Abstract
A 22-year-old woman reports having hirsutism and irregular menses. She describes unpredictable and infrequent menses (five or six per year) since menarche at 11 years of age. Dark, coarse facial hair began to develop at 13 years of age. The symptoms worsened after she gained weight in college. The physical examination includes a body-mass index (BMI; the weight in kilograms divided by the square of the height in meters) of 29, blood pressure of 135/85 mm Hg, and moderate hirsutism without virilization. Laboratory tests reveal a total testosterone level of 65 ng per deciliter (2.3 nmol per liter) (assay reference range, 14 to 53 ng per deciliter [0.5 to 1.8 nmol per liter]), calculated free testosterone level of 15.3 pg per milliliter (53.1 pmol per liter) (assay reference range, 0.6 to 6.8 pg per milliliter [2.1 to 23.6 pmol per liter]), and glycated hemoglobin level of 5.7% (normal value, ≤5.6%). How should this case be evaluated and managed?
THE CLINICAL PROBLEM
The polycystic ovary syndrome is a disorder that is characterized by hyperandrogenism, ovulatory dysfunction, and polycystic ovarian morphologic features. As defined by the diagnostic criteria of the National Institutes of Health (i.e., hyperandrogenism plus ovulatory dysfunction), “classic” polycystic ovary syndrome affects 6 to 10% of women of reproductive age, but the prevalence may be twice as high under the broader Rotterdam criteria (Table 1).1 Manifestations of androgen excess (e.g., hirsutism) may cause substantial distress in patients, and the polycystic ovary syndrome is the most common cause of anovulatory infertility.
Table 1.
Diagnostic Criteria for the Polycystic Ovary Syndrome.
| Variable | National Institutes of Health | Rotterdam | Androgen Excess and PCOS Society |
|---|---|---|---|
| Hyperandrogenism* | Hyperandrogenism required | Any two of the three features (hyperandrogenism, ovulatory dysfunction, polycystic ovarian morphologic features) required | Hyperandrogenism required |
| Oligo-ovulation or anovulation† | Ovulatory dysfunction required | Any two of the three features (hyperandrogenism, ovulatory dysfunction, polycystic ovarian morphologic features) required | Either ovulatory dysfunction or polycystic ovarian morphologic features required |
| Polycystic ovarian morphologic features‡ | Not applicable | Any two of the three features (hyperandrogenism, ovulatory dysfunction, polycystic ovarian morphologic features) required | Either ovulatory dysfunction or polycystic ovarian morphologic features required |
| No. of combinations that meet criteria for the polycystic ovary syndrome | Two (hyperandrogenism plus ovulatory dysfunction; hyperandrogenism plus ovulatory dysfunction plus polycystic ovarian morphologic features) | Four (hyperandrogenism plus ovulatory dysfunction plus polycystic ovarian morphologic features; hyperandrogenism plus ovulatory dysfunction; hyperandrogenism plus polycystic ovarian morphologic features; ovulatory dysfunction plus polycystic ovarian morphologic features) | Three (hyperandrogenism plus ovulatory dysfunction plus polycystic ovarian morphologic features; hyperandrogenism plus ovulatory dysfunction; hyperandrogenism plus polycystic ovarian morphologic features) |
Evidence of hyperandrogenism can be clinical, biochemical, or both.
Ovulatory dysfunction frequently manifests as unpredictable menses at intervals of less than 21 or more than 35 days, but it may also be present in patients who have hyperandrogenism with apparent eumenorrhea.
Polycystic ovarian morphologic features are defined as 12 or more antral follicles (2 to 9 mm in diameter) in either ovary, an ovarian volume that is greater than 10 ml in either ovary, or both.
This complex polygenic disorder has environmental influences (e.g., those that contribute to obesity).1 Many studies suggest that inherent abnormalities of ovarian steroidogenesis and follicular development play a role in the polycystic ovary syndrome. The syndrome is also associated with persistently rapid gonadotropin-releasing hormone pulses, an excess of luteinizing hormone, and insufficient follicle-stimulating hormone (FSH) secretion, which contribute to excessive ovarian androgen production and ovulatory dysfunction. In addition, many women with the polycystic ovary syndrome have insulin resistance, and compensatory hyperinsulinemia enhances ovarian (and adrenal) androgen production and increases androgen bioavailability through reduced levels of sex hormone–binding globulin. Genomewide association studies implicate many genes, including those for gonadotropin receptors, the beta subunit of FSH, insulin receptor, differentially expressed in normal and neoplastic cells domain-containing protein 1A (DENND1A), and thyroid adenoma-associated protein (THADA). “Developmental programming” through environmental or hormonal imprinting may also contribute to the development of the polycystic ovary syndrome. Various pathophysiological factors (Fig. 1, and Fig. S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org) may play different relative roles in individual patients.1
Figure 1. Basic Pathophysiology of Hyperandrogenemia in the Polycystic Ovary Syndrome.
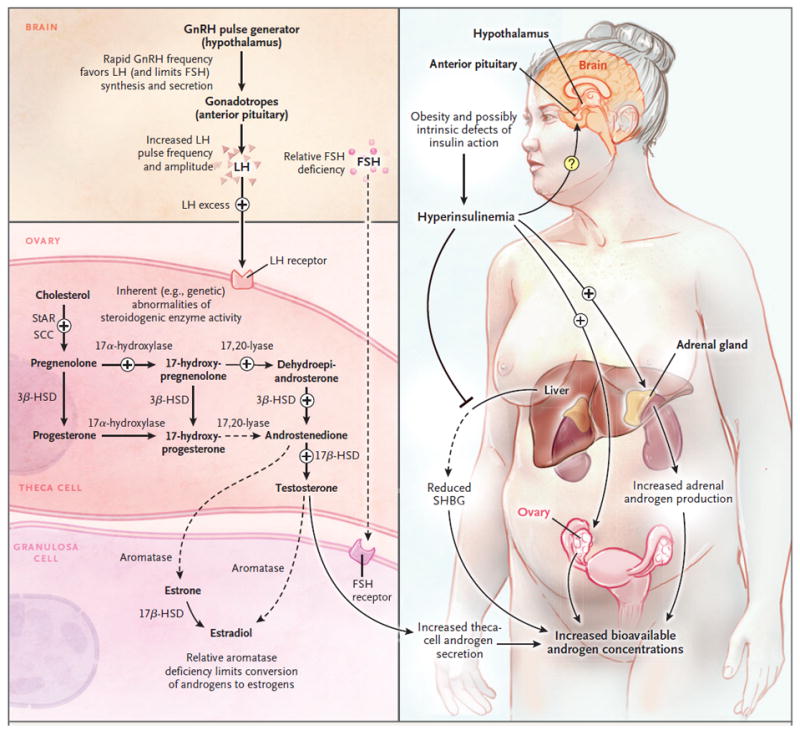
Since ovarian steroidogenesis requires gonadotropin stimulation, luteinizing hormone (LH) is a key factor in the hyperandrogenemia of the polycystic ovary syndrome.2 Progesterone is the primary regulator of gonadotropin-releasing hormone (GnRH) pulse frequency; however, in the polycystic ovary syndrome, the GnRH pulse generator is relatively resistant to the negative feedback effects of progesterone.3 This resistance to progesterone negative feedback appears to be mediated by androgen excess (since it is reversed by the androgen-receptor blocker flutamide).4 Resulting high GnRH pulse frequencies favor production of LH and limit production of follicle-stimulating hormone (FSH), which promote androgen production and interfere with normal follicular development. The polycystic ovary syndrome is associated with inherent abnormalities of ovarian (and adrenal) steroidogenesis: cultured ovarian theca cells from women with the polycystic ovary syndrome secrete excess androgens and precursors,5 and women with the polycystic ovary syndrome have exaggerated ovarian steroidogenic responses to gonadotropin stimulation.6 A recent study suggests that increased expression of a DENND1A splice variant drives a polycystic ovary syndrome–like steroidogenic phenotype in theca cells.7 The polycystic ovary syndrome is associated with insulin resistance — which is at least partly independent of obesity — and compensatory hyperinsulinemia.8 Hyperinsulinemia contributes to hyperandrogenemia in several ways: it augments LH-stimulated androgen production by ovarian theca cells, it potentiates corticotropin-mediated adrenal androgen production, and it inhibits hepatic synthesis of sex hormone–binding globulin (SHBG), which increases free testosterone levels. The precise effects of hyperinsulinemia on gonadotropin secretion remain unclear. 3ß-HSD denotes 3ß-hydroxysteroid dehydrogenase, 17ß-HSD 17ß-hydroxysteroid dehydrogenase, SCC cholesterol side-chain cleavage enzyme, and StAR steroidogenic acute regulatory protein.
The polycystic ovary syndrome is associated with cardiometabolic abnormalities and possibly an increased risk of cardiovascular disease.9 Among women with this syndrome, 50 to 80% are obese.1 Impaired glucose tolerance is reported in 30 to 35% of U.S. women with classic polycystic ovary syndrome, and type 2 diabetes mellitus is reported in 8 to 10%; the risk of these conditions is influenced by age, adiposity, and a family history of diabetes.10,11 Women with the polycystic ovary syndrome have lower high-density lipoprotein cholesterol and higher triglyceride and low-density lipoprotein (LDL) cholesterol levels than women without the syndrome. Differences in LDL cholesterol levels are at least partly independent of differences in BMI.12
Subclinical vascular disease (e.g., impaired endothelial function, increased carotid-artery intima–media thickness, and elevated coronary-artery calcium scores) has also been reported in women with the polycystic ovary syndrome and appears to be at least partly independent of adiposity.1,9 Although some studies suggest a higher incidence of cardiovascular events among postmenopausal women with a presumed history of the polycystic ovary syndrome, data are insufficient to address rates of cardiovascular events among premenopausal women with this syndrome.1,9
The risk of endometrial cancer is estimated to be 2.7 times as high among women with the polycystic ovary syndrome as among women without the syndrome, and the lifetime risk of endometrial cancer among women with the syndrome has been estimated to be as high as 9%.13 Risk factors for endometrial cancer among women with the polycystic ovary syndrome include anovulation, obesity, and insulin resistance; the risk related to chronic anovulation reflects prolonged estrogen-mediated mitogenic stimulation of the endometrium with inadequate progesterone exposure for endometrial differentiation.13 Women with the polycystic ovary syndrome also have increased risks of pregnancy complications (e.g., gestational diabetes and preeclampsia),14 obstructive sleep apnea,15 and emotional distress (e.g., depression and anxiety).16
STRATEGIES AND EVIDENCE
DIAGNOSIS
Three sets of criteria for the polycystic ovary syndrome in women have been developed.17-19 Each set involves different combinations of hyperandrogenism, ovulatory dysfunction, and polycystic ovarian morphologic features (Table 1).
Hyperandrogenism can be clinical (e.g., hirsutism and acne), biochemical (e.g., elevated serum androgen levels), or both. Hirsutism, or excessive growth of terminal hair that appears in a male pattern, may be quantified with the use of the modified Ferriman–Gallwey score. This scoring system involves visual grading of hair growth over nine androgen-sensitive areas (each area graded on a scale from 0 to 4, with 0 indicating no terminal hair growth and 4 indicating marked terminal hair growth), and all individual scores are summed to obtain a final score; a score of 8 or more is typically considered abnormal, although lower thresholds are appropriate for women of eastern Asian descent and higher scores may be more appropriate for women of Hispanic, Mediterranean, and Middle Eastern descent (Fig. S2 in the Supplementary Appendix).1
Assessment of hyperandrogenemia requires reliable androgen assays. Total testosterone assays are relatively inaccurate at the lower levels detected in women, although mass spectrometry–based assays of total testosterone may perform better.20 Free testosterone is the most sensitive test for hyperandrogenemia in women with the polycystic ovary syndrome,1 but direct free testosterone assays are notoriously inaccurate; accordingly, it is more appropriate to calculate free testosterone with the use of measurements of total testosterone and sex hormone–binding globulin.20
Some experts have argued that hyperandrogenism should be required for a diagnosis of the polycystic ovary syndrome because it best identifies women at risk for coexisting metabolic conditions. As a group, women with only ovulatory dysfunction and polycystic ovaries have lower cardiometabolic risk than women with classic polycystic ovary syndrome.19
Ovulatory dysfunction is typically indicated by unpredictable menses that occur at less than 21-day or greater than 35-day intervals. However, regular menses that occur every 21 to 35 days do not confirm normal ovulatory function in women with hyperandrogenism: 15 to 40% of women with hyperandrogenism and regular menses have ovulatory dysfunction.21
Polycystic ovarian morphologic features are currently defined as 12 or more antral follicles (2 to 9 mm in diameter) in either ovary, an ovarian volume that is greater than 10 ml in either ovary, or both.18 A transvaginal transducer with frequencies of 8 MHz or greater commonly detects an antral follicle count in this range in asymptomatic women (in ≥50% of asymptomatic women in some series), and some experts recommend a criterion with a higher antral follicle count (≥25) for sufficient specificity.22 Appropriate use of either criterion requires an experienced ultrasonographer; an unqualified report of “polycystic ovaries” is inadequate for diagnostic purposes. Ovarian ultrasonography is not required for diagnosis when both hyperandrogenism and ovulatory dysfunction are present.
In many patients, the polycystic ovary syndrome manifests in adolescence with the lack of establishment of regular menses and the gradual emergence of hirsutism. However, some findings of the polycystic ovary syndrome (e.g., oligomenorrhea, acne, and polycystic-appearing ovaries) overlap with those of normal puberty. Emerging consensus suggests that a reliable diagnosis of the polycystic ovary syndrome in adolescents may require both unequivocal hyperandrogenism (e.g., moderate-to-severe hirsutism, persistent elevation of serum free testosterone levels, or both) and ovulatory dysfunction that is inappropriate for the developmental stage (e.g., ovulatory dysfunction that persists >2 years after menarche).23
Since features of the polycystic ovary syndrome (hyperandrogenemia, menstrual irregularity, and increases in the antral follicle number and ovarian volume) may improve with advancing age,1 the diagnosis is challenging in perimenopausal or postmenopausal women. However, a clear history of hyperandrogenism and oligomenorrhea may justify a presumptive diagnosis of the polycystic ovary syndrome.24
The polycystic ovary syndrome is a diagnosis of exclusion.17-19 Nonclassic congenital adrenal hyperplasia can closely mimic this syndrome. An early-morning, early follicular-phase plasma level of 17-hydroxyprogesterone of less than 200 ng per deciliter effectively rules out 21-hydroxylase deficiency, which is the most common cause of nonclassic congenital adrenal hyperplasia. Causes of oligomenorrhea and amenorrhea include pregnancy, hyperprolactinemia, hypothyroidism, ovarian failure, and hypogonadotropic hypogonadism (e.g., functional hypothalamic amenorrhea); these entities typically do not cause appreciable hyperandrogenism. Androgen-secreting ovarian or adrenal tumors are rare, but they should be considered in patients with abrupt, rapidly progressive, or severe hyperandrogenism (e.g., virilization, clitoromegaly, a deep voice, and male-pattern baldness), marked hyperandrogenemia (total testosterone level >150 ng per deciliter [5.2 nmol per liter]), or both. Testing for Cushing’s syndrome and acromegaly should be considered in women with other clinical features that indicate these conditions.
Longitudinal screening for cardiometabolic risk factors is recommended in women with the polycystic ovary syndrome.9,24-28 This screening includes measurement of the BMI, waist circumference, and blood pressure at each visit, as well as measurement of fasting lipid levels every 2 years (or sooner if the woman has gained weight).9
Most guidelines regarding the polycystic ovary syndrome suggest screening for impaired glucose tolerance and type 2 diabetes mellitus with a 2-hour oral glucose-tolerance test, repeated every 1 to 5 years depending on the characteristics of the patient (e.g., obesity, a history of gestational diabetes or impaired glucose tolerance, and interval weight gain).9,24-26,28 Measurement of fasting blood glucose levels alone is not recommended for type 2 diabetes screening in women with the polycystic ovary syndrome, although some guidelines suggest that measurement of glycated hemoglobin levels may be an acceptable option.9,24 Laboratory assessments of insulin resistance (e.g., measurements of fasting insulin levels) are not routinely recommended given the imprecision of these assessments and their uncertain clinical usefulness.27-29
Women with the polycystic ovary syndrome should also be screened (by a history, questionnaire, or both) for cigarette smoking, obstructive sleep apnea, depression, and anxiety, with further assessment and treatment as needed.9,24-28 Guidelines do not recommend routine screening for endometrial hyperplasia by means of ultrasonography or biopsy, but they provide support for referral to a gynecologist in patients with prolonged amenorrhea or persistently abnormal vaginal bleeding.24,26,28
MANAGEMENT
Treatment decisions are informed by patient priorities, the likely effectiveness and potential risks of available therapies, and whether the woman wishes to become pregnant (Table 2). Typical therapeutic targets include hirsutism, irregular menses (and the risk of endometrial hyperplasia), and infertility.
Table 2.
Anticipated Effects of Common Therapeutic Options for the Polycystic Ovary Syndrome.*
| Therapeutic Option | Clinical Hyperandrogenism |
Ovulatory Function |
Endometrial Protection |
Reliable Contraception |
Cardiovascular Risk | Practical Considerations |
|---|---|---|---|---|---|---|
| Weight loss (if patient overweight) | Reduced androgen levels likely, but effect on hirsutism uncertain | Variable improvement | Yes, if normal ovulatory function is restored† | No (may increase pregnancy risk) | Expected improvement | No specific diet or exercise regimen has been proved to be superior in the polycystic ovary syndrome. |
| Mechanical hair removal | Improvement expected | — | — | — | — | Typically required even when pharmacologic therapy is used. |
| Oral combined hormonal contraceptives | Improvement expected | Reliable suppression | Yes | Yes | Increased risk of venous thromboembolism; potential increase in blood pressure, triglyceride, and HDL cholesterol levels; possible increase in cardiovascular events (all effects reversed after discontinuation)‡ | One example is ethinyl estradiol (35 μg) plus norgestimate (0.25 mg), but no specific estrogen–progestin combination has been proved to be superior in the polycystic ovary syndrome. Daily ethinyl estradiol doses of 20 to 35 μg are acceptable. Progestins with low androgenic potential include norgestimate, desogestrel, gestodene, and drospirenone; among these, norgestimate may be associated with lower risk of venous thromboembolism.31 Medical eligibility (i.e., potential contraindications) should be considered before and during treatment.§ |
| Spironolactone¶ | Improvement expected | — | — | — | — | Spironolactone may be started at 50 mg twice daily, increasing to 100 mg twice daily as needed. Pregnancy must be strictly avoided in patients who receive spironolactone. |
| Metformin║ | Reduced androgen levels likely, but effect on hirsutism unlikely | Variable improvement | Yes, if normal ovulatory function is restored† | No (may increase pregnancy risk) | Reduced hyperinsulinemia; probable reduced risk of impaired glucose tolerance and type 2 diabetes mellitus; favorable effects on lipid levels possible; possible weight loss (modest); theoretical but unproven benefit with respect to long-term risk of cardiovascular disease | Gastrointestinal side effects of metformin may be limited by starting at a low dose (500 mg daily with a meal), gradually increasing to 1000 mg twice daily with meals. |
| Progestin (episodic administration) | — | — | Yes** | — | — | Oral micronized progesterone (200 mg at bedtime) or oral medroxyprogesterone acetate (5–10 mg daily) for 10–14 days every 1–3 mo. |
| Progestin-only contraceptive pills | — | Variable suppression †† | Yes | Yes | — | Only norethindrone (0.35 mg/day without a hormone-free week) is available in the United States. |
| Levonorgestrel-releasing intrauterine device | — | — | Yes | Yes | — |
Dashes indicate that clinically significant effects are not expected. HDL denotes high-density lipoprotein.
A midluteal progesterone value of 3 to 4 ng per milliliter (10 to 13 nmol per liter) or greater is often described as being adequate evidence of prior ovulation.
Observational data suggest that the use of oral combined hormonal contraceptives may double the risk of myocardial infarction and ischemic stroke. Although any excess risk attributable to oral combined hormonal contraceptives would be minimal among generally healthy women of reproductive age, data to specifically address these risks among women with the polycystic ovary syndrome are lacking.30
According to the Medical Eligibility Criteria for Contraceptive Use of the U.S. Centers for Disease Control and Prevention (2010),32 the risks of combined hormonal contraceptives probably outweigh advantages among women with a number of characteristics and conditions, including (but not limited to) the following: cigarette smoking in women 35 years of age or older, hypertension (even if adequately controlled), hyperlipidemia (depending on the type and severity), diabetes with microvascular disease (e.g., retinopathy, nephropathy, and neuropathy) or a duration of more than 20 years, multiple risk factors for arterial cardiovascular disease, a history of ischemic heart disease or stroke, an increased risk of venous thromboembolism (e.g., a personal history of venous thromboembolism, known thrombophilia, major surgery with prolonged immobilization, and active cancer), a history of malab-sorptive bariatric procedures, migraine headaches in women 35 years of age or older or with an aura, and a history of breast cancer.
The 5α-reductase inhibitor finasteride may also be effective for hyperandrogenism.33,34 As with spironolactone, finasteride must be used with reliable contraception. Use of the androgen-receptor antagonist flutamide is generally inadvisable because of potentially severe hepatotoxicity and high cost.33
Thiazolidinediones may provide similar benefits, but they are not generally recommended for patients with the polycystic ovary syndrome.24,26,28
Induction of withdrawal bleeding every 1 to 3 months is recommended to prevent endometrial hyperplasia.25-27 It is not clear whether a reduction in the risk of endometrial cancer differs according to treatment frequency (e.g., monthly vs. quarterly).
The contraceptive effect of progestin-only contraceptive pills also includes thickening of cervical mucus and endometrial atrophy.
Although subfertility is a characteristic of the polycystic ovary syndrome, pregnancy without medical assistance is common and contraception should be used as indicated. One study suggested that among patients with the polycystic ovary syndrome who had previously attempted to conceive, two thirds had received treatment for infertility at some point in the past and two thirds had had at least one pregnancy without medical assistance.35
Available treatments do not reverse the underlying disorder, although sustained weight loss may be an exception in some obese women with the polycystic ovary syndrome. Weight loss of as little as 5 to 10% can reduce cardiometabolic risk factors and androgen levels and improve menstrual function and possibly fertility.36
Mechanical hair removal (e.g., shaving and plucking) may be adequate to address hirsutism, but when pharmacologic therapy is needed, combined hormonal (estrogen–progestin) oral contraceptives are considered to be first-line agents.24,25,33,37 Combined oral contraceptives suppress gonadotropin secretion and ovarian androgen production, and the estrogen component increases hepatic production of sex hormone–binding globulin, decreasing androgen bioavailability. Combined oral contraceptives reduce new terminal hair growth, although many patients still require concomitant mechanical hair removal for well-established hirsutism. Since changes in the quality of terminal hair begin at the hair root, altered hair shafts may not become visible for up to 6 months. Monophasic combined oral contraceptives containing progestins with low androgenic potential (e.g., ethinyl estradiol [35 μg] plus norgestimate [0.25 mg]) are commonly used in women with the polycystic ovary syndrome, although no particular combined oral contraceptive has proved to be superior for clinical hyperandrogenism.
Other benefits of combined oral contraceptives include amelioration of acne, regular withdrawal bleeding that contributes to prevention of endometrial hyperplasia, and contraception. Although additional delivery methods of combined hormonal contraceptives include extended continuous use of active combined oral contraceptives (i.e., without hormone-free intervals) and nonoral administration (patches and vaginal rings),24 available data on these methods in women with the polycystic ovary syndrome are limited.38,39
Some experts have expressed concern that combined oral contraceptives may increase the risk of cardiovascular disease among women with the polycystic ovary syndrome. Combined oral contraceptives may increase blood pressure and triglyceride levels; their effects on LDL cholesterol levels depend on progestin androgenicity.24 Available data do not support a meaningful effect of combined oral contraceptives on glucose metabolism.24,40,41 Combined oral contraceptives increase the risk of venous thromboembolism; this risk is also influenced by increasing age, smoking, obesity, and the specific progestin used.40 When prescribing combined hormonal contraceptives, providers should carefully consider medical eligibility criteria such as those of the U.S. Centers for Disease Control and Prevention.32
Oral spironolactone is an androgen-receptor antagonist that can reduce the growth of terminal hair.33,34 Spironolactone is typically used as an add-on therapy to combined oral contraceptives. Antiandrogens may disrupt androgen-dependent processes (e.g., formation of external genitalia) in a male fetus; this mandates concomitant use of reliable contraception. Moreover, limited data from randomized trials suggest that combined oral contraceptives and antiandrogens have additive effects on hirsutism.34 Spironolactone antagonizes the mineralocorticoid receptor, so adverse effects can include hyperkalemia.
Metformin reduces hyperinsulinemia and lowers serum testosterone levels by approximately 20 to 25% in women with the polycystic ovary syndrome. However, its effects on hirsutism are modest at best,42 and it is not recommended for this indication.33 Metformin may improve ovulatory function. Meta-analyses of randomized, placebo-controlled trials involving women with the polycystic ovary syndrome have shown increased pregnancy rates but not increased live birth rates among women who received metformin43; this agent is not recommended as a first-line agent for anovulatory infertility.24,27,28 Metformin is recommended for women with the polycystic ovary syndrome and impaired glucose tolerance or type 2 diabetes that does not respond adequately to lifestyle modification.24,27 Given the favorable metabolic effects of metformin, it is plausible that it could provide a long-term cardiovascular benefit to women with the polycystic ovary syndrome, but confirmatory data are lacking.
Prevention of endometrial hyperplasia (endometrial protection) may be achieved with the use of combined hormonal contraceptives, intermittent or continuous progestin therapy, or a levonorgestrel-releasing intrauterine device (IUD) (Table 2). When episodic progestins are used, induction of withdrawal bleeding every 1 to 3 months is recommended.25-27 If regular menses are restored with lifestyle modification, metformin, or both, it may be prudent to seek objective evidence of ovulation (e.g., a plasma level of progesterone ≥3 to 4 ng per milliliter a week before anticipated menses) because apparent eumenorrhea does not confirm regular ovulation in women with the polycystic ovary syndrome.1,21
Clomiphene is generally considered to be the first-line agent for induction of ovulation in women with the polycystic ovary syndrome.24,27,28 A randomized trial of ovulation induction involving women with the polycystic ovary syndrome and infertility showed a higher live birth rate among women who received clomiphene than among women who received metformin alone (22.5% vs. 7.2%).44 However, a subsequent randomized trial showed a higher live birth rate among women who received the aromatase inhibitor letrozole than among women who received clomiphene (27.5% vs. 19.1%)45; this suggests a potential role for letrozole as initial therapy.24 In some cases, ovarian stimulation with exogenous gonadotropins or advanced reproductive techniques (e.g., in vitro fertilization) may be required.46
AREAS OF UNCERTAINTY
The precise role and the best definition of polycystic ovarian morphologic features as a diagnostic criterion is controversial. Although serum antimüllerian hormone levels correlate with the ultrasonographically determined antral follicle count and ovarian volume, the diagnostic usefulness of measurement of these levels in women with the polycystic ovary syndrome is uncertain.22 The practical implications of the various subtypes of the polycystic ovary syndrome allowed by the Rotterdam criteria (e.g., the polycystic ovary syndrome with hyperandrogenism versus ovulatory dysfunction and polycystic ovaries without hyperandrogenism) remain unclear. There is controversy regarding thresholds for diagnosis in adolescents and the most appropriate therapeutic approaches for these patients (e.g., addressing underlying metabolic abnormalities with metformin rather than targeting symptoms with combined oral contraceptives).
It is unclear whether metformin should be used as primary therapy in some patients (e.g., obese adults) or whether the addition of metformin to combined oral contraceptives provides substantial benefits. Most therapeutic studies involving women with the polycystic ovary syndrome have been relatively short (e.g., ≤12 months), and data regarding the potential risks of combined oral contraceptives are derived largely from the general population. The long-term risk–benefit ratios of various treatment approaches in patients with the polycystic ovary syndrome remain uncertain. Best practices for lifestyle management and weight loss in these patients are unknown. The absolute risks of cardiovascular disease and endometrial cancer among women with the polycystic ovary syndrome are unclear, as are the most appropriate screening and prevention strategies.
GUIDELINES
Guidelines, best practices, and consensus statements for the polycystic ovary syndrome have been published by the Endocrine Society,24 the European Society of Endocrinology,27 the PCOS Australian Alliance,25 jointly by the European Society of Human Reproduction and Embryology and the American Society for Reproductive Medicine,26 and jointly by the American Association of Clinical Endocrinologists and the Androgen Excess and PCOS Society.28,37 Most endorse the Rotterdam diagnostic criteria.24,25,37 This review is generally consistent with these recommendations.
CONCLUSIONS AND RECOMMENDATIONS
The patient in the vignette has hyperandrogenism and oligomenorrhea, so ovarian ultrasonography is not needed for diagnosis; however, nonclassic congenital adrenal hyperplasia, hyperprolactinemia, and hypothyroidism should be ruled out. She should also be screened for metabolic abnormalities, including glucose intolerance and dyslipidemia. Although most guidelines suggest screening for impaired glucose tolerance and type 2 diabetes with the use of the 2-hour oral glucose-tolerance test, we favor measurement of the glycated hemoglobin level47 for initial screening given enhanced patient convenience. We would consider a 2-hour oral glucose-tolerance test for patients who have a glycated hemoglobin level that is approaching but below the diagnostic threshold for diabetes of 6.5% (e.g., a glycated hemoglobin level ≥6%). The patient should also be asked about cigarette smoking and symptoms of obstructive sleep apnea, depression, and anxiety, and further assessment and treatment should be provided as needed.
The patient’s primary concerns are hirsutism and irregular menses. Given her borderline glycated hemoglobin level and overweight status, counseling regarding diet, exercise, and weight loss is important. In the absence of contraindications, we would recommend a monophasic combined oral contraceptive containing a low androgenic progestin (e.g., ethinyl estradiol [35 μg] plus norgestimate [0.25 mg]) to ameliorate hirsutism, provide predictable withdrawal bleeding, and prevent endometrial hyperplasia. At 3 months, our clinical assessment would include measurement of blood pressure and fasting lipid levels to rule out adverse effects. If the patient is not satisfied with regression of hirsutism after receiving combined oral contraceptives for 6 to 9 months, we would recommend the addition of spironolactone with monitoring of serum potassium levels. In addition, if normalization of the glycated hemoglobin level has not been achieved with lifestyle modification, we would suggest the addition of metformin.
If the patient has contraindications to or declines combined oral contraceptives, therapeutic options would include mechanical hair removal and spironolactone (with reliable contraception). To prevent endometrial hyperplasia, we would recommend a course of progestin every 1 to 3 months, or, if contraception is required, a daily progestin-only contraceptive pill (e.g., norethindrone [0.35 mg]) or a levonorgestrel-releasing IUD. Alternatively, if eumenorrhea follows weight loss, metformin use, or both, confirmation of ovulatory menses (i.e., luteal progesterone levels) would provide evidence of adequate endometrial protection. The patient can be counseled that live birth can be achieved by a majority of women with the polycystic ovary syndrome, although fertility treatment may be required.
Supplementary Material
KEY CLINICAL POINTS.
POLYCYSTIC OVARY SYNDROME
The polycystic ovary syndrome is diagnosed in women with at least two of the following otherwise unexplained abnormalities: hyperandrogenism (clinical, biochemical, or both), ovulatory dysfunction, and polycystic ovarian morphologic features.
Women with the polycystic ovary syndrome are at increased risk for infertility, endometrial hyperplasia and cancer, abnormal glucose metabolism, dyslipidemia, obstructive sleep apnea, depression, and anxiety.
Nonpharmacologic therapies play key roles in the treatment of the polycystic ovary syndrome. Lifestyle modification is important for patients who are (or are at risk for being) overweight or obese and in those with other coexisting metabolic conditions. Mechanical hair removal (e.g., shaving) is an important treatment strategy in patients with hirsutism.
Combined (estrogen–progestin) oral contraceptives are considered to be the first-line pharmacologic therapy for the classic symptoms of the polycystic ovary syndrome. They ameliorate hyperandrogenism (e.g., hirsutism), result in predictable withdrawal bleeding, and provide reliable endometrial protection and contraception.
Additional pharmacologic therapies may include spironolactone (with appropriate contraception) for hirsutism, episodic or continuous progestin therapy for endometrial protection, metformin for abnormal glucose tolerance, and clomiphene for ovulation induction.
Footnotes
Dr. Marshall reports receiving fees for serving on an advisory board from AstraZeneca and consulting fees from Euroscreen and Millendo Therapeutics (previously Atterocor). No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Dumesic DA, Oberfield SE, Stener-Victorin E, Marshall JC, Laven JS, Legro RS. Scientific statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocr Rev. 2015;36:487–525. doi: 10.1210/er.2015-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burt Solorzano CM, Beller JP, Abshire MY, Collins JS, McCartney CR, Marshall JC. Neuroendocrine dysfunction in polycystic ovary syndrome. Steroids. 2012;77:332–7. doi: 10.1016/j.steroids.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pastor CL, Griffin-Korf ML, Aloi JA, Evans WS, Marshall JC. Polycystic ovary syndrome: evidence for reduced sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab. 1998;83:582–90. doi: 10.1210/jcem.83.2.4604. [DOI] [PubMed] [Google Scholar]
- 4.Eagleson CA, Gingrich MB, Pastor CL, et al. Polycystic ovarian syndrome: evidence that flutamide restores sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab. 2000;85:4047–52. doi: 10.1210/jcem.85.11.6992. [DOI] [PubMed] [Google Scholar]
- 5.Nelson VL, Legro RS, Strauss JF, III, McAllister JM. Augmented androgen production is a stable steroidogenic phenotype of propagated theca cells from polycystic ovaries. Mol Endocrinol. 1999;13:946–57. doi: 10.1210/mend.13.6.0311. [DOI] [PubMed] [Google Scholar]
- 6.Ehrmann DA, Barnes RB, Rosenfield RL. Polycystic ovary syndrome as a form of functional ovarian hyperandrogenism due to dysregulation of androgen secretion. Endocr Rev. 1995;16:322–53. doi: 10.1210/edrv-16-3-322. [DOI] [PubMed] [Google Scholar]
- 7.McAllister JM, Modi B, Miller BA, et al. Overexpression of a DENND1A isoform produces a polycystic ovary syndrome theca phenotype. Proc Natl Acad Sci U S A. 2014;111(15):E1519–27. doi: 10.1073/pnas.1400574111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. 2012;33:981–1030. doi: 10.1210/er.2011-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wild RA, Carmina E, Diamanti-Kandarakis E, et al. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. J Clin Endocrinol Metab. 2010;95:2038–49. doi: 10.1210/jc.2009-2724. [DOI] [PubMed] [Google Scholar]
- 10.Ehrmann DA, Barnes RB, Rosenfield RL, Cavaghan MK, Imperial J. Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care. 1999;22:141–6. doi: 10.2337/diacare.22.1.141. [DOI] [PubMed] [Google Scholar]
- 11.Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab. 1999;84:165–9. doi: 10.1210/jcem.84.1.5393. [DOI] [PubMed] [Google Scholar]
- 12.Wild RA, Rizzo M, Clifton S, Carmina E. Lipid levels in polycystic ovary syndrome: systematic review and meta-analysis. Fertil Steril. 2011;95(3):1073–9.e1. doi: 10.1016/j.fertnstert.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 13.Dumesic DA, Lobo RA. Cancer risk and PCOS. Steroids. 2013;78:782–5. doi: 10.1016/j.steroids.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Boomsma CM, Eijkemans MJ, Hughes EG, Visser GH, Fauser BC, Macklon NS. A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum Reprod Update. 2006;12:673–83. doi: 10.1093/humupd/dml036. [DOI] [PubMed] [Google Scholar]
- 15.Tasali E, Van Cauter E, Ehrmann DA. Polycystic ovary syndrome and obstructive sleep apnea. Sleep Med Clin. 2008;3:37–46. doi: 10.1016/j.jsmc.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Veltman-Verhulst SM, Boivin J, Eijkemans MJ, Fauser BJ. Emotional distress is a common risk in women with polycystic ovary syndrome: a systematic review and meta-analysis of 28 studies. Hum Reprod Update. 2012;18:638–51. doi: 10.1093/humupd/dms029. [DOI] [PubMed] [Google Scholar]
- 17.Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Dunaif A, Givens JR, Haseltine FP, Merriam GR, editors. Polycystic ovary syndrome. Boston: Blackwell Scientific; 1992. pp. 377–84. [Google Scholar]
- 18.The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–7. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 19.Azziz R, Carmina E, Dewailly D, et al. Positions statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J Clin Endocrinol Metab. 2006;91:4237–45. doi: 10.1210/jc.2006-0178. [DOI] [PubMed] [Google Scholar]
- 20.Rosner W, Auchus RJ, Azziz R, Sluss PM, Raff H. Position statement: utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society position statement. J Clin Endocrinol Metab. 2007;92:405–13. doi: 10.1210/jc.2006-1864. [DOI] [PubMed] [Google Scholar]
- 21.Azziz R, Carmina E, Dewailly D, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456–88. doi: 10.1016/j.fertnstert.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 22.Dewailly D, Lujan ME, Carmina E, et al. Definition and significance of polycystic ovarian morphology: a task force report from the Androgen Excess and Polycystic Ovary Syndrome Society. Hum Reprod Update. 2014;20:334–52. doi: 10.1093/humupd/dmt061. [DOI] [PubMed] [Google Scholar]
- 23.Witchel SF, Oberfield S, Rosenfield RL, et al. The diagnosis of polycystic ovary syndrome during adolescence. Horm Res Paediatr. 2015 Apr 1; doi: 10.1159/000375530. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 24.Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565–92. doi: 10.1210/jc.2013-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teede HJ, Misso ML, Deeks AA, et al. Assessment and management of polycystic ovary syndrome: summary of an evidence-based guideline. Med J Aust. 2011;195:S65–112. doi: 10.5694/mja11.10915. [DOI] [PubMed] [Google Scholar]
- 26.Fauser BC, Tarlatzis BC, Rebar RW, et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril. 2012;97(1):28–38.e25. doi: 10.1016/j.fertnstert.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 27.Conway G, Dewailly D, Diamanti-Kandarakis E, et al. The polycystic ovary syndrome: a position statement from the European Society of Endocrinology. Eur J Endocrinol. 2014;171:1–29. doi: 10.1530/EJE-14-0253. [DOI] [PubMed] [Google Scholar]
- 28.Goodman NF, Cobin RH, Futterweit W, Glueck JS, Legro RS, Carmina E. American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome — part 2. Endocr Pract. 2015;21:1415–26. doi: 10.4158/EP15748.DSCPT2. [DOI] [PubMed] [Google Scholar]
- 29.Diamanti-Kandarakis E, Kouli C, Alexandraki K, Spina G. Failure of mathematical indices to accurately assess insulin resistance in lean, overweight, or obese women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89:1273–6. doi: 10.1210/jc.2003-031205. [DOI] [PubMed] [Google Scholar]
- 30.Beller JP, McCartney CR. Cardiovascular risk and combined oral contraceptives: clinical decisions in settings of uncertainty. Am J Obstet Gynecol. 2013;208:39–41. doi: 10.1016/j.ajog.2012.01.037. [DOI] [PubMed] [Google Scholar]
- 31.Vinogradova Y, Coupland C, Hippisley-Cox J. Use of combined oral contraceptives and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2015;350:h2135. doi: 10.1136/bmj.h2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.U.S. medical eligibility criteria for contraceptive use, 2010. MMWR Recomm Rep. 2010;59(RR-4):1–86. [PubMed] [Google Scholar]
- 33.Martin KA, Chang RJ, Ehrmann DA, et al. Evaluation and treatment of hirsutism in premenopausal women: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2008;93:1105–20. doi: 10.1210/jc.2007-2437. [DOI] [PubMed] [Google Scholar]
- 34.Swiglo BA, Cosma M, Flynn DN, et al. Antiandrogens for the treatment of hirsutism: a systematic review and meta-analyses of randomized controlled trials. J Clin Endocrinol Metab. 2008;93:1153–60. doi: 10.1210/jc.2007-2430. [DOI] [PubMed] [Google Scholar]
- 35.Hudecova M, Holte J, Olovsson M, Sundström Poromaa I. Long-term follow-up of patients with polycystic ovary syndrome: reproductive outcome and ovarian reserve. Hum Reprod. 2009;24:1176–83. doi: 10.1093/humrep/den482. [DOI] [PubMed] [Google Scholar]
- 36.Moran LJ, Pasquali R, Teede HJ, Hoeger KM, Norman RJ. Treatment of obesity in polycystic ovary syndrome: a position statement of the Androgen Excess and Polycystic Ovary Syndrome Society. Fertil Steril. 2009;92:1966–82. doi: 10.1016/j.fertnstert.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 37.Goodman NF, Cobin RH, Futterweit W, Glueck JS, Legro RS, Carmina E. American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome — part 1. Endocr Pract. 2015;21:1291–300. doi: 10.4158/EP15748.DSC. [DOI] [PubMed] [Google Scholar]
- 38.Legro RS, Pauli JG, Kunselman AR, et al. Effects of continuous versus cyclical oral contraception: a randomized controlled trial. J Clin Endocrinol Metab. 2008;93:420–9. doi: 10.1210/jc.2007-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White T, Jain JK, Stanczyk FZ. Effect of oral versus transdermal steroidal contraceptives on androgenic markers. Am J Obstet Gynecol. 2005;192:2055–9. doi: 10.1016/j.ajog.2005.02.067. [DOI] [PubMed] [Google Scholar]
- 40.Domecq JP, Prutsky G, Mullan RJ, et al. Adverse effects of the common treatments for polycystic ovary syndrome: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2013;98:4646–54. doi: 10.1210/jc.2013-2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lopez LM, Grimes DA, Schulz KF. Steroidal contraceptives: effect on carbohydrate metabolism in women without diabetes mellitus. Cochrane Database Syst Rev. 2014;4 doi: 10.1002/14651858.CD006133.pub5. CD006133. [DOI] [PubMed] [Google Scholar]
- 42.Cosma M, Swiglo BA, Flynn DN, et al. Insulin sensitizers for the treatment of hirsutism: a systematic review and metaanalyses of randomized controlled trials. J Clin Endocrinol Metab. 2008;93:1135–42. doi: 10.1210/jc.2007-2429. [DOI] [PubMed] [Google Scholar]
- 43.Tang T, Lord JM, Norman RJ, Yasmin E, Balen AH. Insulin-sensitising drugs (metformin, rosiglitazone, pioglitazone, D-chiro-inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database Syst Rev. 2012;5 doi: 10.1002/14651858.CD003053.pub5. CD003053. [DOI] [PubMed] [Google Scholar]
- 44.Legro RS, Barnhart HX, Schlaff WD, et al. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med. 2007;356:551–66. doi: 10.1056/NEJMoa063971. [DOI] [PubMed] [Google Scholar]
- 45.Legro RS, Brzyski RG, Diamond MP, et al. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med. 2014;371:119–29. doi: 10.1056/NEJMoa1313517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perales-Puchalt A, Legro RS. Ovulation induction in women with polycystic ovary syndrome. Steroids. 2013;78:767–72. doi: 10.1016/j.steroids.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 47.American Diabetes Association. 2. Classification and diagnosis of diabetes. Diabetes Care. 2015;38(Suppl):S8–S16. doi: 10.2337/dc15-S005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


