alterations in the fibrin coagulation system. Fibrosis and the altered ECM subsequently provide a permissive setting for the development of cellular dysplasia and HCC. HSCs are the dominant contributors to fibrosis in the liver driven by varied etiology, and upon activation they produce profibrogenic factors such as collagen and smooth muscle actin.110 Although a mechanistic link between apoptosis or necroptosis and HSC activation has been suggested, it is not fully understood.
Hepatic injury in experimental models of liver disease often involves dysregulation of the fibrin cascade, resulting in the formation of fibrin clots that can cause hepatocellular death and induce inflammatory signaling in the liver. Inhibition of fibrinolysis by plasminogen activator inhibitor-1 (PAI-1) can cause fibrin-ECM to accumulate, even in the absence of enhanced fibrin deposition by the thrombin cascade. An imbalance in coagulation factors as well as elevated PAI-1 levels and hypofibrinolysis are common in patients with either NAFLD or ALD.111 Indeed, it has been shown that circulating plasma PAI-1 levels in humans are closely related to the degree of liver steatosis.112 Coagulation cascade activation has also been shown to be critical for liver inflammation and steatosis in Western-diet-induced NAFLD, and PAI-1 levels during disease development are a predictor of later severity.113 Fibrosis is also commonly seen in TAFLD caused by exposure to drugs114 and vinyl chloride.10 Also, exposure of mice to the dioxin TCDD results in hepatic steatosis and fibrosis with up-regulation of profibrogenic genes.115 However, the underlying mechanisms remain undetermined.
Genetics/Epigenetics
Recent studies have shown that both genetic and epigenetic factors are important for disease pathogenesis and progression in steatohepatitis. The genetic variations are often associated with conformational changes in protein structures and functions due to single-nucleotide polymorphisms (SNPs), whereas epigenetic changes are phenotypic changes resulting from altered gene expression without affecting the underlying DNA sequence.
Genomewide association studies have identified around 3.1 million SNPs that can contribute to disease states, and these SNPs may increase or decrease the function of encoded proteins. Specifically, a study conducted by Romeo et al116 showed 9229 SNPs in NAFLD patients as compared with controls. Some of the more important ones appeared to be patatin-like phospholipase domain-containing 3 (PNPLA3), peroxisome proliferator-activated receptor-γ (PPAR-γ), and TNFα.117 In NAFLD, TNFα-238, adiponectin-45, leptin 2548 PPAR-γ-161, and phosphatidylethanolamine N-methyltransferase-175 (PEMT-175) have an increased risk association, and adiponectin-276 and hepatic lipase-514 have a negative or decreased risk association.118 In ALD, polymorphisms of alcohol metabolizing enzymes such as alcohol dehydrogenase and CYP2E1 as well as antioxidant enzymes and cytokine-coding genes have shown a strong correlation with the progression of ALD.119
Epigenetic changes occurring in response to various environmental signals can produce diverse tissue-specific effects. These epigenetic modifications include microRNAs (miR), DNA methylation, and histone modifications. The role of microRNA is well established in both NASH and ASH. Some of these microRNAs play a causal role, as they have targets that are important for the development of disease, but others likely are seen only as associations. A decrease in miR-122 and induction in miR-155 expression has been reported in models of NASH and ASH. Mice deficient in miR-122 develop steatohepatitis and fibrosis. TNF and CEBP are the targets of miR-155. Additionally, other microRNAs such as miR-320, miR-486, miR-705, miR-1224, miR-27b, miR-214, miR-199a, miR-192, and miR-183 likely contribute to both diseases.120 Importantly, there is a strong association between HCC, which can arise from ALD, NAFLD, or TAFLD, and hepatic microRNA alterations.121, 122
The potential role of alcohol induced histone modifications in the development of ALD has been observed in several in vivo and in vitro studies. Increases in histone H3 acetylation have been documented resulting from increased histone acetyltransferase activity and histone deacetylase (HDAC) inhibition. Reduced expression of sirtuin 1 (SIRT 1), a class III HDAC, has been shown in alcohol-exposed hepatocytes and is known to regulate the lipid metabolism pathway. Our own studies support this notion. We have shown that dysregulation of hepatic HDAC expression plays a major role in the binge alcohol-induced hepatic steatosis and liver injury by affecting lipogenesis and fatty acid β-oxidation.123 In DNA methylation studies, hypermethylation has been observed in PPARGC1A (PPAR-γ coactivator 1α) and TFAM (mitochondrial transcription factor A) promoters in NAFLD livers. In ALD, decreased S-adenosyl-l-methionine levels seem to influence DNA methylation. Decreased S-adenosyl-l-methionine is known to induce global hypomethylation and regional hypermethylation of various promoters, and such changes are hypothesized to contribute to ASH.
Our analysis of environmental chemicals associated with TAFLD showed that exposures to chemicals that cause hepatic steatosis also can lead to multigenerational toxic effects in offspring, strongly suggesting that epigenetic alterations may be responsible.124 Long-term exposure of humans to high concentrations of arsenic is hepatotoxic and associated with an increased risk of cancer. Arsenic-induced fatty liver and hepatotoxicity are closely associated with both DNA damage (genetic changes) and DNA methylation (epigenetic changes), and such alterations may lead to the development of liver cancer.125 Interestingly, long-term arsenic exposure has been shown to down-regulate p16 (INK4a) by targeting recruitment of G9a and H3K9 dimethylation without changing DNA methylation in the normal mouse liver, and these changes occurred in the absence of tumorigenesis, suggesting that they may be precursors.126
Cell Death
Defining mechanisms for hepatocyte cell death is a critical area of interest for liver injury of all etiologies, and the common modes of cell death relevant to this article are apoptosis, necrosis, and necroptosis.127 Apoptotic death has been demonstrated in ALD and NAFLD in both animal models and humans; moreover, apoptosis and necrosis frequently coexist in liver pathology. We have observed coexistent apoptosis and necrosis in human ASH and NASH, with necrosis tending to be more dominant in ASH and apoptosis in NASH. Our work has shown that hepatocyte necrosis (rather than apoptosis) is seen in TASH;128 this is also a primary death mechanism with other hepatotoxins such as carbon tetrachloride. Acetaminophen-induced liver injury evokes both necrosis and necroptosis.129, 130 Interestingly, unlike ALD and NAFLD, which are typically associated with elevated aspartate transaminase (AST) and alanine aminotransferase (ALT) activities, TAFLD is commonly associated with normal liver enzymes.10 Alcoholic liver disease classically has AST>ALT whereas NAFLD usually has ALT predominance. These liver enzyme profiles are sometimes helpful in identifying/suggesting an underlying cause of liver injury (eg, alcohol abuse in someone who does not provide a reliable alcohol history, NAFLD in a nonobese patient). Dying hepatocytes, particularly during necrosis/necroptosis, can release pathogenic mediators or damage-associated molecular patterns such as lipid-derived metabolites (eg, aldehydes), HMGB1 (high-mobility group box 1), formyl peptides, and mitochondrial DNA, which trigger inflammation and cell death in neighboring hepatocytes and exacerbate liver damage.
Conclusions
Fatty liver disease (ASH, NASH, TASH) occurs as a result of varied etiologies (see Figure 1) and can progress to histologically identical, more severe liver disease. Disease progression is a result of both direct effects on the liver as well as indirect alterations in other organs/tissues such as intestine, adipose tissue, and the immune system. Although the three diseases share many common pathogenic mechanisms, they also exhibit distinct differences. Both shared and divergent mechanisms can be potential therapeutic targets. Better biomarkers for ASH, NASH, and TASH and improved model systems that more closely resemble human disease will promote future mechanistic investigations and therapeutic development.
Footnotes
Conflicts of interest The authors declare no conflicts.
Funding This work was supported by National Institutes of Health grants R01AA018869 (to C.J.M.), U01AA021893 (to C.J.M.), U01AA021901 (to C.J.M.), R01AA023681 (to C.J.M.), U01AA022489 (to C.J.M.), KO1ES017105 (to S.J.B.), K23AA018399 (to M.C.), R13ES024661 (to M.C.), RO1ES021375 (to M.C.), R21AA020849 (to I.K.), and the Veterans Administration.
References
- 1.Sofair A.N., Barry V., Manos M.M. The epidemiology and clinical characteristics of patients with newly diagnosed alcohol-related liver disease: results from population-based surveillance. J Clin Gastroenterol. 2010;44:301–307. doi: 10.1097/MCG.0b013e3181b3f760. [DOI] [PubMed] [Google Scholar]
- 2.Orholm M., Sorensen T.I., Bentsen K. Mortality of alcohol abusing men prospectively assessed in relation to history of abuse and degree of liver injury. Liver. 1985;5:253–260. doi: 10.1111/j.1600-0676.1985.tb00246.x. [DOI] [PubMed] [Google Scholar]
- 3.Heron M., Hoyert D.L., Murphy S.L. Deaths: final data for 2006. Natl Vital Stat Rep. 2009;57:1–134. [PubMed] [Google Scholar]
- 4.National Center for Health Statistics . Centers for Disease Control and Prevention; Hyattsville, MD: 2009. Health, United States, 2008, with chartbook.http://www.cdc.gov/nchs/data/hus/hus08.pdf tables 68 and 69. Available at: [Google Scholar]
- 5.Lieber C.S., Rubin E. Alcoholic fatty liver in man on a high protein and low fat diet. Am J Med. 1968;44:200–206. doi: 10.1016/0002-9343(68)90151-4. [DOI] [PubMed] [Google Scholar]
- 6.Rubin E., Lieber C.S. Alcohol-induced hepatic injury in nonalcoholic volunteers. N Engl J Med. 1968;278:869–876. doi: 10.1056/NEJM196804182781602. [DOI] [PubMed] [Google Scholar]
- 7.Chedid A., Mendenhall C.L., Gartside P. Prognostic factors in alcoholic liver disease. VA Cooperative Study Group. Am J Gastroenterol. 1991;86:210–216. [PubMed] [Google Scholar]
- 8.Ludwig J., Viggiano T.R., McGill D.B., Oh B.J. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434–438. [PubMed] [Google Scholar]
- 9.Tolman K.G., Sirrine R. Occupational Toxicology. Clin Liver Dis. 1998;2:563–589. [Google Scholar]
- 10.Cave M., Falkner K.C., Ray M. Toxicant-associated steatohepatitis in vinyl chloride workers. Hepatology. 2010;51:474–481. doi: 10.1002/hep.23321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wahlang B., Beier J.I., Clair H.B. Toxicant-associated steatohepatitis. Toxicol Pathol. 2013;41:343–360. doi: 10.1177/0192623312468517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Department of Veterans, A., Payment or reimbursement for certain medical expenses for Camp Lejeune family members. Interim final rule. Fed Regist. 2014;79:57415–57421. [PubMed] [Google Scholar]
- 13.Mendenhall C., Roselle G.A., Gartside P., Moritz T. Relationship of protein calorie malnutrition to alcoholic liver disease: a reexamination of data from two Veterans Administration Cooperative Studies. Alcohol Clin Exp Res. 1995;19:635–641. doi: 10.1111/j.1530-0277.1995.tb01560.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhong W., Zhao Y., Sun X. Dietary zinc deficiency exaggerates ethanol-induced liver injury in mice: involvement of intrahepatic and extrahepatic factors. PLoS One. 2013;8:e76522. doi: 10.1371/journal.pone.0076522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohammad M.K., Zhou Z., Cave M. Zinc and liver disease. Nutr Clin Pract. 2012;27:8–20. doi: 10.1177/0884533611433534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirpich I.A., Feng W., Wang Y. The type of dietary fat modulates intestinal tight junction integrity, gut permeability, and hepatic toll-like receptor expression in a mouse model of alcoholic liver disease. Alcohol Clin Exp Res. 2012;36:835–846. doi: 10.1111/j.1530-0277.2011.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bergheim I., Weber S., Vos M. Antibiotics protect against fructose-induced hepatic lipid accumulation in mice: role of endotoxin. J Hepatol. 2008;48:983–992. doi: 10.1016/j.jhep.2008.01.035. [DOI] [PubMed] [Google Scholar]
- 18.Lanaspa M.A., Ishimoto T., Li N. Endogenous fructose production and metabolism in the liver contributes to the development of metabolic syndrome. Nat Commun. 2013;4:2434. doi: 10.1038/ncomms3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chamorro-Garcia R., Sahu M., Abbey R.J. Transgenerational inheritance of increased fat depot size, stem cell reprogramming, and hepatic steatosis elicited by prenatal exposure to the obesogen tributyltin in mice. Environ Health Perspect. 2013;121:359–366. doi: 10.1289/ehp.1205701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wahlang B., Falkner K.C., Gregory B. Polychlorinated biphenyl 153 is a diet-dependent obesogen that worsens nonalcoholic fatty liver disease in male C57BL6/J mice. J Nutr Biochem. 2013;24:1587–1595. doi: 10.1016/j.jnutbio.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wahlang B., Song M., Beier J.I. Evaluation of Aroclor 1260 exposure in a mouse model of diet-induced obesity and non-alcoholic fatty liver disease. Toxicol Appl Pharmacol. 2014;279:380–390. doi: 10.1016/j.taap.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan M., Schmidt R.H., Beier J.I. Chronic subhepatotoxic exposure to arsenic enhances hepatic injury caused by high fat diet in mice. Toxicol Appl Pharmacol. 2011;257:356–364. doi: 10.1016/j.taap.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan X., Xie G., Sun X. High fat diet feeding exaggerates perfluorooctanoic acid-induced liver injury in mice via modulating multiple metabolic pathways. PLoS One. 2013;8:e61409. doi: 10.1371/journal.pone.0061409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seth R.K., Kumar A., Das S. Environmental toxin-linked nonalcoholic steatohepatitis and hepatic metabolic reprogramming in obese mice. Toxicol Sci. 2013;134:291–303. doi: 10.1093/toxsci/kft104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Das S., Kumar A., Seth R.K. Proinflammatory adipokine leptin mediates disinfection byproduct bromodichloromethane-induced early steatohepatitic injury in obesity. Toxicol Appl Pharmacol. 2013;269:297–306. doi: 10.1016/j.taap.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi X., Wahlang B., Wei X. Metabolomic analysis of the effects of polychlorinated biphenyls in nonalcoholic fatty liver disease. J Proteome Res. 2012;11:3805–3815. doi: 10.1021/pr300297z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wahlang B., Falkner K.C., Clair H.B. Human receptor activation by aroclor 1260, a polychlorinated biphenyl mixture. Toxicol Sci. 2014;140:283–297. doi: 10.1093/toxsci/kfu083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mailloux R.J., Florian M., Chen Q. Exposure to a northern contaminant mixture (NCM) alters hepatic energy and lipid metabolism exacerbating hepatic steatosis in obese JCR rats. PLoS One. 2014;9:e106832. doi: 10.1371/journal.pone.0106832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aschengrau A., Weinberg J.M., Janulewicz P.A. Occurrence of mental illness following prenatal and early childhood exposure to tetrachloroethylene (PCE)-contaminated drinking water: a retrospective cohort study. Environ Health. 2012;11:2. doi: 10.1186/1476-069X-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aschengrau A., Weinberg J.M., Janulewicz P.A. Affinity for risky behaviors following prenatal and early childhood exposure to tetrachloroethylene (PCE)-contaminated drinking water: a retrospective cohort study. Environ Health. 2011;10:102. doi: 10.1186/1476-069X-10-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Massey V.L., Stocke K.S., Schmidt R.H. Oligofructose protects against arsenic-induced liver injury in a model of environment/obesity interaction. Toxicol Appl Pharmacol. 2015;284:304–314. doi: 10.1016/j.taap.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Purohit V., Bode J.C., Bode C. Alcohol, intestinal bacterial growth, intestinal permeability to endotoxin, and medical consequences: summary of a symposium. Alcohol. 2008;42:349–361. doi: 10.1016/j.alcohol.2008.03.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gustot T., Lemmers A., Moreno C. Differential liver sensitization to toll-like receptor pathways in mice with alcoholic fatty liver. Hepatology. 2006;43:989–1000. doi: 10.1002/hep.21138. [DOI] [PubMed] [Google Scholar]
- 34.Gao B., Seki E., Brenner D.A. Innate immunity in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2011;300:G516–G525. doi: 10.1152/ajpgi.00537.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schnabl B., Brenner D.A. Interactions between the intestinal microbiome and liver diseases. Gastroenterology. 2014;146:1513–1524. doi: 10.1053/j.gastro.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bull-Otterson L., Feng W., Kirpich I. Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PLoS One. 2013;8:e53028. doi: 10.1371/journal.pone.0053028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kirpich I.A., Solovieva N.V., Leikhter S.N. Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: a pilot study. Alcohol. 2008;42:675–682. doi: 10.1016/j.alcohol.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aller R., De Luis D.A., Izaola O. Effect of a probiotic on liver aminotransferases in nonalcoholic fatty liver disease patients: a double blind randomized clinical trial. Eur Rev Med Pharmacol Sci. 2011;15:1090–1095. [PubMed] [Google Scholar]
- 39.Wong V.W., Won G.L., Chim A.M. Treatment of nonalcoholic steatohepatitis with probiotics: a proof-of-concept study. Ann Hepatol. 2013;12:256–262. [PubMed] [Google Scholar]
- 40.Shi X., Wei X., Yin X. Hepatic and fecal metabolomic analysis of the effects of Lactobacillus rhamnosus GG on alcoholic fatty liver disease in mice. J Proteome Res. 2015;14:1174–1182. doi: 10.1021/pr501121c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Backhed F., Ding H., Wang T. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Z., Klipfell E., Bennett B.J. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swann J.R., Want E.J., Geier F.M. Systemic gut microbial modulation of bile acid metabolism in host tissue compartments. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4523–4530. doi: 10.1073/pnas.1006734107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cani P.D., Neyrinck A.M., Fava F. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50:2374–2383. doi: 10.1007/s00125-007-0791-0. [DOI] [PubMed] [Google Scholar]
- 45.Ferolla S.M., Armiliato G.N., Couto C.A. The role of intestinal bacteria overgrowth in obesity-related nonalcoholic fatty liver disease. Nutrients. 2014;6:5583–5599. doi: 10.3390/nu6125583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu L., Baker S.S., Gill C. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57:601–609. doi: 10.1002/hep.26093. [DOI] [PubMed] [Google Scholar]
- 47.Henao-Mejia J., Elinav E., Jin C. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miura K., Kodama Y., Inokuchi S. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1beta in mice. Gastroenterology. 2010;139:323–334.e7. doi: 10.1053/j.gastro.2010.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Le Roy T., Llopis M., Lepage P. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut. 2013;62:1787–1794. doi: 10.1136/gutjnl-2012-303816. [DOI] [PubMed] [Google Scholar]
- 50.Lu K., Abo R.P., Schlieper K.A. Arsenic exposure perturbs the gut microbiome and its metabolic profile in mice: an integrated metagenomics and metabolomics analysis. Environ Health Perspect. 2014;122:284–291. doi: 10.1289/ehp.1307429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shi X., Wei X., Koo I. Metabolomic analysis of the effects of chronic arsenic exposure in a mouse model of diet-induced Fatty liver disease. J Proteome Res. 2014;13:547–554. doi: 10.1021/pr400719u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Choi J.J., Eum S.Y., Rampersaud E. Exercise attenuates PCB-induced changes in the mouse gut microbiome. Environ Health Perspect. 2013;121:725–730. doi: 10.1289/ehp.1306534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi Y.J., Seelbach M.J., Pu H. Polychlorinated biphenyls disrupt intestinal integrity via NADPH oxidase-induced alterations of tight junction protein expression. Environ Health Perspect. 2010;118:976–981. doi: 10.1289/ehp.0901751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peverill W., Powell L.W., Skoien R. Evolving concepts in the pathogenesis of NASH: beyond steatosis and inflammation. Int J Mol Sci. 2014;15:8591–8638. doi: 10.3390/ijms15058591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leroux A., Ferrere G., Godie V. Toxic lipids stored by Kupffer cells correlates with their pro-inflammatory phenotype at an early stage of steatohepatitis. J Hepatol. 2012;57:141–149. doi: 10.1016/j.jhep.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 56.Owumi S.E., Corthals S.M., Uwaifo A.O. Depletion of Kupffer cells modulates ethanol-induced hepatocyte DNA synthesis in C57Bl/6 mice. Environ Toxicol. 2014;29:867–875. doi: 10.1002/tox.21814. [DOI] [PubMed] [Google Scholar]
- 57.Miura K., Yang L., van Rooijen N. Hepatic recruitment of macrophages promotes nonalcoholic steatohepatitis through CCR2. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1310–G1321. doi: 10.1152/ajpgi.00365.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maltby J., Wright S., Bird G. Chemokine levels in human liver homogenates: associations between GRO alpha and histopathological evidence of alcoholic hepatitis. Hepatology. 1996;24:1156–1160. doi: 10.1053/jhep.1996.v24.pm0008903391. [DOI] [PubMed] [Google Scholar]
- 59.Dominguez M., Miquel R., Colmenero J. Hepatic expression of CXC chemokines predicts portal hypertension and survival in patients with alcoholic hepatitis. Gastroenterology. 2009;136:1639–1650. doi: 10.1053/j.gastro.2009.01.056. [DOI] [PubMed] [Google Scholar]
- 60.Rensen S.S., Bieghs V., Xanthoulea S. Neutrophil-derived myeloperoxidase aggravates non-alcoholic steatohepatitis in low-density lipoprotein receptor-deficient mice. PLoS One. 2012;7:e52411. doi: 10.1371/journal.pone.0052411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ibusuki R., Uto H., Arima S. Transgenic expression of human neutrophil peptide-1 enhances hepatic fibrosis in mice fed a choline-deficient, L-amino acid-defined diet. Liver Int. 2013;33:1549–1556. doi: 10.1111/liv.12203. [DOI] [PubMed] [Google Scholar]
- 62.Alkhouri N., Morris-Stiff G., Campbell C. Neutrophil to lymphocyte ratio: a new marker for predicting steatohepatitis and fibrosis in patients with nonalcoholic fatty liver disease. Liver Int. 2012;32:297–302. doi: 10.1111/j.1478-3231.2011.02639.x. [DOI] [PubMed] [Google Scholar]
- 63.Joka D., Wahl K., Moeller S. Prospective biopsy-controlled evaluation of cell death biomarkers for prediction of liver fibrosis and nonalcoholic steatohepatitis. Hepatology. 2012;55:455–464. doi: 10.1002/hep.24734. [DOI] [PubMed] [Google Scholar]
- 64.Kopec A.K., D’Souza M.L., Mets B.D. Non-additive hepatic gene expression elicited by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and 2,2′,4,4′,5,5′-hexachlorobiphenyl (PCB153) co-treatment in C57BL/6 mice. Toxicol Appl Pharmacol. 2011;256:154–167. doi: 10.1016/j.taap.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lasram M.M., Dhouib I.B., Bouzid K. Association of inflammatory response and oxidative injury in the pathogenesis of liver steatosis and insulin resistance following subchronic exposure to malathion in rats. Environ Toxicol Pharmacol. 2014;38:542–553. doi: 10.1016/j.etap.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 66.Albano E., Vidali M. Immune mechanisms in alcoholic liver disease. Genes Nutr. 2010;5:141–147. doi: 10.1007/s12263-009-0151-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mottaran E., Stewart S.F., Rolla R. Lipid peroxidation contributes to immune reactions associated with alcoholic liver disease. Free Radic Biol Med. 2002;32:38–45. doi: 10.1016/s0891-5849(01)00757-2. [DOI] [PubMed] [Google Scholar]
- 68.Thiele G.M., Duryee M.J., Willis M.S. Autoimmune hepatitis induced by syngeneic liver cytosolic proteins biotransformed by alcohol metabolites. Alcohol Clin Exp Res. 2010;34:2126–2136. doi: 10.1111/j.1530-0277.2010.01309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tajiri K., Shimizu Y., Tsuneyama K. Role of liver-infiltrating CD3+CD56+ natural killer T cells in the pathogenesis of nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2009;21:673–680. doi: 10.1097/MEG.0b013e32831bc3d6. [DOI] [PubMed] [Google Scholar]
- 70.Sutti S., Jindal A., Locatelli I. Adaptive immune responses triggered by oxidative stress contribute to hepatic inflammation in NASH. Hepatology. 2014;59:886–897. doi: 10.1002/hep.26749. [DOI] [PubMed] [Google Scholar]
- 71.Syn W.K., Oo Y.H., Pereira T.A. Accumulation of natural killer T cells in progressive nonalcoholic fatty liver disease. Hepatology. 2010;51:1998–2007. doi: 10.1002/hep.23599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kumar V. NKT-cell subsets: promoters and protectors in inflammatory liver disease. J Hepatol. 2013;59:618–620. doi: 10.1016/j.jhep.2013.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Syn W.K., Agboola K.M., Swiderska M. NKT-associated hedgehog and osteopontin drive fibrogenesis in non-alcoholic fatty liver disease. Gut. 2012;61:1323–1329. doi: 10.1136/gutjnl-2011-301857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Locatelli I., Sutti S., Vacchiano M. NF-κB1 deficiency stimulates the progression of non-alcoholic steatohepatitis (NASH) in mice by promoting NKT-cell-mediated responses. Clin Sci (Lond) 2013;124:279–287. doi: 10.1042/CS20120289. [DOI] [PubMed] [Google Scholar]
- 75.Lemmers A., Moreno C., Gustot T. The interleukin-17 pathway is involved in human alcoholic liver disease. Hepatology. 2009;49:646–657. doi: 10.1002/hep.22680. [DOI] [PubMed] [Google Scholar]
- 76.Tang Y., Bian Z., Zhao L. Interleukin-17 exacerbates hepatic steatosis and inflammation in non-alcoholic fatty liver disease. Clin Exp Immunol. 2011;166:281–290. doi: 10.1111/j.1365-2249.2011.04471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gadd V.L., Skoien R., Powell E.E. The portal inflammatory infiltrate and ductular reaction in human nonalcoholic fatty liver disease. Hepatology. 2014;59:1393–1405. doi: 10.1002/hep.26937. [DOI] [PubMed] [Google Scholar]
- 78.Miyagi T., Takehara T., Uemura A. Absence of invariant natural killer T cells deteriorates liver inflammation and fibrosis in mice fed high-fat diet. J Gastroenterol. 2010;45:1247–1254. doi: 10.1007/s00535-010-0272-y. [DOI] [PubMed] [Google Scholar]
- 79.Miller A.M., Horiguchi N., Jeong W.I. Molecular mechanisms of alcoholic liver disease: innate immunity and cytokines. Alcohol Clin Exp Res. 2011;35:787–793. doi: 10.1111/j.1530-0277.2010.01399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Winau F., Hegasy G., Weiskirchen R. Ito cells are liver-resident antigen-presenting cells for activating T cell responses. Immunity. 2007;26:117–129. doi: 10.1016/j.immuni.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 81.Mossanen J.C., Tacke F. Role of lymphocytes in liver cancer. Oncoimmunology. 2013;2:e26468. doi: 10.4161/onci.26468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Joshi-Barve S., Barve S.S., Amancherla K. Palmitic acid induces production of proinflammatory cytokine interleukin-8 from hepatocytes. Hepatology. 2007;46:823–830. doi: 10.1002/hep.21752. [DOI] [PubMed] [Google Scholar]
- 83.Mohammad M.K., Avila D., Zhang J. Acrolein cytotoxicity in hepatocytes involves endoplasmic reticulum stress, mitochondrial dysfunction and oxidative stress. Toxicol Appl Pharmacol. 2012;265:73–82. doi: 10.1016/j.taap.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Facchinetti F., Amadei F., Geppetti P. Alpha,beta-unsaturated aldehydes in cigarette smoke release inflammatory mediators from human macrophages. Am J Respir Cell Mol Biol. 2007;37:617–623. doi: 10.1165/rcmb.2007-0130OC. [DOI] [PubMed] [Google Scholar]
- 85.Chen G.Y., Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Beier J.I., McClain C.J. Mechanisms and cell signaling in alcoholic liver disease. Biol Chem. 2010;391:1249–1264. doi: 10.1515/BC.2010.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Meagher E., Barry O., Burke A. Alcohol-induced generation of lipid peroxidation products in humans. J Clin Invest. 1999;104:805–813. doi: 10.1172/JCI5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lin T.H., Huang Y.L., Huang S.F. Lipid peroxidation in liver of rats administrated with methyl mercuric chloride. Biol Trace Elem Res. 1996;54:33–41. doi: 10.1007/BF02785318. [DOI] [PubMed] [Google Scholar]
- 89.Patrick L. Toxic metals and antioxidants: Part II. The role of antioxidants in arsenic and cadmium toxicity. Altern Med Rev. 2003;8:106–128. [PubMed] [Google Scholar]
- 90.Santra A., Maiti A., Das S. Hepatic damage caused by chronic arsenic toxicity in experimental animals. J Toxicol Clin Toxicol. 2000;38:395–405. doi: 10.1081/clt-100100949. [DOI] [PubMed] [Google Scholar]
- 91.Recknagel R.O., Glende E.A., Jr., Dolak J.A. Mechanisms of carbon tetrachloride toxicity. Pharmacol Ther. 1989;43:139–154. doi: 10.1016/0163-7258(89)90050-8. [DOI] [PubMed] [Google Scholar]
- 92.Sanyal A.J., Chalasani N., Kowdley K.V. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ji C. New insights into the pathogenesis of alcohol-induced ER stress and liver diseases. Int J Hepatol. 2014;2014:513787. doi: 10.1155/2014/513787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang X.Q., Xu C.F., Yu C.H. Role of endoplasmic reticulum stress in the pathogenesis of nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:1768–1776. doi: 10.3748/wjg.v20.i7.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Esfandiari F., Villanueva J.A., Wong D.H. Chronic ethanol feeding and folate deficiency activate hepatic endoplasmic reticulum stress pathway in micropigs. Am J Physiol Gastrointest Liver Physiol. 2005;289:G54–G63. doi: 10.1152/ajpgi.00542.2004. [DOI] [PubMed] [Google Scholar]
- 96.Galligan J.J., Smathers R.L., Shearn C.T. Oxidative stress and the ER stress response in a murine model for early-stage alcoholic liver disease. J Toxicol. 2012;2012:207594. doi: 10.1155/2012/207594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tsedensodnom O., Vacaru A.M., Howarth D.L. Ethanol metabolism and oxidative stress are required for unfolded protein response activation and steatosis in zebrafish with alcoholic liver disease. Dis Model Mech. 2013;6:1213–1226. doi: 10.1242/dmm.012195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tsuchiya M., Ji C., Kosyk O. Interstrain differences in liver injury and one-carbon metabolism in alcohol-fed mice. Hepatology. 2012;56:130–139. doi: 10.1002/hep.25641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ramirez T., Tong M., Chen W.C. Chronic alcohol-induced hepatic insulin resistance and endoplasmic reticulum stress ameliorated by peroxisome-proliferator activated receptor-delta agonist treatment. J Gastroenterol Hepatol. 2013;28:179–187. doi: 10.1111/j.1440-1746.2012.07256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tong M., Longato L., Ramirez T. Therapeutic reversal of chronic alcohol-related steatohepatitis with the ceramide inhibitor myriocin. Int J Exp Pathol. 2014;95:49–63. doi: 10.1111/iep.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Boden G., Duan X., Homko C. Increase in endoplasmic reticulum stress-related proteins and genes in adipose tissue of obese, insulin-resistant individuals. Diabetes. 2008;57:2438–2444. doi: 10.2337/db08-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gregor M.F., Yang L., Fabbrini E. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes. 2009;58:693–700. doi: 10.2337/db08-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Puri P., Mirshahi F., Cheung O. Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology. 2008;134:568–576. doi: 10.1053/j.gastro.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 104.Sharma N.K., Das S.K., Mondal A.K. Endoplasmic reticulum stress markers are associated with obesity in nondiabetic subjects. J Clin Endocrinol Metab. 2008;93:4532–4541. doi: 10.1210/jc.2008-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kammoun H.L., Chabanon H., Hainault I. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest. 2009;119:1201–1215. doi: 10.1172/JCI37007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ota T., Gayet C., Ginsberg H.N. Inhibition of apolipoprotein B100 secretion by lipid-induced hepatic endoplasmic reticulum stress in rodents. J Clin Invest. 2008;118:316–332. doi: 10.1172/JCI32752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sun L.P., Seemann J., Goldstein J.L. Sterol-regulated transport of SREBPs from endoplasmic reticulum to Golgi: Insig renders sorting signal in Scap inaccessible to COPII proteins. Proc Natl Acad Sci USA. 2007;104:6519–6526. doi: 10.1073/pnas.0700907104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ji C., Kaplowitz N. Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology. 2003;124:1488–1499. doi: 10.1016/s0016-5085(03)00276-2. [DOI] [PubMed] [Google Scholar]
- 109.Ronis M.J., Korourian S., Blackburn M.L. The role of ethanol metabolism in development of alcoholic steatohepatitis in the rat. Alcohol. 2010;44:157–169. doi: 10.1016/j.alcohol.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mederacke I., Hsu C.C., Troeger J.S. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun. 2013;4:2823. doi: 10.1038/ncomms3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dimova E.Y., Kietzmann T. Metabolic, hormonal and environmental regulation of plasminogen activator inhibitor-1 (PAI-1) expression: lessons from the liver. Thromb Haemost. 2008;100:992–1006. [PubMed] [Google Scholar]
- 112.Alessi M.C., Bastelica D., Mavri A. Plasma PAI-1 levels are more strongly related to liver steatosis than to adipose tissue accumulation. Arterioscler Thromb Vasc Biol. 2003;23:1262–1268. doi: 10.1161/01.ATV.0000077401.36885.BB. [DOI] [PubMed] [Google Scholar]
- 113.Verrijken A., Francque S., Mertens I. Prothrombotic factors in histologically proven nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology. 2014;59:121–129. doi: 10.1002/hep.26510. [DOI] [PubMed] [Google Scholar]
- 114.Kleiner D.E., Chalasani N.P., Lee W.M. Hepatic histological findings in suspected drug-induced liver injury: systematic evaluation and clinical associations. Hepatology. 2014;59:661–670. doi: 10.1002/hep.26709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pierre S., Chevallier A., Teixeira-Clerc F. Aryl hydrocarbon receptor-dependent induction of liver fibrosis by dioxin. Toxicol Sci. 2014;137:114–124. doi: 10.1093/toxsci/kft236. [DOI] [PubMed] [Google Scholar]
- 116.Romeo S., Kozlitina J., Xing C. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li Y.Y. Genetic and epigenetic variants influencing the development of nonalcoholic fatty liver disease. World J Gastroenterol. 2012;18:6546–6551. doi: 10.3748/wjg.v18.i45.6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhou Y.J., Li Y.Y., Nie Y.Q. Influence of polygenetic polymorphisms on the susceptibility to non-alcoholic fatty liver disease of Chinese people. J Gastroenterol Hepatol. 2010;25:772–777. doi: 10.1111/j.1440-1746.2009.06144.x. [DOI] [PubMed] [Google Scholar]
- 119.Stickel F., Osterreicher C.H. The role of genetic polymorphisms in alcoholic liver disease. Alcohol Alcohol. 2006;41:209–224. doi: 10.1093/alcalc/agl011. [DOI] [PubMed] [Google Scholar]
- 120.Dolganiuc A., Petrasek J., Kodys K. MicroRNA expression profile in Lieber-DeCarli diet-induced alcoholic and methionine choline deficient diet-induced nonalcoholic steatohepatitis models in mice. Alcohol Clin Exp Res. 2009;33:1704–1710. doi: 10.1111/j.1530-0277.2009.01007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yu F.J., Zheng J.J., Dong P.H. Long non-coding RNAs and hepatocellular carcinoma. Mol Clin Oncol. 2015;3:13–17. doi: 10.3892/mco.2014.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Buendia M.A., Neuveut C. Hepatocellular carcinoma. Cold Spring Harb Perspect Med. 2015;5:a021444. doi: 10.1101/cshperspect.a021444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kirpich I., Ghare S., Zhang J. Binge alcohol-induced microvesicular liver steatosis and injury are associated with down-regulation of hepatic Hdac 1, 7, 9, 10, 11 and up-regulation of Hdac 3. Alcohol Clin Exp Res. 2012;36:1578–1586. doi: 10.1111/j.1530-0277.2012.01751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Al-Eryani L., Wahlang B., Falkner K.C. Identification of environmental chemicals associated with the development of toxicant-associated fatty liver disease in rodents. Toxicol Pathol. 2015;43:482–497. doi: 10.1177/0192623314549960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bustaffa E., Stoccoro A., Bianchi F., Migliore L. Genotoxic and epigenetic mechanisms in arsenic carcinogenicity. Arch Toxicol. 2014;88:1043–1067. doi: 10.1007/s00204-014-1233-7. [DOI] [PubMed] [Google Scholar]
- 126.Suzuki T., Nohara K. Long-term arsenic exposure induces histone H3 Lys9 dimethylation without altering DNA methylation in the promoter region of p16(INK4a) and down-regulates its expression in the liver of mice. J Appl Toxicol. 2013;33:951–958. doi: 10.1002/jat.2765. [DOI] [PubMed] [Google Scholar]
- 127.Luedde T., Kaplowitz N., Schwabe R.F. Cell death and cell death responses in liver disease: mechanisms and clinical relevance. Gastroenterology. 2014;147:765–783.e4. doi: 10.1053/j.gastro.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Falkner K.C., Hill B.G., Sansbury B. Mitochondrial toxicity of chloroacetaldehyde in HepG2 cells. Hepatology. 2010;52(Suppl 1):121A. [Google Scholar]
- 129.Ramachandran A., McGill M.R., Xie Y. Receptor interacting protein kinase 3 is a critical early mediator of acetaminophen-induced hepatocyte necrosis in mice. Hepatology. 2013;58:2099–2108. doi: 10.1002/hep.26547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Masubuchi Y., Suda C., Horie T. Involvement of mitochondrial permeability transition in acetaminophen-induced liver injury in mice. J Hepatol. 2005;42:110–116. doi: 10.1016/j.jhep.2004.09.015. [DOI] [PubMed] [Google Scholar]