Abstract
Diarrheal infectious diseases represent a major cause of global morbidity and mortality. There is an urgent need for vaccines against diarrheal pathogens, especially parasites. Modern subunit vaccines rely on combining a highly purified antigen with an adjuvant to increase their efficacy. In the present study, we evaluated the ability of a nanoliposome adjuvant system to trigger a strong mucosal immune response to the Entamoeba histolytica Gal/GalNAc lectin LecA antigen. CBA/J mice were immunized with alum, emulsion or liposome based formulations containing synthetic TLR agonists. A liposome formulation containing TLR4 and TLR7/8 agonists was selected based on its ability to generate intestinal IgA, plasma IgG2a/IgG1, IFN-γ and IL-17A. Immunization with a mucosal prime followed by a parenteral boost generated a high mucosal IgA response that inhibited adherence of parasites to mammalian cells. Inclusion of the immune potentiator all-trans retinoic acid in the regimen further improved the mucosal IgA response. Immunization protected from infection with up to 55% efficacy. Our results show that a nanoliposome delivery system coupled to TLR agonists is a promising prospect for the development of vaccines against enteric pathogens, especially when a multifaceted immune response is desired.
Keywords: Liposome, Vaccine, Immune response, Amebiasis, Entamoeba
1. Introduction
Diarrheal diseases account for two million deaths worldwide per year and approximately 750,000 of these are children under the age of five [1]. Repeated infections in the surviving children often lead to lifelong health problems including gut dysfunction, weaker immune response to vaccines, stunting and impaired cognitive growth [2,3]. With regard to global public health issues, enteric diseases probably represent the first cause of infectious morbidity and second cause of infectious mortality worldwide.
Although effective licensed vaccines are available against a few enteric diseases such as cholera and rotavirus, a large number of such diseases remain without any vaccine. Vaccines that can generate an effective mucosal immune activation are beneficial since most pathogens enter the body via mucosal routes. In general, recombinant antigen based modern vaccines in order to be immunogenic require adjuvants such as aluminium salts, emulsions, liposomes and/or TLR agonists. However, it is important to choose the adjuvants rationally as different adjuvants can have substantially different outcomes for the same antigen [4]. Only a handful of adjuvants have been licensed for human use, e.g. MF59 (Novartis) which is a squalene oil-in-water emulsion and AS04 (GSK) that contains a naturally derived TLR4 agonist adsorbed to an aluminium salt. Recently, interest in liposome based adjuvant systems has markedly increased due to advantages such as an improved presentation to immunocompetent cells and versatility to formulate both hydrophobic as well as hydrophilic molecules [5–7].
The toll like receptor (TLR) proteins are a well characterized family of host receptors that upon ligand binding can trigger an appropriate immune reaction in response to pathogen-associate molecular patterns (PAMPs). Different TLRs serve as receptors for diverse ligands, for example TLR4 is activated by lipopolysaccharide; TLR7/8 responds to single stranded RNA and TLR9 detects CpG motifs in DNA. Several well-defined TLR ligands are being developed to serve as adjuvant components. Multiple TLRs can be synergistically triggered for an enhanced immune response and recent studies have successfully used such an approach [6,8].
Entamoeba histolytica, the etiologic agent of amebiasis, is an enteric protozoan human pathogen. Amebiasis is responsible for approximately 100,000 deaths per year mostly in the low-income countries around the world. The disease presentation ranges from a mild diarrhea to severe dysentery and in rare cases liver abscess. The Global Enteric Multicenter Study (GEMS) highlighted E. histolytica as an important pathogen and cause of diarrhea among the pediatric population [9]. E. histolytica associated diarrheal illness was negatively associated with the growth of preschool children; such children were at nearly five times greater risk for stunting [10]. Several studies have been carried out in recent years to develop an effective amebiasis vaccine, mostly using E. histolytica Gal/GalNAc-lectin derived antigens [11,12]. The Gal/GalNAc-lectin has been shown to be essential for contact dependent host cell killing and remains the most characterized virulence factor. LecA is a 577aa region of the lectin and contains all the virulence neutralizing antibody epitopes of the native lectin. A cGMP scalable purification process has also been developed that yields highly pure, stable, non-tagged recombinant protein with acceptably low endotoxin levels [13]. The high purity of subunit vaccines often renders them poorly immunogenic requiring effective adjuvants [14,15].
Resistance to recurrent amebiasis in children has been correlated with the production of IFN-γ by peripheral blood mononuclear cells (PBMCs) upon re-stimulation in vitro, as well as high titers of anti-lectin stool IgA [16,17]. An optimal Th1 response has been shown to be important for the protection against Entamoeba histolytica challenge in the mouse model of intestinal amebiasis [18]. Vaccinated mice also showed a robust IgA response when either whole amebic lectin or recombinant LecA was used for immunization in combination with a strong mucosal adjuvant [18,19]. Our goal was to generate an anti-parasite immune response that included mucosal IgA as well as Th1 cytokine production. We used various pharmaceutically acceptable formulations of TLR agonists, singly or in combination and evaluated their potential to generate antigen specific immune responses. In vitro experiments were used to characterize the magnitude of splenic cytokine response as well as the protective capacity of the gut IgA. In vivo experiments were carried out using a mouse model of intestinal amebiasis to assess the protection efficacy of the novel nanoliposome adjuvant.
2. Materials and methods
2.1. Adjuvants
All adjuvants were prepared at the Infectious Disease Research Institute (IDRI, Seattle, WA) and provided as 2× or 5× concentrated formulations for mixing with the antigen immediately prior to injection. Squalene-in-water emulsions were prepared by microfluidization (M110P, Microfluidics Corp) at 30,000 psi essentially as described earlier [20]. Alum-adsorbed formulations were prepared by mixing an aqueous phospholipid-based suspension of GLA or an aqueous solution of CpG 1826 to Alhydrogel® (Brenntag Biosector) as previously indicated [21]. Liposome formulations based on dipalmitoyl phosphatidylcholine (DPPC), PEGylated dipalmitoyl phosphatidylethanolamine (DPPE-PEG750) and cholesterol at a 18:3:5.5 weight ratio, respectively, were prepared using the thin film technique followed by hydration and water bath sonication and/or microfluidization (M110P) similar to previous descriptions [22]. Emulsion and liposome particle sizes were measured by dynamic light scattering after 1:100 dilution in water. The TLR 7/8 agonist 3M-052 was provided by 3M. Its structure and adjuvant properties have been described previously [23].
2.2. LecA antigen
Details of expression, purification and characterization of recombinant, non-tagged LecA protein have been described previously [13].
2.3. Immunizations
All mouse studies were IACUC approved. Four to six week old CBA/J male mice (Jackson Labs.) were used for all the experiments and 5 μg antigen was used per immunization per mouse. For subcutaneous immunization in the neck region, LecA was mixed with the respective adjuvant formulation (Table 1) and volume brought up to 100 μl with saline for injection. Intranasal immunizations were carried out under anesthesia and typically 10 μl of antigen-adjuvant mixture was used per nostril. A two week interval was maintained between successive immunizations for all the regimens. For the immunogenicity experiments (IgG and cytokines), mice were immunized with three consecutive subcutaneous injections. For the challenge experiments, mice were primed with an intranasal dose followed by a subcutaneous and an intranasal prime dose. For experiments involving weekly dosing of all-trans retinoic acid (RA), each mouse received 150 μg RA (Sigma-Aldrich) dissolved in a 50 μl final volume of DMSO intraperitoneally. RA stock was stored at −80 °C and protected from light. All adjuvants were stored at 4 °C and formulations prepared aseptically just before immunization.
Table 1.
List of adjuvants used in the study. The amounts of active compounds and the IgG2a/IgG1 ratios obtained from the immunogenicity experiment (Fig. 1) are also indicated.
| Adjuvant | Agonists | Formulation | IgG2a/IgG1 ratio |
|---|---|---|---|
| GLA CpG emulsion | TLR-4 (20 mg GLA), TLR-9 (25 mg CpG) | Oil-in-water emulsion (2% v/v squalene) | 0.44 |
| 3M-052 Liposome | TLR-7/8 (2 mg 3M-052) | Liposome (4 mg/ml or 11 mg/ml phospholipid) | 0.22 |
| 3M-052 emulsion | TLR-7/8 (2 mg 3M-052) | Oil-in-water emulsion (2% v/v squalene) | 0.56 |
| GLA 3M-052 Liposome | TLR-4(5 mg GLA), TLR-7/8 (2 mg 3M-052) | Liposome (4 mg/ml or 11 mg/ml phospholipid) | 0.86 |
| GLA 3M-052 Emulsion | TLR-4(5 mg GLA), TLR-7/8 (2 mg 3M-052) | Oil-in-water emulsion (2% v/v squalene) | 0.53 |
| GLA Alum Low dose | TLR-4 (5 μg GLA) | Alum (0.2 mg/ml Al+3) | 0.06 |
| GLA Alum High dose | TLR-4 (20 μg GLA) | Alum (0.2 mg/ml Al+3) | 0.02 |
| CpG Alum | TLR-9 (25 mg CpG) | Alum (0.2 mg/ml Al+3) | 0.18 |
2.4. Measurement of immunogenicity
All antibody titers were measured by ELISA using 96-well plates coated with 0.5 μg LecA per well. Plasma samples were diluted appropriately (see figure legend) and antigen specific IgG subtypes were measured using 1:10,000 diluted horseradish peroxidase conjugated goat anti-mouse IgG1 and IgG2a detection antibodies (Southern Biotechnology). Stool supernatants were prepared as described [18]. Appropriately diluted stool supernatants were used for ELISA (see figure legend); horseradish peroxidase conjugated goat anti-mouse IgA at 1:5000 dilution was used as a secondary antibody (Southern Biotechnology). Antibody units were determined using standard curves.
For the measurement of extracellular cytokines, splenocytes from five mice in each group were re-stimulated with 50 μg/ml LecA in RPMI complete medium for 72 h and supernatants analyzed by a multiplex suspension array system using Luminex beads (Bio-Rad) [18]. Samples were run undiluted as per manufacturer's instructions and measured in picograms per milliliter of supernatant.
2.5. Culture conditions and challenge experiments
Trophozoites originally derived from HM1: IMSS (ATCC) and passed sequentially through mice to maintain animal virulence were used for the challenge experiments. Trophozoites were maintained in a trypsin-yeast extract-iron (TYI-S-33) medium supplemented with 2% Diamond vitamins, 13% heat inactivated bovine serum (Gemini Labs) and 100 U/ml penicillin plus 100 μg/ml streptomycin (Invitrogen) [24]. Mice from immunized and control groups were challenged intracecally four weeks after the final boost with two million trophozoites in 150 μl medium following laparotomy [19]. Mice were euthanized a week after the challenge. Cecal contents were suspended in 1 ml PBS, and 300 μl of was cultured in TYI-S-33 broth for up to five days, with 200 μl used for antigen load ELISA. Vaccine efficacy was calculated as 100 × 1 – (% of vaccinated mice with infection)/(% of sham mice with infection) [18,25].
2.6. Fecal antigen detection
Fecal antigen in the cecal contents was detected using the E. his II ELISA kit (TechLab Inc., Blacksburg, VA). An optical density at 450 nm of ≥0.05 above the negative control was considered positive. A standard curve was generated using purified LecA.
2.7. Adherence assay
Chinese hamster ovary (CHO) cells were grown in α-MEM medium, and E. histolytica trophozoites were grown as described above. E. histolytica trophozoites were pre-incubated with a tenfold dilution of fecal supernatants from control or immunized groups on ice for 1 h. Trophozoites and CHO cells were then mixed at a 1:20 ratio and incubation continued for 90 min on ice in round bottom polystyrene tubes. Just prior to microscopic counting, the tubes were briefly vortexed and cells counted on a hemocytometer. Adherence was measured as the number of trophozoites having at least 3 adherent CHO cells and reported as% rosette formation. Each sample was run in triplicate and a minimum of 100 amebae were counted [13,26].
2.8. Statistical analysis
All analyses were performed using Graph Pad Prism software. Proportions of infected and uninfected mice from challenge trials were analyzed using Fisher's exact test. Antigen loads, antibody titers, cytokine levels and adherence inhibition differences were analyzed using the Mann-Whitney test. P < 0.05 was considered statistically significant.
3. Results
3.1. Evaluation of immunogenicity and selection of a liposome formulation
We used the well-characterized E. histolytica recombinant adhesin LecA as an immunogen. Adjuvants containing synthetic TLR agonists were prepared using emulsion, liposome or alum (Table 1) and their ability to generate an antigen specific humoral response was evaluated. Each of these adjuvants were comprised of pharmaceutically acceptable components and would be suitable for clinical studies; indeed some of the formulations have already been manufactured in IDRI's cGMP facility and are in clinical evaluation with various vaccine antigens. The emulsion containing GLA and CpG 1826 (also known as EM014) demonstrated promising protective efficacy with LecA in previously published work [13,18]. The emulsion and liposome formulations demonstrated an average particle size of 65–130 nm depending on composition and processing method, whereas the Alum-containing formulations contained microparticles (data not shown). Eight formulations were prepared by mixing the adjuvants with LecA protein and mice were immunized subcutaneously as described. Titers of plasma IgG subclasses were determined by ELISA. The liposome formulation containing a mixture of GLA (a TLR-4 agonist) and 3M-052 (a TLR-7/8 agonist) was selected for further studies based on the plasma antibody titer biased away from a Th2 response as indicated by a higher IgG2a/IgG1 ratio (Table 1) as well as the magnitude of IgG2a titer (Fig. 1).
Fig. 1.
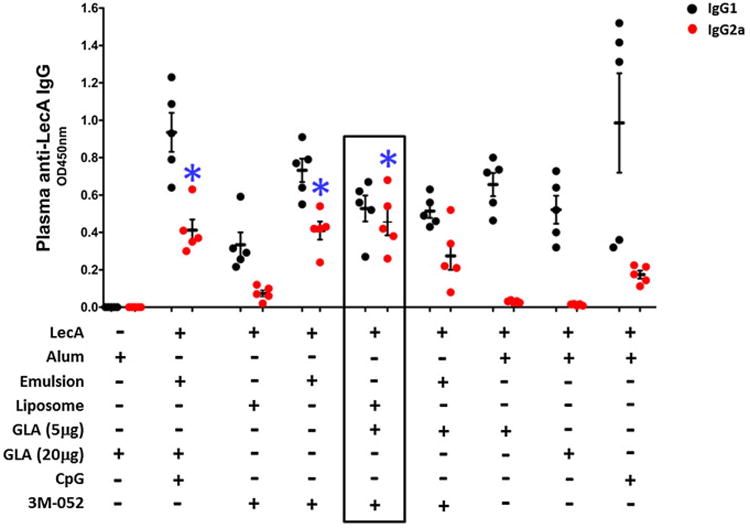
Comparison of plasma IgG titers. Five mice per group were immunized three times subcutaneously at a 2-week interval with LecA antigen mixed with the corresponding adjuvant. Plasma samples collected a week after the final immunization were diluted 256,000-fold and analyzed for LecA specific IgG1 (black) and IgG2a (red) levels by ELISA. GLA-alum without LecA served as a control group. *IgG2a level elicited by these adjuvants were comparable and statistically significant (p < 0.05) in comparison with all other groups. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Next, we tested this adjuvant's ability to elicit an antigen specific cytokine response indicative of a cell mediated immune response [27,28]. Splenocytes from the experimental and control mice were re-stimulated with LecA in vitro and culture supernatants analyzed by Luminex assay. GLA-3M-052 liposome adjuvanted LecA elicited strong IFN-γ and IL-17 responses, which are well established markers of protection in the mouse model (Fig. 2) [18,29]. We also tested the compatibility of all-trans retinoic acid (RA) as a co-adjuvant [30,31]. Mice in the respective groups received a weekly injection of RA. The RA assisted regimen further increased the levels of IFN- γ and IL-17 (Fig. 2). Both (adjuvant + LecA) and (adjuvant + LecA + RA) groups generated an equivalent IL-2 response; whereas the RA assisted regimen resulted in a slightly higher IL-4 response.
Fig. 2.
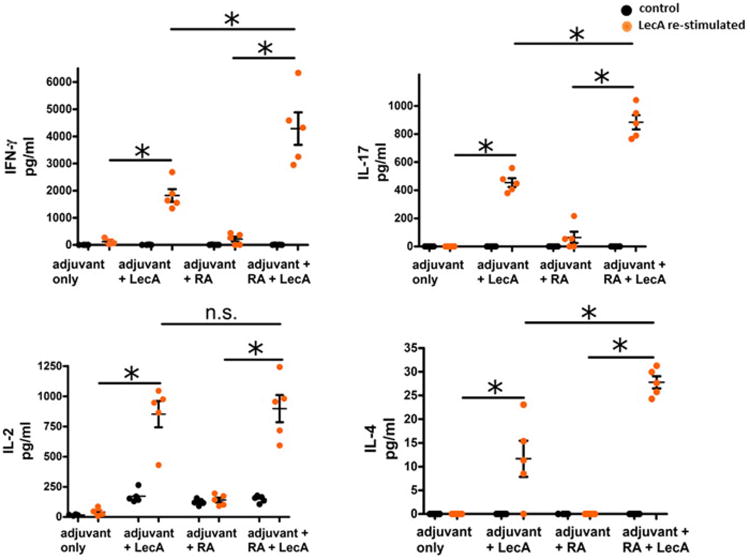
LecA specific cytokine response. Mice were immunized subcutaneously at a two week interval as described. Splenocytes were isolated a week after third immunization and re-stimulated with LecA for 72 h. Production of extracellular IFN- γ, IL-17A, IL-2 and IL-4 were detected in the culture supernatants by Luminex. Black and orange circles denote cytokine levels upon re-stimulation either with medium alone or LecA respectively. * = p < 0.01; n.s. = not significant; RA = all-trans retinoic acid. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2. Mucosal IgA response and its adherence inhibitory potential
Since a liposome-based vaccine is also suitable for mucosal immunization, a mixed mucosal/parenteral immunization regimen was used for the subsequent experiments to test its ability to generate an antigen specific gut IgA response. Mice were primed with an intranasal immunization, followed by a subcutaneous and an intranasal boost. Mice in the RA assisted groups received a weekly injection of RA. As shown in Fig. 3A, adjuvanted LecA generated a robust mucosal IgA response that was antigen specific (p < 0.001). Inclusion of RA increased the IgA titer substantially (p < 0.0001). Adherence of E. histolytica trophozoites to the target cells is the initial and crucial step in the onset of infection. To assess if the mucosal IgA was neutralizing, we tested its ability to block adherence of parasites to the CHO cells in vitro. Preincubation of trophozoites with fecal supernatants from the immunized mice significantly reduced their adherence potential (Fig. 3B). Thus, adjuvanted LecA elicited a high titer gut IgA response that was protective in vitro regardless of use of RA as a co-adjuvant.
Fig. 3.
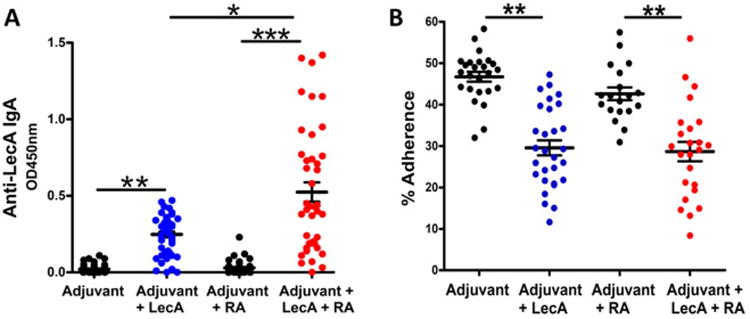
Prechallenge stool IgA response and adherence inhibitory potential. Mice were immunized with GLA 3M-052 liposome adjuvanted LecA using a mixed intranasal (weeks 0 and 4) and subcutaneous (week 2) regimen. Mice in the indicated groups received a weekly intraperitoneal injection of 150 μg RA. Stool samples were collected three weeks post third immunization. (A) Fecal supernatants were diluted 120-fold and prechallenge anti-LecA IgA titer was determined by ELISA; (B) potential of fecal IgA to inhibit adherence of trophozoites to mammalian cells was determined in vitro using adherence inhibition assay as described. * = p < 0.01; ** = p < 0.001; *** = p < 0.0001.
3.3. Nanoliposome formulation containing synergistic TLR agonists protected against intestinal parasitic challenge
Finally, we tested the potential of the GLA-3M-052 liposome adjuvant to protect against E. histolytica challenge using the mouse model of intestinal amebiasis. Mice from the control and experimentally immunized groups were challenged intracecally with a virulent strain of E. histolytica and ceca harvested a week after the challenge to assess the antigen load (Fig. 4A) as well as presence of live parasites (Fig. 4B). Adjuvanted LecA significantly reduced the antigen load compared to control mice and this reduction was even more pronounced with the use of RA as a co-adjuvant. Mice immunized with the GLA/3M-052/liposome-adjuvanted LecA showed a moderate 34% protective efficacy (p = 0.02) that was increased to 55% with the inclusion of RA (p = 0.01) (Fig. 4B). We concluded that the novel nanoliposome formulation containing a mixture of synthetic synergistic TLR agonists with or without RA had the potential to generate an antigen specific protective response.
Fig. 4.
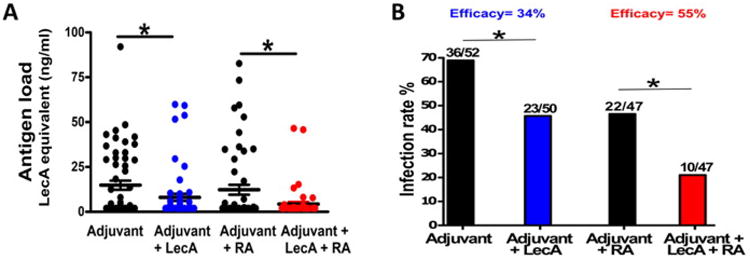
Vaccine mediated protection in a mouse model of intestinal amebiasis. Mice immunized using a mixed regimen as described were challenged intracecally with E. histolytica four weeks post-final immunization. Mice were euthanized a week after the challenge and cecal contents analyzed for (A) antigen load using ELISA and (B) live ameba by culture as a measure of sterile immunity. Number of infected mice from the total challenged are indicated above each column. The efficacies for (adjuvant + LecA) and (adjuvant + LecA + RA) groups were calculated with regard to the corresponding control groups as described. Data from two independent but identical trials was pooled. * = p < 0.05.
4. Discussion
The most important outcome of this study was the identification of a nanoliposome adjuvant formulation containing synthetic TLR agonists that was capable of eliciting a systemic as well as mucosal immune response. We compared immunogenicity of alum, emulsion and liposome based formulations containing various TLR agonists and using Entamoeba histolytica recombinant LecA as a test antigen. The liposome based adjuvant system containing TLR4 (GLA) and TLR7/8 (3M-052) agonists generated a balanced humoral and a strong cytokine response skewed towards a Th1 phenotype. GLA is a synthetic TLR4 ligand that is formulated in lipid-based platforms employed in various Phase 1 and 2 clinical trials [32]. 3M-052 is a synthetic TLR7/8 ligand in advanced preclinical development [23,33,34]. A liposomal formulation of GLA and 3M-052 was suitable for a mixed mucosal/parenteral immunization regimen and elicited an excellent mucosal IgA response as well. Immunized mice challenged with E. histolytica showed a substantial reduction in the antigen load. Finally, the GLA 3M-052 nanoliposome formulation was compatible with the use of all-trans retinoic acid as a co-adjuvant and inclusion of RA resulted in a significant increase in mucosal IgA levels as well as a moderate improvement in the protective efficacy.
Alum and emulsion based adjuvants have been known to generate strong humoral responses but are less potent enhancers of Th1 immune responses [35]. Our initial screening for an optimal adjuvant was based on the nature of the humoral response (Th1/Th2). Interestingly, the alum and emulsion based adjuvants tested generated a somewhat Th2 biased response as seen from a predominance of IgG1 titers. In contrast, the combination of GLA and 3M-052 in liposomes generated a balanced high-titer IgG response. IFN-γ and IL-17 have been shown to be important mediators of protection in immunized mice [18,29]. In human studies, a direct correlation was shown between higher production of parasite specific IFN- γ production by PBMCs and protection from re-infection [17]. The experimental vaccine elicited robust IFN-γ, IL-17 and IL-2 responses as seen from re-stimulation of splenocytes from the immunized mice. A modest increase in the Th2 cytokine IL-4 was also observed similar to the previous studies that did not compromise the protective efficacy [18]. IL-4 played a pathogenic role in the persistence of E. histolytica infection through the suppression of protective IFN-γ [36]. At present we do not know the exact source or trigger for IL-4 production or its effects on the vaccine elicited immune response especially in the RA assisted group. Use of cholera toxin as an adjuvant has similarly induced Th1, Th2 as well as Th17 responses [37].
Two independent groups have reported the use of all-trans retinoic acid as an adjuvant to elicit protective mucosal and cellular responses [30,31]. Six RA injections were administered over a span of 14 days in one study while the second study administered two doses of RA flanking the immunization. We tested compatibility of RA with GLA-3M-052 liposome adjuvant and mice in the respective groups received a weekly RA injection. RA enhanced the production of IFN-γ and IL-17 stimulated by the adjuvanted LecA and acted as an effective co-adjuvant. RA-RAR α signaling was previously shown to elicit proinflammatory CD4+ helper T cell responses to infection as well as mucosal vaccination [38].
Taking advantage of the flexibility offered by liposome adjuvant with respect to the route of immunization, we tested its potential to generate a mucosal IgA response through a heterologous prime-boost regimen. Such an approach has been reported to generate as strong or stronger local and systemic immune response compared to that resulting from homologous mucosal or parenteral vaccination alone [39,40]. Mucosal immunization has been shown to elicit both local and systemic humoral as well as cellular responses in animal models and in humans [41]. Intranasal priming in particular has been shown to influence stronger Th1 polarization along with a higher local IgA response [42]. Similarly, mice receiving either an intranasal prime or boost were shown to also produce higher levels of IL-17A [42]. We saw a robust antigen specific gut IgA response, which can be a major advantage in the context of vaccines against enteric pathogens. An RA assisted regimen showed a further increase in the IgA titer as expected, since RA is known to be important in the generation and enhancement of IgA secreting B cells [30,43]. There was no difference in the adherence inhibitory potential between stool suspensions from groups with or without RA. This was not totally surprising due to a lack of correlation between IgA titer and protection in the mouse model [18]. Anti-lectin IgA response, however, was shown to be associated with protection from E. histolytica colonization in children [16]. A correlation has also been shown between IgA production and clearance of parasites in baboons [44]. In short, GLA-3M-052 liposome generated a robust anti-amebic mucosal IgA response that was protective in vitro. Use of RA further increased the IgA response.
In the cecal challenge trial, immunized mice showed an excellent reduction in antigen load and use of RA helped reduce it even further. Experiments are underway to improve the moderate vaccine efficacy shown by (adjuvant + LecA) group. This may be achieved by using different approaches, such as changing the route of immunization, altering the chemistry of formulation or optimizing the agonist concentrations [6,35]. An emulsion containing GLA and CpG 1826 (EM014) previously showed 50% protective efficacy when a subcutaneous regimen was used [13]. The present formulation showed 34% efficacy with the potential advantage that liposomes are generally considered to be less reactogenic than oil-in-water emulsions [45]. The RA assisted regimen showed an enhancement in the protective efficacy, although we do not know the exact mechanism at present including any degree of non-specific protection. One possible mechanism could be an improved CD4+ and CD8+ T cell response in the gut via upregulation of gut homing receptors on these cells [30,31,46,47]. Retinoic acid's compatibility with the synthetic TLR agonists used offers a way to modulate antigen specific immune response [38,48].
In conclusion, we have shown that the nanoliposome adjuvant containing synergistic TLR agonists successfully elicited a balanced systemic humoral and cellular immune response, as well as a robust mucosal IgA response. It also supported production of IFN-γ and IL-17, two well-established protective cytokines in the mouse model of amebiasis. Important considerations in the development of a successful vaccine include availability of not only highly purified antigen but a potent adjuvant formulation that can be tested in clinical settings after completion of the pre-clinical studies [13]. We hope to improve the efficacy through the approaches mentioned. Future experiments will be designed to generate a potent Th1 cellular and mucosal response in addition to an enhanced local IgA response. Only one class of drugs is available to treat invasive amebiasis, and should resistance develop, vaccination would be a most effective and economical strategy, especially in the low income countries which account for more than 90% of enteric infections. We hope that this exploratory study will provide a basis for the development of prophylactic vaccines against such pathogens.
Acknowledgments
The adjuvant formulation work was funded in part by the Bill and Melinda Gates Foundation grants 42387, OPP1055855, and OPP1130379, and by the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Human and Health Services under grant 5R21AI109118 and contract HHSN272200800045C. We gratefully acknowledge Susan Lin, Sarah Parker, Sandra Sivanantha, Traci Mikasa, Tiep Pham, and Molly Blust for excellent technical assistance.
Footnotes
Conflict of interest: M.A.T and J.E. are employees of 3M and own shares of 3M. W.A.P. is a consultant for TechLab, Inc. and in addition receives royalties for amebiasis diagnostics that are donated in their entirety to the American Society of Tropical Medicine and Hygiene. All other authors declare no potential conflicts of interest.
References
- 1.WHO. Diarrheal disease. WHO; 2013. WHO fact sheet N0 330. [Google Scholar]
- 2.Moore SR, Lima AA, Conaway MR, Schorling JB, Soares AM, Guerrant RL. Early childhood diarrhoea and helminthiases associate with long-term linear growth faltering. Int J Epidemiol. 2001;30(6):1457–64. doi: 10.1093/ije/30.6.1457. [DOI] [PubMed] [Google Scholar]
- 3.Petri WA, Miller M, Binder HJ, Levine MM, Dillingham R, Guerrant RL. Enteric infections, diarrhea, and their impact on function and development. J Clin Invest. 2008;118(4):1277–90. doi: 10.1172/JCI34005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ansong D, Asante KP, Vekemans J, Owusu SK, Owusu R, Brobby NAW, et al. T cell responses to the RTS, S/AS01(E) and RTS, S/AS02(D) malaria candidate vaccines administered according to different schedules to Ghanaian children. PLoS ONE. 2011;6(4):e18891. doi: 10.1371/journal.pone.0018891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwendener RA. Liposomes as vaccine delivery systems: a review of the recent advances. Ther Adv Vacc. 2014;2(6):159–82. doi: 10.1177/2051013614541440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox CB, Sivananthan SJ, Duthie MS, Vergara J, Guderian JA, Moon E, et al. A nanoliposome delivery system to synergistically trigger TLR4 AND TLR7. J Nanobiotechnol. 2014;26(12):17. doi: 10.1186/1477-3155-12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Hagan DT, Fox CB. New generation adjuvants–from empiricism to rational design. Vaccine. 2015;8(33 Suppl 2):B14–20. doi: 10.1016/j.vaccine.2015.01.088. [DOI] [PubMed] [Google Scholar]
- 8.Zhang WW, Matlashewski G. Immunization with a Toll-like receptor 7 and/or 8 agonist vaccine adjuvant increases protective immunity against Leishmania major in BALB/c mice. Infect Immun. 2008;76(8):3777–83. doi: 10.1128/IAI.01527-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet Lond Engl. 2013;382(9888):209–22. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 10.Mondal D, Petri WA, Sack RB, Kirkpatrick BD, Haque R. Entamoeba histolytica-associated diarrheal illness is negatively associated with the growth of preschool children: evidence from a prospective study. Trans R Soc Trop Med Hyg. 2006;100(11):1032–8. doi: 10.1016/j.trstmh.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 11.Quach J, St-Pierre J, Chadee K. The future for vaccine development against Entamoeba histolytica. Hum Vacc Immunother. 2014;10(6):1514–21. doi: 10.4161/hv.27796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roncolato EC, Teixeira JE, Barbosa JE, Zambelli Ramalho LN, Huston CD. Immunization with the Entamoeba histolytica surface metalloprotease EhMSP-1 protects hamsters from amebic liver abscess. Infect Immun. 2015;83(2):713–20. doi: 10.1128/IAI.02490-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barroso L, Abhyankar M, Noor Z, Read K, Pedersen K, White R, et al. Expression, purification, and evaluation of recombinant LecA as a candidate for an amebic colitis vaccine. Vaccine. 2014;32(10):1218–24. doi: 10.1016/j.vaccine.2013.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 15.Moser M, Leo O. Key concepts in immunology. Vaccine. 2010;28(Suppl 3):C2–C13. doi: 10.1016/j.vaccine.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 16.Haque R, Ali IM, Sack RB, Farr BM, Ramakrishnan G, Petri WA. Amebiasis and mucosal IgA antibody against the Entamoeba histolytica adherence lectin in Bangladeshi children. J Infect Dis. 2001;183(12):1787–93. doi: 10.1086/320740. [DOI] [PubMed] [Google Scholar]
- 17.Haque R, Mondal D, Shu J, Roy S, Kabir M, Davis AN, et al. Correlation of interferon-gamma production by peripheral blood mononuclear cells with childhood malnutrition and susceptibility to amebiasis. Am J Trop Med Hyg. 2007;76(2):340–4. [PubMed] [Google Scholar]
- 18.Guo X, Barroso L, Becker SM, Lyerly DM, Vedvick TS, Reed SG, et al. Protection against intestinal amebiasis by a recombinant vaccine is transferable by T cells and mediated by gamma interferon. Infect Immun. 2009;77(9):3909–18. doi: 10.1128/IAI.00487-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Houpt E, Barroso L, Lockhart L, Wright R, Cramer C, Lyerly D, et al. Prevention of intestinal amebiasis by vaccination with the Entamoeba histolytica Gal/GalNac lectin. Vaccine. 2004;22(5–6):611–7. doi: 10.1016/j.vaccine.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Fox CB, Moutaftsi M, Vergara J, Desbien AL, Nana GI, Vedvick TS, et al. TLR4 ligand formulation causes distinct effects on antigen-specific cell-mediated and humoral immune responses. Vaccine. 2013;31(49):5848–55. doi: 10.1016/j.vaccine.2013.09.069. [DOI] [PubMed] [Google Scholar]
- 21.Fox CB. Characterization of TLR4 agonist effects on alhydrogel® sedimentation: a novel application of laser scattering optical profiling. J Pharm Sci. 2012;101(11):4357–64. doi: 10.1002/jps.23307. [DOI] [PubMed] [Google Scholar]
- 22.Misquith A, Fung HWM, Dowling QM, Guderian JA, Vedvick TS, Fox CB. In vitro evaluation of TLR4 agonist activity: formulation effects. Colloids Surf B Biointerfaces. 2014;1(113):312–9. doi: 10.1016/j.colsurfb.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smirnov D, Schmidt JJ, Capecchi JT, Wightman PD. Vaccine adjuvant activity of 3M–052: an imidazoquinoline designed for local activity without systemic cytokine induction. Vaccine. 2011;29(33):5434–42. doi: 10.1016/j.vaccine.2011.05.061. [DOI] [PubMed] [Google Scholar]
- 24.Diamond LS, Harlow DR, Cunnick CC. A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans R Soc Trop Med Hyg. 1978;72(4):431–2. doi: 10.1016/0035-9203(78)90144-x. [DOI] [PubMed] [Google Scholar]
- 25.Soong CJ, Kain KC, Abd-Alla M, Jackson TF, Ravdin JI. A recombinant cysteine-rich section of the Entamoeba histolytica galactose-inhibitable lectin is efficacious as a subunit vaccine in the gerbil model of amebic liver abscess. J Infect Dis. 1995;171(3):645–51. doi: 10.1093/infdis/171.3.645. [DOI] [PubMed] [Google Scholar]
- 26.Ravdin JI, Guerrant RL. Role of adherence in cytopathogenic mechanisms of Entamoeba histolytica. Study with mammalian tissue culture cells and human erythrocytes. J Clin Invest. 1981;68(5):1305–13. doi: 10.1172/JCI110377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin W, Dong C. IL-17 cytokines in immunity and inflammation. Emerg Microbes Infect. 2013;2(9):e60. doi: 10.1038/emi.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thakur A, Pedersen LE, Jungersen G. Immune markers and correlates of protection for vaccine induced immune responses. Vaccine. 2012;30(33):4907–20. doi: 10.1016/j.vaccine.2012.05.049. [DOI] [PubMed] [Google Scholar]
- 29.Guo X, Barroso L, Lyerly DM, Petri WA, Houpt ER. CD4+ and CD8+ T cell- and IL-17-mediated protection against Entamoeba histolytica induced by a recombinant vaccine. Vaccine. 2011;29(4):772–7. doi: 10.1016/j.vaccine.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hammerschmidt SI, Friedrichsen M, Boelter J, Lyszkiewicz M, Kremmer E, Pabst O, et al. Retinoic acid induces homing of protective T and B cells to the gut after subcutaneous immunization in mice. J Clin Invest. 2011;121(8):3051–61. doi: 10.1172/JCI44262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan X, Sande JL, Pufnock JS, Blattman JN, Greenberg PD. Retinoic acid as a vaccine adjuvant enhances CD8+ T cell response and mucosal protection from viral challenge. J Virol. 2011;85(16):8316–27. doi: 10.1128/JVI.00781-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fox CB, Carter D, Kramer RM, Beckmann AM, Reed SG. Current status of TLR4 ligand vaccine adjuvants, immunopotentiators. In: O'Hagan Derek T, Schijns Virgil., editors. Modern vaccines. 2nd. Elsevier; 2016. [Google Scholar]
- 33.Zhao BG, Vasilakos JP, Tross D, Smirnov D, Klinman DM. Combination therapy targeting toll like receptors 7, 8 and 9 eliminates large established tumors. J Immunother Cancer. 2014;2:12. doi: 10.1186/2051-1426-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh M, Khong H, Dai Z, Huang XF, Wargo JA, Cooper ZA, et al. Effective innate and adaptive antimelanoma immunity through localized TLR7/8 activation. J Immunol Baltim Md 1950. 2014;193(9):4722–31. doi: 10.4049/jimmunol.1401160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orr MT, Fox CB, Baldwin SL, Sivananthan SJ, Lucas E, Lin S, et al. Adjuvant formulation structure and composition are critical for the development of an effective vaccine against tuberculosis. [cited 2016 Feb 11];J Control Release Off J Control Release Soc [Internet] 2013 Nov 28;172(1) doi: 10.1016/j.jconrel.2013.07.030. Available from: < http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3871206/>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo X, Stroup SE, Houpt ER. Persistence of Entamoeba histolytica infection in CBA mice owes to intestinal IL-4 production and inhibition of protective IFN-gamma. Mucosal Immunol. 2008;1(2):139–46. doi: 10.1038/mi.2007.18. [DOI] [PubMed] [Google Scholar]
- 37.Mattsson J, Schön K, Ekman L, Fahlén-Yrlid L, Yrlid U, Lycke NY. Cholera toxin adjuvant promotes a balanced Th1/Th2/Th17 response independently of IL-12 and IL-17 by acting on Gsα in CD11b+ DCs. Mucosal Immunol. 2015;8(4):815–27. doi: 10.1038/mi.2014.111. [DOI] [PubMed] [Google Scholar]
- 38.Hall JA, Cannons JL, Grainger JR, Santos LMD, Hand TW, Naik S, et al. Essential role for retinoic acid in the promotion of CD4+ T cell effector responses via retinoic acid receptor alpha. Immunity. 2011;34(3):435–47. doi: 10.1016/j.immuni.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCluskie MJ, Weeratna RD, Payette PJ, Davis HL. Parenteral and mucosal prime-boost immunization strategies in mice with hepatitis B surface antigen and CpG DNA. FEMS Immunol Med Microbiol. 2002;32(3):179–85. doi: 10.1111/j.1574-695X.2002.tb00551.x. [DOI] [PubMed] [Google Scholar]
- 40.Pattani A, McKay PF, Garland MJ, Curran RM, Migalska K, Cassidy CM, et al. Microneedle mediated intradermal delivery of adjuvanted recombinant HIV-1 CN54gp140 effectively primes mucosal boost inoculations. J Control Release. 2012;162(3):529–37. doi: 10.1016/j.jconrel.2012.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lycke N. Recent progress in mucosal vaccine development: potential and limitations. Nat Rev Immunol. 2012;12(8):592–605. doi: 10.1038/nri3251. [DOI] [PubMed] [Google Scholar]
- 42.Fiorino F, Pettini E, Pozzi G, Medaglini D, Ciabattini A. Prime-boost strategies in mucosal immunization affect local IgA production and the type of Th response. [cited 2016 Feb 11];Front Immunol [Internet] 2013 May 29;4 doi: 10.3389/fimmu.2013.00128. Available from: < http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3665932/>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314(5802):1157–60. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 44.Abd Alla MD, Wolf R, White GL, Kosanke SD, Cary D, Verweij JJ, et al. Efficacy of a Gal-lectin subunit vaccine against experimental Entamoeba histolytica infection and colitis in baboons (Papio sp) Vaccine. 2012;30(20):3068–75. doi: 10.1016/j.vaccine.2012.02.066. [DOI] [PubMed] [Google Scholar]
- 45.Owusu-Agyei S, Ansong D, Asante K, Kwarteng Owusu S, Owusu R, Wireko Brobby NA, et al. Randomized controlled trial of RTS, S/AS02D and RTS, S/AS01E malaria candidate vaccines given according to different schedules in Ghanaian children. PLoS ONE. 2009;4(10):e7302. doi: 10.1371/journal.pone.0007302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raverdeau M, Mills KHG. Modulation of T cell and innate immune responses by retinoic acid. J Immunol Baltim Md 1950. 2014;192(7):2953–8. doi: 10.4049/jimmunol.1303245. [DOI] [PubMed] [Google Scholar]
- 47.Mwanza-Lisulo M, Kelly P. Potential for use of retinoic acid as an oral vaccine adjuvant. Phil Trans R Soc B. 2015;370(1671):20140145. doi: 10.1098/rstb.2014.0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ross AC, Chen Q, Ma Y. Augmentation of antibody responses by retinoic acid and costimulatory molecules. Semin Immunol. 2009;21(1):42–50. doi: 10.1016/j.smim.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]


