Abstract
Background & Aims
Dietary factors are likely an important determinant of gallstone development, and difficulty in adapting to lithogenic diets may predispose individuals to gallstone formation. Identification of the critical early diet-dependent metabolic markers of adaptability is urgently needed to prevent gallstone development. We focus on the interaction between diet and genes, and the resulting potential to influence gallstone risk by dietary modification.
Methods
Expression levels of hepatic protein kinase C (PKC) isoforms were determined in lithogenic diet-fed mice, and the relationship of hepatic cholesterol content and PKCβ expression and the effect of hepatic PKCβ overexpression on intracellular signaling pathways were analyzed.
Results
Lithogenic diet feeding resulted in a striking induction of hepatic PKCβ and PKCδ mRNA and protein levels, which preceded the appearance of biliary cholesterol crystals. Unlike PKCβ deficiency, global PKCδ deficiency did not influence lithogenic diet-induced gallstone formation. Interestingly, a deficiency of apolipoprotein E abrogated the diet-induced hepatic PKCβ expression, whereas a deficiency of liver X receptor-α further potentiated the induction, suggesting a potential link between the degree of hepatic PKCβ induction and the intracellular cholesterol content. Furthermore, our results suggest that PKCβ is a physiologic repressor of ileum basal fibroblast growth factor 15 (FGF15) expression and activity of hepatic proto-oncogene serine/threonine-protein kinase Raf-1/mitogen-activated protein (MAP) kinase kinase/extracellular signal-regulated kinases 1/2 (Raf-1/MEK/ERK1/2) cascade proteins, and the complex interactions between these pathways may determine the degree of hepatic ERK1/2 activation, a potent suppressor of cholesterol 7α-hydroxylase and sterol 12α-hydroxylase expression. We found that PKCβ regulated Raf-1 activity by modulating the inhibitory Raf-1Ser259 phosphorylation.
Conclusions
Our results demonstrate a novel interaction between the hepatic PKCβ/Raf-1 regulatory axis and ileum PKCβ/FGF15/ERK axis, which could modulate the bile lithogenecity of dietary lipids. The data presented are consistent with a two-pronged mechanism by which intestine and liver PKCβ signaling converges on the liver ERK1/2 pathway to control the hepatic adaptive response to a lithogenic diet. Elucidating the impact and the underlying mechanism(s) of PKCβ action could help us understand how different types of dietary fat modify the risk of gallstone formation, information that could help to identify novel targets for therapeutic approaches to combat this disease.
Keywords: Hepatic Cholesterol Metabolism, Lithogenic Diet, Signal Transduction, Protein Kinase Cβ
Abbreviations used in this paper: Akt, protein kinase B; ApoE, apolipoprotein E; Cyp7a1, cholesterol 7α-hydroxylase; Cyp8b1, sterol 12α-hydroxylase; ERK1/2, extracellular signal regulated kinase-1/2; FGF15, fibroblast growth factor 15; FXR, farnesoid X receptor; GSK-3, glycogen synthase kinase-3; JNK, c-Jun N-terminal kinase; LDL, low-density lipoprotein; LXR, liver X receptor; MEK, mitogen-activated protein (MAP) kinase kinase; MMLD, modified milk fat lithogenic diet; PKCβ, protein kinase C isoform β; Raf-1, Raf-1 hepatic proto-oncogene serine/threonine-protein kinase; SREBP, sterol response element-binding protein; WT, wild type
Summary.
Examining the basic mechanisms by which lithogenic diet-induced hepatic protein kinase Cβ (PKCβ) expression initiates signal transduction pathways to control cholesterol metabolism reveals novel ileum and liver PKCβ regulatory axes critical for fine-tuning hepatic cholesterol content and bile lithogenicity.
Cholesterol gallstone disease is characterized by the perturbations of the physical-chemical balance of cholesterol solubility in bile, resulting in a nonphysiologic cholesterol saturation level.1 Hypersecretion of nonesterified cholesterol into bile appears to represent the key molecular mechanism of gallstone formation in susceptible mouse models and in humans. The source of cholesterol destined for biliary secretion has not been identified, but a major source is thought to be the preexisting hepatic cholesterol pool and dietary cholesterol.
Whole body cholesterol homeostasis reflects a balance between endogenous cholesterol synthesis, dietary uptake, reverse cholesterol transport, and biliary excretion. The liver plays a critical role in nutrient sensing, the regulation of cholesterol synthesis, the conversion of cholesterol into bile acids, the secretion of cholesterol into bile either directly or as bile salts, the uptake and hydrolysis/metabolism of plasma cholesterol in the form of high-density lipoprotein or low-density lipoprotein (LDL) particles, the esterification of excess free cholesterol, and the secretion of cholesterol into the plasma. Liver cholesterol metabolism relies on an intricate network of cellular regulatory processes and dysregulation can lead to several life-threatening pathologies, such as hypercholesterolemia, cardiovascular disease, and gallstone disease.2 The rate-limiting enzyme for the major pathway of hepatic bile acid synthesis is cholesterol 7α-hydroxylase (Cyp7a1).
The regulation of Cyp7a1 in mice occurs via the nuclear receptors farnesoid X receptor (FXR) and liver X receptor (LXR). FXR responds to endogenous bile acid ligands to induce feedback loops that both repress bile acid synthesis and enhance the hepatic secretion of bile acids into bile. FXR may suppress bile acid synthesis via the up-regulation of a nuclear receptor small heterodimer partner, although fibroblast growth factor (FGF) 15/19 may also play an important regulatory role.3 Oxysterols are the endogenous ligands for LXR, and LXR stimulation in mice increases expression of Cyp7a1. This increases the rate of bile acid synthesis and thus increases the systemic elimination of cholesterol.4 Several previous studies have indicated a role for protein kinase Cs (PKCs) in modulating the functions of multiple nuclear receptors in vitro, including FXR, LXRs, vitamin D receptor, and FGF signaling pathway proteins.5, 6, 7, 8, 9, 10 However, few studies have determined whether PKCs can regulate bile lithogenicity by inducing metabolic adaptations in the liver and intestine. To date, there is no information regarding the direct regulation and activation of PKC isoforms in the liver by a lithogenic diet. Specifically, which particular PKC isoforms are altered in the livers of lithogenic diet-fed animals and their influence on gallstone formation remain unknown.
Our recent study suggests that PKCβ might be a pivotal protein regulating cholesterol homeostasis.11 Interestingly, deficiency of PKCβ sensitized mice to the development of cholesterol gallstone disease and is associated with reduced expression of Cyp7a1 and Cyp8b1, leading to increased biliary cholesterol saturation and hydrophobicity indices. These results suggest that PKCβ could be a target for the study of the pathogenesis of cholesterol gallstones in response to nutritional changes. However, the exact role and signaling pathway(s) by which PKCβ modulates Cyp7a1 and Cyp8b1 gene expression remain unknown. To determine how PKCβ deficiency triggers the development of gallstone in response to lithogenic diet intake, we determined lithogenic diet-PKCβ interaction and the impact of PKCβ signaling on pathways modulating hepatic cholesterol homeostasis. Our results identified a diet-sensitive regulatory axis involving PKCβ, FGF15, and Raf-1 hepatic proto-oncogene serine/threonine-protein kinase (Raf-1) in which the loss of PKCβ altered FGF15 action by modulating its expression, and activity of hepatic Raf-1/mitogen-activated protein (MAP) kinase kinase/extracellular signal regulated kinase-1/2 (Raf-1/MEK/ERK) cascade. This resulted in fine-tuning of the hepatic cholesterol content and bile lithogenicity. Thus, hepatic PKCβ induction and induced changes in signaling and gene transcription may constitute a possible mechanism for the chronic adaptations to lithogenic diets. This study suggests that the modulation of PKCβ activity in the ileum and/or liver may be an effective approach to reduce the risk of gallstone formation.
Materials and Methods
Animals and Diets
PKCβ−/− mice on a C57BL6 background were described previously elsewhere.11 Generation of PKCδ−/− mice on a C57/129 Sv background was described by Miyamoto et al.12 All mice were housed in a temperature-controlled room (22°C) with a 12-hour light/dark cycle, and were maintained on a standard rodent diet (7912 rodent chow; Harland Teklad, Madison, WI). Eight-week-old wild-type (WT) and PKCβ−/− mice were fed a modified-milk fat lithogenic diet (MMLD; Teklad TD-10014; Harland Teklad) containing (by weight) 15% anhydrous milk fat, 1% cholesterol, and 0.5% cholate for the indicated periods. The PKCδ−/− and WT littermates were fed MMLD for 2 or 5 weeks. Mice were fed ad libitum with free access to water. Unless indicated, all experiments were performed on nonfasted male mice, and the mice were euthanized in the morning. All procedures and experiments on mice were approved by institutional animal care and use committee of the Ohio State University.
Experimental Protocol and Tissues and Bile Harvest
For gallbladder bile collection, mice were fasted for 4 hours before euthanasia. The gallbladder was ligated, and the bile was collected through a fundus incision and gently squeezed with forceps. The bile collected was then spread on glass slides and examined immediately under polarizing light microscopy for the presence of cholesterol crystals. The bile flow was determined as previously described elsewhere.11 For tissues collection, the liver, gallbladder, and small intestine excised from each mouse were rinsed briefly with cold phosphate-buffered saline, then aliquoted and snap-frozen in liquid nitrogen.
Assays
Mice were sacrificed at 09:00. Liver tissues (weighing ∼100 mg) were harvested and homogenized in chloroform/methanol (2:1 v/v) using a Polytron tissue grinder (Glen Mills, Clifton, NJ). The extracts were dried under nitrogen flow and resuspended in isopropanol. For the in vitro model of cellular steatosis, mouse primary hepatocyte cells were exposed to palmitate at a concentration of 0.5 mM. Triglyceride concentrations were measured using commercial kits (Sigma-Aldrich, St. Louis, MO; Biovision, Milpitas, CA) as described elsewhere.11 Hepatic lipids were extracted according to the Folch method and separated with silica gel thin-layer chromatography plates as described previously elsewhere.13, 14
Western Blot Analysis
Western blot analysis was performed as previously described elsewhere.15 The blots were incubated overnight at 4°C with following primary antibodies: PKCβ, total ERK1/2, and p38MAPK (Santa Cruz Biotechnology, Dallas, TX); β-actin (Sigma-Aldrich, St. Louis, MO); PKCα, PKCδ, PKCε, phospho-ERK1/2, phospho-Raf-1-Ser259, phospho-protein kinase B (Akt)-Ser308, phospho-Akt-Ser473, phospho-glycogen synthase kinase-α/β (pGSKα/β)-Ser21/9, phospho-c-Jun N-terminal kinase (JNK)-Thr183/Tyr185, phospho-p38MAPK-Thr180/Tyr182, and total Akt (Cell Signaling Biotechnology, Framington, MA); and glycogen synthase (Abcam, MA). The band intensity was quantified using a Bio-Rad GS-800 calibrated densitometer with Quantify One analysis software (Bio-Rad Laboratories, Hercules, CA).
Cell Culture
The H2.35 cell line (purchased from the American Type Culture Collection, Manassas, VA) was routinely grown in Dulbecco’s modified Eagle’s medium with 1 g/L glucose, 200 nM dexamethasone, and 4% fetal bovine serum. To test the effect of sterols on PKCβ expression, the cells were switched to medium containing 5% lipoprotein-deficient serum in the presence of sterols (2 μg/mL 25-hydroxycholesterol plus 10 μg/mL cholesterol) or 30 mM lovastatin. After incubation for 16–20 hours, cell lysates were probed by Western blotting for PKCβ and β-actin.
Isolation and Culture of Mouse Hepatocytes
Mouse hepatocytes were isolated from 12 week-old C57BL/6J male mice from the Jackson Laboratory (Bar Harbor, ME) as described previously for rat hepatocytes.16
Infection of Primary Mouse Hepatocytes With Adenovirus Overexpressing Green Fluorescent Protein or Adenovirus Overexpressing Protein Kinase C Isoform β
Primary mouse hepatocytes were seeded also at a density of 0.3 × 106 per well in a six-well plate with Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum and penicillin/streptomycin. The recombinant adenovirus overexpressing green fluorescent protein and adenovirus overexpressing protein kinase C isoform β were used to overexpress PKCβ, as described previously elsewhere.17 After 36 hours of infection with 1 to 100 multiplicity of infection, cells were harvested with ice-cold phosphate-buffered saline.
After spinning, the cell pellets were resuspended in cell lysis buffer (20 mM Tris buffer, 2 mM ethylenediaminetetraacetic acid [EDTA], 2mM [ethylenebis(oxyethylenenitrilo)]tetraacetic acid [EGTA], 0.1% sodium dodecyl sulfate, proteinase, and phosphatase inhibitors) for 30 minutes on ice, and sonicated briefly. The supernatant was collected after centrifugation. The protein assay was performed before loading for sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
Statistical Analysis
The results are shown as mean ± standard deviation (SD). All statistical analysis was performed by Student’s t test or analysis of variance (ANOVA) in Excel (Microsoft, Redmond, WA). P < .05 was considered statistically significant.
Results
Lithogenic Diet Specifically Induces Expression of Hepatic Protein Kinase C β and δ Isoforms However Unlike PKCβ, PKCδ Deficiency Does Not Promote Gallstone Formation
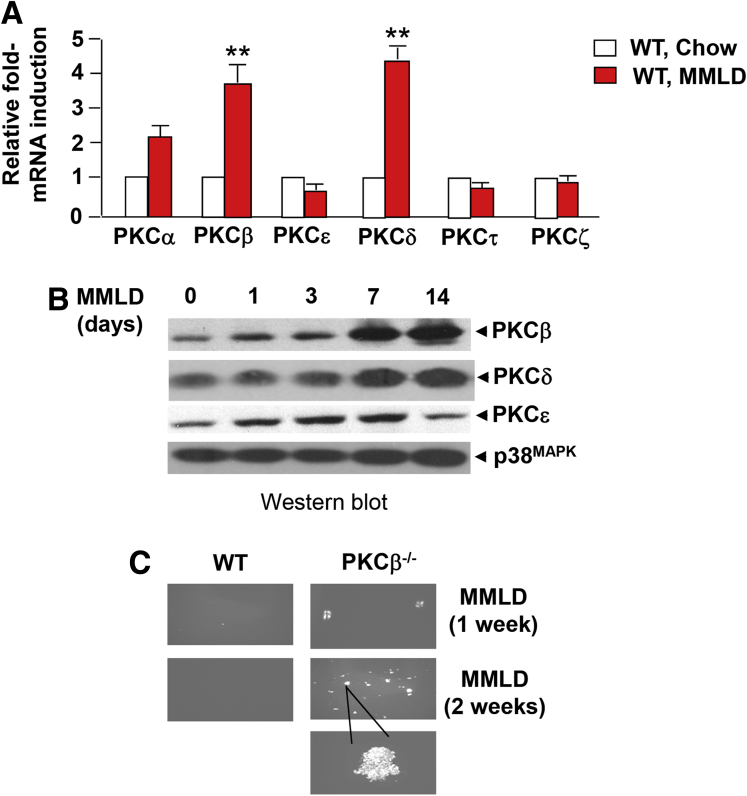
It is well established that the liver plays a critical role in the regulation of cholesterol and bile acid homeostasis, and is targeted by all three constituents of the lithogenic diet. We initially assessed whether metabolic adaptation to the lithogenic diet intake was associated with changes in hepatic expression of PKCβ and other PKC isoforms. Although the mRNA level of PKCβ and PKCδ were quite low in the liver of control mice, they were markedly increased after lithogenic diet intake for 2 weeks. PKCα also showed a slight increase, whereas expression of other PKC isoforms studied remained unaffected (Figure 1A).
Figure 1.
Relative expression of selective protein kinase C (PKC) isoforms in the livers of male C57/BL6 WT mice (n = 6–8 mice per group) fed either a chow or lithogenic diet (MMLD) for the indicated periods. (A) Comparison of mRNA expression levels for the indicated PKC isoforms in the livers of mice fed MMLDL for 2 weeks. The data are expressed relative to corresponding level in chow fed livers. Results are expressed as mean ± standard deviation. **P < .01. (B) Protein levels of indicated PKC isoforms and β-actin were determined using immunoblotting of pooled liver extracts. Equal amounts of protein were loaded per well. (C) Polarizing light microscopy examination of cholesterol crystals in pooled gallbladder bile with the indicated genotype on feeding a lithogenic diet for 1 or 2 weeks.
Likewise, PKCβ and PKCδ proteins levels showed strong increases (>eightfold) after lithogenic diet exposure in a time-dependent manner. A slight induction of the PKCβ isoform was observed at 1 to 3 days, and significant induction at week 1 with a peak at 2 weeks (the last time point measured). The peak induction for PKCδ was observed at 1 week and remained elevated at week 2. A slight induction of the PKCε protein was observed at day 1, reached a peak at 1 week, and returned to normal by 2 weeks (Figure 1B). In agreement with this study, both PKCβ and PKCδ proteins showed greater abundance in the membrane-fractions of lithogenic diet-fed livers (results not shown).
Because lithogenic diet increased levels of both PKCβ and PKCδ, we hypothesized that, like PKCβ deficiency, PKCδ deficiency might influence lithogenic diet-induced gallstone formation. To determine the physiologic relevance of induction in the development of gallstone formation, we compared lithogenic diet-induced gallstone formation using mice deficient in individual isoform.
Interestingly, unlike PKCβ deficiency, PKCδ deficiency did not influence lithogenic diet-induced gallstone formation (Table 1). Gallstones were found in 5/13 PKCβ−/− mice compared with 0/14 wild-type (WT) mice after 2 weeks of lithogenic diet exposure. In contrast, no major difference was observed between WT and PKCδ−/− mice even after 5 weeks of lithogenic diet feeding, whereas, as expected from our previous study,11 all gallbladders from PKCβ−/− mice fed this diet were engorged with gallstones to varying degrees. These results suggest that gallstone susceptibility was specifically increased by the PKCβ deficiency and not affected at all by the PKCδ deficiency.
Table 1.
Comparison of Gallstone Formation
| Genotype | MMLD (wk) | Total mice | Mice with stones | Incidence (%) |
|---|---|---|---|---|
| WT, C57BL6 | 2 | 14 | 0 | 0 |
| PKCβ−/−, C57BL6 | 2 | 13 | 5 | 38 |
| WT, C57BL6 | 5 | 14 | 3 | 21 |
| PKCβ−/−, C57BL6 | 5 | 13 | 13 | 100 |
| WT, C57/129Sv | 2 | 14 | 0 | 0 |
| PKCδ−/−, C57/129Sv | 2 | 15 | 0 | 0 |
| WT, C57/129Sv | 5 | 11 | 2 | 18 |
| PKCβ−/−, C57/129Sv | 5 | 15 | 3 | 20 |
MMLD, modified milk fat lithogenic diet; PKC, protein kinase C; WT, wild type.
We next compared hepatic PKCβ induction relative to the appearance of biliary cholesterol crystals. Establishing the temporal relationship between these variables will not only allow us to more accurately define the potential regulators of gallstone formation in a physiologic setting, but more importantly will help to determine whether alteration in PKCβ expression is an early event in the development of gallstone formation.
WT and PKCβ−/− mice fed a lithogenic diet for 0, 1, and 2 weeks were analyzed for hepatic lipid contents, and also by microscopic examination of the biliary cholesterol crystals. As expected from our previous study,11 PKCβ−/− mice showed an increased bile volume compared with WT mice (results not shown). PKCβ−/− mice also demonstrated markedly larger and more numerous aggregated cholesterol monohydrate crystals in the gallbladder bile at 1 or 2 weeks of lithogenic feeding (Figure 1C). Under higher magnification, the crystals in PKCβ−/− mice after 2 weeks of lithogenic diet appeared to be aggregated, solid cholesterol monohydrate crystals.
Apolipoprotein E Deficiency Attenuates and Liver X Receptor-α Deficiency Potentiates Lithogenic Diet-Induced Hepatic Protein Kinase C Isoform β Expression
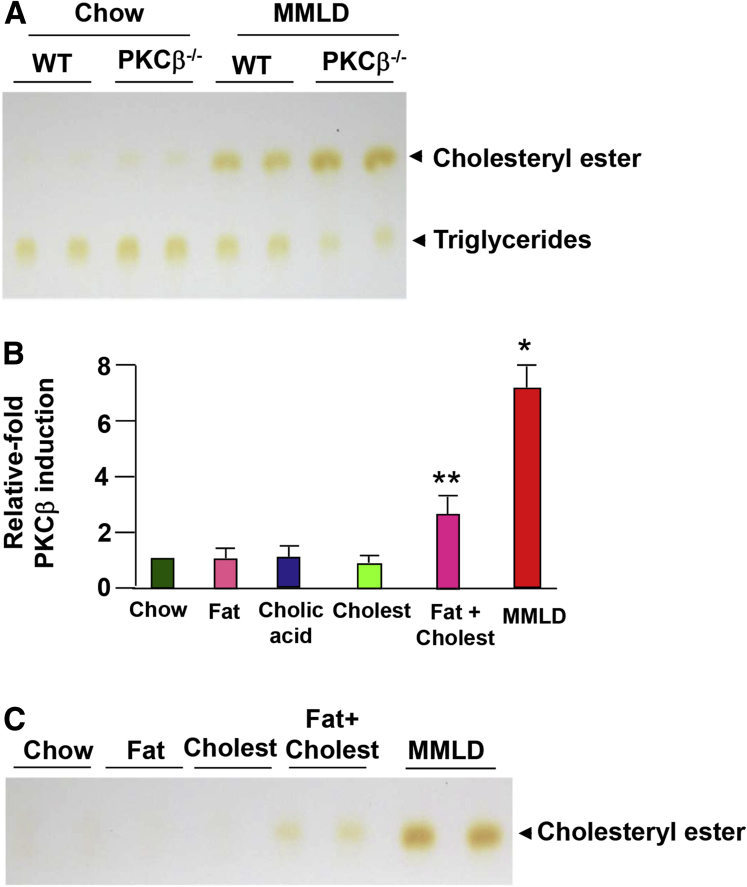
Feeding a lithogenic diet to WT and PKCβ−/− mice resulted in marked differences in hepatic triglyceride and cholesterol contents. As expected, the PKCβ−/− mice exhibited significantly lower hepatic triglyceride content, but the hepatic total cholesterol content was significantly higher (Figure 2A).
Figure 2.
Potential relationship between diet-induced hepatic protein kinase C isoform β (PKCβ) and increased hepatic cholesterol levels. (A) Thin-layer chromatography of total lipid extracts from livers of WT and PKCβ−/− mice (n = 2) after a lithogenic diet feeding for 0 or 2 weeks. (B) Effect of feeding individual components of a lithogenic diet, diet containing high fat and cholesterol, or a lithogenic diet containing all three components for 2 weeks on hepatic PKCβ protein levels (n = 3). (C) Thin-layer chromatography of total lipid extracts from livers of two of the mice fed an indicated diet. The above results are representative of at least three separate experiments. Each value represents the mean ± standard deviation. *P < .05; **P < .01.
To investigate the effect of individual components of the lithogenic diet on hepatic PKCβ induction, WT mice were fed a chow or one of the three modified chows in which cholesterol, cholate, or fat was in excess. As shown in Figure 2B, unlike individual components alone, a diet containing both high fat and cholesterol slightly increased the hepatic PKCβ expression, whereas maximal induction was seen with the MMLD diet containing fat, cholesterol, and cholic acid. The induction was also accompanied by an increase in hepatic cholesterol content (see Figure 2C).
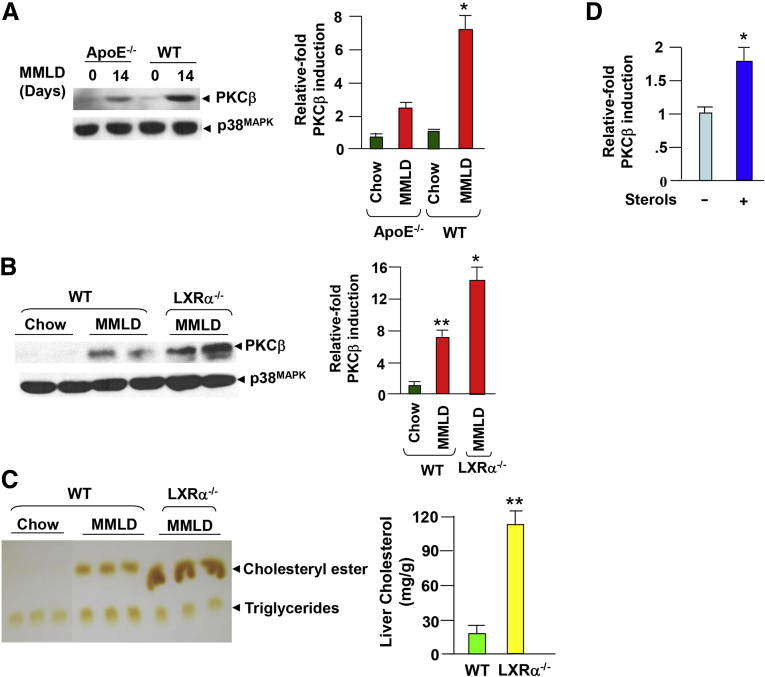
To determine further the role of hepatic cholesterol uptake on the induction process, we examined lithogenic diet-induced hepatic PKCβ expression in ApoE−/− mice. Apolipoprotein E (ApoE) is a major apolipoprotein constituent of chylomicron remnant particles and plays a critical role in mediating the uptake of dietary cholesterol by the liver. As a result, feeding a lithogenic diet to ApoE−/− mice was not expected to dramatically elevate the hepatic cholesterol content, presumably as a result of the impaired delivery of dietary cholesterol to the liver.18 As shown in Figure 3A, lithogenic diet-induced hepatic PKCβ expression was greatly diminished in ApoE−/− mice compared with WT controls, further supporting a possible role of dietary cholesterol in the induction process.
Figure 3.
Effects of apolipoprotein E (ApoE) or liver X receptor-α (LXRα) deficiency on lithogenic diet-induced hepatic protein kinase C isoform β (PKCβ) expression. (A) ApoE−/− deficiency attenuates diet-induced hepatic PKCβ. Livers from ApoE−/− mice (n = 2) fed a lithogenic diet for 2 weeks were examined for PKCβ protein levels by immunoblotting. (B, C) LXRα deficiency potentiates diet-induced hepatic PKCβ expression; the induction is accompanied by an increase in hepatic cholesterol content. Livers from wild-type and LXR−/− mice (n = 2 mice per diet group) fed modified milk fat lithogenic diet (MMLD) for 2 weeks were used to examine hepatic PKCβ protein levels and the lipid contents. (D) Effect of sterols (10 μg/mL cholesterol plus 2 μg/mL 25-hydroxycholesterol, 24 hours) treatment on endogenous PKCβ expression in mouse liver cell line H2.35 cultured in medium supplemented with lipoprotein-deficient serum. Equal amounts of cell extracts were probed for PKCβ expression by immunoblotting. This experiment was repeated twice with a similar outcome. *P < .05; **P < .001.
Several earlier reports have highlighted the role of LXRs in cholesterol efflux in animal cells.19, 20 We next investigated whether lithogenic diet feeding affected the hepatic PKCβ expression in mice lacking LXRα. As shown in Figure 3B, there was a dramatic increase in the induction of PKCβ expression in the livers of these mice compared with those fed a chow diet. As expected, the LXRα−/− mice fed a lithogenic diet showed an excess accumulation of cholesterol in the livers compared with WT mice (see Figure 3C). The parallels between the increase in hepatic cholesterol content and PKCβ induction prompted us to analyze the effects of sterols on endogenous PKCβ expression in cultured liver cell line. Sterols treatment alone for 24 hours slightly increased PKCβ expression (see Figure 3D), lending further support to the idea that an increase in hepatic cholesterol content may contribute, at least in part, to the induction process.
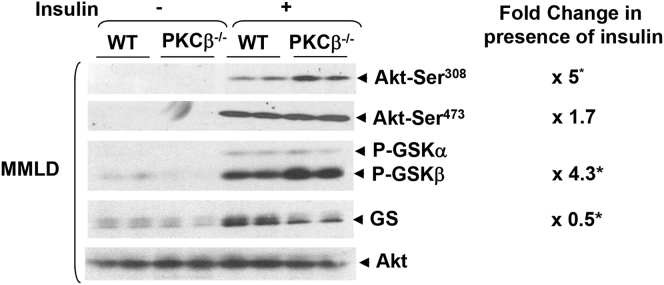
Lithogenic Diet-Fed PKCβ−/− Mice Show Greater Hepatic Insulin Sensitivity Than Wild-Type Mice
Hepatic insulin resistance was recently shown to play a part in gallstone formation through regulation of bile acid synthesis.21 We therefore investigated phosphorylation status of Akt/PKB and downstream GSKs as markers of insulin sensitivity in the livers of lithogenic diet-fed mice. Phosphorylation levels of both Akt-Ser308 (∼fivefold) and GSKs (∼fourfold) in vivo were higher in PKCβ−/− mice compared with WT mice (Figure 4), suggesting that PKCβ−/− deficiency increases hepatic insulin signaling even in mice fed a lithogenic diet. In view of previous demonstration that physiologic concentrations of insulin stimulate CYP7A1 mRNA expression,22 it is evident that alterations in insulin signaling may not account for greater suppression of Cyp7a1 in PKCβ−/− livers.
Figure 4.
Lithogenic diet-fed PKCβ−/−mice exhibit greater liver insulin-sensitivity than control mice. Wild-type (WT) and PKCβ−/− mice (n = 2) fed a lithogenic diet for 0 or 2 weeks were either untreated or injected with insulin (0.9 U/kg body weight), and the livers were analyzed for the insulin-signaling proteins. Western blots are representative of two separate experiments. Fold change shows the band intensity ratio of PKCβ−/− over WT. *P < .05, n = 4.
Protein Kinase C Isoform β Deficiency Stimulates Ileum Fibroblast Growth Factor 15 Expression and Activity
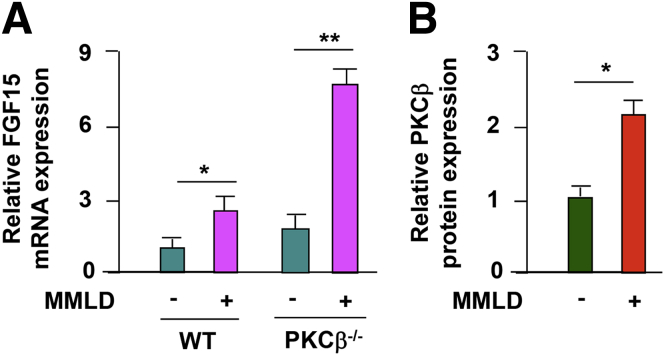
Because the expression levels of genes implicated in bile acid, cholesterol or phospholipids transporters were not significantly altered in the livers of lithogenic-fed PKCβ−/− mice,11 we speculated that changes in hepatic Cyp7a1 expression either were secondary to alterations in the bile acid pool size and composition or were caused by perturbation of signaling factor secreted from the intestine. Ileal FGF15 is the primary mediator of bile acid feedback inhibition of hepatic Cyp7a1 in vivo, so we examined the expression levels of ileum FGF15 between genotypes. Figure 5A shows that mice fed a lithogenic diet for 2 weeks demonstrated significant up-regulation of ileal FGF15 mRNA expression in PKCβ−/− mice compared with WT mice, suggesting that Cyp7a1 suppression may be related, at least in part, to enhanced ileal FGF15 expression by PKCβ deficiency. We also examined the effect of feeding a lithogenic diet on intestinal PKCβ expression and observed a slight elevation in PKCβ expression (Figure 5B).
Figure 5.
Effects of lithogenic diet feeding on ileum fibroblast growth factor 15 (FGF15) and protein kinase C isoform β (PKCβ) expression levels. (A) PKCβ deficiency significantly induces ileum FGF15 expression in response to lithogenic diet intake. FGF15 mRNA expression levels were analyzed in the ileum of WT and PKCβ−/− mice fed a chow or lithogenic diet for 2 weeks. (B) The ilea from the animals were also used to examine PKCβ expression levels by immunoblotting. Levels of FGF15 and PKCβ in WT mice fed a chow diet were assigned a value of 1. Results are expressed as the mean ± standard deviation. *P < .05, **P < .01 (n = 5).
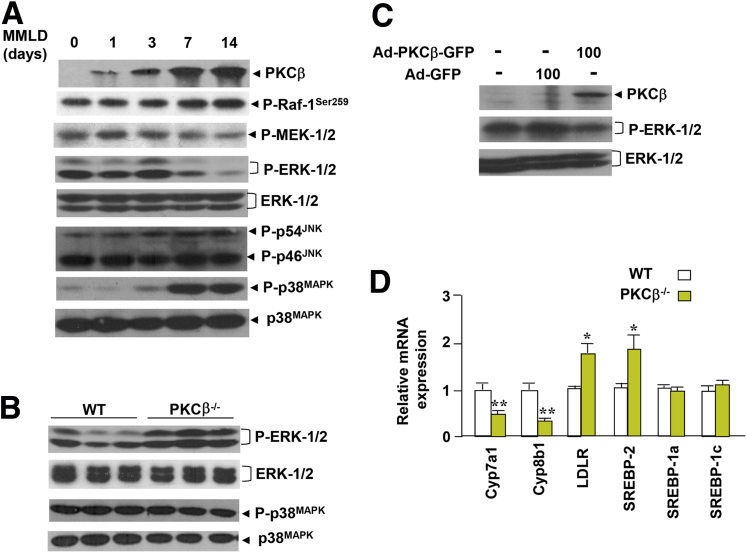
Recent studies have also established that ERK1/2 activation is associated with suppression of hepatic Cyp7a1 gene expression by FGF15.23, 24 Activation of ERK1/2 is catalyzed by the dual specificity kinase MEK1/2. To phosphorylate and activate ERK1/2, MEK1/2 must first itself be serine-phosphorylated by Raf-1. We therefore analyzed Raf-1 and MEK1/2 activations using the indicated phospho-specific antibodies. The results presented in Figure 6A shows an increase in inhibitory serine 259 phosphorylation for Raf-1 in the lithogenic diet-fed livers of WT mice. As expected, it was accompanied by a decrease in MEK1/2 and ERK1/2 activation, suggesting that PKCβ uses Raf-1 inhibition as the main interface for the negative regulation of downstream MEK and ERK1/2 phosphorylation. We also compared phospho-ERK1/2 levels in the livers of lithogenic diet-fed WT and PKCβ−/− mice. The PKCβ−/− livers showed increased ERK1/2 activation compared with WT (see Figure 6B), an observation consistent with our earlier report on feeding mice a lithogenic diet containing cocoa butter.11
Figure 6.
Protein kinase C isoform β (PKCβ) negatively regulates hepatic proto-oncogene serine/threonine-protein kinase Raf-1/mitogen-activated protein (MAP) kinase kinase/extracellular signal regulated kinase-1/2 (Raf-1/MEK/ERK1/2) signaling cascade in the liver. (A) Comparison of expression and phosphorylation levels of MAPKs in the pooled liver extracts of mice (n = 5) fed a lithogenic diet for the indicated periods. (B) Comparison of phosphorylation levels of ERK1/2 and p38MAPK in the pooled liver extracts of wild-type (WT) and PKCβ−/− mice (n = 5) fed a lithogenic diet for 2 weeks. (C) Overexpression of PKCβ reduces basal and FGF15-induced ERK1/2 phosphorylation in mouse hepatocytes. Primary mouse adipocytes were infected with either adenovirus overexpressing green fluorescent protein or adenovirus overexpressing protein kinase C isoform β at an multiplicity of infection of 0 and 100; after 24 hours the PKCβ and phospho-ERKs levels were measured by immunoblotting using anti-phospho-ERK1/2. The above Western blots are representative of two separate experiments. (D) Comparison of hepatic gene expression between lithogenic diet-fed WT and PKCβ−/− mice. Levels for each gene in WT mice were assigned a value of 1. Each value represents the mean ± standard deviation (n = 5 mice/group). *P < .05; **P < .01.
Interestingly, in contrast to ERK1/2, the lithogenic diet induced p38MAPK in a PKCβ-independent manner (see Figure 6A and B). As expected, overexpression of PKCβ protein in mouse primary hepatocytes also reduced ERK1/2 phosphorylation (see Figure 6C), suggesting that the negative influence of PKCβ on ERK1/2 signaling is also operative in the intact hepatocytes. Because ERK1/2 and various PKC isoforms have been implicated in regulating the expression and function of genes involved in various aspects of cholesterol and bile acid homeostasis, we also investigated the expression of these genes. As shown in Figure 6D, expression of both Cyp7a1 and Cyp8b1 genes were dramatically reduced, whereas expression of LDL receptor and sterol response element-binding protein 2 (SREBP-2) genes were significantly up-regulated.
Discussion
The lithogenic diet containing cholesterol, cholic acid, and fat has been used extensively to induce and study the pathology and genetics of gallstone formation in inbred mice.25 Using a well-established mouse model for lithogenesis, we provide the first evidence that lithogenic feeding specifically induces the expression of both PKCβ and PKCδ isoforms in the liver, and that PKCβ induction attenuates gallstone formation. PKCβ induction was dependent on the presence of all three components of the lithogenic diet, whereas slight or no increase in PKCβ expression was observed by feeding diets in which cholesterol, cholate, or fat was in excess. The fact that all three components were required to induce gallstone formation in mice suggests that the lithogenic diet induced distinct intracellular signaling pathway(s) when compared with the individual components of the lithogenic diet. These results, combined with our earlier results showing that PKCβ deficiency stimulates diet-induced gallstone formation,11 provide direct evidence that PKCβ induction represents an adaptive mechanism and plays a protective role in the metabolic adaptation to a lithogenic diet.
The mechanism of nutritional regulation of PKCβ expression/activation with a lithogenic diet remains unknown and requires further investigation. The induction of PKCβ could involve nuclear receptors and transcription factors targeted by the components of this diet, bile acid stimulation of diacylglycerol synthesis,26 or activation of phospholipase C and increased calcium mobilization,27 resulting in the transcriptional induction of PKCβ expression. A recent report that DNA methylation regulates PKCβ expression28 suggests that a lithogenic diet could also influence DNA methylation at the PKCβ promoter level.
The interaction between genes and the environment is an integral component of evolution, and includes cross-talk between a subset of genes and the diet, which results in adaptations to specific nutrients. It is well-established that dietary components can influence the expression of genes controlling metabolic processes via signal transduction pathways affecting transcriptional signal cascades and networks. Given the role of PKCβ in gallstone formation, it is evident that PKCβ may mediate the effects of a lithogenic diet via a variety of downstream signal-transducing pathways critical for bile lithogenicity and the subsequent development of gallstones. It is apparent that pathways involving PKCβ-dependent modifications of nuclear factors are unlikely targets to modify cytochrome P450 enzymes because phosphorylation of LXR and FXR by PKCs is known to suppress and activate transactivation, respectively.
In the present study, we therefore explored other signaling pathways downstream of PKCβ that suppressed the expression of cytochrome P450 isoforms in response to lithogenic diet exposure. Ileal FGF15 is the primary mediator of bile acid feedback inhibition of hepatic cytochrome P450 isoforms.3, 29 Our results suggest that PKCβ is a repressor of ileum FGF15 expression. We found that lithogenic diet-fed mice demonstrated the up-regulation of ileal FGF15 expression. However, the degree of up-regulation was greater in PKCβ−/− mice compared with WT mice, suggesting that cytochrome P450 isoform suppression may be related, at least in part, to enhanced ileal FGF15 expression induced by PKCβ deficiency. The potential mechanism of FGF15 induction may involve alterations in the vitamin D receptor, FXR, and/or RXRα activity, which are required for FGF15 expression.30 Phosphorylation of the vitamin D receptor specifically by PKCβ has been shown to suppress its function and thereby can modulate recruitment, function, and/or affinity of coactivator complex.9 PKC activation is also known to control nuclear localization of nuclear receptors and their stability.7, 10, 31
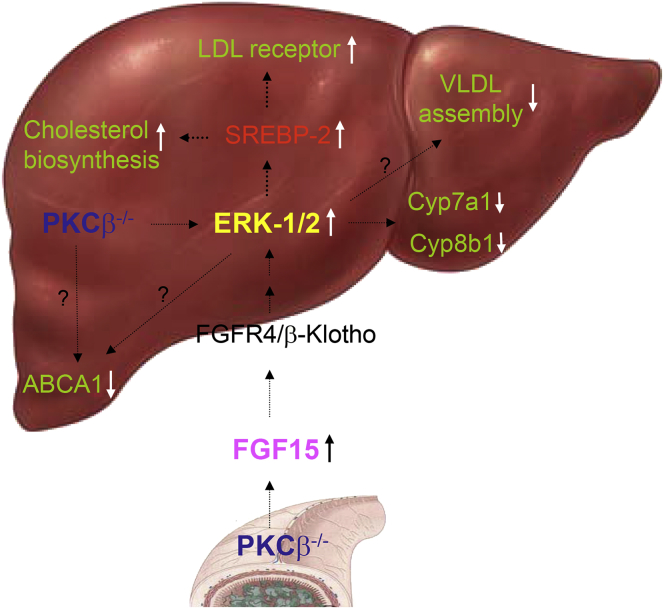
Our results suggest that PKCβ is a critical link in an adaptive response for proper handling of high dietary fat and cholesterol by modulating hepatic ERK1/2 activity to coordinately regulate the cellular cholesterol biosynthesis, uptake, and degradation. Our data are consistent with a two-pronged mechanism by which intestinal and liver PKCβ deficiency converges on liver ERK1/2 to modify the expression of genes involved in the cholesterol and bile acid homeostasis (Figure 7). One pathway may cause the stimulation of intestinal FGF15 expression, leading to an increase in ERK1/2 activity in the liver via FGFR4 and FGFR4 coreceptor β-Klotho, resulting in the suppression of Cyp7a1 and Cyp8b1 gene transcription and the reduction of cholesterol catabolism. The other pathway may rely on the negative regulation of the Raf-1/MEK/ERK signaling axis by PKCβ itself in the liver, resulting in further activation of ERK1/2 to reduce cholesterol catabolism in PKCβ−/− mice. Both mechanisms may cooperate to maximize the effectiveness of PKCβ deficiency on hepatic ERK1/2 activation.
Figure 7.
Proposed model for the mechanism by which protein kinase C isoform β (PKCβ) activation in the liver and ileum contributes to adaptive response for proper handling of a large supply of dietary fat, cholesterol, and bile acids to prevent hepatic overaccumulation of toxic cholesterol. Cholesterol homeostasis is achieved through regulation of cholesterol uptake, cholesterol biosynthesis, cholesterol conversion to bile acids, and excretion of bile acids. The data presented in this study are consistent with a two-pronged mechanism by which PKCβ deficiency contributes to dysfunctional cholesterol homeostasis through disturbing the fibroblast growth factor 15/extracellular signal regulated kinase (FGF15/ERK) and Raf-1 hepatic proto-oncogene serine/threonine-protein kinase/mitogen-activated protein (MAP) kinase kinase/extracellular signal regulated kinase-1/2 (Raf-1/MEK/ERK1/2) regulatory axes.
It is interesting to note from our previous work that ERK1/2 played an important role in controlling hepatic LDL receptor expression.32, 33 PKCβ deficiency is therefore predicted to induce hepatic LDL receptor expression to further promote cholesterol accumulation. In addition, ERK1/2 was reported to regulate the activity of SREBP-2 and consequently its expression due to self-regulation.34, 35 In agreement with this model, lithogenic diet-fed PKCβ deficient livers showed an increase in the expression of hepatic LDL receptors and SREBP-2 (Figure 6). Additionally, in agreement with the protective role of PKCβ activation, the modulation of either PKCα or ERK1/2 has been shown to influence cholesterol efflux.36, 37 Finally, inhibition of ERK1/2 also has been shown to promote very-low-density lipoprotein assembly in HepG2 cells,38 and as a result, PKCβ deficiency should inhibit very-low-density lipoprotein assembly. The net effect of PKCβ deficiency would be to promote hepatic cholesterol accumulation as was observed for PKCβ−/− mice. This model has the potential to explain how PKCβ directly or indirectly through ERK1/2 caused the differential expression of genes involved in the cholesterol uptake, biosynthesis, and catabolic reduction of the hepatic cholesterol burden. Establishing PKCβ as the prime kinase involved in fine-tuning the hepatic ERK1/2 activation in response to lithogenic diet feeding introduces a new model system that can be used to investigate the FGF15 regulatory mechanisms within a functional context. Negative in vivo regulation of the hepatic Raf/MEK/ERK cascade by PKCβ is unexpected due to numerous in vitro studies in cell culture models, suggesting that PKCβ is required for ERK1/2 activation.39, 40
The additional means by which ERK1/2 activation is positively influenced by PKCβ deficiency may be attributable to the differences in hepatic insulin sensitivity. Overexpression of PKCβ in the liver, similar to its overexpression in muscle, may result in hepatic insulin resistance,41 a condition known to affect ERK1/2 activation and the expression of both Cyp7a1 and Cyp8b1 genes. Insulin has been known to initiate signaling cascades through Akt and ERK1/2 signaling pathways and has been shown to act synergistically with FGF15 to activate these critical signaling pathways.42, 43 Considering the physiologically important roles of Cyp7a1 in regulating the pool size and of Cyp8b1 in controlling bile acid profiles, it is possible that these genes may have acquired more sophisticated expression control mechanisms to allow for a greater flexibility in the precise regulation of bile acid homeostasis in response to alterations in nutrition and growth conditions. Furthermore, extremely high PKCβ levels in the livers of lithogenic diet-fed animals could help to control insulin and FGF15 signaling pathways. Further studies are needed to determine how insulin- and FGF15-specific phosphorylation of ERK1/2 is coordinated with PKCβ-dependent signaling events to respond appropriately to environmental conditions.
In conclusion, we have demonstrated that PKCβ activation and signaling represent a critical link between lithogenic diet intake and an adaptive response for proper handling of high dietary fat, cholesterol, and bile acid by the modulation of the hepatic ERK1/2 activity. PKCβ may provide a novel therapeutic target for metabolic disease treatment in the future.
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding This study was funded by the National Institutes of Health [Grant HL79091] (to K.D.M.).
References
- 1.Wang D.H.Q., Cohen D.E., Carey M.C. Biliary lipids and cholesterol gallstone disease. J Lipid Res. 2009;50:S406–S411. doi: 10.1194/jlr.R800075-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tabas I. Consequences of cellular cholesterol accumulation: basic concepts and physiological implications. J Clin Invest. 2002;110:905–911. doi: 10.1172/JCI16452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holt J.A., Luo G., Billin A.N. Definition of a novel growth factor-dependent signal cascade for the suppression of bile acid biosynthesis. Genes Dev. 2003;17:1581–1591. doi: 10.1101/gad.1083503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalaany N.Y., Mangelsdorg D.J. LXRS and FXR: the yin and yang of cholesterol and fat metabolism. Ann Rev Physiol. 2006;68:159–191. doi: 10.1146/annurev.physiol.68.033104.152158. [DOI] [PubMed] [Google Scholar]
- 5.Delvecchio C.J., Capone J.P. Protein kinase Cα modulates liver X receptor alpha transactivation. J Endocrinol. 2008;197:121–130. doi: 10.1677/JOE-07-0525. [DOI] [PubMed] [Google Scholar]
- 6.Gineste R., Sirvent A., Paumelle R. Phosphorylation of farnesoid X receptor by protein kinase C promotes its transcriptional activity. Mol Endocrinol. 2008;22:2433–2447. doi: 10.1210/me.2008-0092. [DOI] [PubMed] [Google Scholar]
- 7.Ye X.F., Liu S., Wu Q. Degradation of retinoid X receptor α by TPA through proteosome pathway in gastric cancer cells. World J Gastroenterol. 2003;9:1915–1919. doi: 10.3748/wjg.v9.i9.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ding X., Staudinger J.L. Repression of PXR-mediated induction of hepatic CYP3A gene expression by protein kinase C. Biochem Pharmacol. 2005;69:867–873. doi: 10.1016/j.bcp.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 9.Hsieh J.C., Jurutka P.W., Makajima S. Phosphorylation of the human vitamin D receptor by protein kinase C: biochemical and functional evaluation of the serine 51 recognition site. J Biol Chem. 1993;268:15118–15126. [PubMed] [Google Scholar]
- 10.Sun K., Montana V., Chellappa K. Phosphorylation of a conserved serine in the deoxyribonucleic acid binding domain of nuclear receptors alters intracellular localization. Mol Endocrinol. 2007;21:1297–1311. doi: 10.1210/me.2006-0300. [DOI] [PubMed] [Google Scholar]
- 11.Huang W., Bansode R.R., Xie Y. Disruption of the murine protein kinase Cβ gene promotes gallstone formation and alters biliary lipid and hepatic cholesterol metabolism. J Biol Chem. 2011;286:22795–22805. doi: 10.1074/jbc.M111.250282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyamoto A., Nakayama K., Imaki H. Increased proliferation of B cells and auto-immunity in mice lacking protein kinase Cδ. Nature. 2002;416:865–869. doi: 10.1038/416865a. [DOI] [PubMed] [Google Scholar]
- 13.Huang W., Bansode R., Mehta M. Loss of protein kinase Cβ function protects mice against diet-induced obesity and development of hepatic steatosis and insulin resistance. Hepatology. 2009;49:1525–1536. doi: 10.1002/hep.22815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang W., Bansode R.R., Bal N. PKCβ deficiency attenuates obesity syndrome of ob/ob mice by promoting white adipose tissue remodeling. J Lipid Res. 2012;53:368–378. doi: 10.1194/jlr.M019687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bansode R., Huang W., Roy S. PKCβ deficiency increases fatty acid oxidation and reduces fat storage. J Biol Chem. 2008;283:231–236. doi: 10.1074/jbc.M707268200. [DOI] [PubMed] [Google Scholar]
- 16.Bocharov A.V., Huang W., Vishniakova T.G. Glucocorticoids upregulate high-affinity, high-density lipoprotein binding sites in rat hepatocytes. Metabolism. 1995;44:730–738. doi: 10.1016/0026-0495(95)90185-x. [DOI] [PubMed] [Google Scholar]
- 17.Patergani S., Marchi S., Rimessi A. PKCB/protein kinase C, beta and the mitochondrial axis as key regulators of autophagy. Autophagy. 2013;9:1–9. doi: 10.4161/auto.25239. [DOI] [PubMed] [Google Scholar]
- 18.Amigo L., Quinones V., Mardones P. Impaired biliary cholesterol secretion and decreased gallstone formation in apolipoprotein E-deficient mice fed a high-cholesterol diet. Gastroenterology. 2000;118:772–779. doi: 10.1016/s0016-5085(00)70147-8. [DOI] [PubMed] [Google Scholar]
- 19.Repa J.J., Mangelsdorf D.J. The role of orphan nuclear receptors in the regulation of cholesterol homeostasis. Ann Rev Cell Dev Biol. 2000;16:459–481. doi: 10.1146/annurev.cellbio.16.1.459. [DOI] [PubMed] [Google Scholar]
- 20.Calkin A.C., Tontonoz P. Transcriptional integration of metabolism by the nuclear sterol-activated receptors LXR and FXR. Nat Rev Mol Cell Biol. 2012;13:213–227. doi: 10.1038/nrm3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biddinger S.B., Haas J.T., Yu B.B. Hepatic insulin resistance directly promotes formation of cholesterol gallstones. Nat Med. 2008;14:778–782. doi: 10.1038/nm1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li T., Kong X., Owsley E. Insulin regulation of cholesterol-7α-hydroxylase expression in human hepatocytes. Roles of forkhead boxO1 and sterol regulatory element-binding protein. J Biol Chem. 2006;281:28745–28754. doi: 10.1074/jbc.M605815200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miao J., Xiao Z., Kanamaluru D. Bile acid signaling pathways increase stability of small heterodimeric partner (SHP) by inhibiting ubiquitin-proteasomal degradation. Genes Dev. 2009;23:986–996. doi: 10.1101/gad.1773909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kong B., Wang L., Chiang J.L. Mechanism of tissue-specific farnesoid X receptor in suppressing the expression of genes in bile-acid biosynthesis in mice. Hepatology. 2012;56:1034–1043. doi: 10.1002/hep.25740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khanuja B., Cheah Y.C., Hunt M. Lith1, a major gene affecting cholesterol gallstone formation among inbred strains of mice. Proc Natl Acad Sci USA. 1995;92:7729–7733. doi: 10.1073/pnas.92.17.7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerasimenko J.V., Flowerdew S.E., Voronina S.G. Bile acids induce Ca2+ release from both the endoplasmic reticulum and acidic intracellular calcium stores through activation of inositol trisphosphate receptors and ryanodine receptors. J Biol Chem. 2006;281:154–163. doi: 10.1074/jbc.M606402200. [DOI] [PubMed] [Google Scholar]
- 27.Lau B.W., Colella M., Ruder W.C. Deoxycholic acid activates protein kinase C and phospholipase C via increased Ca2+ entry at plasma membrane. Gastroenterology. 2005;128:695–707. doi: 10.1053/j.gastro.2004.12.046. [DOI] [PubMed] [Google Scholar]
- 28.Hagiwara K., Ito H., Murate T. PROX1 overexpression inhibits protein kinase C beta II transcription through promoter DNA methylation. Genes Chrom Cancer. 2012;51:1024–1036. doi: 10.1002/gcc.21985. [DOI] [PubMed] [Google Scholar]
- 29.Inagaki T., Choi M., Moschetta Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt D.R., Holmstrom S.R., Tacer K.F. Regulation of bile acid synthesis by fat-soluble vitamins A and D. J Biol Chem. 2010;285:14486–14494. doi: 10.1074/jbc.M110.116004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ye X.F., Liu S., Wu Q. Degradation of retinoid X receptor alpha by TPA through proteosome pathway in gastric cancer cells. World J Gastroentrol. 2003;9:1915–1919. doi: 10.3748/wjg.v9.i9.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar A., Middleton A., Chambers T.C. Differential roles of extracellular signal-regulated kinase-1/2 and p38MAPK in interleukin-1β- and tumor necrosis factor-α-induced low density lipoprotein receptor expression in HepG2 cells. J Biol Chem. 1998;273:15742–15748. doi: 10.1074/jbc.273.25.15742. [DOI] [PubMed] [Google Scholar]
- 33.Kapoor G.S., Atkins B.A., Mehta K.D. Activation of Raf-1/MEK-1/2/p42/44MAPK cascade alone is sufficient to uncouple LDL receptor expression from cell growth. Mol Cell Biol. 2002;236:13–22. doi: 10.1023/a:1016185928871. [DOI] [PubMed] [Google Scholar]
- 34.Kotzka J., Lehr S., Roth G. Insulin-activated Erkmitogen-activated protein kinases phosphorylate sterol regulatory element-binding protein-2 at serine residues 432 and 455 in vivo. J Biol Chem. 2004;283:15224–15231. doi: 10.1074/jbc.M401198200. [DOI] [PubMed] [Google Scholar]
- 35.Sato R., Inoue J., Kawabe Y. Sterol-dependent transcriptional regulation of sterol regulatory element-binding protein-2. J Biol Chem. 1996;271:26461–26464. doi: 10.1074/jbc.271.43.26461. [DOI] [PubMed] [Google Scholar]
- 36.Mulay V., Wood P., Manetsch M. Inhibition of mitogen-activated protein kinase Erk1/2 promotes protein degradation of ATP binding cassette transporters A1 and G1 in CHO and HuH7 cells. PLoS One. 2013;8:e62667. doi: 10.1371/journal.pone.0062667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamauchi Y., Hayashi M., Abe-Dohmae S. Apolipoprotein A-I activates protein kinase C alpha signaling to phosphorylate and stabilize ATP binding cassette transporter A1 for the high density lipoprotein assembly. J Biol Chem. 2003;278:47890–47897. doi: 10.1074/jbc.M306258200. [DOI] [PubMed] [Google Scholar]
- 38.Tsai J., Qiu W., Kohen-Avramoglu R. MEK-ERK inhibition corrects the defect in VLDL assembly in HepG2 cells: potential role of ERK in VLDL-ApoB100 particle assembly. Areterioscler Thromb Vasc Biol. 2007;27:211–218. doi: 10.1161/01.ATV.0000249861.80471.96. [DOI] [PubMed] [Google Scholar]
- 39.Formisano P., Oriente F., Fiory F. Insulin-activated protein kinase Cbeta bypasses Ras and stimulates mitogen-activated protein kinase activity and cell proliferation in muscle cells. Mol Cell Biol. 2000;20:6323–6333. doi: 10.1128/mcb.20.17.6323-6333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo K., Liu Y., Zhou H. Involvement of protein kinase C beta-extracellular signal-regulating kinase 1/2/p38 mitogen-activated protein kinase-heat shock protein 27 activation in hepatocellular carcinoma cell motility and invasion. Cancer Sci. 2008;99:486–496. doi: 10.1111/j.1349-7006.2007.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hennige A.M., Heni M., Machann J. Enforced expression of protein kinase C in skeletal muscle causes physical inactivity, fatty liver and insulin resistance in the brain. J Cell Mol Med. 2010;14:903–913. doi: 10.1111/j.1582-4934.2008.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shin D.J., Osborne T.F. FGF15/FGFR4 integrates growth factor signaling with hepatic bile acid metabolism and insulin action. J Biol Chem. 2009;284:11110–11120. doi: 10.1074/jbc.M808747200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Potthoff M.J., Boney-Montoya J. FGF15/19 regulates hepatic glucose metabolism by inhibiting the CREB-PGC-1α pathway. Cell Metab. 2011;13:729–738. doi: 10.1016/j.cmet.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]