Abstract
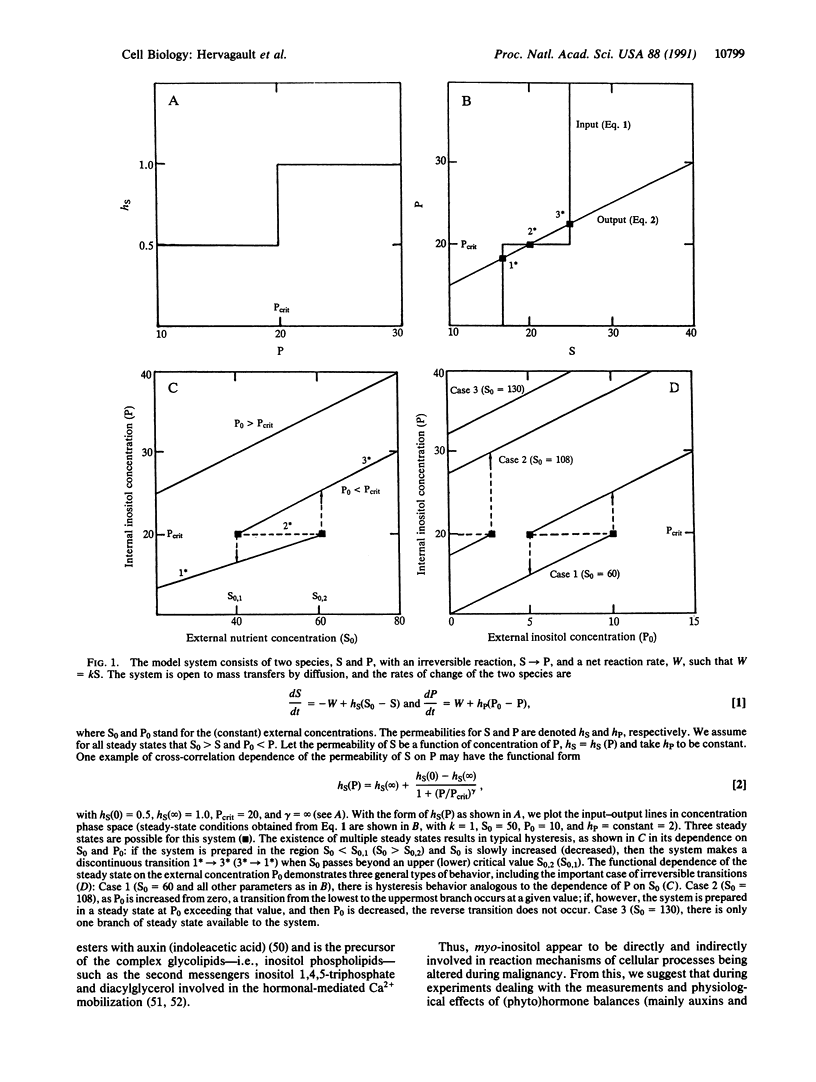
We offer a plausible interpretation of some experiments on the reversal of neoplastic transformations in plants. We suggest that normal cells and tumorous cells represent multiple stable-steady states corresponding to a reaction feedback mechanism. The (autocatalytic) feedback loop is constructed from observations on the role played by myo-inositol: it increases the permeability of ions through the membrane and the biosynthetic pathway to myo-inositol is activated by ions. Provided that the permeabilities of nutrients (sugars and salts) are a product-enhanced function of myo-inositol, then we have a (oversimplified) model that can exhibit multiple stationary stable states, one or two depending on the exogenous nutrients and myo-inositol concentrations, and reversible and irreversible transitions from one of these states to the other are possible. From this model, straightforward simple experiments are suggested. We also propose that recent models dealing with the intracellular calcium regulation by hormones, where one key step requires the hydrolysis of inositol phospholipids, take into account free myo-inositol and endogenous hormone concentrations (e.g., auxins).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amasino R. M., Powell A. L., Gordon M. P. Changes in T-DNA methylation and expression are associated with phenotypic variation and plant regeneration in a crown gall tumor line. Mol Gen Genet. 1984;197(3):437–446. doi: 10.1007/BF00329940. [DOI] [PubMed] [Google Scholar]
- BRAUN A. C., WOOD H. N. On the activation of certain essential biosynthetic systems in cells of Vinca rosea L. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1776–1782. doi: 10.1073/pnas.48.10.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babloyantz A., Nicolis G. Chemical instabilities and multiple steady state transitions in Monod-Jacob type models. J Theor Biol. 1972 Jan;34(1):185–192. doi: 10.1016/0022-5193(72)90062-8. [DOI] [PubMed] [Google Scholar]
- Babloyantz A., Sanglier M. Chemical instabilities of "all-or-none" type in beta - galactosidase induction and active transport. FEBS Lett. 1972 Jul 1;23(3):364–366. doi: 10.1016/0014-5793(72)80317-x. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Braun A. C. A DEMONSTRATION OF THE RECOVERY OF THE CROWN-GALL TUMOR CELL WITH THE USE OF COMPLEX TUMORS OF SINGLE-CELL ORIGIN. Proc Natl Acad Sci U S A. 1959 Jul;45(7):932–938. doi: 10.1073/pnas.45.7.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN M., HORIBATA K. Inhibition by glucose of the induced synthesis of the beta-galactoside-enzyme system of Escherichia coli. Analysis of maintenance. J Bacteriol. 1959 Nov;78:601–612. doi: 10.1128/jb.78.5.601-612.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T. Y. Induction of indoleacetic Acid synthetases in tobacco pith explants. Plant Physiol. 1972 Dec;50(6):723–727. doi: 10.1104/pp.50.6.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Macphee C. H. myo-inositol metabolites as cellular signals. Eur J Biochem. 1990 Oct 5;193(1):1–18. doi: 10.1111/j.1432-1033.1990.tb19297.x. [DOI] [PubMed] [Google Scholar]
- Durand-Tardif M., Broglie R., Slightom J., Tepfer D. Structure and expression of Ri T-DNA from Agrobacterium rhizogenes in Nicotiana tabacum. Organ and phenotypic specificity. J Mol Biol. 1985 Dec 5;186(3):557–564. doi: 10.1016/0022-2836(85)90130-5. [DOI] [PubMed] [Google Scholar]
- Eisen H., Brachet P., Pereira da Silva L., Jacob F. Regulation of repressor expression in lambda. Proc Natl Acad Sci U S A. 1970 Jul;66(3):855–862. doi: 10.1073/pnas.66.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelvin S. B. Crown gall disease and hairy root disease : a sledgehammer and a tackhammer. Plant Physiol. 1990 Feb;92(2):281–285. doi: 10.1104/pp.92.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn H. S., Ortoleva P. J., Ross J. Chemical oscillations and multiple steady states due to variable boundary permeability. J Theor Biol. 1973 Oct;41(3):503–521. doi: 10.1016/0022-5193(73)90058-1. [DOI] [PubMed] [Google Scholar]
- Halpaap-Wood K., Horning E. C., Horning M. G. The effect of phenobarbital and beta-naphthoflavone induction on the metabolism of biphenyl in the rat and mouse. Drug Metab Dispos. 1981 Mar-Apr;9(2):97–102. [PubMed] [Google Scholar]
- John M. C., Amasino R. M. Extensive changes in DNA methylation patterns accompany activation of a silent T-DNA ipt gene in Agrobacterium tumefaciens-transformed plant cells. Mol Cell Biol. 1989 Oct;9(10):4298–4303. doi: 10.1128/mcb.9.10.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmers M., De Beuckeleer M., Holsters M., Zambryski P., Depicker A., Hernalsteens J. P., Van Montagu M., Schell J. Internal organization, boundaries and integration of Ti-plasmid DNA in nopaline grown gall tumours. J Mol Biol. 1980 Dec 15;144(3):353–376. doi: 10.1016/0022-2836(80)90095-9. [DOI] [PubMed] [Google Scholar]
- MONOD J., JACOB F. Teleonomic mechanisms in cellular metabolism, growth, and differentiation. Cold Spring Harb Symp Quant Biol. 1961;26:389–401. doi: 10.1101/sqb.1961.026.01.048. [DOI] [PubMed] [Google Scholar]
- Marco Y., Rochaix J. D. Organization of the nuclear ribosomal DNA of Chlamydomonas reinhardii. Mol Gen Genet. 1980;177(4):715–723. doi: 10.1007/BF00272684. [DOI] [PubMed] [Google Scholar]
- Meins F., Jr Habituation: heritable variation in the requirement of cultured plant cells for hormones. Annu Rev Genet. 1989;23:395–408. doi: 10.1146/annurev.ge.23.120189.002143. [DOI] [PubMed] [Google Scholar]
- Meins F., Jr Stability of the tumor phenotype in crown gall tumors of tobacco. Prog Exp Tumor Res. 1972;15:93–109. doi: 10.1159/000392510. [DOI] [PubMed] [Google Scholar]
- Meyer T., Stryer L. Molecular model for receptor-stimulated calcium spiking. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5051–5055. doi: 10.1073/pnas.85.14.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanney D. L. EPIGENETIC CONTROL SYSTEMS. Proc Natl Acad Sci U S A. 1958 Jul 15;44(7):712–717. doi: 10.1073/pnas.44.7.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick A., Weiner M. ENZYME INDUCTION AS AN ALL-OR-NONE PHENOMENON. Proc Natl Acad Sci U S A. 1957 Jul 15;43(7):553–566. doi: 10.1073/pnas.43.7.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooms G., Hooykaas P. J., Van Veen R. J., Van Beelen P., Regensburg-Tuïnk T. J., Schilperoort R. A. Octopine Ti-plasmid deletion mutants of agrobacterium tumefaciens with emphasis on the right side of the T-region. Plasmid. 1982 Jan;7(1):15–29. doi: 10.1016/0147-619x(82)90023-3. [DOI] [PubMed] [Google Scholar]
- Otten L., De Greve H., Hernalsteens J. P., Van Montagu M., Schieder O., Straub J., Schell J. Mendelian transmission of genes introduced into plants by the Ti plasmids of Agrobacterium tumefaciens. Mol Gen Genet. 1981;183(2):209–213. doi: 10.1007/BF00270619. [DOI] [PubMed] [Google Scholar]
- Shaffer B. M. Secretion of cyclic AMP induced by cyclic AMP in the cellular slime mould Dictyostelium discoideum. Nature. 1975 Jun 12;255(5509):549–552. doi: 10.1038/255549a0. [DOI] [PubMed] [Google Scholar]
- Tepfer D. Transformation of several species of higher plants by Agrobacterium rhizogenes: sexual transmission of the transformed genotype and phenotype. Cell. 1984 Jul;37(3):959–967. doi: 10.1016/0092-8674(84)90430-6. [DOI] [PubMed] [Google Scholar]
- Thomashow M. F., Hugly S., Buchholz W. G., Thomashow L. S. Molecular basis for the auxin-independent phenotype of crown gall tumor tissues. Science. 1986 Feb 7;231(4738):616–618. doi: 10.1126/science.3511528. [DOI] [PubMed] [Google Scholar]
- Turgeon R., Wood H. N., Braun A. C. Studies on the recovery of crown gall tumor cells. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3562–3564. doi: 10.1073/pnas.73.10.3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOD H. N., BRAUN A. C. Studies on the regulation of certain essential biosynthetic systems in normal and crown-gall tumor cells. Proc Natl Acad Sci U S A. 1961 Dec 15;47:1907–1913. doi: 10.1073/pnas.47.12.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood H. N., Braun A. C. Studies on the net uptake of solutes by normal and crown-gall tumor cells. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1532–1538. doi: 10.1073/pnas.54.6.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wullems G. J., Molendijk L., Ooms G., Schilperoort R. A. Retention of tumor markers in F1 progeny plants from in vitro induced octopine and nopaline tumor tissues. Cell. 1981 Jun;24(3):719–727. doi: 10.1016/0092-8674(81)90098-2. [DOI] [PubMed] [Google Scholar]
- Yang F., Montoya A. L., Merlo D. J., Drummond M. H., Chilton M. D., Nester E. W., Gordon M. P. Foreign DNA sequences in crown gall teratomas and their fate during the loss of the tumorous traits. Mol Gen Genet. 1980;177(4):707–714. doi: 10.1007/BF00272683. [DOI] [PubMed] [Google Scholar]



