Highlight
This study demonstrates the importance of the wheat ABCC transporter (TaABCC13) for achieving low phytic acid in grains, and substantiates its previously speculated role during heavy metal detoxification.
Keywords: ABC-type transporters, cadmium stress, cadmium tolerance, lateral roots, phytic acid, Triticum aestivum.
Abstract
Low phytic acid is a trait desired in cereal crops and can be achieved by manipulating the genes involved either in its biosynthesis or its transport in the vacuoles. Previously, we have demonstrated that the wheat TaABCC13 protein is a functional transporter, primarily involved in heavy metal tolerance, and a probable candidate gene to achieve low phytate wheat. In the current study, RNA silencing was used to knockdown the expression of TaABCC13 in order to evaluate its functional importance in wheat. Transgenic plants with significantly reduced TaABCC13 transcripts in either seeds or roots were selected for further studies. Homozygous RNAi lines K1B4 and K4G7 exhibited 34–22% reduction of the phytic acid content in the mature grains (T4 seeds). These transgenic lines were defective for spike development, as characterized by reduced grain filling and numbers of spikelets. The seeds of transgenic wheat had delayed germination, but the viability of the seedlings was unaffected. Interestingly, early emergence of lateral roots was observed in TaABCC13-silenced lines as compared to non-transgenic lines. In addition, these lines also had defects in metal uptake and development of lateral roots in the presence of cadmium stress. Our results suggest roles of TaABCC13 in lateral root initiation and enhanced sensitivity towards heavy metals. Taken together, these data demonstrate that wheat ABCC13 is functionally important for grain development and plays an important role during detoxification of heavy metals.
Introduction
In plants, phytic acid (myo-inositol 1,2,3,4,5,6-hexakisphosphate; PA; IP6) is a major storage form of seed phosphate that chelates important micronutrients (Ca, Zn, Mg, Fe, Mn, etc.) and therefore reduces their bio-availability (Sandberg and Andersson, 1988; Raboy, 2001, 2009). One strategy for enhancing the bioavailability of micronutrients is by lowering the PA content in cereal grains (Raboy, 2009). PA biosynthesis takes place by sequential phosphorylation of the primary substrate, myo-inositol, in the cytosol (Greenwood and Bewley, 1984; Rasmussen et al., 2010). PA is subsequently compartmentalized into vacuoles by multidrug resistance-associated transporters (Raboy, 2002; Regvar et al., 2011). Previous studies suggest that lowering the seed PA level can be achieved either by targeting genes involved in its biosynthesis or its compartmentalization (Raboy, 2001; Rasmussen et al., 2010; Sparvoli and Cominelli, 2015).
Multiple genes involved in different steps of PA biosynthesis have been targeted to achieve low phytate content in diverse cereals (Shi et al., 2005; Kim and Tai, 2011; Ali et al., 2013a,b). The maize lpa1-1 and lpa2-1 lines were the first mutants studied, and produced seeds that had 50% to 66% lower PA levels and high inorganic phosphate (Raboy et al., 2000). The lpa1-1 mutation was mapped to a locus encoding an ATP-binding cassette transporter C (MRP4) with a function in PA transportation and the lpa2-1 mutation was mapped to a locus encoding an inositol phosphate kinase (IPK1) with a function in PA biosynthesis (Shi et al., 2003, 2005). Mutants similar to maize lpa1 with defects in MRP (ABCC type) transporter activity have also been recovered from Arabidopsis (ABCC5; Nagy et al., 2009), barley (ABCC1; Dorsch et al., 2003), soybean (ABCC1; Panzeri et al., 2011), and rice (MRP5; Xu et al., 2009). These observations have encouraged reverse genetic approaches in crop plants to further validate the role of ABCC transporters in lowering the PA content. For example, MRP4 in Zea mays, MRP1 in Phaseolus vulgaris, and MRP5 in Oryza sativa have been targeted to generate low phytate content in grain (Shi et al., 2007; Li et al., 2014). Among all lpa mutants, the lpa1 mutations have been most predominantly examined, with breeding and genetic engineering efforts in multiple plants (Supplementary Table S1 at JXB online).
Initially, plant ABCC transporters were known for their roles in heavy metal sequestration and transport of glutathione conjugates, but subsequently they have been examined for their role in multiple physiological responses (Ishikawa et al., 1997; Gaedeke et al., 2001; Martinoia et al., 2002). Correlations were then observed between ABCC transporter-mediated lowering of PA in cereal grains and their pleiotropic effects. For example, MRP5 knockout in Arabidopsis plants not only results in a decrease of seed PA content, but also makes the plant more sensitive in its response towards hormones (Gaedeke et al., 2001; Nagy et al., 2009). Similarly, Arabidopsis ABCC1, ABCC2, and ABCC3 are well-known active glutathione conjugate transporters. In addition, ABCC1 is a potential folate and metal transporter whereas ABCC2 and ABCC3 are involved in chlorophyll catabolite transport (Lu et al., 1997, 1998; Tommasini et al., 1998; Frelet-Barrand et al., 2008; Raichaudhuri et al., 2009; Song et al., 2010). Similarly, maize ABCC4/MRP4 performs multiple functions including flavonoid transportation and PA compartmentalization, and ZmMRP4 mutants have altered root morphology (Goodman et al., 2004; Shi et al., 2007; Badone et al., 2012).
Hexaploid wheat is one of the important food crops that has been improved for numerous traits through breeding efforts. The grains of wheat and other cereals store several essential inorganic nutrients and micronutrients compartmentalized in the aleurone (Regvar et al., 2011; Singh et al., 2013; Bhati et al., 2014). Wheat mutants and landraces have been studied for seed PA, iron bioavailability, and agronomic performance (Guttieri et al., 2004; Salunke et al., 2012; Gupta et al., 2015). Surprisingly, the pleiotropic effects observed in wheat due to lowering of PA are more drastic compared to other crop species with either lpa1/2/3 mutations (Guttieri et al., 2004; Gupta et al., 2015).
Unlike in other crops, transgenic-based approaches have not been utilized and assessed to address the roles of candidate genes to develop low phytate in wheat. Wheat genes involved in PA biosynthesis or its possible transport,have been studied previously (Bhati et al., 2014; Aggarwal et al., 2015). It has also been demonstrated that expression of TaABCC13 in a heterologous system conferred tolerance to heavy metals via the utilization of glutathione conjugates as substrates. In the present work, the functional role of ABCC13 in wheat was investigated by RNAi-mediated gene silencing by using a constitutive promoter. Silencing of ABCC13 in transgenic wheat reduced PA in mature grains, and it caused developmental defects during grain filling. Furthermore, wheat transgenic roots showed sensitivity for the presence of cadmium (Cd) and phenotypic defects were observed. These results along with previous information for TaABCC13 suggest its important role in wheat.
Materials and methods
RNAi vector preparation and Agrobacterium-mediated transformation
A monocot-specific RNAi vector pMCG161 (TAIR stock-CD3-459) was used for silencing of the wheat (Triticum aestivum) ABCC13 gene (Zalewski et al., 2010; Gasparis et al., 2011). This vector confers selection of transgenic plants in the presence of the herbicide BASTA, and the RNAi cassette is expressed ubiquitously under the control of the CaMV 35S promoter. Primers were designed to amplify and clone a 394-bp conserved sequence from TaABCC13 that was confirmed by sequencing (Chr 4B, 4D, and 5A; Supplementary Fig. S1). This nucleotide fragment was used to clone sense (AscI/AvrII) and anti-sense (SpeI/SgfI) strands following a one-step cloning method. The primer sequences used are listed in Supplementary Table S2. The cloning of sense and antisense sequences on either side of a rice waxy intron was subsequently confirmed using multiple restriction digestions and sequencing. Final constructs with proper insertion of sense and antisense sequences was transformed into Agrobacterium tumefaciens strain AGL-1. Positive colonies were screened and a single Agrobacterium clone harboring a confirmed TaABCC13:pMCG161 RNAi construct was used for wheat transformation.
Wheat transformation experiments were performed using Agrobacterium-mediated transformation with minor modifications (Przetakiewicz et al., 2004), while the growth and culture media were used as per Patnaik and Khurana (2003) and Gasparis et al. (2011). Bread wheat (cv C306) was grown in an experimental field of the National Agri-food Biotechnology Institute, Mohali, India. Immature seeds were collected at 12–16 d after anthesis (DAA) and sterilized aseptically using HgCl2 (0.01% w/v). These sterilized seeds were used to rescue immature embryos using manual dissection under a laminar flow hood. About 700 dissected embryos, with their scutella facing up, were cultured on callus-induction medium consisting of MS medium with 2mg L–1 of 2,4-Dichlorophenoxyacetic acid (2,4-D). After 48h of incubation at 22 °C in the dark, explants were infected with an Agrobacterium suspension (OD600 = 0.4) carrying the TaABCC13:pMCG161 construct. The Agrobacterium suspension in liquid MS medium with Silwet (0.001% v/v) was poured drop by drop over the explants (Gasparis et al., 2011). Following 3 d of co-cultivation, growing calli were rinsed 4–5 times with autoclaved MilliQ water to remove excess bacterial suspension, and sub-cultured for 4 weeks on callus-induction medium containing cefotaxime (200mg L–1). Healthy calli were transferred to selection and regeneration medium supplemented with 1mg L–1 Zeatin and 2mg L–1 of the herbicide BASTA (Gasparis et al., 2011). At least four rounds of selection, each for 3–4 weeks, were performed for the regeneration of putative transformants. The plantlets were rooted in MS medium containing 2.5mg L–1 BASTA. Healthy and well-rooted plants were transferred to soil-pots for hardening. The shoots regenerated from independent calli were considered as independent integration events and assigned as T0 putative plants.
Transgenic integration and genetic analysis
The plants those survived after hardening were selected for gene integration analysis through PCR. Total genomic DNA was isolated from fully expanded leaves of putative transgenic and non-transformed (control) plants using the DNeasy Plant Mini Kit–QIAGEN following manufacturer’s protocol. Two sets of specific primers were designed for amplification of the bar gene and the OCS1 terminator region of RNAi cassette (Gasparis et al., 2011) (Supplementary Table S2). Amplicon generated by PCR from T0 plants was purified and sequenced for confirmation. An individual tiller from each T0 plant was also screened by PCR to eliminate the possibility of chimera. Subsequently, seeds developed from the PCR-positive T0 plants were subjected to segregation analysis on 2mg L–1 BASTA that was optimized for cv C306 (control wheat) in hydroponic media. The T1 progenies derived from positive transformation events that fitted a 3:1 Mendelian segregation ratio based on BASTA selection and bar gene amplification were cultivated up to the T2 and T3 generations. Homozygous lines were selected using PCR in the T2 and T3 generations. These homozygous TaABCC13 RNAi lines were maintained up to the T4 generation with consistent BASTA selection as well as by PCR-based screening. At the onset of flowering, the spikes were tagged on the day of anthesis for each line for subsequent observation on days post anthesis. The mature seeds from wild-type C306 and the RNAi lines were collected at approximately 56 d post anthesis and stored in cool and dry conditions.
RNA extraction and confirmation of gene silencing using qRT-PCR
Tissue samples from selected homozygous T4 lines were snap-frozen in liquid nitrogen and stored at −80 °C until further analysis. Total RNA was extracted using the RNeasy Plant MiniKit (Qiagen, Valencia, CA, USA), as per the manufacturer’s instructions. Two micrograms of DNA-free total RNA were used for cDNA preparation. Reverse transcription reactions were performed using the Transcriptor First-Strand cDNA Synthesis Kit RT-PCR (Roche, USA) according to the manufacturer’s instructions. Primers used in the expression study are noted in Supplementary Table S2. Quantitative real-time PCR analysis was performed by following SYBR Green (QuantiFastTM SYBR Green PCR kit, QIAGEN) chemistry using the ABI PRISM 7500 Fast Realtime Platform (Applied Biosystems). Target genes were amplified by a two-step PCR reaction with an initial denaturation at 95 °C for 5min followed by 40 cycles of 95 °C for 30s and annealing/extension at 60 °C for 30s. Relative quantification for fold-changes were calculated and Ct values were normalized against wheat ARF (ADP-ribosylation factor, AB050957.1) as defined previously by Bhati et al. (2014) and Alok et al. (2015) using the 2–ΔΔCT method (Livak and Schmitteng, 2001). The specificity of the amplification was also assessed for each gene by dissociation curve analysis. A unique peak on the dissociation curve was confirmed for each gene.
Phytic acid, free phosphate and total protein estimation
Total phytate in seeds was estimated by a colorimetric method using a K-PHYT kit (Megazyme, Inc, Bray, Ireland). Mature seeds from T4 homozygous lines were ground into a fine powder and extracted using 0.66N HCl for 6h with continuous stirring. Reactions were processed as described previously (Bhati et al., 2014). Free phosphate in mature seeds of selected RNAi lines was measured by the ascorbate and ammonium molybedate method (Ames, 1966). Total protein was estimated by using the Bradford method (Bradford, 1976) with comparison against a BSA standard curve.
Elemental analysis and cadmium uptake assays
Sterilized seeds of homozygous RNAi lines and non-transformed plants were germinated on Hoagland liquid media (Hoagland and Arnon, 1950) supplemented with 50 µM CdCl2. Sample collection, phenotypic observations, and Cd estimation were performed after 7 d. Root and leaf samples were heat-dried and microwave-digested with HNO3 (SuraPureTM, Merck). Total Cd uptake was measured by using Inductive Coupled Plasma-MS (ICP-MS) as described previously (Bhati et al., 2014). In the figures the error bars indicate the standard deviation across two independent experiments.
Results
Homoeologous specific expression pattern of TaABCC13
TaABCC13 was mapped to the long arm of the 4B, 4D, and 5A chromosomes with a similarity score of 99% and 97% (4B allele with respect to 4D and 5A). Fine mapping of these homoeologous sequences in the wheat genome assembly from the Ensembl plant server (http://plants.ensembl.org/Triticum_aestivum /Info/Annotation) helped in identification of their physical location on the respective chromosomes (Supplementary Table S3). The wheat ABCC13 transporter was conserved among orthologs from other cereals that share phylogenetic proximity (Supplementary Fig. S2). TaABCC13 was differentially expressed during the developmental stages of wheat grains (Bhati et al., 2014). To check the homoeolog-based expression of TaABCC13, gene-specific primers were used to determine the contribution from different wheat genomes. Expression of each of the TaABCC13 homoeologs at two developmental stages during grain filling suggested preferentially high expression of transcripts arising from the B genome (Supplementary Fig. S3). The expression of all TaABCC13 homoeologous transcripts was higher at 14 DAA than at 21 DAA. The transcript accumulation derived from the B genome was ~13-fold higher at 14 DAA compared to 21 DAA.
Selection and screening of transgenic wheat lines
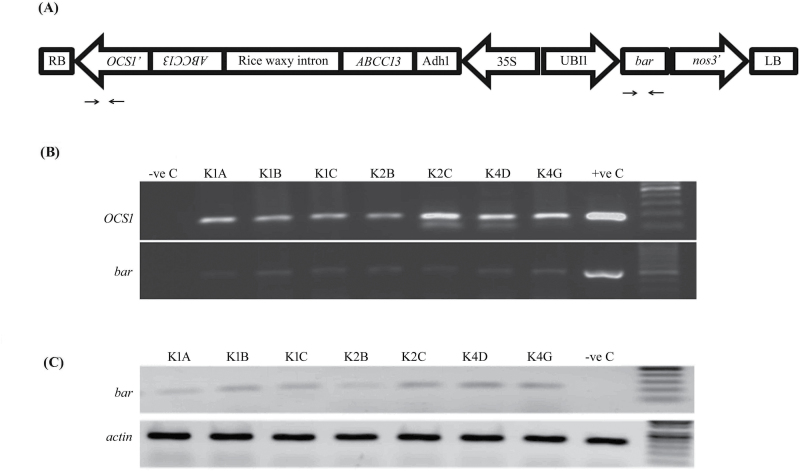
To gain insights into the function of TaABCC13 in wheat, a binary vector pMCG161 with a constitutive promoter was designed to target a conserved region of the homoeologous gene sequences (Fig. 1A and Supplementary Fig. S1). The pMCG161 binary vector was previously shown to be an efficient vector for gene silencing in monocots such as barley, wheat, and maize (Zalewski et al., 2010; Gasparis et al., 2011). Nine independent putative transgenic events survived during multiple rounds of selection on BASTA (Supplementary Fig. S4), but only four of the putative transgenic plants survived the hardening procedure. These four putative transformants were subsequently confirmed for the presence of the transgene by amplifying and sequencing the bar and OCS1 terminator sequences (Fig. 1B; Supplementary Fig. S5A, B). Additionally, mosaicism of transgenic integration in the wheat callus was also ruled out by screening each tiller by amplification and sequencing of bar (Supplementary Fig. S5C), and the flag leaves of T0–1 plants were found positive for bar transcript expression (Fig. 1C). The T1 progenies from the third event (K3) failed to survive due to reduced seed setting and subsequent failure of seed germination. Eventually, three independent transgenic events (K1, K2, and K4) showed healthy growth and seed germination for further analysis. The lines from these three independent events were propagated to the T4 generation, which was analysed in detail. Two transgenic plants from lines K1 (K1B4-2–5, K1A13-8-2), K2 (K2C4-6–8, K2C9-2–3), and K4 (K4G3-5-1, K4G7-10–3) selected randomly for further study.
Fig. 1.
Designing the TaABCC13 RNAi construct and selection of transgenic plants. (A) Schematic image of the pMCG161 vector backbone with inverted repeats of TaABCC13 and the binding site for representative primers used for screening and selection of progeny positive for T-DNA integration. (B) Genomic DNA was isolated from the second leaf of T1 progeny. PCR reactions using primers targeting the OCS terminator (190bp) and the bar (480bp) gene were performed to confirm the presence of the transgene. The amplification products were visualized by using agarose gel electrophoresis. (C) cDNA was prepared from RNA isolated from the second leaf of PCR-positive plants from representative lines and bar gene-based primers were used for PCR amplification up to 30 cycles; the control reaction was run using the actin gene.
Silencing of TaABCC13 in wheat RNAi lines
The selected T3 or T4 transgenic plants developed from three events (K1, K2, and K4) were subjected to qRT-PCR to assess the level of gene silencing in varying tissues. Transcript abundance of TaABCC13 was quantified in T4 seeds at 14 DAA in the grains and flag leaf of the same tiller (Supplementary Fig. S6). The fold-decrease in the TaABCC13 transcript for the T4 generation seed is presented in Fig. 2A and Supplementary Fig. S6. The silencing of TaABCC13 resulted in 20–60% reduction in the transcript levels in the seed tissue, while 20–70% of silencing was observed in the flag leaf (Fig. 2A and Supplementary Fig. S6A, B). Maximum seed tissue silencing was observed for lines K4G7-10–3, K4G3-5-1, K1A13-8-2, and K1B4-2–5 in both T3 and T4 developing seeds. The representative silenced lines were further used for detailed characterization and phenotypic studies. As noted above, chromosome 4B is the major contributor of the TaABCC13 transcripts (Supplementary Fig. S3), and we wanted to know how the silencing construct affected the expression of each of the homoeologs. To study the relative expression of the transcripts of TaABCC13, homoeolog-specific primers were employed and expression was compared to respective non-transgenic plants. The results confirmed that the construct was able to reduce the expression of the transcripts derived from the 4B, 4D, and 5A chromosomes by 40–72% (Supplementary Fig. S6C). In addition, to check whether other closely related ABCC genes (TaABCC3 and TaABCC4) were affected by silencing of TaABCC13, expression analysis was performed for them. No significant change in the transcript of TaABCC3 and TaABCC4 was observed in the non-transgenic and transgenic lines (Supplementary Fig. S6D). This suggests that the silencing construct was specific for TaABCC13 and probably does not affect the expression of the other studied ABCC genes.
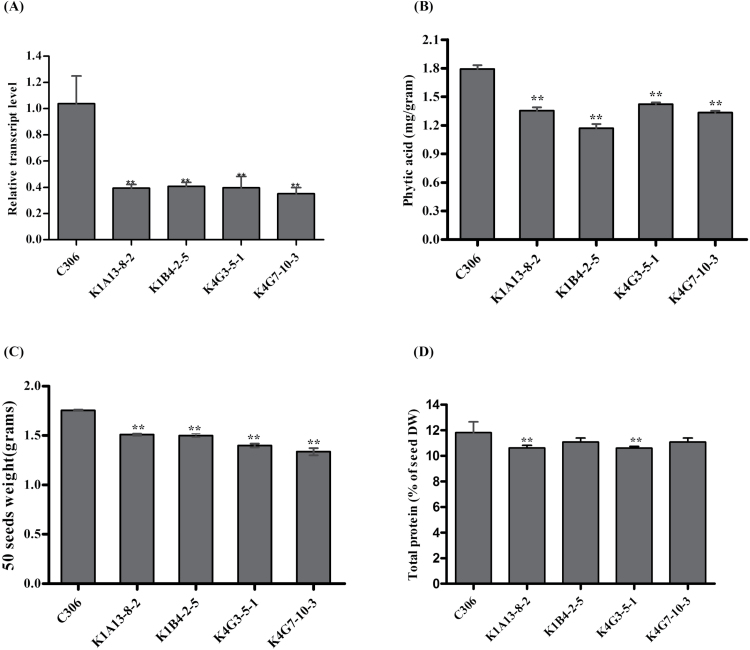
Fig. 2.
Silencing in seeds, phytic acid estimation, and seed quality of TaABCC13:RNAi lines. (A) Relative transcript levels of TaABCC13 in different RNAi lines of wheat. Total RNA was isolated at 14 d after anthesis of the primary tiller of non-segregating T4 RNAi lines, cDNA was prepared from 2 µg of RNA (DNA free) and qRT-PCR analysis was performed using a SYBR Green-based assay. (B) Estimation of total phytic acid in mature wheat grains of transgenic lines. Seeds were collected from the primary tiller of each line. (C) Average seed weight of the TaABCC13:RNAi lines was determined by weighing 50 random seeds. (D) The total protein content of seeds from the TaABCC13:RNAi lines and non-transgenic parent was determined by using the Bradford method. Each bar indicates the mean of three biological replicates (three technical replicates). ** indicates significant differences at P<0.05 (t-test).
TaABCC13 silencing reduces grain total PA content
To examine the effect of silencing of TaABCC13 in seeds, PA content was measured in the mature grains. Significant differences in the accumulation of PA were observed among the transgenic lines that ranged from 22% to 34% reduction when compared to the non-transformed mature seeds. The maximal reduction in seed PA was observed in line K1B4-2–5 (~34%) followed by K4G7-10–3, which had a ~22% reduction in the PA level (Fig. 2B). No significant changes in total seed phosphorus (P) was observed for the ABCC13:RNAi lines as compared to C306 (Supplementary Fig. S7). However, the lowering of PA content was accompanied by a increase in free phosphate (Pi) for these transgenic lines (data not shown). Silencing of TaABCC13 in the selected transgenic lines resulted in a slight reduction in seed weight (Fig. 2C). Although this slight reduction was observed consistently in other lines, it was most apparent in the K1B4-2–5 and K4G7-10–3 lines. The calculated protein content range for C306 was 11–12%. Although our transgenic lines varied in protein content from 10.3–11.2%, it was within the usual range expected for C306 (Fig. 2D).
Reduction in PA is often accompanied by remobilization or a change in the content of micronutrients (Ali et al., 2013a). Transgenic wheat seeds showed increases in the accumulation of Ca, but no significant changes were observed for micronutrients such as Zn and Fe. The accumulation of Ca was ~1.7–1.8-fold greater than in the non-transformed control seeds (Table 1). Results of Perl’s staining showed a decreased density of iron at the crease region in the transgenic seed as compared to C306 (Supplementary Fig. S8). Enhanced colouration was also observed on the germinating coleoptile region, suggesting a possible early remobilization of iron in transgenic seeds.
Table 1.
Estimation of metal content as analyzed by ICP-MS in the T4 grains of two wheat transgenic lines and the non-transgenic control (C306). The data are means ±SD with three biological replicates.
| Metal (µg g −1) | C306 | K1B4-2–5 | K4G7-10–3 |
|---|---|---|---|
| Fe | 47.2±0.15 | 55.8±0.21 | 45.9±0.05 |
| Ca | 75.2±1.09 | 80.2±0.35 | 82.38±0.30 |
| Zn | 22.6±0.20 | 25.22±0.56 | 23.2±0.06 |
| Mn | 48.06±0.77 | 45.42±0.50 | 48.3±3.00 |
TaABCC13 affects grain filling, spike characteristics, and lateral root formation
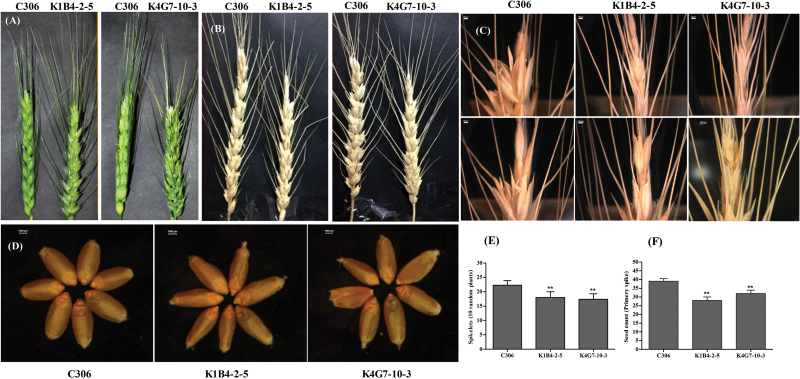
Previously, it was shown that wheat ABC transporters affect anther development and subsequently the process of grain maturation (Niu et al., 2013; Walter et al., 2015). Reports from multiple studies have also suggested that lowering PA generally results in altered grain morphology (Guttieri et al., 2004; Li et al., 2014). To assess the contribution of ABCC13 to grain development, developing spikes after heading were examined for phenotypic changes in C306 and transgenic plants. An altered spikelet arrangement was noticed in the developing spikes of transgenic lines. In these lines, the outer glumes are more exposed, thus causing spikes with an altered spikelet arrangement that is always accompanied by a reduction in total spikelet counts, when compared to the primary tiller of the control plants (Fig. 3A, B, E). These observations were consistently observed for both the silenced lines. The occurrence of head sterility was also observed in these RNAi lines in T2 to T4 progenies (Fig. 3C). Therefore, seed setting and grain filling were affected in transgenic lines, but the head development was normal at the pedicel region in both plants. Taking into account the pleiotropic effects of silencing TaABCC13, a reduction in the number of seeds recovered from each primary spike was generally observed (Fig. 3F). These results suggest the possibility that grain development directly involves ABCC13, or it could be affected by PA levels.
Fig. 3.
TaABCC13 silencing affects grain filling and spike characteristics. (A) Representative images of the developing wheat caryopsis at the onset of flowering for C306 (control) and representative TaABCC13:RNAi lines. (B) Decreased length of spikes upon maturation for control (C306) and RNAi lines. (C) Detailed grain filling at the spike head on control (C306) and RNAi lines. (D) Representative images of seeds collected from C306 (control) and RNAi lines. (E) Total spikelet counts from RNAi lines as compared to C306. (F) Seed count from the primary spikes of the C306 and RNAi lines. Observations presented here were collected from T4 progenies that were consistent with the non-segregating lines of the T3 generation. The data in (E) and (F) are means ±SD. Ten spikes from each TaABCC13:RNAi line (only from the primary tiller) and the respective controls were considered for above observations. ** indicates significant differences at P<0.05 (t-test).
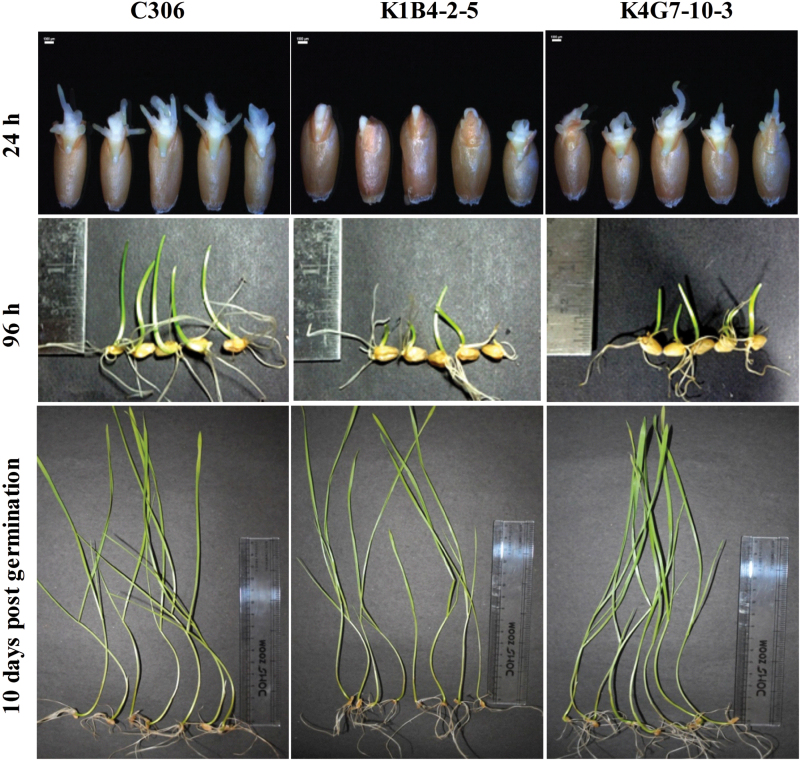
A reduction in PA often correlates with the rate of germination for cereal grains. The germination rate of transgenic wheat (T4) with low PA levels was reduced by 5–7% when compared to the non-transgenic wheat seeds. Interestingly, a slow rate of germination was observed for low-PA seeds, which was apparent at the early times points (24 and 96h) after imbibition. In addition, we also observed that the germination rate was comparatively slow in transgenic compared to non-transgenic seeds during the early hours of germination. (Fig. 4). At a later stage of development (10 d), no significant difference in the height of germinated seedlings was noted between transgenic or non-transgenic lines (Fig. 4). These data confirm that silencing of ABCC13 in wheat is related to lowering of total PA and to grain development.
Fig. 4.
Germination of seeds from the TaABCC13:RNAi lines. Twenty seeds from mature plants (56 d after anthesis) from each transgenic line and control C306 plants were collected, sterilized, and soaked overnight in sterilized water. Seeds were then transferred to a growth chamber for germination. Representative images were photographed at 24h, 96h and at 10 d post germination.
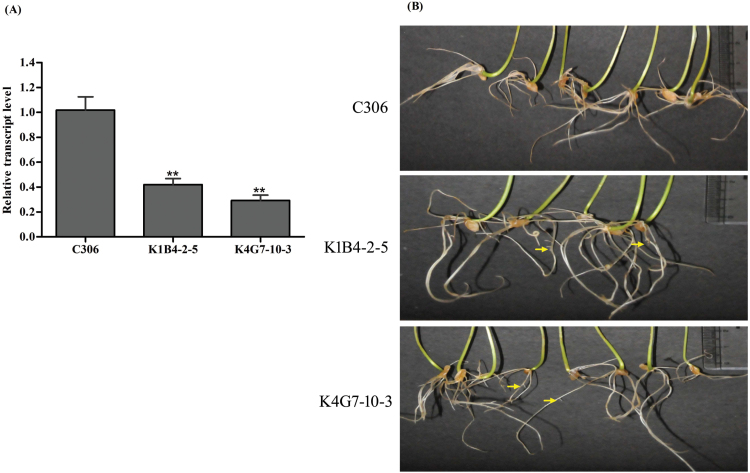
Plant ABCC transporters are also known for their role during root development under stress conditions (Gaedeke et al., 2001). The K1B4-2–5 and K4G7-10–3 transgenic lines were selected to determine the effect of TaABCC13 silencing on root development. In general, TaABCC13 transcript was reduced by 30–65% in the roots of the transgenic lines (Fig. 5A), but there was no difference in the root length compared to the non-transgenic plants. Interestingly, early emergence of lateral roots was observed in transgenic seedlings in contrast to non-transgenic plants (Fig. 5B). The formation of lateral roots in the transgenic lines was higher as compared to the non-transgenic, suggesting that TaABCC13 might be involved in controlling the emergence of lateral roots in wheat.
Fig. 5.
Emergence of lateral roots in TaABCC13:RNAi lines. (A) Relative transcript level of TaABCC13 in different RNAi lines of T4 wheat seedlings. cDNA was prepared from 2 µg RNA (DNA free) and qRT-PCR analysis was performed using a SYBR Green-based assay. (B) Phenotypic analysis of the roots of C306 and transgenic RNAi lines. Ten seeds from TaABCC13:RNAi and C306 were germinated on half-strength Hoagland media in a hydroponic system and observations were recorded at 10 d after germination. Each bar indicates the mean of three biological replicates (10 technical replicates).
Influence of cadmium toxicity on the wheat transgenic lines
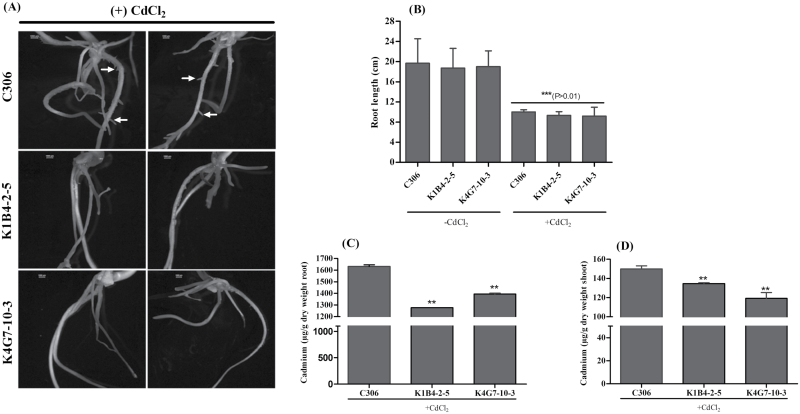
Heterologous expression of TaABCC13 in yeast has suggested a role in Cd tolerance that could utilize glutathione conjugates as substrates (Bhati et al., 2014, 2015). In order to further characterize the role of TaABCC13 in heavy metal detoxification, wheat RNAi transgenic seedlings were exposed to Cd. As expected, the emergence of multiple lateral roots was observed in control plants treated with Cd. Transgenic lines showed phenotypic sensitivity when exposed to Cd compared to non-transgenic (Fig. 6A). Interestingly, the TaABCC13:RNAi lines did not develop lateral roots when exposed to Cd (Fig. 6A). This phenotype was noted in two different transgenic lines (K1B4-2–5 and K4G7-10–3). In general, a decrease in the root length was observed in Cd-exposed plants when compared to untreated seedlings (Fig. 6B). The shoot biomass was significantly higher in C306 plants as compared to TaABCC13:RNAi seedlings exposed to Cd (Supplementary Fig. S9). The TaABCC13 silencing and altered root phenotype under Cd stress resulted into differences in the root and shoot uptake of Cd (Fig. 6C, D). This in planta evidence shows that Cd uptake is reduced and sensitivity to Cd is decreased in TaABCC13:RNAi lines, thus signifying the importance of ABC transporters for Cd detoxification.
Fig. 6.
Differential root architecture and cadmium uptake assay in RNAi seedlings. (A) Phenotypic differences were observed on the roots of C306 and TaABCC13:RNAi lines. Ten seedlings from transgenic and control lines were exposed to 50 µM CdCl2 for 7 d. After sprouting, seedlings were transferred to a hydroponic system for 7 d of heavy metal stress treatment. Roots were observed under a light microscope. Representative images are presented with two replicate plantlets after 7 d exposure to Hoagland media supplemented with cadmium. (B) Primary root length was measured at 7 d after C306 and RNAi plantlets were exposed to heavy metal stress. Seedlings without heavy metal exposure were used as the experimental control. (C, D) Cadmium uptake in roots (C) and shoots (D) at 7 d after exposure to CdCl2. Samples were washed using ICP-MS grade water after collection from the hydroponic system, heat-dried and digested in HNO3 using high-intensity microwaves. PPM outputs from the ICP-MS run were normalized with ICP-MS values of the controls with cadmium stress. The data in (C) and (D) are means ±SD. ** indicates significant differences at P<0.05 (t-test).
Discussion
The present study provides insights into multiple physiological roles of ABCC13 in wheat. Previous studies have proposed high expression levels of TaABCC13 in immature seeds and roots, thus advocating TaABCC13 as a potential candidate gene regulating seed and root phenotypes in wheat (Bhati et al., 2015). The constitutive expression of the RNAi cassette targeting TaABCC13 enabled the functional validation of this gene not only in seeds but also in the root tissue. TaABCC13 is evolutionarily conserved with orthologs of other candidate genes from maize (ZmMRP4) and Arabidopsis (AtMRP5) that were demonstrated to have multiple functions in earlier studies (Gaedeke et al., 2001; Badone et al., 2012). In the present study, we observed that ABCC13-silenced wheat transgenic lines had reduced PA and altered heavy metal detoxification.
ABCC13 as a candidate to achieve low phytic acid in wheat
Multiple approaches have been considered to generate a low-phytate trait in crop plants such as soybean, rice, and maize (Shi et al., 2007; Kim and Tai, 2011; Ali et al., 2013a,b; Li et al., 2014). However, such studies have not previously been undertaken for important crops such as wheat where most of the PA–along with micronutrients–accumulates in the aleurone layer (Bohn et al., 2008; Bhati et al., 2014). Utilizing a transgenic approach to modify the PA in wheat is important since genetic variation for this trait in this species is very limited. Although the transcript arising from chromosome 4B was highly expressed, to evaluate the functionality of TaABCC13 a conserved region of the three homoeologous genes was targeted (Supplementary Figs S1 and S6). In general, we observed that this construct was able to reduce relative expression levels of all the transcripts of TaABCC13 irrespective of the origin.
Recently, wheat genes involved in biosynthesis of PA have been reported, and these could be potential candidates for developing low-PA traits (Bhati et al., 2014). ABCC transporters are involved in the transport or compartmentalization of the PA after its synthesis in grains. Loss-of-function mutations for ABCC transporters have been characterized with varying levels of reduction in seed phytate (Raboy et al., 2000; Pilu et al., 2003; Raboy, 2009; Panzeri et al., 2011). Moreover, ABCC13 also shares close homology with ZmMRP4 and AtMRP5 (90.5 and 73.5%, respectively), with a similar exon–intron arrangement (Bhati et al., 2014). These studies strongly support this ABCC transporter as a strong candidate for the development of a lpa phenotype in agronomically important crops.
Wheat RNAi lines targeting this ABCC transporter gene (K1B4-2–5 and K4G7-10–3) showed 34–22% reduction in the PA level with a concomitant increase in calcium. Depending on the crop and the candidate genes targeted, lowering of PA is often accompanied by an increase in micronutrients, as demontsrated in other studies (Shi et al., 2007; Ali et al., 2013a). In our transgenic seeds, although no significant increase in the iron content was observed, our preliminary screening suggested its faster remobilization in other tissues. This phenomenon was consistently observed in most of our RNAi seeds, reinforcing the correlation between the rate of iron remobilization and lowering of PA. Studies utilizing spectroscopy-based methods could be further used to validate these redistribution patterns.
The only previous report for reduced PA in wheat was a line with mutations in two independent lpa1-related loci that caused a 37% reduction (Guttieri et al., 2004). Similar, the lpa-1 mutation in barley and maize showed reduction of 50–95% and 50–60%, respectively (Raboy et al., 2000; Dorsch et al., 2003). Reduction of PA due to a defective PA transport mechanism causes significant pleiotropic effects that results in unacceptable agronomic field performance in wheat, maize, and rice (Guttieri et al., 2004; Badone et al., 2012; Li et al., 2014). Some of these negative impacts were also observed in the current study in TaABCC13:RNAi lines, including reduced seed weight, slightly delayed germination, and slow coleoptile growth. However, these defects were not as drastic as reported in the first wheat lpa mutant and in the low field performance by the lpa1-7 mutation in ZmMRP4 (Guttieri et al., 2004; Badone et al., 2012). Similar agronomical impacts of reduced PA have been observed in other crop plants silenced for genes involved in either the early or late phase of PA biosynthesis (Raboy, 2009; Ali et al., 2013a,b; Sparvoli and Cominelli, 2015). Thus these impacts may vary from crop to crop, and with the choice of the candidate gene. Phenotypic effects on the lpa crops could be reduced by utilizing tissue-specific targeting of the PA biosynthetic pathway (Shi et al., 2007; Ali et al., 2013a). Such strategies could also be designed to target ABCC13 in the aleurone to develop an lpa trait in wheat, because the aleurone is the tissue where the TaABCC13 transcript is abundant (Bhati et al., 2014).
Role of TaABCC13 in grain development and physiological characteristics
The phenomenon of multi-functionality is common among plant ABCC transporters (Gaedeke et al., 2001; Nagy et al., 2009; Walter et al., 2015). Functional studies of the wheat ABCC transporter have established its roles in responses to fungal pathogens, and in grain formation and maturation (Walter et al., 2015). Subsequently, multiple wheat ABCC transporters were reported that are highly expressed in developing grains (Bhati et al., 2015). These reports have emphasized the need to expand our knowledge of the role of ABCC transporters in wheat. Our data confirm the importance of TaABCC13 and generally support the emerging roles of ABCC transporters in grain development and maturation. The early development of wheat spikes is regulated by abscisic acid (ABA) and gibberellic acid (GA) responses (Thiel et al., 2008; Pearce et al., 2013). Previously we have reported that the exogenous application of GA significantly stimulated TaABCC6, TaABCC8, and TaABCC13 transcript levels in wheat seeds (Bhati et al., 2015). Plant ABCC transporters are known to have a role in the transport of derivatives in hormone biosynthesis to further facilitate localized hormonal response (Gaedeke et al., 2001; Ko et al., 2014; Borghi et al., 2015). Inositol phosphate biosynthesis pathways control signaling responses in eukaryotic cells (Gillaspy, 2013). In plants PA also acts as a signaling molecule or a co-factor that controls the physiological response to hormones or oxidative stress (Lemtiri-Chlieh et al., 2003; Tan et al., 2007; Doria et al., 2009; Sparvoli and Cominelli, 2015). The biosynthesis of wheat PA starts at the early stages of seed development. Therefore, one could speculate that the phenotypes observed during wheat spike development are possibly effects of reduced PA signaling and/or perturbation in the ABCC13-dependent transport of hormonal derivatives.
Plant roots may develop different phenotypes and anatomical features when exposed to metal stress. Higher plants commonly develop lateral roots in an attempt to block the radial transport of heavy metals (Hu et al., 2013; Sofo et al., 2013). In our experiments, non-transgenic wheat lines also developed lateral roots under Cd stress, reinforcing the conserved mechanism of sensitivity in plants. Plant ABCC transporters may have direct or indirect roles in regulation of plant development. Interestingly, the untreated transgenic lines silenced for TaABCC13 showed an early emergence of lateral roots, but they were inhibited in the presence of Cd (Figs 5 and 6). Low PA mutants of maize (zmmrp4) and Arabidopsis (atmrp5) also have altered root phenotypes that include the early emergence of lateral roots (Gaedeke et al., 2001; Nagy et al., 2009; Badone et al., 2012). The early emergence of lateral roots on TaABCC13:RNAi lines suggests a conserved role for TaABCC13 in wheat root development. These observations coupled with previous evidence suggest a correlation for the possible homeostatic role of TaABCC13 in lateral root formation and metal stress.
It may be speculated that altered root development might occur as a result of changes in auxin flux (Gaedeke et al., 2001). If this is the case, then we could propose that TaABCC13 may function in the transport of auxin or a derivative; however, our experiments do not directly test this hypothesis. In our study, 10-d-old C306 seedlings formed lateral roots when exposed to Cd, whereas TaABCC13-silenced plants were not able to develop lateral roots. This surprising reversal of lateral root development on TaABCC13:RNAi seedlings under Cd stress suggests that there is a complex interaction between the processes regulating root development and responses to Cd stress. In plants, the response activated by metals is mediated through the biosynthesis of signalling molecules such as phytohormones. Multiple reports have suggested that Cd interferes with the maintenance of auxin homeostasis (Xu et al., 2010; Elobeid et al., 2012; Hu et al., 2013). Previous reports have also suggested that Cd alters the expression of multiple genes responsible for auxin biosynthesis and distribution, which leads to increased lateral root density (Hu et al., 2013, Yuan and Huang, 2015). Our data not only validate the role of TaABCC13 during lateral root formation, but also link with the possibility for metal–auxin homeostasis.
The current study provides in planta evidence for the previously speculated role that TaABCC13 has in yeast. Taken together, our data reinforce the importance of targeting ABCC transporters to achieve low PA, and we further demonstrate their roles in heavy metal detoxification. This research lays the groundwork for further examination for the functions of ABCC transporters in wheat, especially those that are highly expressed during grain development.
Supplementary data
Supplementary data are available at JXB online.
Table S1. List of ABCC transporters reported from different plant systems together with the approaches used to develop the low phytic acid trait.
Table S2. List of primers used in the present study.
Table S3. Physical coordinates of TaABCC13 on the wheat genome on the Ensembl Plants genome database.
Figure S1. Multi-alignment of nucleotide sequence of TaABCC13 transcripts used to design RNAi targets.
Figure S2. TaABCC13 is evolutionary close to lpa1 orthologs from other cereals.
Figure S3. Differential expression analysis of three homoeologous of TaABCC13 at developmental stages of the seed.
Figure S4. Representative images of regenerated transgenic seedling selected over herbicide BASTA with respect to the control.
Figure S5. Confirmation of RNAi integration in T0 transgenic plants.
Figure S6. Primary screening for relative transcript level for TaABCC13 in multiple RNAi lines.
Figure S7. Estimation of total phosphorus in flour of TaABCC13:RNAi seeds and cv C306.
Figure S8. Perl’s staining for iron localization in cv C306 and transgenic seeds (low phytic acid content).
Figure S9. Effect of Cd stress on seedling biomass.
Acknowledgements
The authors would like to thank the Executive Director of NABI for facilities and support. This research was funded by the Department of Biotechnology (DBT), Government of India (BT/PR5989/AGII/106/867/2012) to AKP and ST. The authors would like to thank Dr Sebastian Gasparis, Department of Functional Genomics, Instytut Hodowli i Aklimatyzacji Roslin, Poland for his suggestions relating to the screening of wheat RNAi plants. We also thank Dr Steven Whitham (Iowa State University, Ames, Iowa, USA) for critically reading the manuscript. KKB was supported by a Senior Research Fellowship from the DBT, AA was supported by a fellowship from the NABI-CORE fund, and AK was supported by a fellowship from the DBT grant. Technical assistance from Pankaj Pandey was greatly appreciated.
References
- Aggarwal S, Shukla V, Bhati KK, Kaur M, Sharma S, Singh A, Mantri S, Pandey AK. 2015. Hormonal regulation and expression profiles of wheat genes involved during phytic acid biosynthesis pathway. Plants 4, 298–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali N, Paul S, Gayen D, Sarkar SN, Datta K, Datta SK. 2013. a Development of low phytate rice by RNAi mediated seed-specific silencing of inositol 1,3,4,5,6-pentakisphosphate 2-kinase gene (IPK1). PLoS ONE 8, e68161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali N, Paul S, Gayen D, Sarkar SN, Datta SK, Datta K. 2013. b RNAi mediated down regulation of myo-inositol-3-phosphate synthase to generate low phytate rice. Rice 6:12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames BN. 1966. Assay of inorganic phosphate, total phosphate and phosphatases. Methods in Enzymology 8, 115–118. [Google Scholar]
- Alok A, Kaur H, Bhati KK, Kumar J, Pandey P, Upadhyay SK, Pandey A, Sharma NC, Pandey AK, Tiwari S. 2015. Biochemical characterization and spatio-temporal expression of myo-inositol oxygenase (MIOX) from wheat (Triticum aestivum L.). Plant Gene 4, 10–19. [Google Scholar]
- Badone CF, Amelotti M, Cassani E, Pilu R. 2012. Study of low phytic acid1-7 (lpa1-7), a new ZmMRP4 mutation in maize. Journal of Heredity 103, 598–605. [DOI] [PubMed] [Google Scholar]
- Bhati KK, Aggarwal S, Sharma S, et al. 2014. Differential expression of structural genes for the late phase of phytic acid biosynthesis in developing seeds of wheat (Triticum aestivum L.). Plant Science 224, 74–85. [DOI] [PubMed] [Google Scholar]
- Bhati KK, Sharma S, Aggarwal S, Kaur M, Shukla V, Kaur J, Mantri S, Pandey AK. 2015. Genome-wide identification and expression characterization of ABCC-MRP transporters in hexaploid wheat. Frontiers in Plant Science 6, 488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn L, Meyer AS, Rasmussen SK. 2008. Phytate: impact on environment and human nutrition. A challenge for molecular breeding. Journal of Zhejiang University Science B 9, 165–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghi L, Kang J, Ko D, Lee Y, Martinoia E. 2015. The role of ABCG-type ABC transporters in phytohormone transport. Biochemical Society Transactions 43, 924–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. 1976. A rapid and sensitive for the quantitation of microgram quantitites of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72, 248–254. [DOI] [PubMed] [Google Scholar]
- Doria E, Galleschi L, Calucci L, Pinzino C, Pilu R, Cassani E, Nielsen E. 2009. Phytic acid prevents oxidative stress in seeds: Evidence from a maize (Zea mays L.) low phytic acid mutant. Journal of Experimental Botany 60 967–978. [DOI] [PubMed] [Google Scholar]
- Dorsch JA, Cook A, Young KA, Anderson JM, Bauman AT, Volkmann CJ, Murthy PPN, Raboy V. 2003. Seed phosphorusand inositol phosphate phenotype of barley low phytic acid genotypes. Phytochemistry 62, 691–706. [DOI] [PubMed] [Google Scholar]
- Elobeid M, Gobel C, Feussner I, Polle A. 2012. Cadmium interferes with auxin physiology and lignification in poplar. Journal of Experimental Botany 63, 1413–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frelet-Barrand A, Kolukisaoglu HU, Plaza S, Ruffer M, Azevedo L, Hortensteiner S, Marinova K, Weder B, Schulz B, Klein M. 2008. Comparative mutant analysis of Arabidopsis ABCC-type ABC transporters: AtMRP2 contributes to detoxification, vacuolar organic anion transport and chlorophyll degradation. Plant Cell Physiology 49, 557–569. [DOI] [PubMed] [Google Scholar]
- Gaedeke N, Klein M, Kolukisaoglu U, et al. 2001. The Arabidopsis thaliana ABC transporter AtMRP5 controls root development and stomata movement. EMBO Journal 20, 1875–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparis S, Orczyk W, Zalewski W, Nadolska-Orczyk A. 2011. The RNA-mediated silencing of one of the Pin genes in allohexaploid wheat simultaneously decreases the expression of the other, and increases grain hardness. Journal of Experimental Botany 62, 4025–4036. [DOI] [PubMed] [Google Scholar]
- Gillaspy GE. 2013. The role of phosphoinositides and inositol phosphates in plant cell signaling. In Capelluto D, ed. Lipid-mediated protein signaling, volume 991 . Dordrecht, The Netherlands: Springer,141–157. [DOI] [PubMed] [Google Scholar]
- Goodman CD, Casati P, Walbot V. 2004. A multidrug resistance-associated protein involved in anthocyanin transport in Zea mays . Plant Cell 16, 1812–1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood JS, Bewley JD. 1984. Subcellular distribution of phytin in the endosperm of developing castor bean: a possibility for its synthesis in the cytoplasm prior to deposition within protein bodies. Planta 160, 113–120. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Gangoliya SS, Singh NK. 2015. Screening and characterization of wheat germplasms for phytic acid and iron content. Journal of Agriculture Science Technology 17, 747–756. [Google Scholar]
- Guttieri M, Bowen D, Dorsch JA, Raboy V, Souza E. 2004. Identification and characterization of a low phytic acid wheat. Crop Science 44, 418–424. [Google Scholar]
- Hoagland DR, Arnon DI. 1950. The water-culture method for growing plants without soil. California Agricultural Experiment Station Circular 347, 1–32. [Google Scholar]
- Hu YF, Zhou G, Na XF, Yang L, Nan WB, Liu X, Zhang YQ, Li JL, Bi YR. 2013. Cadmium interferes with maintenance of auxin homeostasis in Arabidopsis seedlings. Journal of Plant Physiology 170, 965– 975. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Li ZS, Lu YP, Rea PA. 1997. The GS‐X pump in plant, yeast, and animal cells: structure, function, and gene expression. BioScience Reports 17, 189–207. [DOI] [PubMed] [Google Scholar]
- Kim S, Tai TH. 2011. Identification of genes necessary for wild-type levels of seed phytic acid in Arabidopsis thaliana using a reverse genetics approach. Molecular Genetics Genomics 286, 119–133. [DOI] [PubMed] [Google Scholar]
- Ko D, Kang J, Kiba T, et al. 2014. Arabidopsis ABCG14 is essential for the root-to-shoot translocation of cytokinin. Proceedings of the National Academy of Sciences, USA 111, 7150–7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemtiri-Chlieh F, MacRobbie EAC, Webb AAR, et al. 2003. Inositol hexakisphosphate mobilizes an endomembrane store of calcium in guard cells. Proceedings of the National Academy of Sciences, USA 100, 10091–10095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WX, Zhao HJ, Pang WQ, Cui HR, Poirier Y, Shu QY. 2014. Seed-specific silencing of OsMRP5 reduces seed phytic acid and weight in rice. Transgenic Research 23, 585–599. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Lu YP, Li ZS, Drozdowicz YM, Hortensteiner S, Martinoia E, Rea PA. 1998. AtMRP2, an Arabidopsis ATP binding cassette transporter able to transport glutathione S-conjugates and chlorophyll catabolites: functional comparisons with AtMRP1. Plant Cell 10, 267–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YP, Li ZS, Rea PA. 1997. AtMRP1 gene of Arabidopsis encodes a glutathione S-conjugate pump: isolation and functional definition of a plant ATP-binding cassette transporter gene. Proceedings of the National Academy of Sciences, USA 94, 8243–8248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinoia E, Klein M, Geisler M, Bovet L, Forestier C, Kolukisaoglu U, Muller-Rober B, Schulz B. 2002. Multifunctionality of plant ABC transporters–more than just detoxifiers. Planta 214, 345–355. [DOI] [PubMed] [Google Scholar]
- Nagy R, Grob H, Weder B, Green P, Klein M, Frelet-Barrand A, Schjoerring JK, Brearley C, Martinoia E. 2009. The Arabidopsis ATP-binding cassette protein AtMRP5/AtABCC5 is a high affinity inositol hexakisphosphate transporter involved in guard cell signalling and phytate storage. Journal of Biological Chemistry 284, 33614–33622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu BX, He FR, He M, Ren D, Chen LT, Liu YG. 2013. The ATP-binding cassette transporter OsABCG15 is required for anther development and pollen fertility in rice. Journal of Integrative Plant Biology 55, 710–720. [DOI] [PubMed] [Google Scholar]
- Panzeri D, Cassani E, Doria E, et al. 2011. A defective ABC transporter of the MRP family, responsible for the bean lpa1 mutation, affects the regulation of the phytic acid pathway, reduces seed myo-inositol and alters ABA sensitivity. New Phytologist 191, 70–83. [DOI] [PubMed] [Google Scholar]
- Patnaik D, Khurana P. 2003. Genetic transformation of Indian bread (T. aestivum) and pasta (T. durum) wheat by particle bombardment of mature embryo-derived calli. BMC Plant Biology 3, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce S, Vanzetti LS, Dubcovsky J. 2013. Exogenous gibberellins induce wheat spike development under short days only in the presence of VERNALIZATION1 . Plant Physiology 163, 1433–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilu R, Panzeri D, Gavazzi G, et al. 2003. Phenotypic, genetic and molecular characterization of a maize low phytic acid mutant (lpa 241). Theoretical Applied Genetics 107, 980–987. [DOI] [PubMed] [Google Scholar]
- Przetakiewicz A, Karas A, Orczyk W, Nadolska-Orczyk A. 2004. Agrobacterium-mediated transformation of polyploid cereals. The efficiency of selection and transgene expression in wheat. Cell and Molecular Biology Letters 9, 903–917. [PubMed] [Google Scholar]
- Raboy V. 2001. Seeds for a better future: ‘Low phytate’, grains help to overcome malnutrition and reduce pollution. Trends Plant Science 6, 458–462. [DOI] [PubMed] [Google Scholar]
- Raboy V. 2002. Progress in breeding low phytate crops. Journal of Nutrition 132, 503S–505S. [DOI] [PubMed] [Google Scholar]
- Raboy V. 2009. Approaches and challenges to engineering seed phytate and total phosphorus. Plant Science 177, 281–296. [Google Scholar]
- Raboy V, Gerbasi PF, Young KA, Stoneberg SD, Pickett SG, Bauman AT, Murthy PP, Sheridan WF, Ertl DS. 2000. Origin and seed phenotype of maize low phytic acid 1-1 and low phytic acid 2-1 . Plant Physiology 124, 355–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichaudhuri A, Peng M, Naponelli V, Chen S, Sanchez-Fernandez R, Gu H, Gregory JF, Hanson AD, Rea PA. 2009. Plant vacuolar ATP-binding cassette transporters that translocate folates and antifolates in vitro and contribute to antifolate tolerance in vivo . Journal of Biological Chemistry 284, 8449–8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen SR, Ingvardsen CR, Torp AM. 2010. Mutations in genes controlling the biosynthesis and accumulation of inositol phosphates in seeds. Biochemical Society Transactions 38, 689–694. [DOI] [PubMed] [Google Scholar]
- Regvar M, Eichert D, Kaulich B, Gianoncelli A, Pongrac P, Vogel-Mikus K, Kreft I. 2011. New insights into globoids of protein storage vacuoles in wheat aleurone using synchrotron soft X-ray microscopy. Journal of Experimental Botany 62, 3929–3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salunke R, Nidhi R, Tiwari VK, Kumari N, Randhawa GS, Dhaliwal HS, Roy P. 2012. Determination of bioavailable-zinc from biofortified wheat using a coupled in vitro digestion/Caco-2 reporter-gene based assay. Journal of Food Composition and Analysis 25, 49–159. [Google Scholar]
- Sandberg AS, Andersson H. 1988. Effect of dietary phytase on the digestion of phytate in the stomach and small intestine of humans. Journal of Nutrition 118, 469–473. [DOI] [PubMed] [Google Scholar]
- Shi JR, Wang HY, Hazebroek J, Ertl DS, Harp T. 2005. The maize low-phytic acid 3 encodes a myo-inositol kinase that plays a role in phytic acid biosynthesis in developing seeds. Plant Journal 42, 708–719. [DOI] [PubMed] [Google Scholar]
- Shi J, Wang H, Schellin K, Li B, Faller M, Stoop JM, Meeley RB, Ert DS, Ranch JP, Glassman K. 2007. Embryo-specific silencing of a transporter reduces phytic acid content of maize and soybean seeds. Nature Biotechnology 25, 930–937. [DOI] [PubMed] [Google Scholar]
- Shi J, Wang H, Wu Y, Hazebroek J, Meeley RB, Ertl DS. 2003. The maize low-phytic acid mutant lpa2 is caused by mutation in an inositol phosphate kinase gene. Plant Physiology 131, 507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SP, K Vogel-Mikuš Arčon I, Vavpetič P, Jeromel L, Pelicon P, Kumar J, Tuli R. 2013. Pattern of iron distribution in maternal and filial tissues in wheat grains with contrasting levels of iron. Journal of Experimental Botany 64, 3249–3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofo A, Vitti A, Nuzzaci M, et al. 2013. Correlation between hormonal homeostasis and morphogenic responses in Arabidopsis thaliana seedlings growing in a Cd/Cu/Zn multi-pollution context. Physiologia Plantarum 149, 487–498. [DOI] [PubMed] [Google Scholar]
- Song WY, Park J, Mendoza-Cozatl D, et al. 2010. Arsenic tolerance in Arabidopsis is mediated by two ABCC-type phytochelatin transporters. Proceedings of the National Academy of Sciences, USA 107, 21187–21192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparvoli F, Cominelli E. 2015. Seed biofortification and phytic acid reduction: a conflict of interest for the plant? Plants 4, 728–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X, Calderon-Villalobos LI, Sharon M, Zheng C, Robinson CV, Estelle M, Zheng N. 2007. Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature 446, 640–645. [DOI] [PubMed] [Google Scholar]
- Thiel J, Weier D, Sreenivasulu N, et al. 2008. Different hormonal regulation of cellular differentiation and function in nucellar projection and endosperm transfer cells: a micro dissection-based transcriptome study of young barley grains. Plant Physiology 148, 1436–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommasini R, Vogt E, Fromenteau M, et al. 1998. An ABC-transporter of Arabidopsis thaliana has both glutathione-conjugate and chlorophyll catabolite transport activity. Plant Journal 13, 773–780. [DOI] [PubMed] [Google Scholar]
- Walter S, Kahla A, Arunachalam C, et al. 2015. A wheat ABC transporter contributes to both grain formation and mycotoxin tolerance. Journal of Experimental Botany 66, 2583–2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Wang W, Yin H, Liu X, Sun H, Mi Q. 2010. Exogenous nitric oxide improves antioxidative capacity and reduces auxin degradation in roots of Medicago truncatula seedlings under cadmium stress. Plant Soil 326, 321–330. [Google Scholar]
- Xu XH, Zhao HJ, Liu QL, Frank T, Engel KH, An G, Shu QY. 2009. Mutations of the multi-drug resistance-associated protein ABC transporter gene 5 result in reduction of phytic acid in rice seeds. Theoretical and Applied Genetics 119, 75–83. [DOI] [PubMed] [Google Scholar]
- Yuan H-M, Huang X. 2015. Inhibition of root meristem growth by cadmium involves nitric oxide-mediated repression of auxin accumulation and signaling in Arabidopsis. Plant Cell Environment 39, 120–135. [DOI] [PubMed] [Google Scholar]
- Zalewski W, Galuszka P, Gasparis S, Orczyk W, Nadolska-Orczyk A. 2010. Silencing of the HvCKX1 gene decreases the cytokinin oxidase/dehydrogenase level in barley and leads to higher plant productivity. Journal of Experimental Botany 61, 1839–1851. [DOI] [PubMed] [Google Scholar]