Abstract
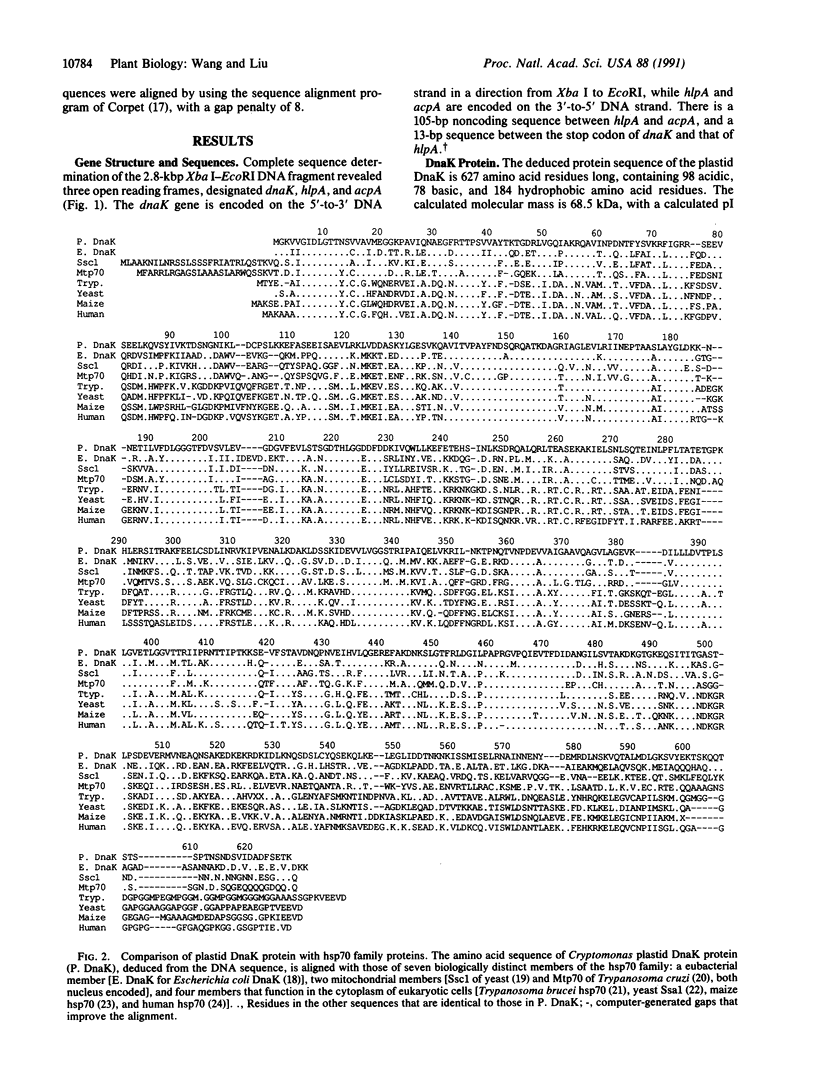
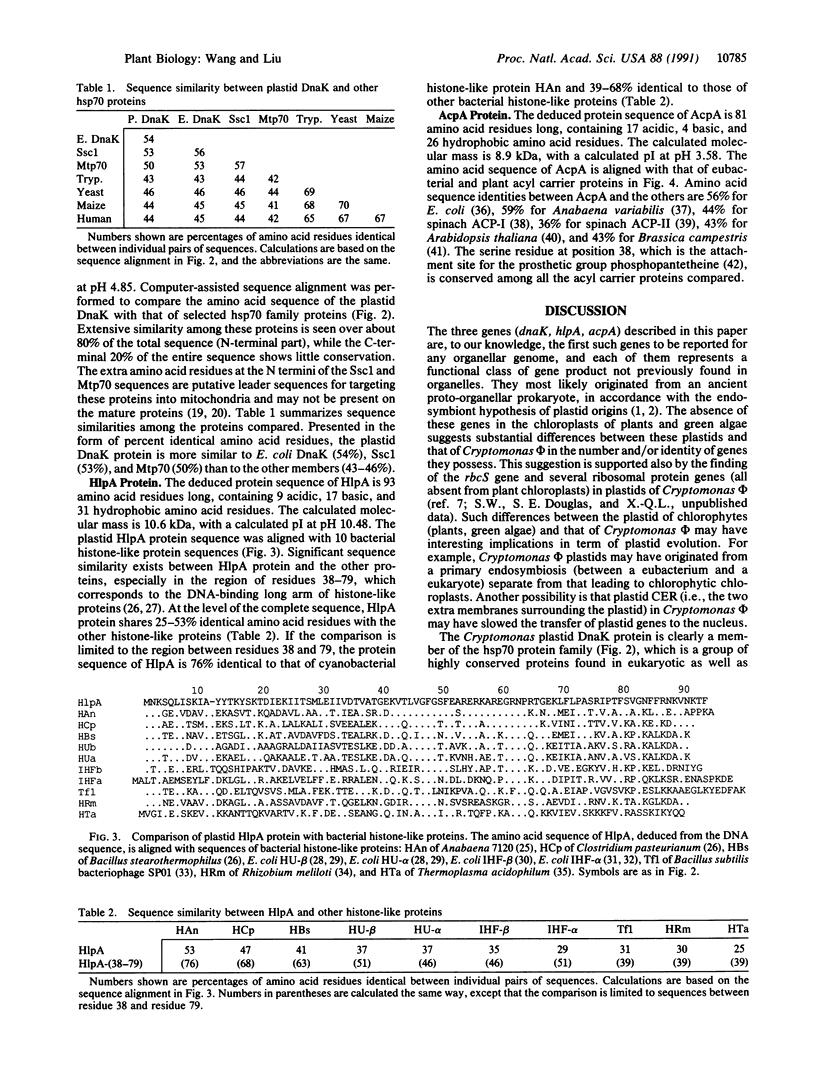
The plastid genome of Cryptomonas phi, a cryptomonad alga, contains three genes that have not previously been found in any organellar genome. Each of these genes encodes a functional class of organellar gene product not previously reported. The first gene, dnaK, encodes a polypeptide of the hsp70 heat shock protein family. The predicted amino acid sequence of the DnaK protein is 54% identical to that of the Escherichia coli hsp70 protein (DnaK), 50-53% identical to that of two nucleus-encoded mitochondrial hsp70 proteins, and 43-46% identical to that of several eukaryotic cytoplasmic members of the hsp70 protein family. The second gene, hlpA, encodes a polypeptide resembling bacterial histone-like proteins. The predicted amino acid sequence of the HlpA protein is 25-53% identical to that of several bacterial histone-like proteins, and the identity increases to 39-76% over a conserved region corresponding to the long arm that binds DNA. The third gene, acpA, encodes an acyl carrier protein, which is a key cofactor in the synthesis and metabolism of fatty acids. Its predicted amino acid sequence is 36-59% identical to that of eubacterial and plant chloroplast (nucleus-encoded) acyl carrier proteins.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman E., Kumamoto C. A., Emr S. D. Heat-shock proteins can substitute for SecB function during protein export in Escherichia coli. EMBO J. 1991 Feb;10(2):239–245. doi: 10.1002/j.1460-2075.1991.tb07943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir-Shapira D., Leustek T., Dalie B., Weissbach H., Brot N. Hsp70 proteins, similar to Escherichia coli DnaK, in chloroplasts and mitochondria of Euglena gracilis. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1749–1752. doi: 10.1073/pnas.87.5.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldauf S. L., Manhart J. R., Palmer J. D. Different fates of the chloroplast tufA gene following its transfer to the nucleus in green algae. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5317–5321. doi: 10.1073/pnas.87.14.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardwell J. C., Craig E. A. Major heat shock gene of Drosophila and the Escherichia coli heat-inducible dnaK gene are homologous. Proc Natl Acad Sci U S A. 1984 Feb;81(3):848–852. doi: 10.1073/pnas.81.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnefoy E., Rouvière-Yaniv J. HU and IHF, two homologous histone-like proteins of Escherichia coli, form different protein-DNA complexes with short DNA fragments. EMBO J. 1991 Mar;10(3):687–696. doi: 10.1002/j.1460-2075.1991.tb07998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyles S. S., Pettijohn D. E. Interaction of the Escherichia coli HU protein with DNA. Evidence for formation of nucleosome-like structures with altered DNA helical pitch. J Mol Biol. 1986 Jan 5;187(1):47–60. doi: 10.1016/0022-2836(86)90405-5. [DOI] [PubMed] [Google Scholar]
- Corpet F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 1988 Nov 25;16(22):10881–10890. doi: 10.1093/nar/16.22.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig E. A., Gross C. A. Is hsp70 the cellular thermometer? Trends Biochem Sci. 1991 Apr;16(4):135–140. doi: 10.1016/0968-0004(91)90055-z. [DOI] [PubMed] [Google Scholar]
- Craig E. A., Kramer J., Shilling J., Werner-Washburne M., Holmes S., Kosic-Smithers J., Nicolet C. M. SSC1, an essential member of the yeast HSP70 multigene family, encodes a mitochondrial protein. Mol Cell Biol. 1989 Jul;9(7):3000–3008. doi: 10.1128/mcb.9.7.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLange R. J., Williams L. C., Searcy D. G. A histone-like protein (HTa) from Thermoplasma acidophilum. II. Complete amino acid sequence. J Biol Chem. 1981 Jan 25;256(2):905–911. [PubMed] [Google Scholar]
- Douglas S. E., Durnford D. G. The small subunit of ribulose-1,5-bisphosphate carboxylase is plastid-encoded in the chlorophyll c-containing alga Cryptomonas phi. Plant Mol Biol. 1989 Jul;13(1):13–20. doi: 10.1007/BF00027331. [DOI] [PubMed] [Google Scholar]
- Douglas S. E., Murphy C. A., Spencer D. F., Gray M. W. Cryptomonad algae are evolutionary chimaeras of two phylogenetically distinct unicellular eukaryotes. Nature. 1991 Mar 14;350(6314):148–151. doi: 10.1038/350148a0. [DOI] [PubMed] [Google Scholar]
- Drlica K., Rouviere-Yaniv J. Histonelike proteins of bacteria. Microbiol Rev. 1987 Sep;51(3):301–319. doi: 10.1128/mr.51.3.301-319.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engman D. M., Kirchhoff L. V., Donelson J. E. Molecular cloning of mtp70, a mitochondrial member of the hsp70 family. Mol Cell Biol. 1989 Nov;9(11):5163–5168. doi: 10.1128/mcb.9.11.5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamm E. L., Weisberg R. A. Primary structure of the hip gene of Escherichia coli and of its product, the beta subunit of integration host factor. J Mol Biol. 1985 May 25;183(2):117–128. doi: 10.1016/0022-2836(85)90206-2. [DOI] [PubMed] [Google Scholar]
- Flashner Y., Gralla J. D. DNA dynamic flexibility and protein recognition: differential stimulation by bacterial histone-like protein HU. Cell. 1988 Aug 26;54(5):713–721. doi: 10.1016/s0092-8674(88)80016-3. [DOI] [PubMed] [Google Scholar]
- Froehlich J. E., Poorman R., Reardon E., Barnum S. R., Jaworski J. G. Purification and characterization of acyl carrier protein from two cyanobacteria species. Eur J Biochem. 1990 Nov 13;193(3):817–825. doi: 10.1111/j.1432-1033.1990.tb19405.x. [DOI] [PubMed] [Google Scholar]
- Glass D. J., Polvere R. I., Van der Ploeg L. H. Conserved sequences and transcription of the hsp70 gene family in Trypanosoma brucei. Mol Cell Biol. 1986 Dec;6(12):4657–4666. doi: 10.1128/mcb.6.12.4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M. W., Doolittle W. F. Has the endosymbiont hypothesis been proven? Microbiol Rev. 1982 Mar;46(1):1–42. doi: 10.1128/mr.46.1.1-42.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene J. R., Brennan S. M., Andrew D. J., Thompson C. C., Richards S. H., Heinrikson R. L., Geiduschek E. P. Sequence of the bacteriophage SP01 gene coding for transcription factor 1, a viral homologue of the bacterial type II DNA-binding proteins. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7031–7035. doi: 10.1073/pnas.81.22.7031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiratsuka J., Shimada H., Whittier R., Ishibashi T., Sakamoto M., Mori M., Kondo C., Honji Y., Sun C. R., Meng B. Y. The complete sequence of the rice (Oryza sativa) chloroplast genome: intermolecular recombination between distinct tRNA genes accounts for a major plastid DNA inversion during the evolution of the cereals. Mol Gen Genet. 1989 Jun;217(2-3):185–194. doi: 10.1007/BF02464880. [DOI] [PubMed] [Google Scholar]
- Hodges-Garcia Y., Hagerman P. J., Pettijohn D. E. DNA ring closure mediated by protein HU. J Biol Chem. 1989 Sep 5;264(25):14621–14623. [PubMed] [Google Scholar]
- Huisman O., Faelen M., Girard D., Jaffé A., Toussaint A., Rouvière-Yaniv J. Multiple defects in Escherichia coli mutants lacking HU protein. J Bacteriol. 1989 Jul;171(7):3704–3712. doi: 10.1128/jb.171.7.3704-3712.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt C., Morimoto R. I. Conserved features of eukaryotic hsp70 genes revealed by comparison with the nucleotide sequence of human hsp70. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6455–6459. doi: 10.1073/pnas.82.19.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S. R., Tabita F. R. Acyl carrier protein-derived sequence encoded by the chloroplast genome in the marine diatom Cylindrotheca sp. strain N1. J Biol Chem. 1991 Jul 25;266(21):13492–13494. [PubMed] [Google Scholar]
- Jaworski J. G., Post-Beittenmiller M. A., Ohlrogge J. B. Site-directed mutagenesis of the spinach acyl carrier protein-I prosthetic group attachment site. Eur J Biochem. 1989 Oct 1;184(3):603–609. doi: 10.1111/j.1432-1033.1989.tb15056.x. [DOI] [PubMed] [Google Scholar]
- Kang P. J., Ostermann J., Shilling J., Neupert W., Craig E. A., Pfanner N. Requirement for hsp70 in the mitochondrial matrix for translocation and folding of precursor proteins. Nature. 1990 Nov 8;348(6297):137–143. doi: 10.1038/348137a0. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y., Wada C., Yura T. Roles of Escherichia coli heat shock proteins DnaK, DnaJ and GrpE in mini-F plasmid replication. Mol Gen Genet. 1990 Jan;220(2):277–282. doi: 10.1007/BF00260494. [DOI] [PubMed] [Google Scholar]
- Kuo T. M., Ohlrogge J. B. The primary structure of spinach acyl carrier protein. Arch Biochem Biophys. 1984 Oct;234(1):290–296. doi: 10.1016/0003-9861(84)90351-5. [DOI] [PubMed] [Google Scholar]
- Laine B., Bélaïche D., Khanaka H., Sautière P. Primary structure of the DNA-binding protein HRm from Rhizobium meliloti. Eur J Biochem. 1983 Mar 15;131(2):325–331. doi: 10.1111/j.1432-1033.1983.tb07265.x. [DOI] [PubMed] [Google Scholar]
- Laine B., Kmiecik D., Sautiere P., Biserte G., Cohen-Solal M. Complete amino-acid sequences of DNA-binding proteins HU-1 and HU-2 from Escherichia coli. Eur J Biochem. 1980 Feb;103(3):447–461. doi: 10.1111/j.1432-1033.1980.tb05968.x. [DOI] [PubMed] [Google Scholar]
- Marshall J. S., DeRocher A. E., Keegstra K., Vierling E. Identification of heat shock protein hsp70 homologues in chloroplasts. Proc Natl Acad Sci U S A. 1990 Jan;87(1):374–378. doi: 10.1073/pnas.87.1.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W., Lagrange T., Li Y. F., Bisanz-Seyer C., Mache R. Hypothesis for the evolutionary origin of the chloroplast ribosomal protein L21 of spinach. Curr Genet. 1990 Dec;18(6):553–556. doi: 10.1007/BF00327027. [DOI] [PubMed] [Google Scholar]
- Mechulam Y., Fayat G., Blanquet S. Sequence of the Escherichia coli pheST operon and identification of the himA gene. J Bacteriol. 1985 Aug;163(2):787–791. doi: 10.1128/jb.163.2.787-791.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mende L., Timm B., Subramanian R. Primary structures of two homologous ribosome-associated DNA-binding proteins of Escherichia coli. FEBS Lett. 1978 Dec 15;96(2):395–398. doi: 10.1016/0014-5793(78)80446-3. [DOI] [PubMed] [Google Scholar]
- Miller H. I. Primary structure of the himA gene of Escherichia coli: homology with DNA-binding protein HU and association with the phenylalanyl-tRNA synthetase operon. Cold Spring Harb Symp Quant Biol. 1984;49:691–698. doi: 10.1101/sqb.1984.049.01.078. [DOI] [PubMed] [Google Scholar]
- Ostermann J., Voos W., Kang P. J., Craig E. A., Neupert W., Pfanner N. Precursor proteins in transit through mitochondrial contact sites interact with hsp70 in the matrix. FEBS Lett. 1990 Dec 17;277(1-2):281–284. doi: 10.1016/0014-5793(90)80865-g. [DOI] [PubMed] [Google Scholar]
- Pettijohn D. E. Histone-like proteins and bacterial chromosome structure. J Biol Chem. 1988 Sep 15;263(26):12793–12796. [PubMed] [Google Scholar]
- Phillips G. J., Silhavy T. J. Heat-shock proteins DnaK and GroEL facilitate export of LacZ hybrid proteins in E. coli. Nature. 1990 Apr 26;344(6269):882–884. doi: 10.1038/344882a0. [DOI] [PubMed] [Google Scholar]
- Post-Beittenmiller M. A., Hlousek-Radojcić A., Ohlrogge J. B. DNA sequence of a genomic clone encoding an Arabidopsis acyl carrier protein (ACP). Nucleic Acids Res. 1989 Feb 25;17(4):1777–1777. doi: 10.1093/nar/17.4.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochester D. E., Winer J. A., Shah D. M. The structure and expression of maize genes encoding the major heat shock protein, hsp70. EMBO J. 1986 Mar;5(3):451–458. doi: 10.1002/j.1460-2075.1986.tb04233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose R. E., DeJesus C. E., Moylan S. L., Ridge N. P., Scherer D. E., Knauf V. C. The nucleotide sequence of a cDNA clone encoding acyl carrier protein (ACP) from Brassica campestris seeds. Nucleic Acids Res. 1987 Sep 11;15(17):7197–7197. doi: 10.1093/nar/15.17.7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer P. E., Krieg U. C., Hwang S. T., Vestweber D., Schatz G. A precursor protein partly translocated into yeast mitochondria is bound to a 70 kd mitochondrial stress protein. EMBO J. 1990 Dec;9(13):4315–4322. doi: 10.1002/j.1460-2075.1990.tb07880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid K. M., Ohlrogge J. B. A root acyl carrier protein-II from spinach is also expressed in leaves and seeds. Plant Mol Biol. 1990 Nov;15(5):765–778. doi: 10.1007/BF00016126. [DOI] [PubMed] [Google Scholar]
- Schmid M. B. More than just "histone-like" proteins. Cell. 1990 Nov 2;63(3):451–453. doi: 10.1016/0092-8674(90)90438-k. [DOI] [PubMed] [Google Scholar]
- Sheffield W. P., Shore G. C., Randall S. K. Mitochondrial precursor protein. Effects of 70-kilodalton heat shock protein on polypeptide folding, aggregation, and import competence. J Biol Chem. 1990 Jul 5;265(19):11069–11076. [PubMed] [Google Scholar]
- Shimada H., Sugiura M. Fine structural features of the chloroplast genome: comparison of the sequenced chloroplast genomes. Nucleic Acids Res. 1991 Mar 11;19(5):983–995. doi: 10.1093/nar/19.5.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K., Ohme M., Tanaka M., Wakasugi T., Hayashida N., Matsubayashi T., Zaita N., Chunwongse J., Obokata J., Yamaguchi-Shinozaki K. The complete nucleotide sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO J. 1986 Sep;5(9):2043–2049. doi: 10.1002/j.1460-2075.1986.tb04464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater M. R., Craig E. A. The SSA1 and SSA2 genes of the yeast Saccharomyces cerevisiae. Nucleic Acids Res. 1989 Jan 25;17(2):805–806. doi: 10.1093/nar/17.2.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. J., Yaffe M. P. A mutation in the yeast heat-shock factor gene causes temperature-sensitive defects in both mitochondrial protein import and the cell cycle. Mol Cell Biol. 1991 May;11(5):2647–2655. doi: 10.1128/mcb.11.5.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smooker P. M., Kruft V., Subramanian A. R. A ribosomal protein is encoded in the chloroplast DNA in a lower plant but in the nucleus in angiosperms. Isolation of the spinach L21 protein and cDNA clone with transit and an unusual repeat sequence. J Biol Chem. 1990 Sep 25;265(27):16699–16703. [PubMed] [Google Scholar]
- Stirdivant S. M., Crossland L. D., Bogorad L. DNA supercoiling affects in vitro transcription of two maize chloroplast genes differently. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4886–4890. doi: 10.1073/pnas.82.15.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka I., Appelt K., Dijk J., White S. W., Wilson K. S. 3-A resolution structure of a protein with histone-like properties in prokaryotes. Nature. 1984 Aug 2;310(5976):376–381. doi: 10.1038/310376a0. [DOI] [PubMed] [Google Scholar]
- Vanaman T. C., Wakil S. J., Hill R. L. The complete amino acid sequence of the acyl carrier protein of Escherichia coli. J Biol Chem. 1968 Dec 25;243(24):6420–6431. [PubMed] [Google Scholar]
- Wickner S. H. Three Escherichia coli heat shock proteins are required for P1 plasmid DNA replication: formation of an active complex between E. coli DnaJ protein and the P1 initiator protein. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2690–2694. doi: 10.1073/pnas.87.7.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner S., Hoskins J., McKenney K. Function of DnaJ and DnaK as chaperones in origin-specific DNA binding by RepA. Nature. 1991 Mar 14;350(6314):165–167. doi: 10.1038/350165a0. [DOI] [PubMed] [Google Scholar]
- Yamada E. W., Dotzlaw H., Huzel N. J. Isolation of histone-like proteins from mitochondria of bovine heart. Prep Biochem. 1991;21(1):11–23. doi: 10.1080/10826069108021512. [DOI] [PubMed] [Google Scholar]



