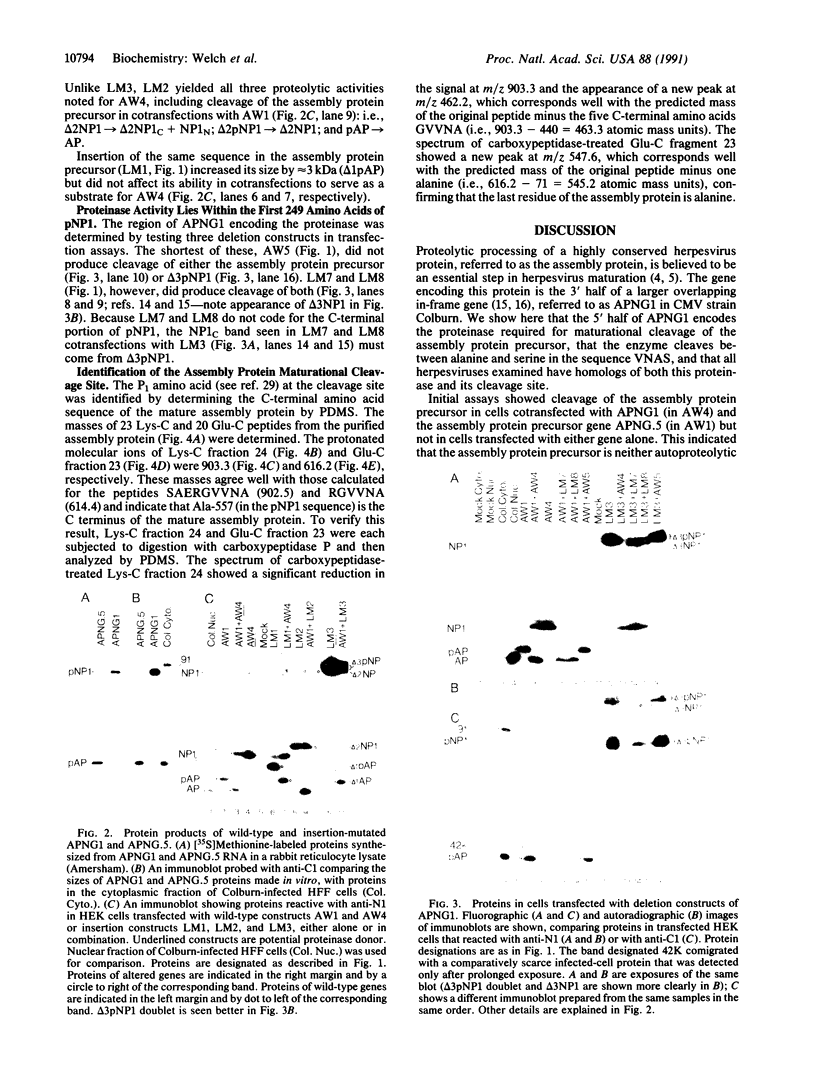
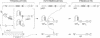Abstract
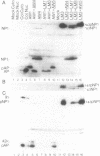
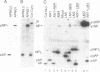
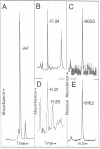
A herpesvirus proteinase activity has been identified and partially characterized by using the cloned enzyme and substrate genes in transient transfection assays. Evidence is presented that the proteinase gene of cytomegalovirus strain Colburn encodes a 590-amino acid protein whose N-terminal 249 residues contain the proteolytic activity and two domains that are highly conserved in the homologous protein of other herpesviruses. Insertion of a short amino acid sequence between these domains abolished proteinase activity, suggesting that this region constitutes part or all of the enzyme active site. Plasma desorption mass spectrometry was used to identify the C terminus of the mature assembly protein as alanine, enabling the recognition of a consensus proteinase cleavage sequence of V/L-X-A decreases S/V, near the C-terminal end of all herpesvirus assembly protein homologs. Interestingly, the proteinase and its substrate, the assembly protein precursor, are encoded by opposite halves of the same open reading frame.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bazan J. F., Fletterick R. J. Viral cysteine proteases are homologous to the trypsin-like family of serine proteases: structural and functional implications. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7872–7876. doi: 10.1073/pnas.85.21.7872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun D. K., Roizman B., Pereira L. Characterization of post-translational products of herpes simplex virus gene 35 proteins binding to the surfaces of full capsids but not empty capsids. J Virol. 1984 Jan;49(1):142–153. doi: 10.1128/jvi.49.1.142-153.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casjens S., King J. Virus assembly. Annu Rev Biochem. 1975;44:555–611. doi: 10.1146/annurev.bi.44.070175.003011. [DOI] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W., Marcy A. I., Comolli J. C., Lee J. Identification of precursor to cytomegalovirus capsid assembly protein and evidence that processing results in loss of its carboxy-terminal end. J Virol. 1990 Mar;64(3):1241–1249. doi: 10.1128/jvi.64.3.1241-1249.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W., Roizman B. Proteins specified by herpes simplex virus. 8. Characterization and composition of multiple capsid forms of subtypes 1 and 2. J Virol. 1972 Nov;10(5):1044–1052. doi: 10.1128/jvi.10.5.1044-1052.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W., Roizman B. Proteins specified by herpes simplex virus. Staining and radiolabeling properties of B capsid and virion proteins in polyacrylamide gels. J Virol. 1974 Jan;13(1):155–165. doi: 10.1128/jvi.13.1.155-165.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W. Structural and nonstructural proteins of strain Colburn cytomegalovirus. Virology. 1981 Jun;111(2):516–537. doi: 10.1016/0042-6822(81)90354-8. [DOI] [PubMed] [Google Scholar]
- Gorbalenya A. E., Donchenko A. P., Blinov V. M., Koonin E. V. Cysteine proteases of positive strand RNA viruses and chymotrypsin-like serine proteases. A distinct protein superfamily with a common structural fold. FEBS Lett. 1989 Jan 30;243(2):103–114. doi: 10.1016/0014-5793(89)80109-7. [DOI] [PubMed] [Google Scholar]
- Griffin A. M. The complete sequence of the capsid p40 gene from infectious laryngotracheitis virus. Nucleic Acids Res. 1990 Jun 25;18(12):3664–3664. doi: 10.1093/nar/18.12.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irmiere A., Gibson W. Isolation of human cytomegalovirus intranuclear capsids, characterization of their protein constituents, and demonstration that the B-capsid assembly protein is also abundant in noninfectious enveloped particles. J Virol. 1985 Oct;56(1):277–283. doi: 10.1128/jvi.56.1.277-283.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladin B. F., Ihara S., Hampl H., Ben-Porat T. Pathway of assembly of herpesvirus capsids: an analysis using DNA+ temperature-sensitive mutants of pseudorabies virus. Virology. 1982 Jan 30;116(2):544–561. doi: 10.1016/0042-6822(82)90147-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee J. Y., Irmiere A., Gibson W. Primate cytomegalovirus assembly: evidence that DNA packaging occurs subsequent to B capsid assembly. Virology. 1988 Nov;167(1):87–96. doi: 10.1016/0042-6822(88)90057-8. [DOI] [PubMed] [Google Scholar]
- Liu F. Y., Roizman B. The promoter, transcriptional unit, and coding sequence of herpes simplex virus 1 family 35 proteins are contained within and in frame with the UL26 open reading frame. J Virol. 1991 Jan;65(1):206–212. doi: 10.1128/jvi.65.1.206-212.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long E. O., Rosen-Bronson S., Karp D. R., Malnati M., Sekaly R. P., Jaraquemada D. Efficient cDNA expression vectors for stable and transient expression of HLA-DR in transfected fibroblast and lymphoid cells. Hum Immunol. 1991 Aug;31(4):229–235. doi: 10.1016/0198-8859(91)90092-n. [DOI] [PubMed] [Google Scholar]
- Mullis K., Faloona F., Scharf S., Saiki R., Horn G., Erlich H. Specific enzymatic amplification of DNA in vitro: the polymerase chain reaction. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):263–273. doi: 10.1101/sqb.1986.051.01.032. [DOI] [PubMed] [Google Scholar]
- O'Callaghan D. J., Randall C. C. Molecular anatomy of herpesviruses: recent studies. Prog Med Virol. 1976;22:152–210. [PubMed] [Google Scholar]
- Preston V. G., Coates J. A., Rixon F. J. Identification and characterization of a herpes simplex virus gene product required for encapsidation of virus DNA. J Virol. 1983 Mar;45(3):1056–1064. doi: 10.1128/jvi.45.3.1056-1064.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rixon F. J., Cross A. M., Addison C., Preston V. G. The products of herpes simplex virus type 1 gene UL26 which are involved in DNA packaging are strongly associated with empty but not with full capsids. J Gen Virol. 1988 Nov;69(Pt 11):2879–2891. doi: 10.1099/0022-1317-69-11-2879. [DOI] [PubMed] [Google Scholar]
- Robson L., Gibson W. Primate cytomegalovirus assembly protein: genome location and nucleotide sequence. J Virol. 1989 Feb;63(2):669–676. doi: 10.1128/jvi.63.2.669-676.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter I., Berger A. On the size of the active site in proteases. I. Papain. Biochem Biophys Res Commun. 1967 Apr 20;27(2):157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- Schenk P., Woods A. S., Gibson W. The 45-kilodalton protein of cytomegalovirus (Colburn) B-capsids is an amino-terminal extension form of the assembly protein. J Virol. 1991 Mar;65(3):1525–1529. doi: 10.1128/jvi.65.3.1525-1529.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman G., Bachenheimer S. L. Characterization of intranuclear capsids made by ts morphogenic mutants of HSV-1. Virology. 1988 Apr;163(2):471–480. doi: 10.1016/0042-6822(88)90288-7. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch A. R., McNally L. M., Gibson W. Cytomegalovirus assembly protein nested gene family: four 3'-coterminal transcripts encode four in-frame, overlapping proteins. J Virol. 1991 Aug;65(8):4091–4100. doi: 10.1128/jvi.65.8.4091-4100.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wychowski C., van der Werf S., Siffert O., Crainic R., Bruneau P., Girard M. A poliovirus type 1 neutralization epitope is located within amino acid residues 93 to 104 of viral capsid polypeptide VP1. EMBO J. 1983;2(11):2019–2024. doi: 10.1002/j.1460-2075.1983.tb01694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]