Highlight
YUCCA family genes act in response to multiple signals in leaf explants and contribute to de novo auxin biogenesis for fate transition of regeneration-competent cells.
Key words: Arabidopsis, auxin biogenesis, de novo root organogenesis, plant regeneration, WOX11, YUCCA.
Abstract
Many plant organs have the ability to regenerate a new plant after detachment or wounding via de novo organogenesis. During de novo root organogenesis from Arabidopsis thaliana leaf explants, endogenic auxin is essential for the fate transition of regeneration-competent cells to become root founder cells via activation of WUSCHEL-RELATED HOMEOBOX 11 (WOX11). However, the molecular events from leaf explant detachment to auxin-mediated cell fate transition are poorly understood. In this study, we used an assay to determine the concentration of indole-3-acetic acid (IAA) to provide direct evidence that auxin is produced after leaf explant detachment, a process that involves YUCCA (YUC)-mediated auxin biogenesis. Inhibition of YUC prevents expression of WOX11 and fate transition of competent cells, resulting in the blocking of rooting. Further analysis showed that YUC1 and YUC4 act quickly (within 4 hours) in response to wounding after detachment in both light and dark conditions and promote auxin biogenesis in both mesophyll and competent cells, whereas YUC5, YUC8, and YUC9 primarily respond in dark conditions. In addition, YUC2 and YUC6 contribute to rooting by providing a basal auxin level in the leaf. Overall, our study indicates that YUC genes exhibit a division of labour during de novo root organogenesis from leaf explants in response to multiple signals.
Introduction
Plants have powerful abilities to regenerate. Many detached or wounded plant organs can undergo de novo organogenesis—the regeneration of adventitious shoots and roots upon wounding—allowing the individual to survive various levels of damage (Duclercq et al., 2011; Sugimoto et al., 2011; Xu and Huang, 2014). The natural ability of plants to spontaneously regenerate has also been exploited for tissue culture and is widely used in modern agriculture (Sussex, 2008).
In a previous study, we used a simple method to examine de novo root organogenesis by culturing Arabidopsis thaliana leaf explants on B5 medium without added exogenous hormones: our results suggested that two steps are involved in cell fate transition during the regeneration of adventitious roots (Chen et al., 2014; Liu et al., 2014). Activation of WUSCHEL-RELATED HOMEOBOX 11 (WOX11) is involved in the first step of cell fate transition from regeneration-competent cells (i.e. procambium and vascular parenchyma cells) to root founder cells. Cell division occurs in the second step of cell fate transition as root founder cells transition into becoming root primordium cells, which is marked by WOX5. Finally, adventitious roots are differentiated from the root primordium (Liu et al., 2014).
Auxin is a critical hormone that controls de novo root organogenesis (Greenwood et al., 2001; De Klerk, 2002; Ahkami et al., 2009; Gutierrez et al. 2009; Correa Lda et al., 2012; Sukumar et al. 2013; Liu et al., 2014; Xu and Huang, 2014). In this rooting system, we previously observed that endogenous auxin is essential for cell fate transition because blocking auxin polar transport resulted in the loss of the auxin maximum in competent cells near the wound and thus blocked regeneration (Liu et al., 2014). The auxin maximum in competent cells was assumed to directly trigger expression of WOX11, because mutations of the auxin response elements on the WOX11 promoter resulted in partial blocking of its expression in competent cells (Liu et al., 2014).
The behaviour of auxin seems to be activated after detachment of the leaf from the source plant during the early stage of adventitious root regeneration from leaf explants (Liu et al., 2014). However, the molecular events between leaf detachment and auxin-mediated cell fate transition are barely known. Thus, the important question is how endogenous auxin accumulates in leaf explants after detachment.
Auxin biosynthesis is involved in multiple developmental processes (Chandler, 2009). The main natural auxin, indole-3-acetic acid (IAA), is biosynthesized primarily via two chemical reactions (Zhao, 2012). First, the amino acid tryptophan (Trp) is catalysed by the TAA/SAC family of tryptophan aminotransferases to become indole-3-puruvate (IPA) (Stepanova et al., 2008; Tao et al., 2008). Next, IPA is converted to IAA by the catalysis of flavin-containing monooxygenases encoded by YUCCA (YUC) genes, and this is a rate-limiting step (Zhao et al., 2001; Mashiguchi et al., 2011; Won et al., 2011). In this study, we provide insight into the multiple roles of YUC-mediated endogenous auxin biosynthesis during de novo root organogenesis.
Materials and methods
Plant materials and culture conditions
Wild-type Arabidopsis thaliana Columbia-0 (Col-0) was used in this study. yuc mutants were in the Col-0 background and have been previously described (Cheng et al., 2006; Robert et al., 2013). The DR5 pro :GUS line in Landsberg erecta (Ulmasov et al., 1997) and the WOX11 pro :GUS, WOX5 pro :GUS, and 35S pro :WOX11 lines in Col-0 (Liu et al., 2014) have also been described previously.
Our previous protocols describe the growth conditions of plants and culture conditions of leaf explants for de novo root organogenesis in either light or dark conditions (Chen et al., 2014). All leaf explants used in this study were from the first pair of rosette leaves from 12-day-old seedlings, except those from 15-day-old seedlings that were used for thin sectioning. Yucasin (cat. 573760, Sigma, USA) treatment was carried out as previously described (Nishimura et al., 2014).
YUC4 pro :GUS, YUC1 pro :GUS, YUC2 pro :GUS, and YUC9 pro :GUS were constructed by insertion of a 3.7-kb promoter of YUC4, a 3.6-kb promoter of YUC1, a 3.0-kb promoter of YUC2, and a 4.0-kb promoter of YUC9 into the pBI101 vector, respectively. These constructs were verified by sequencing and were introduced into wild-type Col-0 plants by Agrobacterium-mediated transformation. Primers for cloning are listed in Supplementary Table S1 at JXB online.
Determination of IAA concentrations
The first pair of rosette leaves of 12-day-old seedlings at 0, 4, and 12 hours after culture (HAC) on B5 medium in dark or light conditions were harvested and frozen in liquid nitrogen; each sample contained ~160–170 fresh leaves. The [2H5]-IAA measurement was carried out as previously described (Zhang et al., 2014).
GUS staining, reverse transcription (RT)-PCR, quantitative RT-PCR, and ChIP
GUS staining was performed as previously described (Chen et al., 2014; Liu et al., 2014) and observed using Nikon Eclipse 80i and Nikon SMZ1500 microscopes (Nikon, Japan).
For reverse transcription (RT)-PCR and quantitative (q)RT-PCR, RNA extraction and reverse transcription were performed as described previously (He et al., 2012). The qRT-PCR results represented the relative expression levels, which were normalized against those produced by the primers for ACTIN, whose values were arbitrarily fixed at 1.0.
ChIP was performed as previously described (He et al., 2012) using the antibody against of histone H3 lysine 27 trimethylation (H3K27me3; cat. 07-449, Millipore, USA). The ChIP results were normalized against those produced by the primers for AG, and the values of time-0 leaf explants were arbitrarily fixed at 1.0. Primers for RT-PCR, qRT-PCR, and ChIP are listed in Supplementary Table S1.
Results
Auxin production during de novo root organogenesis
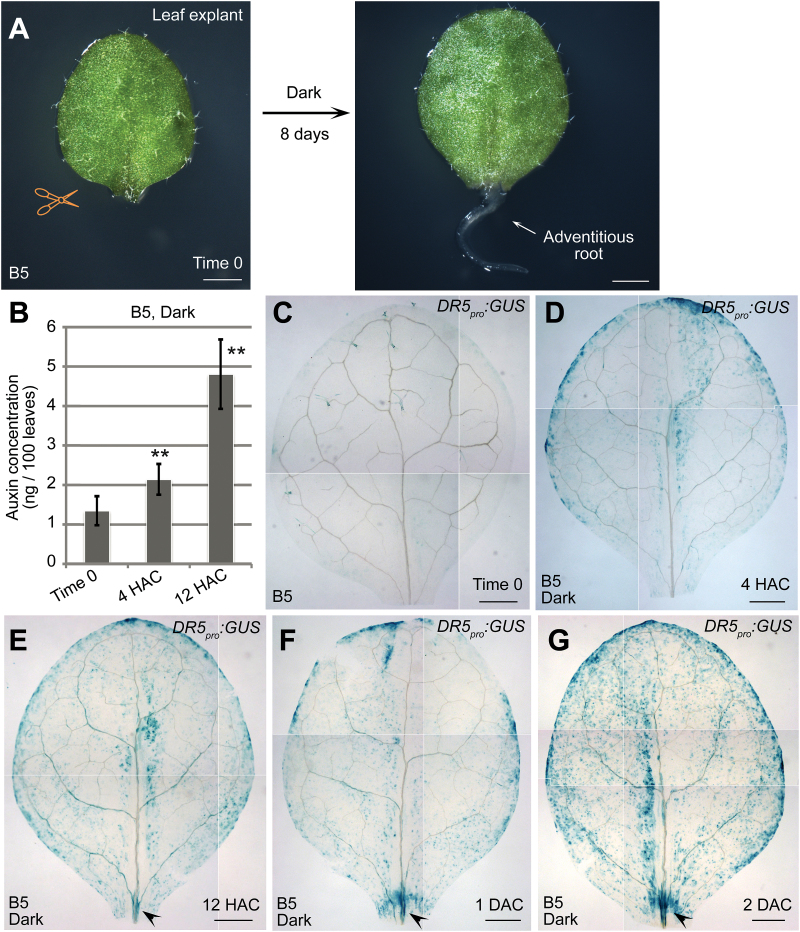
To study the early events after detachment of leaf explants, we cultured leaf explants on B5 medium without added hormones in dark conditions (Fig. 1A) (Chen et al., 2014). We previously showed that endogenous auxin plays important role in the regeneration of adventitious roots (Liu et al., 2014). To directly test the auxin levels in leaf explants during regeneration, we performed an assay to determine the IAA concentration. The results showed that the level of auxin was gradually and significantly elevated in leaf explants within 12 HAC on B5 medium compared with those prior to culture (time 0) (Fig. 1B), indicating that auxin is quickly produced after the detachment of leaf explants from source plants.
Fig. 1.
Auxin production in leaf explants after detachment. (A) System of de novo root organogenesis on B5 medium with sucrose in darkness. (B) Auxin concentration in leaf explants from time 0 to 12 HAC on B5 medium. Bars show SEM with three biological replicates. Each biological replicate was performed with three technical replicates. **P < 0.01 in two-sample t tests compared with time-0 control.
(C–G) Observations of the GUS signal in leaf explants from DR5 pro :GUS reporter line at time 0 (C), 4 HAC (D), 12 HAC (E), 1 DAC (F), and 2 DAC (G). Arrowheads in E–G indicate the GUS signal in vasculature near the wound.
The data in C–G were pasted together from small pictures of the same leaf explant because the microscope was unable to capture the entire leaf explant in a single visual field. Scale bars, 1mm in A and 500 μm in C–G.
To further observe the spatiotemporal pattern of auxin production, we conducted the analysis using the DR5 pro :GUS reporter line, which reflects the auxin level by monitoring auxin responsiveness (Ulmasov et al., 1997). Our data showed that the GUS signal quickly became elevated in mesophyll cells of leaf explants within 4 HAC (Fig. 1C, D). The GUS signal then gradually increased and began to accumulate in the vasculature near the wound at 12 HAC (Fig. 1E). The GUS signal continued to concentrate in the vasculature near the wound at 1–2 days after culture (DAC) (Fig. 1F, G) (Liu et al., 2014). Therefore, auxin levels rose quickly in mesophyll cells after detachment of leaf explants, and the auxin was then polar transported into the competent cells in the vasculature near the wound, where it triggers cell fate transition (Liu et al., 2014).
Identification of YUC4 and YUC1 in regeneration
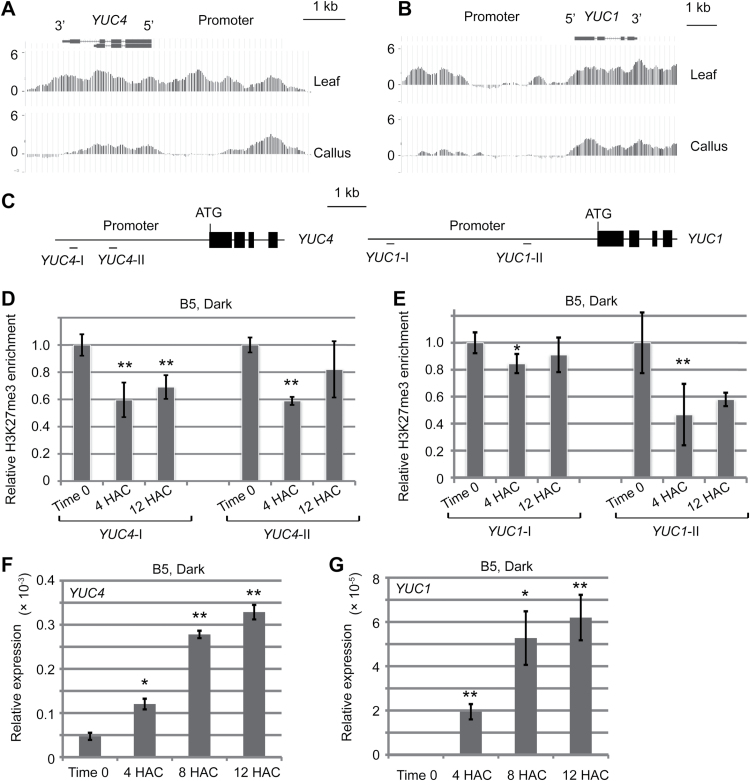
Our previous study showed that de novo root organogenesis shares a similar genetic pathway with callus formation (Liu et al., 2014). To reveal the mechanism of auxin behaviour in leaf explants, we therefore searched our previous ChIP-chip data of histone H3K27me3, a marker of the repressive chromatin state of gene expression (Schatlowski et al., 2008; He et al., 2013), during the leaf-to-callus transition on callus-inducing medium (He et al., 2012). We noted that the H3K27me3 levels at the YUC4 and YUC1 loci were reduced during the leaf-to-callus transition (Fig. 2A, B) (He et al., 2012). This implies that YUC expression is upregulated during regeneration and this upregulation is associated with H3K27 demethylation.
Fig. 2.
Identification of YUC4 and YUC1 in regeneration. (A, B) Levels of epigenetic marker H3K27me3 at YUC4 (A) and YUC1 (B) loci were reduced in 20-DAC leaf explants that produced callus on callus-inducing medium compared with levels in the time-0 leaf explants. The original ChIP-chip data are from our previous study (He et al., 2012). (C) Diagram of YUC4 and YUC1 loci with primer positions (YUC4-I, YUC4-II, YUC1-I, and YUC1-II) in ChIP analysis in D and E. (D, E) ChIP analysis of the H3K27me3 levels at YUC4 (D) and YUC1 (E) loci in time-0, 4-HAC, and 12-HAC leaf explants of wild-type Col-0 cultured on B5 medium. (F, G) qRT-PCR analysis of expression levels of YUC4 (F) and YUC1 (G) within 12 HAC on B5 medium.
Bars show SEM with three biological replicates in D–G. Each biological replicate was performed with three technical replicates. *P < 0.05 and **P < 0.01 in two-sample t tests compared with time-0 control.
To test this hypothesis, we first measured the H3K27me3 levels at YUC4 and YUC1 loci during de novo root organogenesis from leaf explants on B5 medium. Our results showed that H3K27me3 levels at YUC4 and YUC1 loci were reduced in 4-HAC and 12-HAC leaf explants compared with those at time 0 (Fig. 2C–E), indicating that the two YUC genes may also be activated in de novo root organogenesis.
qRT-PCR results showed that expression of YUC4 was at a low level in time-0 leaf explants, became elevated at 4 HAC, and continued to increase at 8 and 12 HAC during culture on B5 medium (Fig. 2F). YUC1 expression was barely detected in time-0 leaf explants, and gradually upregulated in leaf explants on B5 medium from 4 to 12 HAC (Fig. 2G). Hence, we suppose that the two YUC genes are responsible for endogenous auxin production in leaf explants.
YUC-mediated auxin biosynthesis is involved in de novo root organogenesis from leaf explants
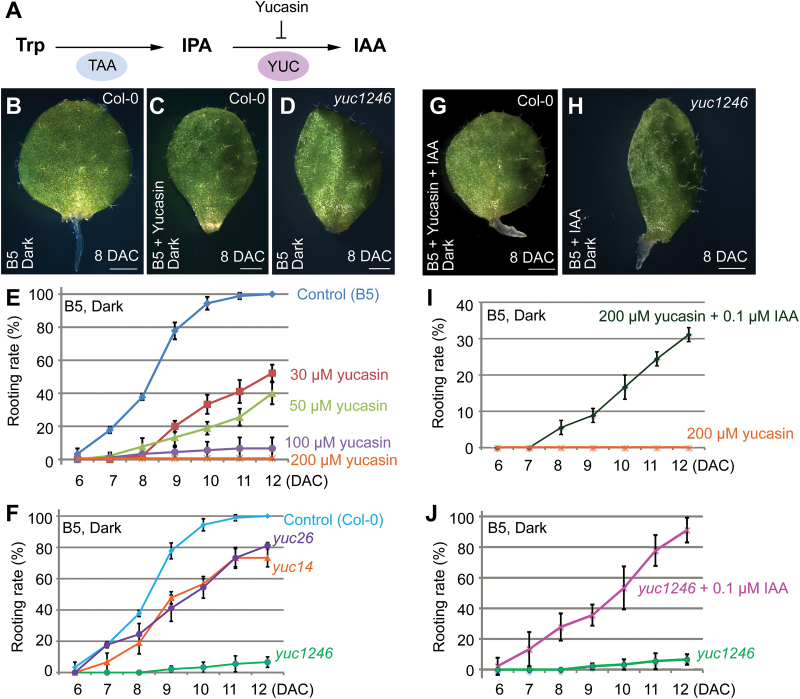
To study whether YUC-mediated auxin biogenesis is required for rooting from leaf explants, we used an auxin biosynthesis inhibitor, yucasin, which specifically inhibits the function of YUC proteins (Fig. 3A) (Nishimura et al., 2014) and thereby reduces auxin levels during regeneration on B5 medium (see Supplementary Fig. S1 at JXB online). The wild-type leaf explants started regenerating adventitious roots by 6–8 DAC on B5 medium (Fig. 3B, E). By contrast, regeneration was blocked when yucasin was supplemented in B5 medium (Fig. 3C, E). To confirm this result, we tested the rooting ability in yuc1 yuc2 yuc4 yuc6 (called yuc1246) quadruple mutants, yuc1 yuc4 (called yuc14) double mutants, and yuc2 yuc6 (called yuc26) double mutants. Our results showed that rooting was partially blocked in yuc14 and yuc26, and was severely blocked in yuc1246 (Fig. 3D, F).
Fig. 3.
YUC is required for de novo root organogenesis. (A) Yucasin inhibits the YUC protein function in the auxin biogenesis pathway. (B, C) Leaf explants cultured on B5 medium (B) or B5 medium with 200 μM yucasin (C) at 8 DAC. Note that yucasin is sufficient to block rooting. (D) Leaf explants from yuc1246 mutant on B5 medium, showing rooting defect at 8 DAC. (E) Rooting rate of leaf explants (percentage of leaf explants with regenerated adventitious roots) on B5 medium with different concentration of yucasin treatment. (F) Rooting rate of leaf explants from wild-type Col-0, yuc14 double mutant, yuc26 double mutant, and yuc1246 quadruple mutant on B5 medium. This experiment was performed together with the yucasin treatment experiment in E, therefore using the same control data. (G) Addition of 0.1 μM IAA partially eliminated the rooting defects caused by 200 μM yucasin treatment. (H) Addition of 0.1 μM IAA eliminated the rooting defects caused by simultaneous mutations in YUC1, YUC2, YUC4, and YUC6. (I) Rooting rate of leaf explants on B5 medium with 200 μM yucasin and 0.1 μM IAA treatment. This experiment was performed together with the yucasin treatment experiment in E, therefore using the same data for 200 μM yucasin treatment. (J) Rooting rate of leaf explants from yuc1246 quadruple mutant on B5 medium with 0.1 μM IAA treatment. This experiment was performed together with the experiment in F, therefore using the same data for yuc1246. Bars in E, F, I, and J show SD with three biological replicates. n = 30 in each replicate. Scale bars, 1mm in B–D, G, and H.
To confirm that the rooting defect was caused by the reduced endogenous auxin production as a result of yucasin treatment, we added a low concentration of IAA (0.1 μM) to the yucasin-containing B5 medium. The addition of IAA partially eliminated the rooting defects caused by yucasin (Fig. 3G, I). Likewise, the addition of IAA also eliminated the rooting defects caused by YUC mutations (Fig. 3H, J), suggesting that the phenotype of rooting defect is caused by the lack of auxin in leaf explants of yuc1246 mutants and is unrelated to the developmental defects of yuc1246 leaves.
The data from yuc mutants, together with yucasin treatment, reveal that YUC-mediated auxin biosynthesis is critical for de novo root organogenesis.
YUC-mediated auxin biosynthesis acts upstream to cell fate transition
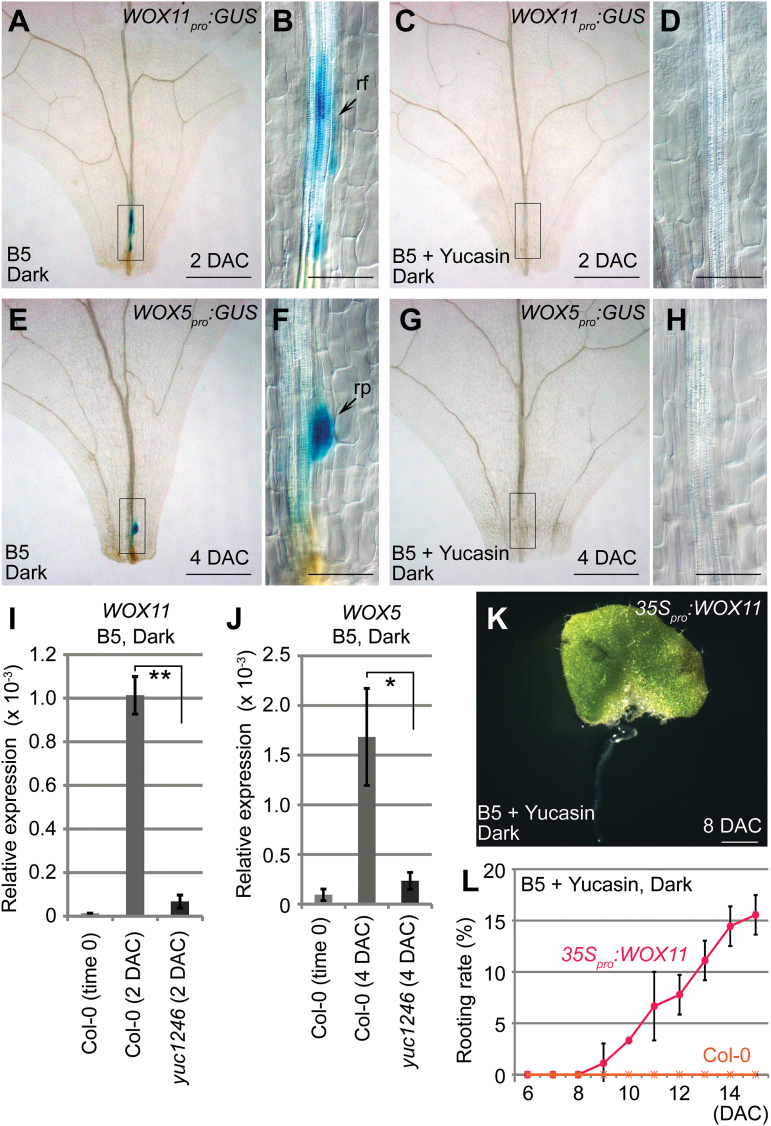
To identify the step in which auxin biosynthesis functions, we used two marker genes, WOX11 and WOX5, to monitor two steps of cell fate transition. In wild-type leaf explants, expression of WOX11 appeared around 2 DAC, suggesting the completion of the first step of cell fate transition from competent cells to root founder cells (Fig. 4A, B) (Liu et al., 2014). Expression of WOX5 was observed at around 4 DAC when the second step of cell fate transition from root founder cells to root primordium cells was completed (Fig. 4E, F) (Liu et al., 2014). By contrast, when yucasin was added to B5 medium to inhibit auxin biosynthesis, the expression of neither WOX11 nor WOX5 was observed in leaf explants during culture (Fig. 4C, D, G, H), suggesting that auxin biosynthesis acts upstream to the first step of cell fate transition. Because auxin production was only partially blocked by yucasin treatment (see Supplementary Fig. S1 at JXB online), it is possible that a certain auxin concentration is required to induce WOX11 expression. In addition, qRT-PCR analysis of WOX11 and WOX5 expression during the process of rooting confirmed that the first step of cell fate transition was severely blocked in the yuc1246 mutant (Fig. 4I, J).
Fig. 4.
Auxin biogenesis is required for the first step of cell fate transition. (A–D) GUS staining of 2-DAC leaf explants from WOX11 pro :GUS cultured on B5 medium (A and B) and B5 medium with 200 μM yucasin (C and D). (E–H) GUS staining of 4-DAC leaf explants from WOX5 pro :GUS cultured on B5 medium (E and F) and B5 medium with 200 μM yucasin (G and H). (I, J) qRT-PCR analysis of WOX11 (I) and WOX5 (J) in wild type and yuc1246 mutant leaf explants on B5 medium. Bars show SEM with three biological replicate. Each biological replicate was performed with three technical replicates. *P < 0.05 and **P < 0.01 in two-sample t tests. (K) Overexpression of WOX11 partially reverses the rooting defects caused by 200 μM yucasin treatment. (L) Rooting rate of leaf explants from wild-type Col-0 and 35S pro :WOX11 on B5 medium with 200 μM yucasin treatment. Bars show SD with three biological replicates. n = 30 in each repeat.
B, D, F, and H are close-up views of the boxed regions in A, C, E, and G, respectively. Scale bars, 500 μm in A, C, E, G; 100 μm in B, D, F, H; and 1mm in K.
To test whether blocking the first step of cell fate transition is a major downstream event caused by a defect in YUC-mediated auxin biosynthesis, we overexpressed the WOX11 gene (see Supplementary Fig. S2 at JXB online) in leaf explants cultured on B5 medium supplemented with yucasin. Our results showed that overexpression of WOX11 was able to partially eliminate the regeneration defects caused by yucasin (Fig. 4K, L), suggesting that WOX11-mediated cell fate transition is a target of auxin in de novo root organogenesis.
YUC4 and YUC1 are dynamically expressed in mesophyll and competent cells in response to wounding
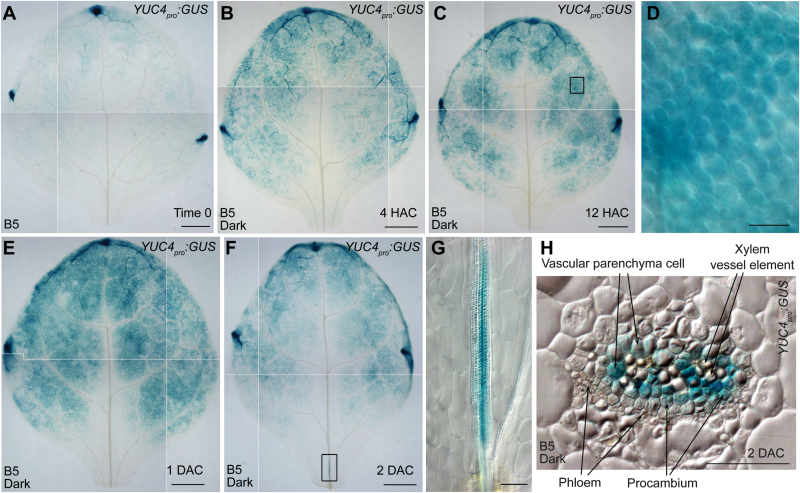
To further analyse the spatial expression patterns of YUC genes, we constructed the YUC4 pro :GUS and YUC1 pro :GUS transgenic reporter lines to monitor the activities of their promoters. In leaf explants of YUC4 pro :GUS, there was a low GUS signal in mesophyll cells and relatively strong expression in the hydathode at time 0 (Fig. 5A). During regeneration, the GUS signal became elevated in mesophyll cells at 4 and 12 HAC (Fig. 5B–D), and continued to climb at 1 DAC (Fig. 5E). Interestingly, we also observed a GUS signal of YUC4 pro :GUS in the vasculature near the wound at 2 DAC (Fig. 5F, G). Therefore, YUC4 is expressed in mesophyll cells prior to its expression in vasculature during regeneration.
Fig. 5.
Spatial expression patterns of YUC4. (A–C) GUS staining of time-0 (A), 4-HAC (B), and 12-HAC (C) leaf explants from YUC4 pro :GUS cultured on B5 medium. (D) Close-up of the boxed regions in C, showing YUC4 expression in mesophyll. (E, F) GUS staining of 1-DAC (E) and 2-DAC (F) leaf explants from YUC4 pro :GUS cultured on B5 medium. (G) Close-up of the boxed regions in F, showing YUC4 expression in the vasculature near a wound. (H) Thin sectioning of 2-DAC leaf explants from YUC4 pro :GUS cultured on B5 medium at the leaf base, showing the midrib of vasculature as indicated in G. Note that the GUS signal was shown in competent cells. The data in A, B, C, E, and F were pasted together from small pictures of the same leaf explant because the microscope was unable to capture the entire leaf explant in a single visual field. Scale bars, 500 μm in A, B, C, E, F; and 50 μm in D, G, H.
To further examine the expression of YUC4 in the vasculature, we performed thin sectioning to show the GUS signals of YUC4 pro :GUS at 2 DAC. Our data showed that YUC4 was expressed in the procambium and vascular parenchyma cells (Fig. 5H), which serve as competent cells during regeneration (Liu et al., 2014).
Compared with YUC4 pro :GUS, there was a weak GUS signal in YUC1 pro :GUS. The GUS signal from YUC1 pro :GUS was barely detected in time-0 leaf explants, and was elevated in mesophyll cells as well as the vasculature near the wound at 1 DAC (see Supplementary Fig. S3 at JXB online).
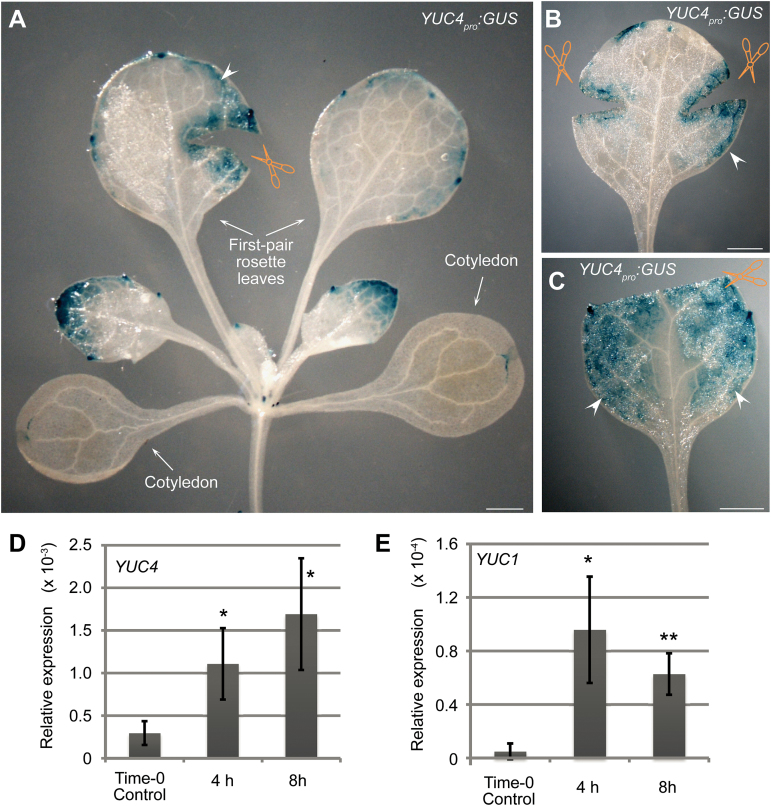
In addition, we showed that the expression of YUC4 and YUC1 was induced by wounding. The GUS signal of YUC4 pro :GUS was observed in mesophyll cells in and near the wounded region of leaves that were not detached from the plant (Fig. 6A–C; for control, see Supplementary Fig. S4 at JXB online). A GUS signal was also observed in the mesophyll that was not near the wound (arrowheads in Fig. 6A–C; for control, see Supplementary Fig. S4), suggesting that the wound signal that triggers YUC expression is able to spread within leaves. The qRT-PCR analysis showed that the expression levels of YUC4 and YUC1 were quickly induced in the attached leaves within 8 hours after wounding (Fig. 6D, E). These data indicate that the previously proposed wound signal (Liu et al., 2014) may have an effect on the upregulation of expression of the two YUC genes.
Fig. 6.
YUC4 and YUC1 were induced by wounding. (A–C) GUS staining of wounded leaves from YUC4 pro :GUS at 4 hours after wounding, showing one (A) and two (B) wounds, or removal of the leaf tip (C). Arrowheads indicate the GUS signal that spreads in the mesophyll. (D, E) qRT-PCR analysis of YUC4 (D) and YUC1 (E) expression levels in wounded leaves. The tissue materials for RNA extraction are from attached leaves with one wound as indicated in A. Bars show SEM from three biological replicates. Each biological replicate was performed with three technical replicates. *P < 0.05 and **P < 0.01 in two-sample t tests compared with time-0 control. Scale bars, 1mm in A–C.
YUC5, YUC8, and YUC9 act primarily in response to darkness
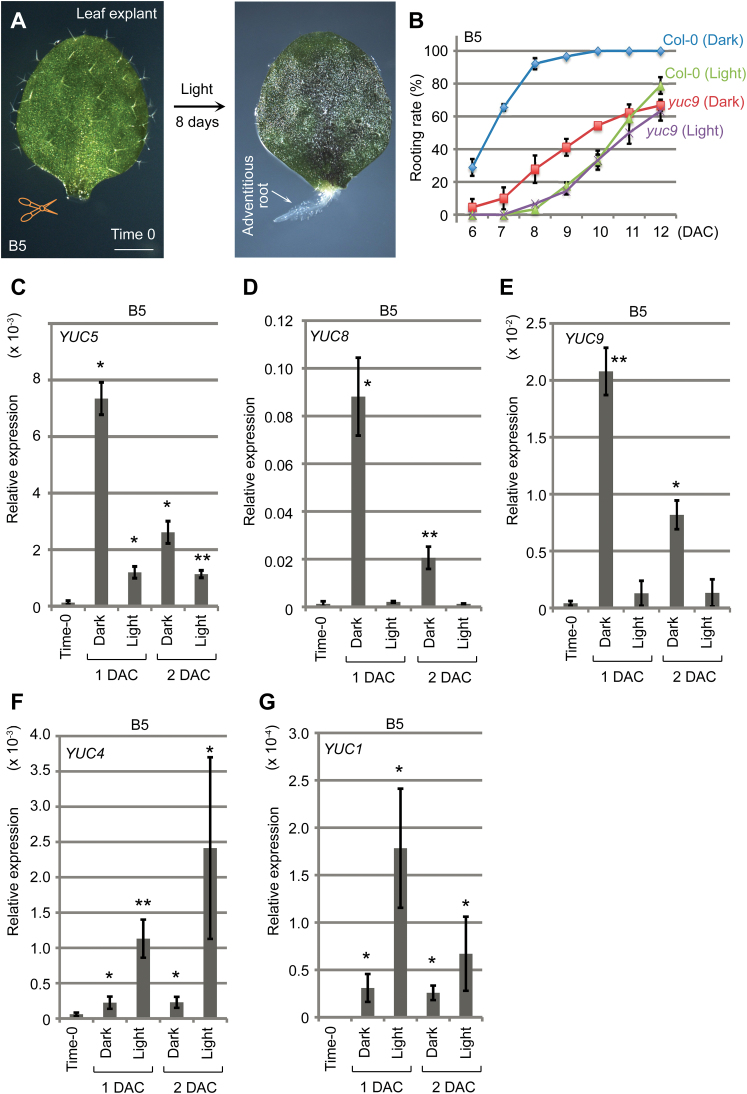
Regeneration of explants in natural conditions may occur in either dark or light conditions. The regeneration system described above was in dark conditions. We also tested regeneration of adventitious roots in light conditions by culturing leaf explants on B5 medium without sucrose (Fig. 7A) (Chen et al., 2014). The rooting rate of wild-type leaf explants was slower in light conditions than that in dark conditions (Fig. 7B). The auxin level was also elevated in wild-type leaf explants cultured in light conditions during regeneration (see Supplementary Fig. S5 at JXB online). However, increase in auxin levels in leaf explants cultured in light conditions was lower than that in leaf explants cultured in dark conditions (Supplementary Fig. S5 compared with Fig. 1B–G). Therefore, leaf explants produce more auxin and have a higher regenerative efficiency in the dark.
Fig. 7.
YUC expression during regeneration in dark and light. (A) De novo root organogenesis on B5 medium without sucrose in light. (B) Rooting rate of leaf explants from wild-type Col-0 and yuc9 on B5 medium in light and dark conditions. Bars show SD with three biological replicates. n = 30 in each repeat. (C–G) qRT-PCR analysis of YUC5 (C), YUC8 (D), YUC9 (E), YUC4 (F), and YUC1 (G) expression during regeneration on B5 medium in either dark or light conditions. Bars show SEM from three biological replicates. Each biological replicate was performed with three technical replicates. *P < 0.05 and **P < 0.01 in two-sample t tests compared with time-0 control.
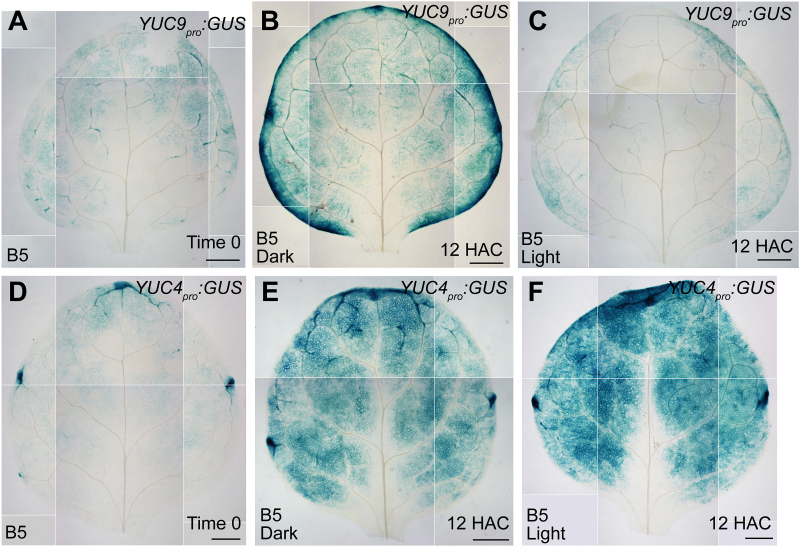
Previous studies revealed that the expression of YUC5, YUC8, and YUC9 is upregulated by dark treatment (Tao et al., 2008; Hornitschek et al., 2012); therefore, they could contribute to the regeneration system in darkness. We performed qRT-PCR analysis of the three closely related YUC genes (see Supplementary Fig. S6 at JXB online) in both dark and light conditions. The results showed that expression of YUC5, YUC8, and YUC9 was dramatically elevated during regeneration in dark conditions within 2 DAC (Fig. 7C–E). Expression of YUC5 was also elevated in light conditions during regeneration, but its increase was much lower in light conditions than in dark conditions (Fig. 7C). To analyse the spatial expression pattern of YUC9, we constructed the YUC9 pro :GUS transgenic reporter line. In leaf explants of YUC9 pro :GUS, the GUS signal became elevated in leaf margin and mesophyll cells at 12 HAC in dark conditions compared with that at time 0 (Fig. 8A, B). In contrast, we did not observe an increase in GUS signal at 12 HAC when leaf explants of YUC9 pro :GUS were cultured in light conditions (Fig. 8C compared with Fig. 8A, B).
Fig. 8.
Expression patterns of YUC9 and YUC4 in dark and light conditions. (A) GUS staining of the time-0 leaf explant from YUC9 pro :GUS. (B, C) GUS staining of 12-HAC leaf explants from YUC9 pro :GUS cultured on B5 medium in dark (B) or light (C) conditions. (D) GUS staining of the time-0 leaf explant from YUC4 pro :GUS. (E, F) GUS staining of 12-HAC leaf explants from YUC4 pro :GUS cultured on B5 medium in dark (E) or light (F) conditions. The data in A–F were pasted together from small pictures of the same leaf explant because the microscope was unable to capture the entire leaf explant in a single visual field. Scale bars, 500 μm in A–F.
We also analysed the rooting ability of the yuc9 mutant. The result showed that rooting was partially defective in yuc9 leaf explants compared with that in wild-type leaf explants when they were cultured in dark conditions (Fig. 7B). In contrast, we did not observe a clear rooting defect of yuc9 leaf explants compared with the rooting ability of wild-type leaf explants when they were cultured in light conditions (Fig. 7B).
The data from YUC5, YUC8, and YUC9 suggest that the three genes may act predominantly in response to darkness but might also have a minor role in response to wounding as indicated by the previous study (Hornitschek et al., 2012).
Expression of YUC4 and YUC1 was elevated during regeneration in both light and dark conditions, although the two genes had a more severely elevated level of expression in light than in dark within 2 DAC (Fig. 7F, G). This might occur because of the low expression levels of YUC5, YUC8, and YUC9 in light, which may result in feedback that stimulates the expression of YUC4 and YUC1 for regeneration. In addition, in leaf explants of YUC4 pro :GUS, the GUS signal became elevated in mesophyll cells at 12 HAC compared with the signal at time 0 in both dark and light conditions (Fig. 8D–F). Because sucrose was not added to the B5 medium when leaf explants were cultured in light conditions, the elevated YUC4 expression level could not have been related to the presence of sucrose in the medium (Fig. 8F). The data from YUC4 and YUC1 suggest that the two genes may act predominantly in response to wounding, regardless of light and dark conditions.
Discussion
De novo root organogenesis commonly occurs when plant organs are detached or wounded, and auxin is known to have an important role in this process ( Greenwood et al., 2001; De Klerk, 2002; Ahkami et al., 2009; Correa Lda et al., 2012; Chen et al., 2014; Liu et al., 2014; Xu and Huang, 2014). In this study, we show that YUC-mediated de novo synthesis of auxin is enhanced after detachment of leaf explants, and this in turn is required for de novo root organogenesis from leaf explants.
The Arabidopsis genome encodes at least 11 YUC genes (see Supplementary Fig. S6 at JXB online) (Cheng et al., 2006), and our data showed that mutations in YUC1, YUC2, YUC4, and YUC6 severely block rooting from leaf explants, suggesting that these four genes have primary roles in regeneration among all the YUC family. However, expression of YUC2 and YUC6 was not significantly induced in leaf explants within 2 DAC (see Supplementary Fig. S7 at JXB online) and these two gene loci are not regulated by the H3K27me3 epigenetic marker (He et al., 2012); this suggests that these two genes may contribute to the basal auxin level in the leaf. In contrast to YUC2 and YUC6, YUC4 and YUC1 were induced by wounding. We propose that both basally expressed YUC2 and YUC6 and wounding-induced YUC4 and YUC1 contribute to the auxin level during rooting from leaf explants, but that it is YUC4 and YUC1 that primarily contribute to the rapid increase in auxin in leaf explants after detachment (see the model in Fig. 9). This may explain the phenotype analysis that yuc14 and yuc26 are partially defective in rooting whereas rooting in yuc1246 is severely blocked.
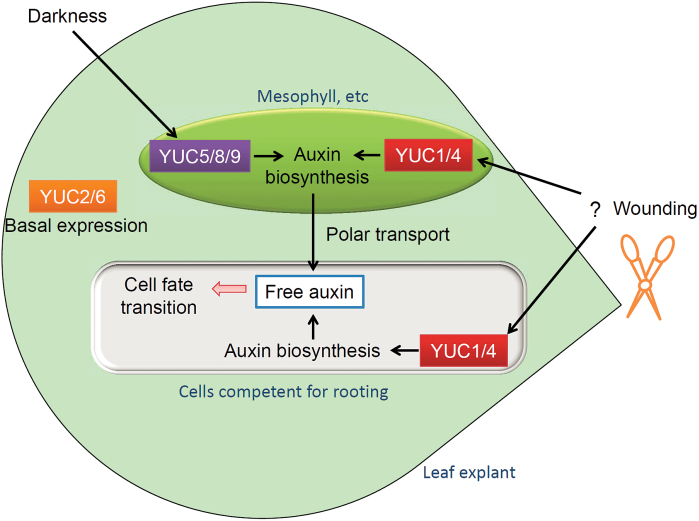
Fig. 9.
Model for YUC-mediated auxin biogenesis in de novo root organogenesis. YUC1/4-mediated auxin biogenesis produces auxin in both mesophyll cells and competent cells primarily in response to wounding. The wound signal that induces YUC1/4 expression in mesophyll cells is not clear (question mark). YUC5/8/9-mediated auxin biogenesis produces auxin in mesophyll cells primarily in response to darkness. YUC2/6-mediated auxin biogenesis contributes to the basal auxin level in the leaf and also contributes to regeneration.
The expression patterns of YUC4 and YUC1 suggest that they have dual roles (see the model in Fig. 9). First, they rapidly produce auxin in mesophyll cells within and after 4 HAC, and the auxin is then polar transported toward competent cells near the wound (Liu et al., 2014). Second, they produce auxin in competent cells near the wound around 1–2 DAC when the cell fate transition is occurring. We propose that the first role of YUC (auxin production in mesophyll cells) is to increase the total auxin level in leaf explants and that some of this auxin is then polar transported to competent cells near the wound, triggering WOX11 expression and the first step of cell fate transition. This supposition is based on the fact that inhibition of polar auxin transport from the mesophyll to the vasculature severely blocks rooting (Liu et al., 2014). The second role of YUC (auxin production in competent cells) might involve the maintenance of the maximum auxin level in competent cells, because this occurs later than auxin production in the mesophyll.
The auxin production levels in leaf explants are different in dark and light conditions. Expression levels of YUC4 and YUC1 were upregulated in both dark and light conditions in leaf explants, and yuc1246 had defective rooting in both dark conditions (Fig. 3F) and light conditions (data not shown). Therefore, YUC1, YUC2, YUC4, and YUC6 contribute to rooting regardless of whether the leaves are in light or dark conditions. In contrast, induction of YUC5, YUC8, and YUC9 expression was primarily in response to darkness, and yuc9 has a rooting defect in the dark but not in the light. Therefore, the major role of YUC5, YUC8, and YUC9 is to produce auxin in response to darkness (see the model in Fig. 9).
Expression of YUC10 and YUC11 was not detected during rooting from leaf explants in our conditions (see Supplementary Fig. S8 at JXB online). YUC3 was expressed in time-0 leaf explants, while its expression level was reduced when leaf explants were cultured on B5 medium (Supplementary Fig. S8). These data indicate that YUC10, YUC11, and YUC3 might not be involved in rooting from leaf explants. YUC7 was expressed in leaf explants during regeneration (Supplementary Fig. S8). However, the role of YUC7 in regeneration is not clear.
Currently, it remains unclear how wounds induce YUC1 and YUC4 expression. Whether YUC1 and YUC4 are the direct targets of wounding is also not known. Wounds may produce many physical and chemical signals, and may also induce hormone actions and wounding-sensitive gene expression (Leon et al., 2001; Maffei et al., 2007; Iwase et al., 2011; Xu and Huang, 2014). However, the concept of a ‘wound signal’ is still very poorly understood. It is possible that the factors triggering YUC1 and YUC4 expression in leaf explants upon wounding may cooperate with epigenetic factors, such as those in the H3K27me3 modification, because our data suggested that upregulation of YUC genes is accompanied by the removal of H3K27me3. Gaining an understanding the complex wound signal and epigenetic regulation of YUC genes will improve our knowledge of the mechanisms that occur at the very start of regeneration.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Yucasin inhibits auxin production in regeneration.
Figure S2. WOX11 expression in 35S pro :WOX11 transgenic plants.
Figure S3. Spatial expression patterns of YUC1 in regeneration.
Figure S4. Expression of WOX5 is not in response to wounding within 4h.
Figure S5. Auxin production in leaf explants in light conditions.
Figure S6. YUC family in Arabidopsis.
Figure S7. Expression of YUC2 and YUC6 genes in regeneration.
Figure S8. YUC3, YUC7, YUC10, YUC11, and YUC9 expression during rooting from leaf explants.
Table S1. List of primers used in this study.
Acknowledgements
We thank Y. Zhao, T. J. Guilfoyle, and ABRC for Arabidopsis seeds used in this work. This work was supported by grants from National Basic Research Program of China (973 Program, 2012CB910500/2014CB943500), the National Natural Science Foundation of China (91419302/31422005/91317312), and Youth Innovation Promotion Association CAS.
References
- Ahkami AH, Lischewski S, Haensch KT, et al. 2009. Molecular physiology of adventitious root formation in Petunia hybrida cuttings: involvement of wound response and primary metabolism. New Phytologist 181, 613–625. [DOI] [PubMed] [Google Scholar]
- Chandler JW. 2009. Local auxin production: a small contribution to a big field. Bioessays 31, 60–70. [DOI] [PubMed] [Google Scholar]
- Chen X, Qu Y, Sheng L, Liu J, Huang H, Xu L. 2014. A simple method suitable to study de novo root organogenesis. Frontiers in Plant Science 5, 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Dai X, Zhao Y. 2006. Auxin biosynthesis by the YUCCA flavin monooxygenases controls the formation of floral organs and vascular tissues in Arabidopsis . Genes & Development 20, 1790–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa Lda R, Troleis J, Mastroberti AA, Mariath JE, Fett-Neto AG. 2012. Distinct modes of adventitious rooting in Arabidopsis thaliana . Plant Biology 14, 100–109. [DOI] [PubMed] [Google Scholar]
- De Klerk G-J. 2002. Rooting of microcuttings: theory and practice. In Vitro Cellular & Developmental Biology--Plant 38, 415–422. [Google Scholar]
- Duclercq J, Sangwan-Norreel B, Catterou M, Sangwan RS. 2011. De novo shoot organogenesis: from art to science. Trends in Plant Science 16, 597–606. [DOI] [PubMed] [Google Scholar]
- Greenwood MS, Cui X, Xu F. 2001. Response to auxin changes during maturation-related loss of adventitious rooting competence in loblolly pine (Pinus taeda) stem cuttings. Physiologia Plantarum 111, 373–380. [DOI] [PubMed] [Google Scholar]
- Gutierrez L, Bussell JD, Pacurar DI, et al. 2009. Phenotypic plasticity of adventitious rooting in Arabidopsis is controlled by complex regulation of AUXIN RESPONSE FACTOR transcripts and microRNA abundance. The Plant Cell 21, 3119–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C, Chen X, Huang H, Xu L. 2012. Reprogramming of H3K27me3 is critical for acquisition of pluripotency from cultured Arabidopsis tissues. PLoS Genetics 8, e1002911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C, Huang H, Xu L. 2013. Mechanisms guiding Polycomb activities during gene silencing in Arabidopsis thaliana . Frontiers in Plant Science 4, 454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornitschek P, Kohnen MV, Lorrain S, et al. 2012. Phytochrome interacting factors 4 and 5 control seedling growth in changing light conditions by directly controlling auxin signaling. The Plant Journal 71, 699–711. [DOI] [PubMed] [Google Scholar]
- Iwase A, Mitsuda N, Koyama T, et al. 2011. The AP2/ERF transcription factor WIND1 controls cell dedifferentiation in Arabidopsis . Current Biology 21, 508–514. [DOI] [PubMed] [Google Scholar]
- Leon J, Rojo E, Sanchez-Serrano JJ. 2001. Wound signalling in plants. Journal of Experimental Botany 52, 1–9. [DOI] [PubMed] [Google Scholar]
- Liu J, Sheng L, Xu Y, Li J, Yang Z, Huang H, Xu L. 2014. WOX11 and 12 are involved in the first-step cell fate transition during de novo root organogenesis in Arabidopsis . The Plant Cell 26, 1081–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei ME, Mithofer A, Boland W. 2007. Before gene expression: early events in plant-insect interaction. Trends in Plant Science 12, 310–316. [DOI] [PubMed] [Google Scholar]
- Mashiguchi K, Tanaka K, Sakai T, et al. 2011. The main auxin biosynthesis pathway in Arabidopsis. Proceedings of the National Academy of Sciences of the United States of America 108, 18512–18517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T, Hayashi K, Suzuki H, et al. 2014. Yucasin is a potent inhibitor of YUCCA, a key enzyme in auxin biosynthesis. The Plant Journal 77, 352–366. [DOI] [PubMed] [Google Scholar]
- Robert HS, Grones P, Stepanova AN, Robles LM, Lokerse AS, Alonso JM, Weijers D, Friml J. 2013. Local auxin sources orient the apical-basal axis in Arabidopsis embryos. Current Biology 23, 2506–2512. [DOI] [PubMed] [Google Scholar]
- Schatlowski N, Creasey K, Goodrich J, Schubert D. 2008. Keeping plants in shape: polycomb-group genes and histone methylation. Seminars in Cell and Developmental Biology 19, 547–553. [DOI] [PubMed] [Google Scholar]
- Stepanova AN, Robertson-Hoyt J, Yun J, Benavente LM, Xie DY, Dolezal K, Schlereth A, Jurgens G, Alonso JM. 2008. TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell 133, 177–191. [DOI] [PubMed] [Google Scholar]
- Sugimoto K, Gordon SP, Meyerowitz EM. 2011. Regeneration in plants and animals: dedifferentiation, transdifferentiation, or just differentiation? Trends in Cell Biology 21, 212–218. [DOI] [PubMed] [Google Scholar]
- Sukumar P, Maloney GS, Muday GK. 2013. Localized induction of the ATP-binding cassette B19 auxin transporter enhances adventitious root formation in Arabidopsis. Plant Physiology 162, 1392–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussex IM. 2008. The scientific roots of modern plant biotechnology. The Plant Cell 20, 1189–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Ferrer JL, Ljung K, et al. 2008. Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell 133, 164–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ. 1997. Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. The Plant Cell 9, 1963–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won C, Shen X, Mashiguchi K, Zheng Z, Dai X, Cheng Y, Kasahara H, Kamiya Y, Chory J, Zhao Y. 2011. Conversion of tryptophan to indole-3-acetic acid by TRYPTOPHAN AMINOTRANSFERASES OF ARABIDOPSIS and YUCCAs in Arabidopsis . Proceedings of the National Academy of Sciences of the United States of America 108, 18518–18523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Huang H. 2014. Genetic and epigenetic controls of plant regeneration. Current Topics in Developmental Biology 108, 1–33. [DOI] [PubMed] [Google Scholar]
- Zhang S, Wu J, Yuan D, Zhang D, Huang Z, Xiao L, Yang C. 2014. Perturbation of auxin homeostasis caused by mitochondrial FtSH4 gene-mediated peroxidase accumulation regulates Arabidopsis architecture. Molecular Plant 7, 856–873. [DOI] [PubMed] [Google Scholar]
- Zhao Y. 2012. Auxin biosynthesis: a simple two-step pathway converts tryptophan to indole-3-acetic acid in plants. Molecular Plant 5, 334–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Christensen SK, Fankhauser C, Cashman JR, Cohen JD, Weigel D, Chory J. 2001. A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science 291, 306–309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.