Abstract
Objective
To report the long-term safety and efficacy data of a third generation drug eluting stent (DES) with biodegradable polymer in the complex patient population of diabetes mellitus after a follow-up period of 5 years.
Background
After percutaneous coronary intervention patients with diabetes mellitus are under higher risk of death, restenosis and stent thrombosis (ST) compared to non-diabetic patients.
Methods
In 126 centers worldwide 3067 patients were enrolled in the NOBORI 2 registry, 888 patients suffered from diabetes mellitus (DM), 213 of them (14%) being insulin dependent (IDDM). Five years follow-up has been completed in this study.
Results
At 5 years, 89.3% of the patients were available for follow-up. The reported target lesion failure (TLF) rates at 5 years were 12.39% in DM group and 7.34% in non-DM group; (p < 0.0001). In the DM group, the TLF rate in patients with IDDM was significantly higher than in the non-IDDM subgroup (17.84 vs. 10.67%; p < 0.01). The rate of ST at 5 years was not different among diabetic versus non-diabetic patients or IDDM versus NIDDM. Only 10 (<0.4%) very late stent thrombotic events beyond 12 months occurred.
Conclusions
The Nobori DES performed well in patients with DM. As expected patients with DM, particularly those with IDDM, had worse outcomes. However, the very low rate of very late stent thrombosis in IDDM patients might have significant clinical value in the treatment of these patients.
Clinical trial registration ISRCTN81649913; http://www.controlled-trials.com/isrctn/search.html?srch=81649913&sort=3&dir=desc&max=10
Background
Upon introduction, drug-eluting stents (DES) with their anti-restenotic properties clearly paved the new path in interventional cardiology and directed future device improvements for the clinical benefit of patients [1]. Initial enthusiasm was suppressed by long-term follow-up data that depicted some late side effects, later proven to be mainly related to the unwanted persistence of only initially necessary anti-proliferative drug or durable polymer carrier [2, 3]. Since then, numerous stent design enhancements, known as “new stent generations” were marketed, succeeding not only to improve anti-restenotic efficacy but also to eliminate downsides of their predecessors [4]. Biodegradable polymer drug-eluting stents, were designed to provide polymer and drug free surroundings at the treatment site after early “vulnerable” restenotic period, thereby eliminating the potentially dangerous effects of persistent inflammatory stimulus [5]. With immediate efficacy evident, remote safety assumptions could not be proven until results of very long-term outcomes were available [6]. Recent developments in poly- and monomer technology demonstrated thromboresistance in blood-contact studies [7]. Potential biodegradable polymer technology advantages over durable polymer drug-eluting stents could be especially valuable in clinically complex patient subgroups, like patients with diabetes mellitus in whom results of percutaneous revascularization are known to be worse compared with non-diabetic population [8, 9]. In patients with diabetes, the use of DES was associated with a significant reduction of target lesion restenosis without an increase in adverse events compared to bare metal stents and the use of a polymer-free sotarolimus- and probutol eluting showed comparable long-term efficacy and safety as second-generation durable polymer zotarolimus-eluting stents [10, 11]. As previously reported, Nobori Biolimus A9™ eluting stent (Terumo corporation, Tokyo, Japan) with biodegradable polymer technology was associated with relatively low rates of adverse events in diabetic subgroup and no stent thrombosis up to 2 years of follow-up in insulin-dependent diabetes mellitus patients—a finding that demanded additional attention and investigation [12]. Therefore, the aim of this predefined sub-study was to further investigate long-term outcome up to 5 years of drug-eluting biodegradable polymer technology in high-risk population.
Methods
Patient population
Study design was previously reported in details [12, 13]. Briefly, the NOBORI 2 study was prospective, single-arm, multi-centre, worldwide registry designed to further validate safety and efficacy of the Nobori stent in real-world patients. Only exclusion criterion was patient inability or unwillingness to provide informed consent to participate. The studied population consisted of 3067 patients enrolled between April 2008 and March 2009 in a total of 126 centers across Europe and Asia. Through data entry in the electronic case report form, based on prior diagnosis, patients were automatically assigned to predefined analysis group of diabetic patients, and if they were on insulin therapy they were allocated to IDDM subgroup. Diabetes mellitus was diagnosed based on previous medical records of the patient and IDDM and NIDDM were differentiated based on presenting drug regimen of glucose lowering therapy. Age at diagnosis of DM was not recorded. No specific laboratory confirmation was requested for confirmation of DM. The study was conducted according to the Declaration of Helsinki, ISO 14155 and respecting all country-specific regulatory requirements. The protocol was reviewed and approved by the ethics committee of each participating hospital and all patients gave written informed consent.
The Nobori Biolimus A9-eluting stent
The Nobori DES system that was used in the study was described in detail previously [14] and comprises four components: (1) the bare metal stent platform; (2) the delivery catheter; (3) the biodegradable drug carrier (polylactic acid); (4) an anti-proliferative substance, Biolimus A9™. Contrary to other DES, the drug polymer matrix is applied only abluminally (toward the vessel wall).
Coronary stent procedure
Patients’ medication regimen, percutaneous access, lesion preparation, and stent implantations were performed according to hospital routine practice. The treatment of multiple target vessels and staged procedures were allowed. Peri-procedural dual antiplatelet and anticoagulation regimen were given at the discretion of the operators. A post-procedural electrocardiogram and the measurement of cardiac enzymes were recommended. Additional assessment of comorbidities was done using the Charlson comorbidity index [15].
Patient follow-up
All patients were followed through hospital discharge and were scheduled for follow-up evaluations (hospital visit or telephone assessment) at 1, 6, and 12 months, and annually up to 5 years post-procedure. No mandatory angiographic follow-up was planned in this study. During the follow-up contacts, information about patients’ clinical condition, adverse events, hospitalizations, and changes to concomitant (cardiac and antiplatelet) medications were collected.
Study management
Data were collected through standardized electronic case report forms (KIKA Medical, Boston, MA, USA). Noteworthy was to highlight a very high rate of data monitoring through online and on site check-ups. All major adverse cardiac events were assessed by independent clinical event committee. All baseline angiograms were analyzed by an independent core laboratory (CorExpert, Belgrade, Serbia).
Study endpoints
The primary endpoint was Academic Research Consortium (ARC) defined, device oriented endpoint, a composite of cardiac death, myocardial infarction (MI) (Q-wave and non-Q-wave not clearly attributable to a non-target vessel) and target lesion revascularization (TLR), also known as target lesion failure (TLF). Secondary endpoints included: (1) TLF, (2) major adverse cardiac events (MACE) defined as cardiac death, MI, or any clinically driven target vessel revascularization (TVR), (3) death and MI, (4) TLR and TVR, (5) ARC defined, patient oriented composite endpoint (POCE) that included any death, any MI and any coronary revascularization (6) stent thrombosis according to ARC definitions [16]. All outcomes were evaluated at 12 months and yearly thereafter for 5 years.
Statistical analysis
Data were presented as percentages and 95% confidence intervals for categorical variables, and means and standard deviations for continuous variables. All analyses were performed by an independent statistical office (SBD Analytics, Bekkevoort, Belgium) using SAS software, version 9.13 (SAS Institute Inc., Cary, NC, USA). All statistical tests were two-tailed with p < 0.05 considered to be statistically significant. Differences between IDDM and non-IDDM (NIDDM) patients were analyzed using Fisher’s exact test for binary variables, and Wilcoxon rank sum test for continuous variables.
Results
DM versus non-DM
The final study population included 3067 patients, among which 888 patients suffered from DM, with 213 of them (14%) being insulin-dependent DM (IDDM). Patient characteristics, baseline, procedural and quantitative coronary angiography analysis (QCA) were reported earlier and here are reported in Tables 1 and 2. BMI in patients with DM was significantly higher compared to patients without DM (28.9 vs. 27.2 kg/m2; p < 0.001). At 5 years, 89.3% of patients were available for follow-up. TLF rate in diabetic patients was significantly higher from year 1 (5.97 vs. 3.03%; p < 0.0001) up to end of the 5 years follow-up period (12.39 vs. 7.34%; p < 0.0001; Fig. 1; Table 3). As observed earlier this difference was driven mainly by cardiac death and TLR rates, while target vessel related MI rate did not differ in diabetic patients compared to non-diabetic (Figs. 2, 3). Rate of POCE was also significantly higher in DM group (22.86 vs. 13.72%; p < 0.0001) with half of the accumulated 5 years difference being generated within 12 months (11.60 vs. 6.79%; p < 0.0001; Fig. 4). Anginal status showed no differences between groups during study period with 87.87% of diabetic patients and 89.20% of non-diabetic patients being symptom free at the 5 years follow-up (p = 0.411; Table 3).
Table 1.
Baseline characteristics
| % | IDDM N = 213 |
Non-IDDMN N = 675 |
P
(IDDM vs non-IDDM) |
DM N = 888 |
Non-DM N = 2179 |
P
(DM vs non-DM) |
|---|---|---|---|---|---|---|
| Age (years) (mean ± SD) | 66.10 ± 10.13 | 66.56 ± 10.13 | 0.740 | 66.45 ± 10.12 | 63.53 ± 11.16 | <0.001 |
| Male sex | 67.14 | 76.93 | 0.065 | 72.30 | 80.27 | <0.001 |
| Previous PCI | 36.15 | 33.13 | 0.455 | 33.86 | 31.40 | 0.199 |
| Previous CABG | 13.62 | 8.06 | 0.021 | 9.40 | 8.57 | 0.481 |
| Previous MI | 33.01 | 32.83 | 1.000 | 32.87 | 33.27 | 0.864 |
| Current smoker | 16.94 | 18.12 | 0.826 | 17.85 | 28.66 | <0.001 |
| Previous smoker | 31.15 | 37.73 | 0.115 | 36.20 | 33.97 | 0.269 |
| Hypercholesterolemia | 78.57 | 76.30 | 0.573 | 76.85 | 68.65 | <0.001 |
| Hypertension | 86.38 | 79.70 | 0.034 | 81.31 | 64.03 | <0.001 |
| Family history of CAD | 29.27 | 29.65 | 1.000 | 29.56 | 38.93 | <0.001 |
| Peripheral vascular disease | 15.08 | 10.46 | 0.098 | 11.57 | 4.61 | <0.001 |
| Congestive heart failure | 12.12 | 4.60 | <0.001 | 6.39 | 2.89 | <0.001 |
| Charlson comorbidity index (mean ± SD) | 2.61 ± 1.67 | 2.10 ± 1.27 | <0.001 | 2.21 ± 1.39 | 0.84 ± 0.91 | <0.001 |
| Baseline anginal status | ||||||
| Stable angina | 46.23 | 44.66 | 0.693 | 45.03 | 46.12 | 0.603 |
| Unstable angina | 33.49 | 38.87 | 0.168 | 37.58 | 39.74 | 0.271 |
| Silent ischemia | 20.28 | 16.47 | 0.213 | 17.38 | 14.13 | 0.026 |
| ACS | 50.70 | 52.44 | 0.694 | 52.03 | 54.06 | 0.318 |
| STEMI in ACS | 8.3 | 16.1 | 0.042 | 14.3 | 15.5 | 0.59 |
PCI percutaneous coronary intervention, CABG coronary artery bypass graft, CAD coronary artery disease, DM diabetes mellitus, IDDM insulin-dependent diabetes mellitus, ACS acute coronary syndrome, SD standard deviation, vs versus, MI myocardial infarction, N number of patients
Table 2.
Procedural and QCA results
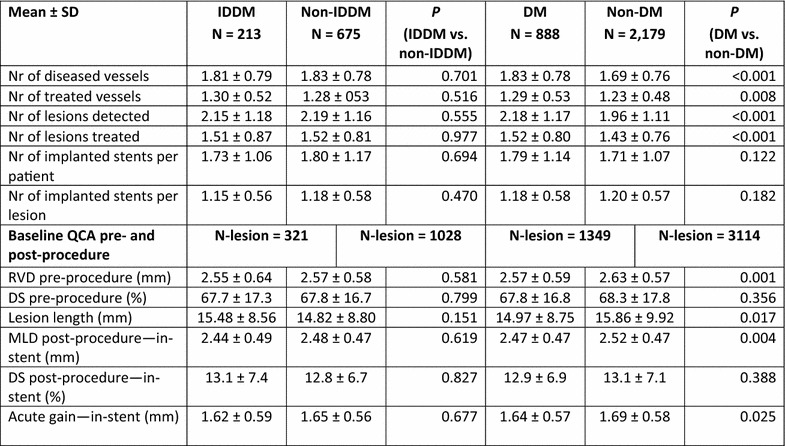
QCA qualitative comparative analysis, SD standard deviation, N number of patients, DM diabetes mellitus, IDDM insulin-dependent diabetes mellitus, RVD reference vessel diameter, DS diameter stenosis, MLD minimal luminal diameter, Nr number, vs versus
Fig. 1.
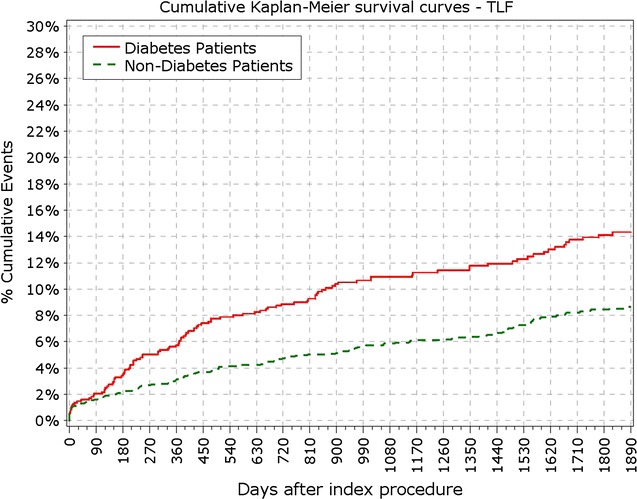
Primary endpoint: TLF incidence. TLF target lesion failure
Table 3.
Clinical outcomes at 1–5 years of follow-up
| % | 12 months | 2 years | 3 years | 4 years | 5 years | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DM | NDM | P | DM | NDM | P | DM | NDM | P | DM | NDM | P | DM | NDM | P | |
| Cardiac death | 1.91 | 0.92 | 0.028 | 3.60 | 1.42 | <.000 | 4.73 | 1.76 | <.000 | 5.29 | 2.34 | <.000 | 5.97 | 2.66 | <.000 |
| Non-cardiac death | 0.90 | 0.41 | 0.111 | 2.14 | 1.10 | 0.040 | 3.38 | 1.7 | 0.006 | 4.84 | 2.11 | <.000 | 5.41 | 2.57 | <.000 |
| Target vessel MI | 1.80 | 1.33 | 0.324 | 2.82 | 1.88 | 0.130 | 3.15 | 2.16 | 0.121 | 3.49 | 2.25 | 0.060 | 3.83 | 2.52 | 0.057 |
| TLR | 3.49 | 1.65 | 0.003 | 4.39 | 2.71 | 0.023 | 4.73 | 3.12 | 0.032 | 5.41 | 3.63 | 0.028 | 5.86 | 4.08 | 0.036 |
| TVR | 4.62 | 2.57 | 0.004 | 2.14 | 1.65 | 0.369 | 7.21 | 5.05 | 0.025 | 8.33 | 5.78 | 0.012 | 2.82 | 2.25 | 0.365 |
| MACE | 7.8 | 4.5 | <.000 | 11.2 | 6.4 | <.000 | 13.1 | 7.9 | <.000 | 14.5 | 9.0 | <.000 | 15.8 | 10.1 | <.000 |
| POCE | 11.60 | 6.79 | <.000 | 15.2 | 8.44 | <.000 | 18.24 | 10.6 | <.000 | 21.06 | 12.25 | <.000 | 22.86 | 13.72 | <.000 |
| TLF | 5.97 | 3.03 | <.000 | 8.67 | 4.77 | <.000 | 10.25 | 5.6 | <.000 | 11.15 | 6.52 | <.000 | 12.39 | 7.34 | <.000 |
| Anginal status | |||||||||||||||
| Stable angina | 10.54 | 9.97 | 0.680 | 11.07 | 9.71 | 0.292 | 11.4 | 9.76 | 0.255 | 10.41 | 9.50 | 0.590 | 10.57 | 9.69 | 0.602 |
| Unstable angina | 0.86 | 1.08 | 0.684 | 1.03 | 0.91 | 0.827 | 0.15 | 0.71 | 0.127 | 0.41 | 0.96 | 0.373 | 1.37 | 0.96 | 0.454 |
| Silent ischemia | 0.74 | 1.52 | 0.102 | 0.90 | 1.42 | 0.346 | 1.06 | 1.54 | 0.440 | 0.41 | 0.48 | 1 | 0.20 | 0.15 | 1 |
| No angina | 87.87 | 87.43 | 0.802 | 87.00 | 87.96 | 0.519 | 87.39 | 87.99 | 0.674 | 88.78 | 89.06 | 0.865 | 87.87 | 89.20 | 0.411 |
DM diabetes mellitus, NDM patient without DM, IDDM insulin-dependent diabetes mellitus, N number of patients, MI myocardial infarction, TLR target lesion revascularization, TVR target vessel revascularization, MACE major adverse cardiovascular events, POCE patient-oriented composite endpoint, TLF target lesion failure, vs versus
Fig. 2.
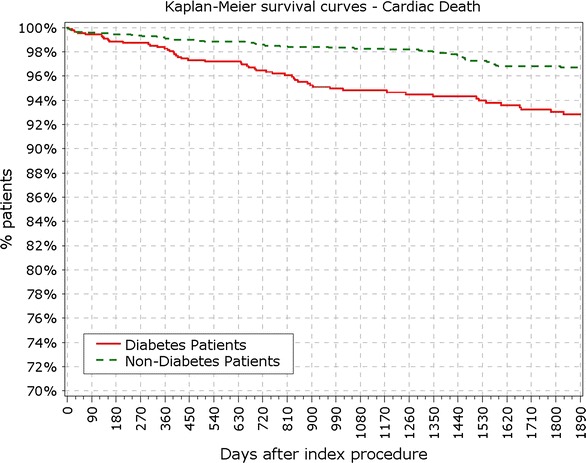
Secondary endpoint: survival from cardiac death
Fig. 3.
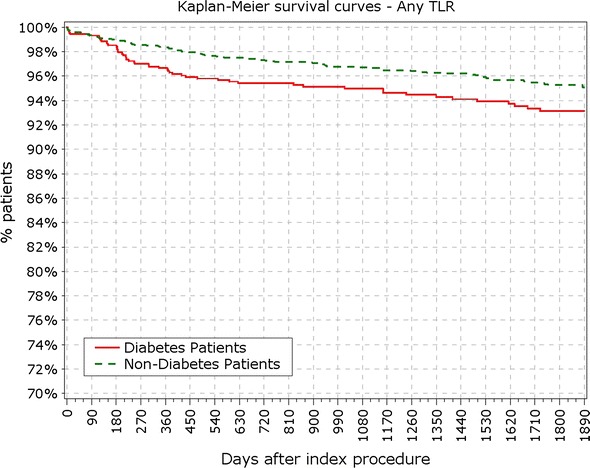
Secondary endpoint: survival from TLR. TLR target lesion revascularization
Fig. 4.
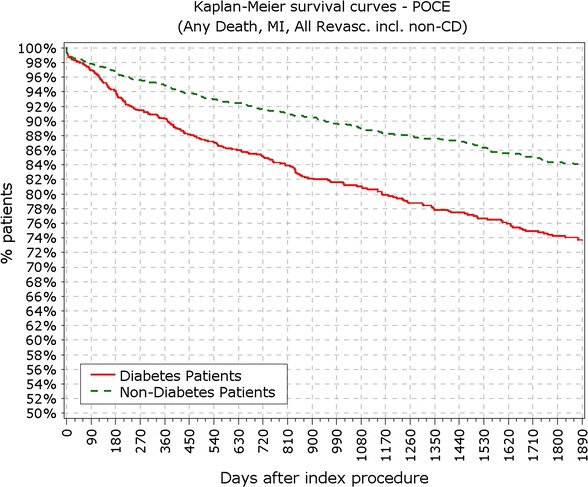
Survival from patient oriented composite endpoints. POCE patient oriented composite endpoints
IDDM versus NIDDM
BMI in patients with IDDM was significantly higher compared to patients with NIDDM (29.5 vs. 28.7 kg/m2; p < 0.001). Most of the difference in primary endpoints between non- and diabetic patients was driven by events occurring in IDDM patient subgroup (213 pts). Patients with IDDM had higher rate of TLF from 12 months (9.86 vs. 5.48%; p = 0.037) up to 5 years (17.84 vs. 10.67; p < 0.01; Fig. 5). Contrasting the findings between DM and non-DM, insulin dependence did not significantly increase the rate of TLR among DM patients not at 1 (5.63 vs. 3.11%; p = 0.09) or at the end of 5 years period (8.45 vs. 5.04%; p = 0.09). Rate of cardiac death and target vessel MI was not significantly increased in the IDDM group at 5 years or at any follow-up point, while rate of POCE showed early increment that persisted through the study period (Figs. 6, 7, 8).
Fig. 5.
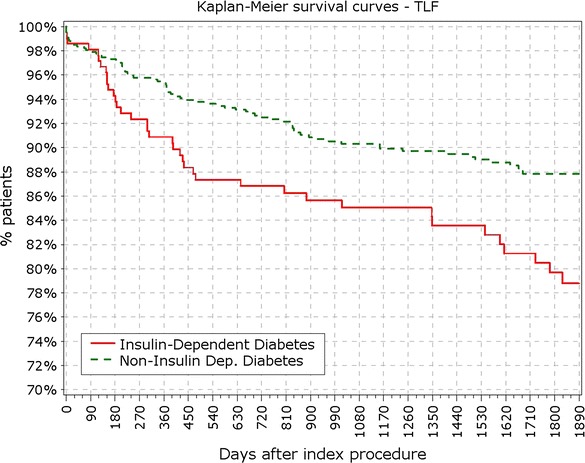
Survival from TLF in non-IDDM versus IDDM. TLR target lesion failure
Fig. 6.
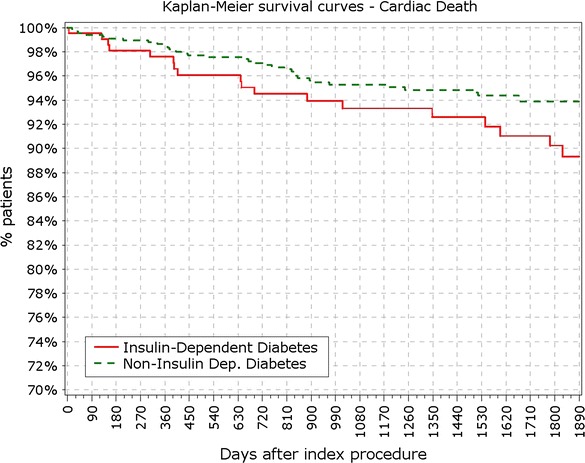
Survival from cardiac death in IDDM versus non-IDDM
Fig. 7.
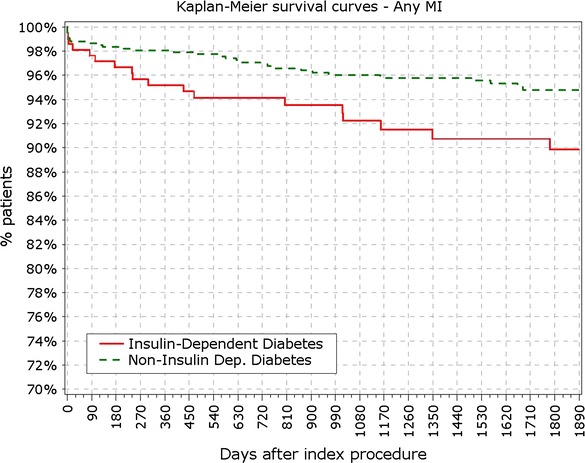
Survival from myocardial infarction in IDDM versus non-IDDM
Fig. 8.
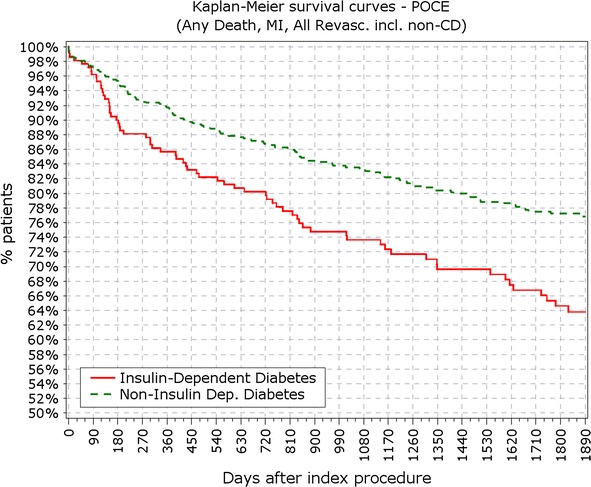
Survival from POCE in IDDM versus non-IDDM
Stent thrombosis
Rate of study ST (definite and probable according to the ARC definitions) was not different among diabetic versus non-diabetic patients or IDDM versus NIDDM at any time point (Table 4). Most of the ST occurred within 30 days of implantation (19/34 ST; 55%) and only 10 (<0.4%) very late stent thrombotic events beyond 12 months were observed in whole study population.
Table 4.
Stent thrombosis
| Definite + probable study stent thrombosis up to | DM N = 888 |
Non-DM N = 2179 |
P
(DM vs. non-DM) |
IDDM N = 213 |
Non-IDDM N = 675 |
P
(IDDM vs. non-IDDM) |
|---|---|---|---|---|---|---|
| 30 days (early ST) | 0.90% (8/888) | 0.50% (11/2179) | 0.2106 | 0.94% (2/213) | 0.89% (6/675) | 1 |
| 6 months | 0.90% (8/888) | 0.64% (14/2179) | 0.480 | 0.94% (2/213) | 0.89% (6/675) | 1 |
| 12 months (late ST) | 0.90% (8/888) | 0.73% (16/2.179) | 0.653 | 0.94% (2/213) | 0.89% (6/675) | 1 |
| 2 years | 1.01% (9/888) | 0.78% (17/2.179) | 0.519 | 0.94% (2/213) | 1.04% (7/675) | 1 |
| 3 years | 1.13% (10/888) | 0.92% (20/2.179) | 0.685 | 0.94% (2/213) | 1.19% (8/675) | 1 |
| 4 years | 1.35% (12/888) | 0.92% (20/2.179) | 0.326 | 1.41% (3/213) | 1.33% (9/675) | 1 |
| 5 years | 1.35% (12/888) | 1.01% (22/2.179) | 0.447 | 1.41% (3/213) | 1.33% (9/675) | 1 |
Antiplatelet therapy
Mean loading dose of Clopidogrel was 415 mg in DM patients versus 442 mg in non-DM patients (p = 0.02) and 412 mg in IDDM patients versus 416 mg in NIDDM patients (p = 0.84). Dual antiplatelet therapy duration at 1 year was 77.4% in DM patients versus 71.4% in non-DM patients (p = 0.01), while for IDDM patients it was 77.3% compared to 77.4% in NIDDM patients (P = 1).
Discussion
Present study shows long-term outcomes of biodegradable polymer drug eluting stent (BP-DES) treatment in highly unselected set of patients, especially in high-risk subgroup of patients with DM. Main findings of our analysis are: (1) Patients with DM have worse outcomes than non-diabetic patients with significantly higher rates of TLF, cardiac death and POCE throughout the whole study period; (2) Presence of insulin-dependent therapy among diabetic patients relates with more repeat revascularization events but not in higher rates of harder clinical endpoints, cardiac death and target vessel MI; (3) Cumulative rate of ST in whole population for the 5 years period was relatively low, especially rate of very-late ST; (4) Rate of stent thrombosis was similar between patients with and without DM, and insulin therapy did not increase the incidence of thrombotic events; Overall, our results confirm findings of previous reports that patients with DM, and especially those with IDDM, are at higher risk of adverse events following PCI [17]. Study results also demonstrated the overall very good performance of the BP-DES system in this high-risk patient population.
Clinical outcomes of Nobori stent implantation have been demonstrated in previous studies [12–14, 18]. This is the first study with a 5 years follow up specifically assessing its long-term performance in unselected group of patients with high-risk features for adverse prognosis like DM, especially IDDM.
Patients with DM compared to non-DM patients have less favorable outcomes in general, especially with percutaneous revascularization and with longer duration of follow-up [17]. Although the magnitude of restenosis reduction achieved with earlier DES platforms was impressive compared to BMS in most of the early randomized trials, this effect was not less evident in real world practice among patients with DM [19]. Differential clinical responses in patients with and without DM was documented even with new stent platforms highlighting the need for further opportunity to improve the treatment of CAD in patients with DM, particularly in those treated with insulin [20]. Meta-analyses showed that DES, compared to bare metal stents, were efficacious without compromising safety and assumed a potential highest benefit for patients with diabetes mellitus after treatment with everolimus eluting stents [11]. As expected, patients with DM had more adverse events in our analysis with higher TLR (5.8 vs. 4.1; p = 0.036), cardiac death (5.97 vs. 2.66%; p < 0.001) and composite endpoints MACE (15.8 vs. 10.1%; p < 0.001) and POCE (22.9 vs. 13.72%; p < 0.001).
Since results from long-term follow-up in diabetic patients treated with available biodegradable polymer stent platforms are lacking, it is justifiable to compare our findings to results of earlier DES platforms and durable polymer DES generations, demonstrating overall good performance of Nobori Biolimus A9 stent with low event rates in patients with or without DM (Table 4) [21–30]. Rate of MACE in DM group was lower than previously reported (15.8 vs. 17.0–40.5%) while rates of TLR (5.9 vs. 4.6–18.3%) cardiac death (5.9 vs. 2.4–15%) and MI (3.8 vs. 1.3–13.6%) also compared favorably with historical reports residing in lower edge of rates ranges. Very recently, the 5-years follow-up data of the diabetes subgroup of the ISAR Test 5 trial showed non-inferiority of a polymer-free sirolimus- and probucol-eluting stent compared to a second-generation durable polymer zotarolimus-eluting stent. Nevertheless, the rate of MACE, TLR, cardiac, and MI was higher in both arms of this study compared to our results. If these findings assume a superiority of BP-DES needs further investigation [10].
Long-term efficacy and safety of DES was subject of considerable debate with reports mainly from large-scale registries conflicting the excellent results of randomized trials focusing on immediate efficacy with shorter clinical evaluation periods. SCAAR study group drew attention of scientific community with results from large-scale registry showing increase in mortality in patients with DES compared with BMS before (HR 1.20) and after 6 months up to 3 years period (HR 1.32). High or prolonged antiproliferative drug delivery and persistent inflammatory propensities of durable polymers were heavily related with inadequate vessel healing, predisposing treated vessels to late adverse events [31–33]. Conceptually, design of biodegradable polymer stents were attractive solution since ultimately their long-term effects resemble BMS-like interaction with the vessel wall with less inflammatory stimulus. Recent reports showed that biodegradable polymers offer clear academic but equivocal clinical advantage. In large comparison, meta-analysis of 20.005 pts, treatment with BP-DES significantly reduced LLL and LST rates, without clear benefits on harder endpoints compared to durable polymer (DP)-DES [6]. High-risk population like STEMI patients also could benefit from BP-DES. In 497 patients with STEMI at 4 years, MACE was significantly reduced following treatment with BP-DES (hazard ratio [HR] 0.59, 95% CI 0.39–0.90; p = 0.01). Effect was driven by reduced TLR (HR 0.54, 95% CI 0.30–0.98; p = 0.04). Trends were also seen for cardiac death or MI (HR 0.63, 95% CI 0.37–1.05; p = 0.07) and definite or probable stent thrombosis (3.6 vs. 7.1%; HR 0.49, 95% CI 0.22–1.11; p = 0.09). Similar conclusions were drawn from pooled individual patient-level data from 3 randomized clinical trials [34] comparing biodegradable polymer DES with durable polymer (DP) DES. Clinical outcomes at 4 years were assessed. Out of 1094 patients with diabetes included in the analysis, 657 received BP-DES and 437 DP-DES. At 4 years, the incidence of the primary end point was similar with BP-DES versus DP-DES (HR 0.95, 95% CI 0.74–1.21, P = 0.67). But, rate of definite or probable stent thrombosis was significantly reduced in patients treated with BP-DES (HR 0.52, 95% CI 0.28–0.96, P = 0.04), and this difference was driven by significantly lower stent thrombosis rate with BP-DES after 1 and up to 4 years (HR 0.15, 95% CI 0.03–0.70, P = 0.02) eliminating the fear from late “catch-up” phenomenon with biodegradable polymers [35]. And indeed, serial optical coherence studies at 6, 12 and 24 months from implantation of BP-DES, Nobori Biolimus stent, did show that favorable features like, small gradual increase in neointimal thickness, with a nonsignificant decrease in the lumen area, lowering frequency of uncovered struts to almost none with very low percentage of detectable thrombi and peri-strut low-intensity area, could explain such low thrombotic risk beyond 1 year period. In addition, atherogenic neointima was not observed in the event-free OCT cohort [36]. When compared to Sirolimus eluting stent (SES) and BMS, BP-DES lies somewhere in-between according to recent OCT analysis. Biodegradable polymer Biolimus eluting stent showed a favorable coronary arterial response compared with SES, but different response with BMS at 5 years follow-up. The observed frequency of in-stent neoatherosclerosis within BP-DES was similar to BMS and tented to be lower than SES [37]. These obvious pathological and angiographically described potential advantages could explain low rates of ST occurring in 1.01, 1.35 and 1.41% in non-DM, DM and IDDM patients respectively up to 5 years with 24 (70%) occurring within 12 months. Rate of ST compared favorably to historical reports (0.8–10.2%). Results of trials with long term follow up after drug eluting stent implantation in patient with diabetes mellitus are summarized in Table 5.
Table 5.
Overview of trials evaluating long-term outcomes of various DES types in diabetic subpopulation
| (%) | Year | FU | Stent | N of pts DM | MACE | Cardiac death | MI | TVR | TLR | ST |
|---|---|---|---|---|---|---|---|---|---|---|
| Olesen et al. | 2015 | 5 years | ZES | 169 | 28.4 | 15 | 13.6 | 24.8 | 18.3 | 1 |
| SES | 168 | 18.5 | 5 | 13.6 | 13.6 | 8.3 | 2.3 | |||
| p = 0.032 | p < 0.01 | p = ns | p < 0.01 | p = 0.006 | p = 0.02 | |||||
| Billinger et al. | 2012 | 5 years | SES and PES combined | SES (503), PES (509) | 25.9 vs. 19.2 | 11.4 vs. 4.3 | 6.5 vs. 6.8 | 14.4 vs. 14.1 | 6 vs. 4.6 | |
| p = 0.02 | p < 0.0001 | p = ns | p = ns | p = ns | ||||||
| Onuma et al. | 2011 | 5 years | BMS | 112 | 53.6 | 11 | ||||
| SES | 159 | 40.5 | 4.8 | 33.2 | ||||||
| CABG | 96 | 23.4 | 5.2 | 10.7 | ||||||
| p < 0.01 | p = ns | p = 0.04 | p < 0.001 | |||||||
| Maeng et al. | 2015 | 4 years | EES | 108 | 20.4 | 8.3 | 1.9 | 12 | 5.6 | 0.9 |
| SES | 105 | 23.8 | 4.8 | 7.6 | 16.2 | 9.5 | 1.9 | |||
| p = 0.55 | p = 0.3 | p = 0.067 | p = 0.39 | p = 0.28 | p = 0.55 | |||||
| Simsek et al. | 2013 | 3 years | EES | 804 | 18 | 12 | 3.1 | 10.3 | 5.6 | 6.8 |
| SES | 512 | 21.7 | 8.4 | 5.7 | 17.4 | 11.5 | 8.7 | |||
| PES | 547 | 20.6 | 9.6 | 7.4 | 16.8 | 11.3 | 10.2 | |||
| Stiermaier et al. | 2014 | 5 years | SES | 120 | 29.7 | 7.6 | 9.3 | 18.6 | 15.3 | 1.6 |
| PES | 116 | 31.6 | 8.8 | 7.9 | 23.7 | 15.8 | 0.9 | |||
| p = 0.86 | p = 0.94 | p = 0.88 | p = 0.44 | p = 1.0 | p = 1.0 | |||||
| Sinning et al. | 2014 | 5 years | SES | 95 | 36 | 15 | 8 | NA | 13 | 5 |
| MBS | 95 | 52 | 13 | 9 | NA | 29 | 6 | |||
| p = 0.02 | p = ns | p = ns | p = 0.003 | p = ns | ||||||
| Sato et al. | 2012 | 5 years | SES DM | 85 | 27.1 | 2.4 | 4.7 | 15.3 | 9.4 | 3.5 |
| SES non-DM | 112 | 16.1 | 2.7 | 0 | 14.3 | 8.9 | 0 | |||
| p = 0.06 | p = 0.635 | p = 0.827 | p = 0.843 | p = 0.886 | p = 0.883 | |||||
| Park et al. | 2012 | 5 years | SES | 3101 | 17.5 vs. 12.3 | 1.8 vs. 2.0 | 6.8 vs. 4.2 | 9.6 vs. 6.9 | 8.8 vs. 6.0 | 2.3 vs. 2.2 |
| PES | ||||||||||
| p < 0.001 | p = 0.623 | p < 0.001 | p < 0.001 | p < 0.001 | p = 0.820 | |||||
| Jensen et al. | 2015 | 5 years | EES | 194 | 17 | 8.2 | 2.6 | 4.6 | 2 pts | |
| SES | 196 | 26.5 | 10.2 | 5.6 | 10.2 | 5 pts | ||||
| Vardi et al. | 2013 | 5 years | ZES | 268 | 17.7 | 3.7 | 1.3 | 17.2 | 11.2 | 1.2 |
| SES and PES | 268 | 26.6 | 7 | 5.1 | 20 | 12 | 0.8 | |||
| p = 0.012 | p = 0.06 | p = 0.011 | p = ns | p = ns | p = ns |
Study limitations
Our study has several limitations. First, the diagnosis of DM was performed only on the bases of patients medical history without any confirmatory tests, potentially leading to lower incidence. Additionally, the diagnosis of diabetes was self reported and uncontrolled. Thus, the “non-DM” group could contain patients with unreported diabetes. Furthermore, NOBORI 2 is the non-randomized registry and the comparison with other DES is limited to historical data. Under-reporting of adverse events during follow-up is also possible. However, this registry is based on close online and on-site source data monitoring and relatively high follow-up compliance rate, we can assume that adverse event non-reporting can be considered as a matter of exception.
Conclusion
This analysis of the 5-years outcomes suggests that the Nobori biodegradable polymer DES is a suitable treatment option for overall but especially for high risk population subset like DM patients with clinical outcomes and safety profile that compare favourably to different DES platforms.
Authors’ contributions
All authors made substantial contributions to acquisition of data and included a substantial number of patients and performed coronary intervention in this study. GBD was the PI of the study. MW, SS and AS were involved in drafting the manuscript. All authors revised the manuscript critically for important intellectual content. All authors gave final approval of the version to be published. Each author participated sufficiently in this work to take public responsibility for appropriate portions of the content; and all authors agree to be accountable for all aspects of this work in ensuring that questions related to the accuracy or integrity of any part of this work are appropriately investigated and resolved. All authors read and approved the final manuscript.
Acknowledgements
Not applicable.
Competing interests
No author has any conflict of interest to declare in relation to this manuscript, except being investigators in NOBORI 2 study. JK has no competing interests except the research grant received in the frame of the NOBORI 2 study. DHS accepted consultancy fees from Terumo for speaking.
Availability of data and materials
The data that support the findings of this study are available from Terumo Cooperation but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Terumo Cooperation.
Ethics approval and consent to participate
The study was conducted according to the Declaration of Helsinki, ISO 14155 and respecting all country-specific regulatory requirements. The protocol was reviewed and approved by the ethics committee of each participating hospital and all patients gave written informed consent.
Funding
Funding for the study was provided by Terumo Corporation, Tokyo, Japan.
Abbreviations
- ARC
Academic Research Consortium
- BMI
body mass index
- BMS
bare metal stent
- BP-DES
biodegradable polymer drug eluting stent
- DES
drug eluting stent
- DM
diabetes mellitus
- DP-DES
durable polymer drug eluting stent
- IDDM
insulin dependent diabetes mellitus
- MACE
major adverse cardiovascular event
- MI
myocardial infarction
- NIDDM
non-insulin dependent diabetes mellitus
- POCE
patient-oriented composite endpoint
- SES
sirolimus eluting stent
- ST
stent thrombosis
- TLF
target lesion failure
- TVR
target vessel revascularization
Contributor Information
Marcus Wiemer, Phone: 0571-790 3101, Email: marcus.wiemer@muehlenkreiskliniken.de.
Sinisa Stoikovic, Email: sstojkovi@mts.rs.
Alexander Samol, Email: Alexander.Samol@muehlenkreiskliniken.de.
Zisis Dimitriadis, Email: zdimitriadis@hdz-nrw.de.
Juan M. Ruiz-Nodar, Email: ruiz_jmi@gva.es
Ralf Birkemeyer, Email: ralf.birkenmeyer@herzklinik-ulm.de.
Jacques Monsegu, Email: j.monsegu@ghm-grenoble.fr.
Gérard Finet, Email: gerard.finet@creatis.univ-lyon1.fr.
David Hildick-Smith, Email: David.Hildick-Smith@bsuh.nhs.uk.
Damras Tresukosol, Email: sidts@mahodol.ac.th.
Enrique Garcia Novo, Email: enovog@yahoo.es.
Jacques J. Koolen, Email: jacques.koolen@cze.nl
Emanuele Barbato, Email: barba22@hotmail.com.
Gian Battista Danzi, Email: gbdanzi@tin.it.
References
- 1.Sousa JE, Costa MA, Abizaid A, Abizaid AS, Feres F, Pinto IM, Seixas AC, Staico R, Mattos LA, Sousa AG, et al. Lack of neointimal proliferation after implantation of sirolimus-coated stents in human coronary arteries: a quantitative coronary angiography and three-dimensional intravascular ultrasound study. Circulation. 2001;103(2):192–195. doi: 10.1161/01.CIR.103.2.192. [DOI] [PubMed] [Google Scholar]
- 2.Lagerqvist B, James SK, Stenestrand U, Lindback J, Nilsson T, Wallentin L, SCAAR/SWEDEHEART Study Group Long-term outcomes with drug-eluting stents versus bare-metal stents in Sweden. N Engl J Med. 2007;356(10):1009–1019. doi: 10.1056/NEJMoa067722. [DOI] [PubMed] [Google Scholar]
- 3.Finn AV, Kolodgie FD, Harnek J, Guerrero LJ, Acampado E, Tefera K, Skorija K, Weber DK, Gold HK, Virmani R. Differential response of delayed healing and persistent inflammation at sites of overlapping sirolimus- or paclitaxel-eluting stents. Circulation. 2005;112(2):270–278. doi: 10.1161/CIRCULATIONAHA.104.508937. [DOI] [PubMed] [Google Scholar]
- 4.Sarno G, Lagerqvist B, Nilsson J, Frobert O, Hambraeus K, Varenhorst C, Jensen UJ, Todt T, Gotberg M, James SK. Stent thrombosis in new-generation drug-eluting stents in patients with STEMI undergoing primary PCI: a report from SCAAR. J Am Coll Cardiol. 2014;64(1):16–24. doi: 10.1016/j.jacc.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 5.Chevalier B, Silber S, Park SJ, Garcia E, Schuler G, Suryapranata H, Koolen J, Hauptmann KE, Wijns W, Morice MC, et al. Randomized comparison of the Nobori Biolimus A9-eluting coronary stent with the Taxus Liberte paclitaxel-eluting coronary stent in patients with stenosis in native coronary arteries: the NOBORI 1 trial—Phase 2. Circ Cardiovasc Interv. 2009;2(3):188–195. doi: 10.1161/CIRCINTERVENTIONS.108.823443. [DOI] [PubMed] [Google Scholar]
- 6.Lupi A, Gabrio Secco G, Rognoni A, Lazzero M, Fattori R, Sheiban I, Sante Bongo A, Bolognese L, Agostoni P, Porto I. Meta-analysis of bioabsorbable versus durable polymer drug-eluting stents in 20,005 patients with coronary artery disease: an update. Catheter Cardiovasc Interv. 2014;83(6):E193–E206. doi: 10.1002/ccd.25416. [DOI] [PubMed] [Google Scholar]
- 7.Liu T-Y, Lin W-C, Huang L-Y, Chen S-Y, Yang M-C. Surface characteristics and hemocompatibility of PAN/PVDF blend membranes. Polym Adv Technol. 2005;16(5):413–419. doi: 10.1002/pat.592. [DOI] [Google Scholar]
- 8.Armstrong EJ, Rutledge JC, Rogers JH. Coronary artery revascularization in patients with diabetes mellitus. Circulation. 2013;128(15):1675–1685. doi: 10.1161/CIRCULATIONAHA.113.002114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Luca G, Dirksen MT, Spaulding C, Kelbaek H, Schalij M, Thuesen L, van der Hoeven B, Vink MA, Kaiser C, Musto C, et al. Impact of diabetes on long-term outcome after primary angioplasty: insights from the DESERT cooperation. Diabetes Care. 2013;36(4):1020–1025. doi: 10.2337/dc12-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harada Y, Colleran R, Kufner S, Giacoppo D, Rheude T, Michel J, Cassese S, Ibrahim T, Laugwitz KL, Kastrati A, et al. Five-year clinical outcomes in patients with diabetes mellitus treated with polymer-free sirolimus- and probucol-eluting stents versus second-generation zotarolimus-eluting stents: a subgroup analysis of a randomized controlled trial. Cardiovasc Diabetol. 2016;15(1):124. doi: 10.1186/s12933-016-0429-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bangalore S, Kumar S, Fusaro M, Amoroso N, Kirtane AJ, Byrne RA, Williams DO, Slater J, Cutlip DE, Feit F. Outcomes with various drug eluting or bare metal stents in patients with diabetes mellitus: mixed treatment comparison analysis of 22,844 patient years of follow-up from randomised trials. BMJ. 2012;345:e5170. doi: 10.1136/bmj.e5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiemer M, Danzi GB, West N, Voudris V, Koning R, Hoffmann S, Lombardi M, Mauri J, Babic R, Witherow F. Drug-eluting stents with biodegradable polymer for the treatment of patients with diabetes mellitus: clinical outcome at 2 years in a large population of patients. Med Devices (Auckl) 2015;8:153–160. doi: 10.2147/MDER.S67399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Danzi GB, Chevalier B, Urban P, Fath-Ordoubadi F, Carrie D, Wiemer M, Serra A, Wijns W, Kala P, Stabile A, et al. Clinical performance of a drug-eluting stent with a biodegradable polymer in an unselected patient population: the NOBORI 2 study. EuroIntervention. 2012;8(1):109–116. doi: 10.4244/EIJV8I1A17. [DOI] [PubMed] [Google Scholar]
- 14.Danzi GB, Chevalier B, Ostojic M, Hamilos M, Wijns W. Nobori drug eluting stent system: clinical evidence update. Minerva Cardioangiol. 2010;58(5):599–610. [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 16.Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, Steg PG, Morel MA, Mauri L, Vranckx P, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115(17):2344–2351. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 17.Fanari Z, Weiss SA, Zhang W, Sonnad SS, Weintraub WS. Short, intermediate and long term outcomes of CABG vs. PCI with DES in patients with multivessel coronary artery disease: meta-analysis of six randomized controlled trials. Eur J Cardiovasc Med. 2014;3(1):382–389. [PMC free article] [PubMed] [Google Scholar]
- 18.Kadota K, Muramatsu T, Iwabuchi M, Saito S, Hayashi Y, Ikari Y, Nanto S, Fujii K, Inoue N, Namiki A, et al. Randomized comparison of the Nobori biolimus A9-eluting stent with the sirolimus-eluting stent in patients with stenosis in native coronary arteries. Catheter Cardiovasc Interv. 2012;80(5):789–796. doi: 10.1002/ccd.23280. [DOI] [PubMed] [Google Scholar]
- 19.Stenestrand U, James SK, Lindback J, Frobert O, Carlsson J, Schersten F, Nilsson T, Lagerqvist B, SCAAR/SWEDEHEART Study Group Safety and efficacy of drug-eluting vs. bare metal stents in patients with diabetes mellitus: long-term follow-up in the Swedish Coronary Angiography and Angioplasty Registry (SCAAR) Eur Heart J. 2010;31(2):177–186. doi: 10.1093/eurheartj/ehp424. [DOI] [PubMed] [Google Scholar]
- 20.Stone GW, Kedhi E, Kereiakes DJ, Parise H, Fahy M, Serruys PW, Smits PC. Differential clinical responses to everolimus-eluting and Paclitaxel-eluting coronary stents in patients with and without diabetes mellitus. Circulation. 2011;124(8):893–900. doi: 10.1161/CIRCULATIONAHA.111.031070. [DOI] [PubMed] [Google Scholar]
- 21.Olesen KK, Tilsted HH, Jensen LO, Kaltoft A, Krusell LR, Ravkilde J, Christiansen EH, Madsen M, Thayssen P, Sorensen HT, et al. Long-term outcome of sirolimus-eluting and zotarolimus-eluting coronary stent implantation in patients with and without diabetes mellitus (a Danish organization for randomized trials on clinical outcome III substudy) Am J Cardiol. 2015;115(3):298–302. doi: 10.1016/j.amjcard.2014.10.038. [DOI] [PubMed] [Google Scholar]
- 22.Billinger M, Raber L, Hitz S, Stefanini GG, Pilgrim T, Stettler C, Zanchin T, Pulver C, Pfaffli N, Eberli F, et al. Long-term clinical and angiographic outcomes of diabetic patients after revascularization with early generation drug-eluting stents. Am Heart J. 2012;163(5):876–886. doi: 10.1016/j.ahj.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 23.Onuma Y, Wykrzykowska JJ, Garg S, Vranckx P, Serruys PW, Arts I, Investigators II. 5-Year follow-up of coronary revascularization in diabetic patients with multivessel coronary artery disease: insights from ARTS (arterial revascularization therapy study)-II and ARTS-I trials. JACC Cardiovasc Interv. 2011;4(3):317–323. doi: 10.1016/j.jcin.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Maeng M, Baranauskas A, Christiansen EH, Kaltoft A, Holm NR, Krusell LR, Ravkilde J, Tilsted HH, Thayssen P, Jensen LO. A 10-month angiographic and 4-year clinical outcome of everolimus-eluting versus sirolimus-eluting coronary stents in patients with diabetes mellitus (the DiabeDES IV randomized angiography trial) Catheter Cardiovasc Interv. 2015;86(7):1161–1167. doi: 10.1002/ccd.25875. [DOI] [PubMed] [Google Scholar]
- 25.Simsek C, Raber L, Magro M, Boersma E, Onuma Y, Stefanini GG, Zanchin T, Kalesan B, Wenaweser P, Juni P, et al. Long-term outcome of the unrestricted use of everolimus-eluting stents compared to sirolimus-eluting stents and paclitaxel-eluting stents in diabetic patients: the Bern-Rotterdam diabetes cohort study. Int J Cardiol. 2013;170(1):36–42. doi: 10.1016/j.ijcard.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 26.Stiermaier T, Heinz A, Schloma D, Kleinertz K, Danschel W, Erbs S, Linke A, Boudriot E, Lauer B, Schuler G, et al. Five-year clinical follow-up of a randomized comparison of a polymer-free sirolimus-eluting stent versus a polymer-based paclitaxel-eluting stent in patients with diabetes mellitus (LIPSIA Yukon trial) Catheter Cardiovasc Interv. 2014;83(3):418–424. doi: 10.1002/ccd.25131. [DOI] [PubMed] [Google Scholar]
- 27.Sinning JM, Baumgart D, Werner N, Klauss V, Baer FM, Hartmann F, Drexler H, Motz W, Klues H, Voelker W, et al. Five-year results of the multicenter randomized controlled open-label study of the CYPHER sirolimus-eluting stent in the treatment of diabetic patients with de novo native coronary artery lesions (SCORPIUS) study: a German multicenter investigation on the effectiveness of sirolimus-eluting stents in diabetic patients. Am Heart J. 2012;163(3):446–453. doi: 10.1016/j.ahj.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 28.Sato T, Ono T, Morimoto Y, Kawai H, Fuke S, Ikeda T, Saito H. Five-year clinical outcomes after implantation of sirolimus-eluting stents in patients with and without diabetes mellitus. Cardiovasc Interv Ther. 2012;27(3):189–195. doi: 10.1007/s12928-012-0115-2. [DOI] [PubMed] [Google Scholar]
- 29.Park K, Park KW, Rha SW, Bae JH, Hur SH, Park JS, Yoon JH, Jang Y, Jeong MH, Kim HS. Comparison of 5-year clinical outcomes between sirolimus-versus paclitaxel-eluting stent: Korean multicenter network analysis of 9000-patient cohort. Circ Cardiovasc Interv. 2012;5(2):174–184. doi: 10.1161/CIRCINTERVENTIONS.111.964650. [DOI] [PubMed] [Google Scholar]
- 30.Vardi M, Burke DA, Bangalore S, Pencina MJ, Mauri L, Kandzari DE, Leon MB, Cutlip DE. Long-term efficacy and safety of Zotarolimus-eluting stent in patients with diabetes mellitus: pooled 5-year results from the ENDEAVOR III and IV trials. Catheter Cardiovasc Interv. 2013;82(7):1031–1038. doi: 10.1002/ccd.25045. [DOI] [PubMed] [Google Scholar]
- 31.Yahagi K, Joner M, Virmani R. Insights into very late stent thrombosis from the wisdom of pathology. J Invasive Cardiol. 2014;26(9):417–419. [PubMed] [Google Scholar]
- 32.Byrne RA, Joner M, Kastrati A. Polymer coatings and delayed arterial healing following drug-eluting stent implantation. Minerva Cardioangiol. 2009;57(5):567–584. [PubMed] [Google Scholar]
- 33.Nakazawa G, Finn AV, Vorpahl M, Ladich ER, Kolodgie FD, Virmani R. Coronary responses and differential mechanisms of late stent thrombosis attributed to first-generation sirolimus- and paclitaxel-eluting stents. J Am Coll Cardiol. 2011;57(4):390–398. doi: 10.1016/j.jacc.2010.05.066. [DOI] [PubMed] [Google Scholar]
- 34.Stefanini GG, Byrne RA, Serruys PW, de Waha A, Meier B, Massberg S, Juni P, Schomig A, Windecker S, Kastrati A. Biodegradable polymer drug-eluting stents reduce the risk of stent thrombosis at 4 years in patients undergoing percutaneous coronary intervention: a pooled analysis of individual patient data from the ISAR-TEST 3, ISAR-TEST 4, and LEADERS randomized trials. Eur Heart J. 2012;33(10):1214–1222. doi: 10.1093/eurheartj/ehs086. [DOI] [PubMed] [Google Scholar]
- 35.de Waha A, Stefanini GG, King LA, Byrne RA, Serruys PW, Kufner S, Meier B, Juni P, Kastrati A, Windecker S. Long-term outcomes of biodegradable polymer versus durable polymer drug-eluting stents in patients with diabetes a pooled analysis of individual patient data from 3 randomized trials. Int J Cardiol. 2013;168(6):5162–5166. doi: 10.1016/j.ijcard.2013.07.263. [DOI] [PubMed] [Google Scholar]
- 36.Konishi A, Shinke T, Otake H, Takaya T, Osue T, Kinutani H, Kuroda M, Takahashi H, Terashita D, Shite J, et al. Serial optical coherence tomography evaluation at 6, 12, and 24 months after Biolimus A9-eluting biodegradable polymer-coated stent implantation. Can J Cardiol. 2015;31(8):980–988. doi: 10.1016/j.cjca.2015.02.036. [DOI] [PubMed] [Google Scholar]
- 37.Kuramitsu S, Sonoda S, Yokoi H, Iwabuchi M, Nishizaki Y, Shinozaki T, Domei T, Hyodo M, Inoue K, Shirai S, et al. Long-term coronary arterial response to biodegradable polymer biolimus-eluting stents in comparison with durable polymer sirolimus-eluting stents and bare-metal stents: five-year follow-up optical coherence tomography study. Atherosclerosis. 2014;237(1):23–29. doi: 10.1016/j.atherosclerosis.2014.08.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from Terumo Cooperation but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Terumo Cooperation.


