Abstract
Objective
Non-alcoholic fatty liver diseases (NAFLD) are related to development of liver fibrosis which currently has few therapeutic options. Rodent models of NAFLD inadequately model the fibrotic aspects of the disease and fail to demonstrate the spectrum of cardiometabolic diseases without genetic manipulation. We aimed to document a monkey model of fatty liver and fibrosis, which naturally develop cardiometabolic disease pathophysiologies.
Methods
We studied 27 cynomologus monkeys (Macaca fascicularis) fed diets either low or high in simple carbohydrates, supplied as fructose, (CTL and HFr), on low fat, cholesterol-free background. The HFr was consumed for up to 7 years and liver tissue was histologically evaluated for fat and fibrosis extent.
Results
The HFr diet increased steatosis, and its extent was related to duration of fructose exposure. Lipid droplet size also increased with HFr duration, however compared to CTL the lipid droplets were smaller on average. Fibrosis extent was significantly greater with fructose feeding and was predicted by fructose exposure, extent of fatty liver, and age.
Conclusions
These data are the first to demonstrate that high carbohydrate diets alone can generate both liver fat and fibrosis and thus allows further study of mechanisms and therapeutic options in this translational animal model.
Keywords: fatty liver, liver fibrosis, fructose
Introduction
Non-alcoholic fatty liver diseases (NAFLD) are prevalent in developed and developing countries (1) and increase risk for cardiometabolic diseases (2). Fatty liver has been attributed to calories excess, and specifically to calories supplied as fructose (3). Controversy exists regarding the role fructose plays, as it is typically consumed within diets characterized by excess calories, high dietary fat and cholesterol. These combined dietary factors drive fatty liver and liver fibrosis in human populations, however they do not consistently produce representative disease in rodents or produce NAFLD through pathways that are implicated in human disease (4).
Rodent studies indicate fructose and high carbohydrate diets potently drive fatty liver, however rodents have far greater lipogenesis rates and thus dietary effects in this model may be exaggerated (5). Rodents also have dissimilar primary sites of lipogenesis, inflammatory responses, toll like receptor expression patterns and do not readily develop hepatic fibrosis, which is the disease feature that is the most difficult to induce experimentally and is without therapeutic options in people (4, 6–8). We have previously shown that high carbohydrate consumption as fructose rapidly causes hepatitis and over time drives fatty liver development in monkeys (9). The current study was undertaken to extend these findings by examining hepatic fibrosis and steatosis in fructose-fed monkeys.
Methods
All procedures were approved and followed the guidelines of the Institutional Animal Care and Use Committee of Wake Forest University Health Sciences. Primates, Macaca fasciculus (n=27), were fed one of two diets: a control diet (CTL; Diet 5038, LabDiet, Purina, St. Louis, MO, n=10) or a high fructose diet (HFr; n=17) which have been previously described in detail (9). The CTL diet was high in carbohydrates (69% of calories), with less than 3% glucose and 0.5% fructose supplying the calories. The HFr diet was equivalent in the percentage of calories supplied by carbohydrates however 24% of the total caloric intake was supplied from fructose. The HFr cohort was fed the diet for up to seven years (mean 2.75 years, range 0.27 – 6.6 years; Figure 1A), which approximates 1 to 20 human years, and were older and heavier than CTL monkeys, as previously described (9). Control monkeys had lifelong exposure to their diet condition.
Figure 1.
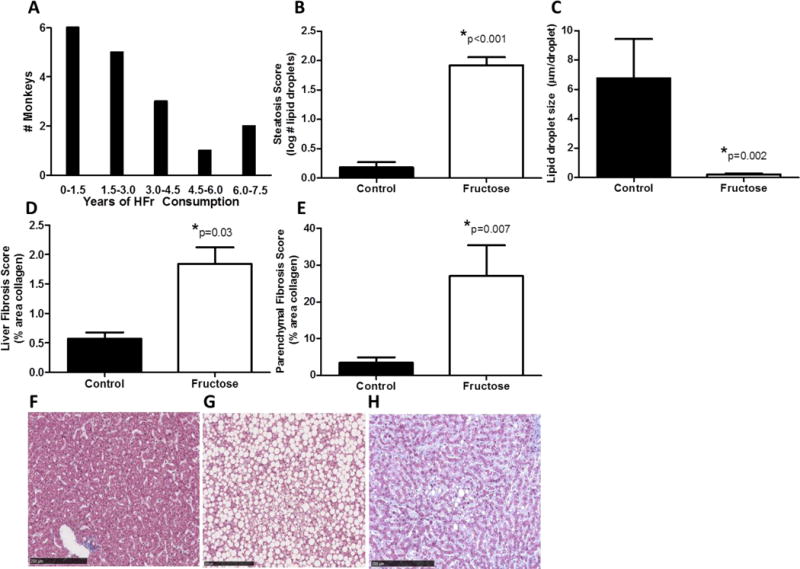
Liver tissue was evaluated from 17 monkeys consuming fructose for 0.27 – 6.6 years, with the number assessed depicted in A. Fructose consumption increased the number of lipid droplets (B) however reduced average lipid droplet size (C) in the liver as compared to monkeys eating a fructose-free control diet. Fibrosis was increased in fructose fed monkeys when measured from the entire histological section (D) and from triplicate parenchymal regions of interest (E). Representative histological examples of liver stained for collagen are shown from a control monkey (F, a severely steatotic monkey fed fructose (G), and a monkey with high fibrosis and moderate steatosis (H).
* indicates significance, with the adjusted p-values (age and bodyweight used as covariates) displayed
Liver tissue collected at necropsy from monkeys was fixed and stained using H&E and Masson’s trichrome (MTC). Sections were assessed for steatosis by lipid droplet number and size and for fibrosis (additional detail available in Supplementary Information). Data were analyzed by analysis of covariance using age and bodyweight as covariates, associations were assessed Pearson’s correlation coefficients, and multiple regression modeling was employed using Statistica (StatSoft Inc., Tulsa, OK). A p<0.05 was used to denote significance. Data are reported as mean ± standard error.
Results
Fructose-fed monkeys were heavier than CTL animals (3.03±0.09kg vs. 5.42±0.83kg; p=0.04). Fructose diets increased the number of lipid droplets, or steatosis score (p<0.001; Figure 1B, graph modified from (9)) but the droplets were of smaller average diameter (p=0.002) (Figure 1C). Fructose consumption significantly increased liver collagen content. Figures 1D and E show that the area stained for collagen in hepatic tissue was increased with fructose consumption when measured as either a percentage of the entire histological section (p=0.03) or the liver parenchyma that was distributed between the portal triads (p=0.007). Representative sections (Figure 1 F–H) illustrate pathological features resembling those seen in human NAFLD. Association analyses from data obtained from the fructose-consuming animals only (n=17; Figure 2) show that the number and diameter of the lipid droplets increased with longer consumption of fructose-containing diet. The steatosis score, or number of droplets, also had a significant positive relationship with hepatic fibrosis. CD3 cell counts were non-significantly higher in HFr monkeys (p=0.36) and not related to liver fat or fibrosis.
Figure 2.
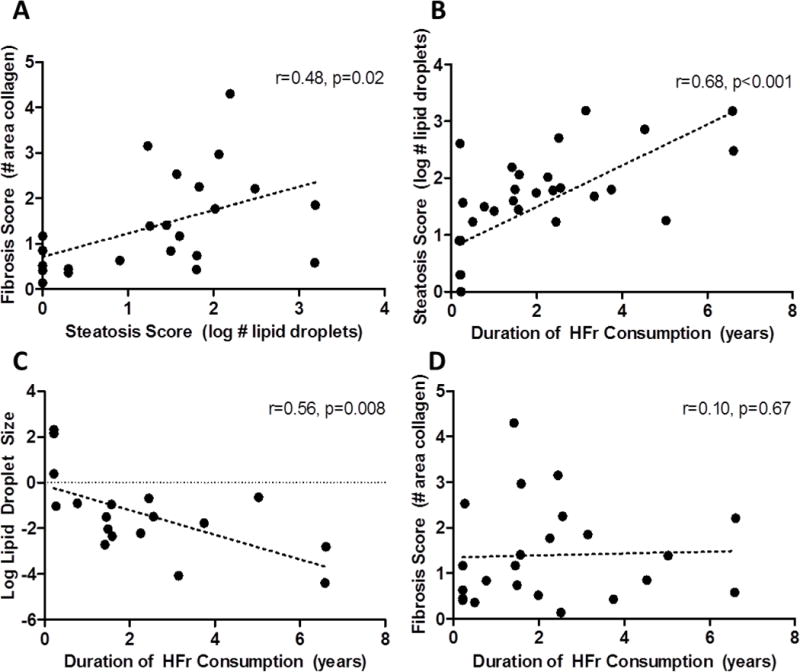
Associations between fibrosis and steatosis (A), steatosis (B) lipid droplet size (C), and fibrosis (D) and duration of high fructose consumption in monkeys. Pearson’s correlation coefficients and p-values are shown on each graph.
To examine the relative contributions of steatosis score, age, weight, duration of consumption, sex, and the experimental diets fed on liver fibrosis, a multiple regression analysis was conducted, with percent area of the liver staining positive for collagen as the outcome variable. The overall model was highly significant (Table 1; p<0.001) only with diet type (HFr vs. CTL), the extent of steatosis and age and being retained as significant predictors. Fructose exposure had the greatest magnitude of effect as compared to steatosis score, with age having a nominal effect on fibrosis.
Table 1.
Multivariate regression modeling results for liver fibrosis show that fructose exposure, the extent of liver fat and age all significantly influence collagen deposition.
| Model Variable | R2 | ß-coefficient | p-value |
|---|---|---|---|
| Fructose Diet | 0.33 | 0.64 | 0.004 |
| Steatosis Score | 0.23 | 0.52 | 0.02 |
| Age | 0.44 | 0.088 | 0.0006 |
Discussion
This report is the first to demonstrate, using a non-human primate animal model, that a high carbohydrate diet not augmented with dietary fat or cholesterol drives the important features of NAFLD including fibrosis (4, 9, 10). The demonstration of these pathologies in a non-human primate is relevant to the human disease as they have both comparable gastrointestinal and hepatic anatomies and functions, and immunological responses (11).
While there has been controversy on the exact cause of steatosis, whether excess calories, fat, fructose, or a combination of the three, this study produced clear evidence that just carbohydrate consumption as fructose induces fatty liver and also promotes fibrotic processes. These results further confirm that non-human primates, unlike rodents, develop fibrosis as part of their NAFLD expression in response to a carbohydrate diet challenge. Only one other primate model, Macaca radiata, describes fibrosis accompanying steatosis (12). In this monkey model, older age was also identified as a risk factor for disease however fibrosis extent was described categorically and the interactions of age and fat were not explored. In our larger cohort of monkeys, the fibrosis extent measured was variable among individuals, as reflected by lack of a direct significant relationship between the duration of diet consumption and liver fibrosis, while steatosis development with the fructose diet was more consistent. This represents an opportunity to further investigate the mechanisms of fibrotic processes and why certain individuals are more vulnerable to this negative consequence of consuming a high simple carbohydrate or fructose-containing diet.
Confidence in our histological results is high as the steatosis and fibrosis outcomes were measured by two different methods and produced consistent results: increased fat and fibrosis in the fructose-fed monkeys. However, these results do not implicate fructose specifically in fibrosis induction, as this study did not include a group of monkeys consuming comparable amounts of glucose, and it is possible that dextrose may also produce similar results. In rodent and human studies, fructose is more potent in inducing fatty liver (13, 14) which should drive fibrosis in a vulnerable clinical population. This hypothesis is supported by clinical reports of fructose being related to liver fibrosis in patients with nonalcoholic hepatosteatitis, even when controlling for caloric intake in statistical models (15). Our monkey cohort was predominantly female, and studies have shown that NAFLD prevalence is higher in males than females (16). Thus, our findings may underestimate the effects of a high carbohydrate diet on fat and fibrosis in the liver of a larger population. The predominant strength for this study was the use of cynologus monkeys as the animal model, and the long duration of dietary manipulation which to date has not yet been reported. Ectopic fat deposition in the liver is linked to the development of metabolic diseases (17). In this regard, monkeys are uniquely translational as rodents do not spontaneously develop diabetes or cardiovascular disease in the absence of genetic manipulation, while monkeys will develop both without experimental intervention (18, 19). Human clinical trials would be ideal, however long-term controlled dietary studies are difficult and liver samples cannot be routinely collected. We conclude that the monkey is the optimal model to study NAFLD etiologies and progression, and to evaluate potential therapeutic options for reversal of fat and fibrosis deposition with amelioration of related cardiometabolic diseases.
Supplementary Material
Study importance.
Diets high in fructose-containing sugars cause fatty liver in animal models but evidence for fibrosis development in animals and human experiments is lacking in the former or confounded by other dietary factors in the latter species.
We demonstrate for the first time that fructose consumption alone can drive increased collagen deposition, indicative of liver fibrosis, in a relevant primate model over a multi-year period of fructose exposure.
Acknowledgments
Funding: Funding was supplied in part by NIH NCATS UL1 TR001420.
Footnotes
Disclosure: The authors report no conflicts of interest.
References
- 1.Sayiner M, Koenig A, Henry L, Younossi ZM. Epidemiology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis in the United States and the Rest of the World. Clinics in liver disease. 2016;20(2):205–14. doi: 10.1016/j.cld.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363(14):1341–50. doi: 10.1056/NEJMra0912063. [DOI] [PubMed] [Google Scholar]
- 3.Sievenpiper JL, de Souza RJ, Mirrahimi A, Yu ME, Carleton AJ, Beyene J, et al. Effect of fructose on body weight in controlled feeding trials: a systematic review and meta-analysis. Ann Intern Med. 2012;156(4):291–304. doi: 10.7326/0003-4819-156-4-201202210-00007. [DOI] [PubMed] [Google Scholar]
- 4.Sanches SC, Ramalho LN, Augusto MJ, da Silva DM, Ramalho FS. Nonalcoholic Steatohepatitis: A Search for Factual Animal Models. Biomed Res Int. 2015;2015:574832. doi: 10.1155/2015/574832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sievenpiper JL, de Souza RJ, Kendall CW, Jenkins DJ. Is fructose a story of mice but not men? J Am Diet Assoc. 2012;111(2):219–20. doi: 10.1016/j.jada.2010.12.001. author reply 20–2. [DOI] [PubMed] [Google Scholar]
- 6.Bergen WG, Mersmann HJ. Comparative aspects of lipid metabolism: impact on contemporary research and use of animal models. J Nutr. 2005;135(11):2499–502. doi: 10.1093/jn/135.11.2499. [DOI] [PubMed] [Google Scholar]
- 7.Ketloy C, Engering A, Srichairatanakul U, Limsalakpetch A, Yongvanitchit K, Pichyangkul S, et al. Expression and function of Toll-like receptors on dendritic cells and other antigen presenting cells from non-human primates. Veterinary immunology and immunopathology. 2008;125(1–2):18–30. doi: 10.1016/j.vetimm.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2013;110(9):3507–12. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kavanagh K, Wylie AT, Tucker KL, Hamp TJ, Gharaibeh RZ, Fodor AA, et al. Dietary fructose induces endotoxemia and hepatic injury in calorically controlled primates. American Journal of Clinical Nutrition. 2013;98(2):349–57. doi: 10.3945/ajcn.112.057331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basciano H, Miller AE, Naples M, Baker C, Kohen R, Xu E, et al. Metabolic effects of dietary cholesterol in an animal model of insulin resistance and hepatic steatosis. Am J Physiol Endocrinol Metab. 2009;297(2):E462–73. doi: 10.1152/ajpendo.90764.2008. [DOI] [PubMed] [Google Scholar]
- 11.Haberthur K, Engelman F, Barron A, Messaoudi I. Immune senescence in aged nonhuman primates. Exp Gerontol. 2010;45(9):655–61. doi: 10.1016/j.exger.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagarajan P, Venkatesan R, Kumar M, Usmani A, Majumdar SS. Macaca radiata (bonnet monkey): a spontaneous model of nonalcoholic fatty liver disease. Liver Int. 2008;28(6):856–64. doi: 10.1111/j.1478-3231.2008.01706.x. [DOI] [PubMed] [Google Scholar]
- 13.Bergheim I, Weber S, Vos M, Kramer S, Volynets V, Kaserouni S, et al. Antibiotics protect against fructose-induced hepatic lipid accumulation in mice: role of endotoxin. J Hepatol. 2008;48(6):983–92. doi: 10.1016/j.jhep.2008.01.035. [DOI] [PubMed] [Google Scholar]
- 14.Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. 2009;119(5):1322–34. doi: 10.1172/JCI37385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdelmalek MF, Suzuki A, Guy C, Unalp-Arida A, Colvin R, Johnson RJ, et al. Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology. 2010;51(6):1961–71. doi: 10.1002/hep.23535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graff M, North KE, Franceschini N, Reiner AP, Feitosa M, Carr JJ, et al. PNPLA3 gene-by-visceral adipose tissue volume interaction and the pathogenesis of fatty liver disease: the NHLBI family heart study. Int J Obes (Lond) 2013;37(3):432–8. doi: 10.1038/ijo.2012.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW, et al. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci U S A. 2009;106(36):15430–5. doi: 10.1073/pnas.0904944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kavanagh K, Fairbanks LA, Bailey JN, Jorgensen MJ, Wilson M, Zhang L, et al. Characterization and heritability of obesity and associated risk factors in vervet monkeys. Obesity (Silver Spring) 2007;15(7):1666–74. doi: 10.1038/oby.2007.199. [DOI] [PubMed] [Google Scholar]
- 19.Bremer AA, Stanhope KL, Graham JL, Cummings BP, Wang W, Saville BR, et al. Fructose-fed rhesus monkeys: a nonhuman primate model of insulin resistance, metabolic syndrome, and type 2 diabetes. Clin Transl Sci. 2012;4(4):243–52. doi: 10.1111/j.1752-8062.2011.00298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


