Abstract
As highlighted by several contributions to this special issue, there is still ongoing debate about the number, exact location, and boundaries of the visual areas located in cortex immediately rostral to the second visual area (V2), i.e., the “third tier” visual cortex, in primates. In this review, we provide a historical overview of the main ideas that have led to four models of third tier cortex organization, which are at the center of today's debate. We formulate specific predictions of these models, and compare these predictions with experimental evidence obtained primarily in New World primates. From this analysis, we conclude that only one of these models (the “multiple-areas” model) can accommodate the breadth of available experimental evidence. According to this model, most of the third tier cortex in New World primates is occupied by two distinct areas, both representing the full contralateral visual quadrant: the dorsomedial area (DM), restricted to the dorsal half of the third visual complex, and the ventrolateral posterior area (VLP), occupying its ventral half and a substantial fraction of its dorsal half. DM belongs to the dorsal stream of visual processing, and overlaps with macaque parietooccipital (PO) area (or V6), whereas VLP belongs to the ventral stream and overlaps considerably with area V3 proposed by others. In contrast, there is substantial evidence that is inconsistent with the concept of a single elongated area V3 lining much of V2. We also review the experimental evidence from macaque monkey and humans, and propose that, once the data are interpreted within an evolutionary-developmental context, these species share a homologous (but not necessarily identical) organization of the third tier cortex as that observed in New World monkeys. Finally, we identify outstanding issues, and propose experiments to resolve them, highlighting in particular the need for more extensive, hypothesis-driven investigations in macaque and humans.
Keywords: Dorsomedial area, Ventrolateral posterior area, Area V3, Marmoset, Area 19
Introduction
The cerebral cortex of primates and other mammals includes a mosaic of areas that process visual information. There is universal agreement about the boundaries and topographic organization of the areas that form the earliest stages of visual processing in the primate cortex: the first (V1), second (V2), and middle temporal (MT) visual areas. However, as highlighted by several contributions to this special issue, there is still ongoing debate about the exact configuration of the areas located immediately rostral to V2, which Allman and Kaas (1975) referred to as the “third tier” visual cortex. Controversy remains about seemingly simple questions such as how many areas exist in this part of the cortex, where their boundaries are, and what criteria can be used to identify them.
In this review, we summarize what we regard as well-established aspects of the organization of the third tier areas. The core points in our argument will be exemplified using the results of experiments performed in marmoset monkeys (genus Callithrix), which is the primate model upon which most of our contributions to this debate have been based. Marmosets, together with other species of small New World monkeys (e.g., the owl monkey, genus Aotus) provide key advantages for experiments aimed at understanding the organization and function of the third tier visual cortex, primarily due to the fact that the most controversial parts of this complex are exposed on the dorsal surface of the brain, rather than being located deep within sulci, as in larger primates. We will also comment on experimental evidence obtained in other species, in particular the Old World macaque monkey (genus Macaca) and humans, and will attempt to identify points that deserve further study.
The main conclusions stemming from our analysis of the experimental evidence can be summarized in the following three points:
First, unlike 20–30 years ago, there is now substantial agreement among most research groups on the fact that a single cortical area occupies most (at least two-thirds) of the third tier visual cortex, forming a spatially reduced mirror-symmetrical representation of the V2 visuotopic map. Although this area is similar to area 19 (or V3) mapped in carnivores, to avoid confusion while we develop this argument we will refer to this area as the ventrolateral posterior (VLP) area, while recognizing that it overlaps considerably with area V3 proposed by others; to emphasize the latter point, the abbreviation “VLP/V3” will be used.
Second, there is substantial evidence that VLP/V3 does not extend all the way along the dorsal border of V2. Instead, the third tier cortex near the dorsal midline is formed by an area that is different from VLP/V3 according to most of the criteria that are usually used to define a visual area, including the topographic organization of receptive fields, histological appearance, and pattern of connections with other areas of the cortex. In New World monkeys, this area is usually referred to as the dorsomedial area (DM).
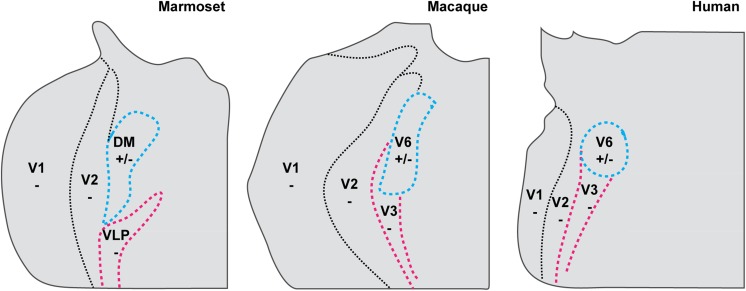
Third, even though the experimental support for distinct dorsal (DM) and ventral (VLP) areas in the third tier cortex is clearest in New World monkeys, it is likely that a homologous organization exists in New and Old World monkeys, and humans. In particular, we propose that the parietooccipital (PO) or sixth (V6) visual area identified in these species is homologous to the New World monkey DM. This, however, does not imply an identical configuration of visual areas within the third tier cortex of New and Old World primates: for example, the human homologue of V3 is relatively larger, and shares a larger fraction of the rostral border of V2. Further, we propose that the differences in configuration of the third tier cortex across primate species can be understood in a developmental-evolutionary context, whereby later-developing areas become relatively larger in larger-brained species. Testing this hypothesis will require more extensive investigations in Old World primates and humans.
Having set out the main points of our thesis, we now proceed to review its basis.
A brief history of the third tier visual cortex
To understand the current controversies, it is useful to step back in time, and review the historical context from which the present definitions of third tier areas have emerged. This account is necessarily colored by our perceptions developed not as main protagonists, but as research students and early career researchers at the time when most of these key events in the evolution of ideas about the third tier complex unfolded.
One area or multiple areas?
By the early 1980s, understanding of the organization of this part of the primate brain was divided along two clearly defined “camps”, with some of the world's leading neuroscience research groups espousing seemingly incompatible views. Although neither of these models has stood the test of time intact, they each proved correct in some aspects, and continue to shape current thinking.
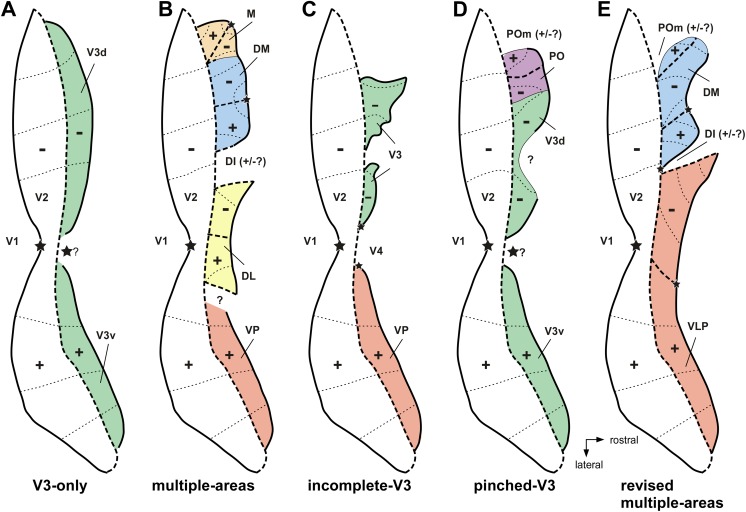
On the one hand, most groups working in Old World macaque monkeys advocated the view that the cortex rostral to V2 was formed by a single area, V3, which formed mirror-symmetrical representations of the lower (dorsally) and upper (ventrally) contralateral quadrants (Fig. 1A). This model was largely inspired by a series of studies by Zeki and colleagues (e.g., Zeki, 1969, 1971, 1977, 1978a,b; Zeki & Sandeman, 1976; Van Essen & Zeki, 1978; see also Cragg, 1969). For simplicity, in the discussion below, we will refer to this as the “V3-only” model.
Fig. 1.
Different models of third tier cortex organization. Partitioning of the primate third tier cortex according to different models, shown onto a schematic representation of unfolded and flattened primate area V2 and cortex immediately rostral to it. Thick solid and dashed contours: representations of the vertical and horizontal meridians, respectively, of the visual field; thin solid contours in (B, D, and E) indicate uncertainties of meridian representation; stars: foveal representations; thin dotted contours: iso-eccentricity lines; “+, −” signs: upper and lower, respectively, visual quadrant representations. (A) The “V3-only” model originally proposed for the macaque by Zeki (1969) and Cragg (1969) on the basis of microelectrode mapping studies, and subsequently espoused by Lyon and Kaas (2001, 2002a,b) on the basis of connectional studies in macaque and several species of New World primates. (B) The original “multiple-areas” model, initially proposed for owl monkey, on the basis of the electrophysiological mapping studies of Allman and Kaas (1975) and Newsome and Allman (1980), and the connectional studies of Krubitzer and Kaas (1993), and later extended to other species of New World primates and to the macaque based on connectional studies (Stepniewska & Kaas, 1996; Beck & Kaas, 1998a, 1999). (C) The “incomplete-V3” model proposed for the macaque on the basis of anatomical (Van Essen et al., 1982, 1986; Felleman et al., 1997) and electrophysiological characterization of receptive field properties and topography (Burkhalter & Van Essen, 1986; Newsome et al., 1986; Felleman & Van Essen, 1987). (D) The “pinched-V3” model proposed in macaque by Gattass et al. (1988) on the basis of microelectrode mapping studies. (E) The “revised multiple-areas” model initially proposed for marmoset monkey by Rosa and Schmid (1995) and Rosa and Tweedale (2000), based on microelectrode mapping, later supported by denser retinotopic mapping as well as by connectional studies in marmosets (Rosa et al., 2005; Jeffs et al., 2013; Jeffs et al. 2015 in this special issue).
On the other hand, a series of electrophysiological mapping studies in the New World owl monkey had reached a rather different conclusion: rather than a single elongated V3, there were multiple smaller areas within the dorsal aspect of the third tier cortex, each containing a representation of the visual field that included the upper and lower quadrants, and distinct in terms of myeloarchitectural appearance (Fig. 1B). According to the original nomenclature, these were named the dorsolateral (DL), dorsomedial (DM), and medial (M) visual areas (Allman & Kaas, 1975, 1976). Subsequent work by Newsome and Allman (1980) proposed a fourth area along the ventral surface of the third tier cortex, which was named the ventral posterior (VP) area. Again, for simplicity, we will refer to this as the original “multiple-areas” model. As indicated in Fig. 1B, some aspects of this organization have never been described in full, including the nature of the transition between areas DL and VP, and the organization of the region between DL and DM (“dorsointermediate area”, DI, of Allman & Kaas, 1975).
Differences between dorsal and ventral cortex are recognized
The first chinks in the armor of the V3-only model in Old World monkeys (Fig. 1A) came from work by Van Essen and colleagues (Van Essen et al., 1982, 1986; Burkhalter et al., 1986; Burkhalter & Van Essen, 1986; Newsome et al., 1986; Felleman & Van Essen, 1987, Felleman et al., 1997), who made a series of important anatomical and physiological observations in studies of the macaque cortex. First, they reported that at least some of the cortex located rostral to dorsal V2 was heavily myelinated, which is in contrast with the ventral cortex in the corresponding position. Second, this densely myelinated portion of the third tier cortex received clear connections from V1, which originated primarily from layer 4B [according to the nomenclature of Brodmann (1994), and corresponding to layer 3C of Hassler (1996)], i.e., the same layer known to project to area MT. In contrast, these investigators found no clear evidence of projections from V1 to the ventral portion of putative V3, at least using the neuroanatomical tracers that were available at that time. Third, anatomical tracing and physiological studies by the same group revealed that neurons in the densely myelinated portion of V3 had response properties and connections that suggested affiliation with the “dorsal stream” of visual processing (Ungerleider & Mishkin, 1982), while the ventral third tier cortex showed clearer affiliation with the ventral stream. To emphasize these differences, they adopted the designation VP for the ventral component of the third tier cortex [following the nomenclature suggested by Newsome and Allman for the owl monkey cortex (Van Essen et al., 1982)], and reserved the designation V3 for the dorsal component. The above observations formed the basis of the model illustrated in Fig. 1C (“incomplete-V3” model), whereby the third tier visual cortex was formed by two areas, each only representing one half of the contralateral visual field (lower quadrant in V3, upper quadrant in VP).
The work of these investigators, in addition, provided other lines of evidence in favor of different areas in the region rostral to dorsal and ventral V2. For example, the pattern of interhemispheric connections [which preferentially connect representations of parts of the visual field near the vertical meridian; (Rosa & Manger, 2005)] was found to be regular and reproducible in ventral extrastriate cortex rostral to V2, suggesting a simple visual topography for VP, but irregular and discontinuous in the dorsal cortex rostral to V2, which included the densely myelinated V3 (Van Essen et al., 1982). In addition, the myeloarchitecturally defined V3 was reported to be irregularly shaped (being often wider near the midline, as depicted in Fig. 1C), and somewhat variable in shape across individuals, in many cases being formed by multiple “islands” of cortex that were separated by histologically distinct tissue (see also Lewis & Van Essen, 2000).
The visual topography of dorsal V3 is redefined
A second, but in our view usually overlooked, demonstration of the problems with the V3-only model (Fig. 1A) was revealed by the first study that attempted to map the topographic organization of the third tier cortex in the macaque (Gattass et al., 1988). Up to that point in time, there had been no attempt to map the receptive field organization across the entire cortex rostral to V2 in Old World monkeys – in contrast with studies in the owl monkey, in which receptive field mapping was the main criterion used to define the third tier areas. Instead, the entire evidence in the macaque was based on recordings across the small portions of the putative V3 strip (in particular, the dorsal component, in the lunate sulcus), which showed that crossing the rostral border of V2 (which represents the horizontal meridian), and moving the electrode to progressively more rostral sites, revealed receptive fields that progressively moved toward the vertical meridian of the visual field, in the lower quadrant (e.g., Van Essen & Zeki, 1978). These observations fulfilled the expectations based on earlier studies in cat area 19 (Hubel & Wiesel, 1965; Tusa et al., 1979; Albus & Beckmann, 1980), and were taken as evidence of a similar organization in the two species. However, it is important to note that they were compatible with both the V3-only model shown in Fig. 1A and the multiple-areas model shown in Fig. 1B. Instead, the main point of distinction between the two models is that, according to the multiple-areas model, recordings at some levels of dorsal extrastriate cortex would not follow this topographic pattern: in these regions (i.e., in the lateral part of area DM), receptive fields are expected to move into the upper visual field as the electrode progresses rostrally in the cortex, while in other locations they could remain close to the horizontal meridian (e.g., at the border region between DL and DI, or at the border between upper and lower field DM – see Fig. 1B).
When Gattass et al. (1988) recorded receptive fields across several mediolateral levels of the third tier cortex, they found that the organization of the dorsal component (dorsal V3 or V3d, in their nomenclature) did not, in fact, meet the expectations of the V3-only model illustrated in Fig. 1A. Instead, in the majority of animals, the expected representation of the lower visual field vertical meridian was only found in relatively small parts of the region where the anterior border of V3d was expected (near its lateral and medial extremities). Looking back, and in light of the evidence of the contemporary studies by the Van Essen group (see above), this appears (to us) as strong indication that the V3-only model did not provide a satisfactory account of the complexity of the organization of the dorsal component of the third tier complex in the macaque. However, to account for these observations, Gattass and colleagues proposed what we refer to as the “pinched-V3d” model (Fig. 1D), in which this area becomes narrower at its midpoint and has a rostral border that represents portions of the visual field closer to the horizontal meridian (e.g., “In fact, in one case V3d was so narrow at one point as to be almost divided into two portions”; Gattass et al., 1988). A more traditional configuration of V3d (i.e., with a continuous representation of the lower vertical meridian) was mentioned to occur, albeit rarely, but receptive field sequences in support of this claim were not illustrated. As reviewed below, subsequent work in macaques has confirmed that the representation of the vertical meridian in the dorsal cortex rostral to V2 is indeed, as a rule, not continuous (see Arcaro et al., 2011, for more recent evidence in this respect).
Further support for multiple areas
The original formulation of the multiple-areas model (Fig. 1B) was based on the electrophysiological studies of Allman and Kaas (1975) in the owl monkey. Throughout the 1980s and 1990s, a series of anatomical studies, primarily from Kaas and colleagues, accumulated evidence suggesting that this organization also applied to many other species of primate, including the Old World macaque (for a review, see Kaas, 1996). One of the key pieces of evidence was the presence of a densely myelinated, V1-recipient region in the dorsal cortex immediately rostral to V2, which was deemed homologous to area DM mapped in owl monkeys (Lin et al., 1982; Cusick et al., 1984; Krubitzer & Kaas, 1990, 1993; Weller et al., 1991; Kaas & Morel, 1993; Stepniewska & Kaas, 1996; Beck & Kaas, 1998a,b, 1999). During this same period, electrophysiological studies provided evidence for area DM being adjacent to V2 in two additional primate species, the New World marmoset monkey and the prosimian Galago (Rosa & Schmid, 1995; Rosa et al., 1997a), albeit with revisions to the topographic map originally proposed for the owl monkey by Allman and Kaas (1975). It was also proposed that area DM overlapped, at least in part, with the densely myelinated component of the macaque dorsal V3, proposed by Van Essen and colleagues (e.g., Beck & Kaas, 1999).
Another line of evidence against the V3-only model originated from subsequent anatomical and electrophysiological studies in the macaque and capuchin monkeys, which revealed an additional area adjacent to the lower quadrant representation of V2, on the medial wall of the hemisphere. This was named the parietooccipital area (PO; Fig. 1D) (Ungerleider & Desimone, 1986; Colby et al., 1988; Boussaoud et al., 1991; Rosa et al., 1993; Neuenschwander et al., 1994; Gattass et al., 1997) or area V6 (Galletti et al., 1999). Area PO shared with the dorsal component of V3 two key characteristics: dense myelination and topographically organized projections from V1 layer 4B. Moreover, while dorsal V3 only represented the visual field up to 30° eccentricity, area PO emphasized the far periphery of the visual field (Colby et al., 1988; Gattass et al., 1988). In capuchin monkeys, at least one additional area, named parietooccipital medial area (POm) was identified along the peripheral lower quadrant representation of V2 (Neuenschwander et al., 1994), which differed from PO by being more lightly myelinated (Fig. 1D).
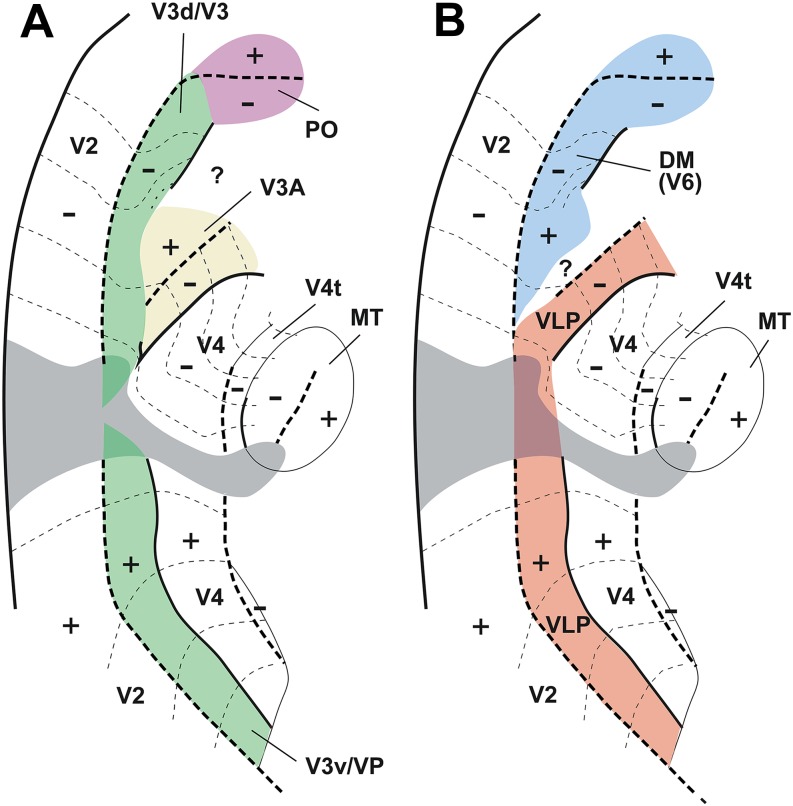
In view of the variety of nomenclatures, and incomplete evidence, ascertaining homologies between different species remained a challenge. One of the ideas that emerged at this time (Fig. 2) was that the Old World monkey homologue of DM included parts of three of the areas then recognized in the macaque: the densely myelinated component of V3 proposed by Van Essen and colleagues, the medially adjacent area PO/V6, and a representation of the central upper visual field which has been traditionally assigned to area V3A, located rostrolateral to V3/V3d (Van Essen & Zeki, 1978; Gattass et al., 1988). Like DM, this joint territory was characterized by dense myelination, direct afferent projections from layer 4B of V1, and neurons with receptive fields that covered both the upper and the lower quadrants of the visual field, from center to periphery (Rosa & Tweedale, 2001). Another complete representation of the visual field was proposed to form most of the remainder of the third tier cortex, by combining the upper quadrant representation of area VP of Newsome and Allman (1980) and Van Essen et al. (1982) (or V3v of Zeki, 1971, and Gattass et al., 1988) with a caudal strip of area DL/V4, which contained a lower quadrant representation (Maguire & Baizer, 1984; Rosa & Tweedale, 2000). This extended VP/V3v was dubbed the ventrolateral posterior area (VLP; Figs. 1E and 2B), and has been hypothesized as the true primate homologue of area 19 found in most mammals (Rosa & Manger, 2005). We refer to this as the “revised multiple-areas model” (Fig. 1E).
Fig. 2.
A hypothesis on the organization of dorsal extrastriate cortex in Old World monkeys, based on studies in New World monkeys. Rosa and Tweedale's (2001) proposal about the organization of third tier cortex in macaque based on the observation that the data on which the accepted subdivision of the macaque cortex is based (shown in panel A) are equally compatible with another interpretation (shown in panel B). (A) Original interpretation of boundaries of visual areas in macaque dorsal extrastriate cortex, based on Gattass et al. (1988) and Colby et al. (1988). Redrawn from Figure 5 of Gattass et al. (1988), with the exception of the organization of area V3A (which was based on Figs. 3, 8, 11, and 13 of the same publication) and area PO [which was based on Colby et al. (1988)]. (B) A re-interpretation of the same data, based on the studies of marmoset monkeys by Rosa and colleagues, and on the studies by Maguire and Baizer (1984) in the macaque. In this model, a lower quadrant representation previously assigned to V3A (corresponding to area PM of Maguire and Baizer) forms the continuation of V3v/VP into the rostral bank of the lunate sulcus and prelunate gyrus. This would result in a VLP/V3 forming a complete representation of the visual field, similar to the New World monkey VLP. The most medial part of the original V3d/V3, combined with area PO, forms the homologue of the New World monkey DM (or V6 of Galletti et al., 1999). Gray area: central 1° of the visual field; other symbols are as in Fig. 1. Here, the thin solid contours indicate areal boundaries which were interpolated based on myeloarchitectural evidence.
The resurgence of the V3-only model
By the turn of the century, the V3-only model appeared discredited. There was strong evidence of a distinct densely myelinated, striate-recipient area which adjoined dorsal V2; moreover, even groups that continued to support the idea of a long V3, with the upper and lower quadrant representations adjacent to those in V2, acknowledged that V3d had a complex visual topography (without the expected representation of the lower quadrant vertical meridian forming most of its rostral border), and that the third tier cortex contained at least one additional area near the midline (PO/V6) and possibly more. It was then that, bringing us back full circle, a series of anatomical studies claimed evidence for a single elongated V3 adjacent to V2, which had a relatively simple topographic organization (similar to the model shown in Fig. 1A). This was reported initially in New World monkeys (Lyon & Kaas, 2001, 2002b), and subsequently, in macaque monkeys (Lyon & Kaas, 2002a).
According to the above studies, a homologue of area DM does exist in the dorsal extrastriate cortex, albeit displaced rostral to dorsal V3, without a shared border with V2 (see for example Fig. 3B) – in other words, DM was no longer considered a third tier area as in the original formulation by Allman and Kaas (1975). The resurgence of the V3-only model promoted by the studies of Lyon and Kaas has, however, remained controversial, prompting several studies addressing the issue of whether or not a single elongated area V3 separates dorsal V2 from DM (Lyon et al., 2002; Rosa et al., 2005, 2009, 2013; Fan et al., 2012; Lyon & Connolly, 2012; Jeffs et al., 2013; see also the article by Jeffs et al., 2015 in this special issue).
Fig. 3.
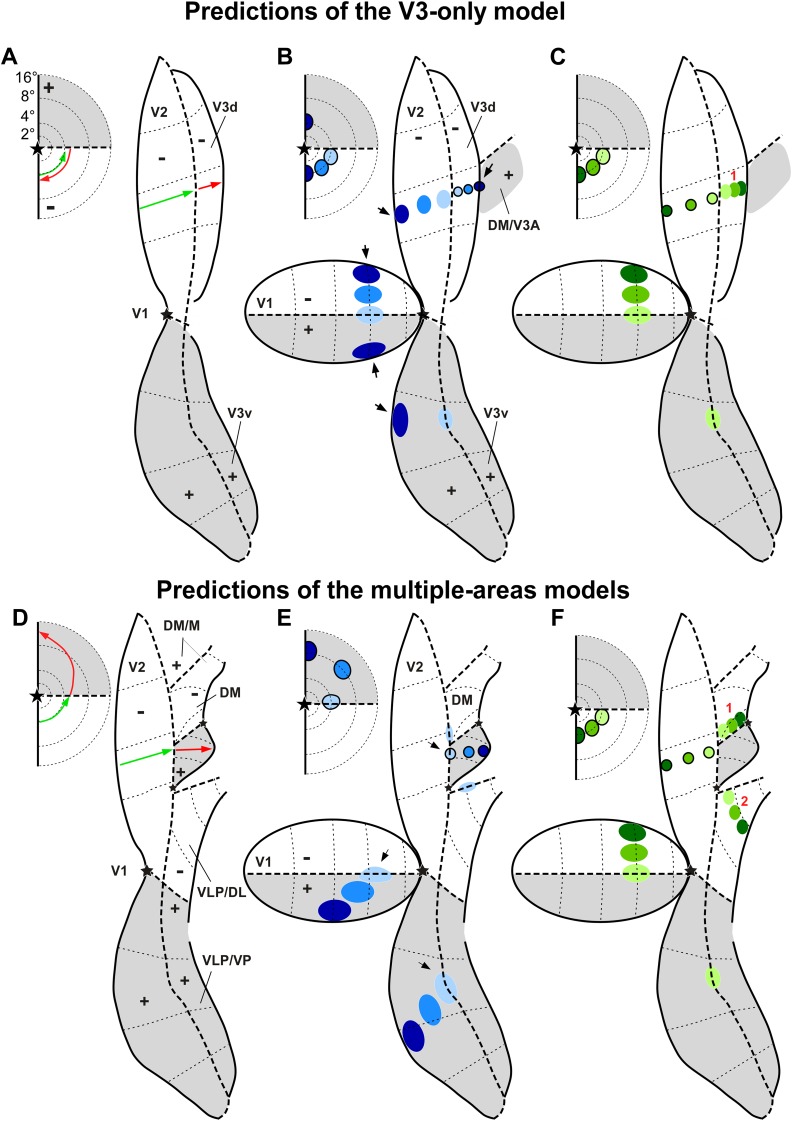
Model Predictions. Topography of receptive field locations (A, D) and labeled interareal connections in V1 and V2 (B-C, E-F) predicted by the V3-only model (A–C) and by the multiple-areas models (D–F). Conventions are as in Fig. 1, but here gray shaded regions indicate regions representing the upper visual quadrant, and white regions those representing the lower quadrant. (A, D) The arrows represent a caudorostral progression of recording sites in V2 (green) and cortex rostral to V2 (red). In each panel, the arrows in the inset to the left indicate the predicted trajectories in the visual field of neuronal receptive fields recorded at the respective cortical sites, according to each model. (B) A hypothetical series of tracer injections (outlined circles), starting near the caudal border of V3d (light blue) and ending with an injection (darkest blue) that straddles the border between V3d and the upper field representation of an area rostral to it (DM or V3A). Here and in panels (C, E-F), the inset to the left indicates the eccentricities of the hypothetical injection sites projected onto the visual field. Only the darkest blue injection site is expected to produce label in upper field V1 and V2, as it straddles a region of the upper visual field; all the other injection sites are expected to produce label only in lower field V1 and V2. Black arrows point at the injection site that straddles the caudal border of upper field DM/V3A, as well as to the label at the vertical meridian representations of V1 and V2 resulting from this injection. (C, F) A hypothetical series of tracer injections across the width of dorsal V2, and expected location of resulting label in V1 and third tier cortex, according to each model. The V3-only model predicts a single label reversal (indicated as 1) in third tier cortex resulting from the injection series, whereas the multiple-areas models predict two label reversals (indicated as 1 and 2). (E) A series of tracer injections across the width of upper field DM, starting caudally with an injection straddling the border between dorsal V2 and DM, and ending near the rostral border of DM. All injections are in upper field cortex and, therefore, are expected to produce label in upper field V1 and V2; however, the caudalmost injection (light blue), which straddles into adjacent V2, is expected to also produce label at the lower horizontal meridian representations of V1 and V2. Black arrows point at an injection site that straddles the caudal border of upper field DM, as well as to the label at the horizontal meridian representations in V1 and V2 resulting from this injection.
Points of agreement and controversy
Before we review the experimental evidence for the different models of organization of the third tier cortex, it is worth identifying the current points of agreement, so we can focus on the more controversial issues.
First, it is important to recognize that there is no longer debate about the organization of the ventral component of the third tier visual cortex. This part of the third tier complex is occupied by a representation of the upper visual quadrant, which has a relatively simple visual topography that mirrors that found in the ventral part of V2. This region is variously referred to as area VP, the ventral portion of area V3 or V3v, or the ventral portion of area VLP. Thus, the remainder of our argument will focus on the dorsal component of the third tier cortex.
Second, there is also agreement on the fact that the representations of the lower visual field vertical meridian form at least part of the rostral border of third tier areas. This feature is common to all the models (Fig. 1A–1E). However, only the V3-only model advocates that this representation is continuous and ordered: sequences of recording sites obtained at any mediolateral level of the dorsal third tier cortex should always result in receptive fields that drift from the horizontal meridian to the vertical meridian of the contralateral lower visual field (Fig. 3A). As a result, this is the model that can be most easily disproven, since any evidence of a different representation pattern in dorsal cortex automatically rules it out. Differentiation between the other models requires not only the analysis of visual topography, preferably across large expanses of dorsal cortex, but also consideration of other criteria, such as architectural characteristics, detailed connectivity, and, ideally, physiological response properties.
A third point of agreement between all, but the incomplete V3 model (Fig. 1C), is that the upper quadrant representation in ventral cortex (VP, V3v, or ventral VLP) is complemented by a lower quadrant representation which borders dorsal V2 rostrally. The actual disagreement is on how far medially this lower quadrant representation extends: it could occupy the entire cortex rostral to dorsal V2 (or at least its vast majority; Fig. 1A), about two-thirds of this region (Fig. 1D), or a smaller part of it (Figs. 1E and 2).
Keeping the above in mind, we will now proceed to review the predictions of each model, and then compare these predictions with the experimental evidence obtained in New World monkeys, Old World macaques, and humans. In the analyses below, we have abstained from including extensive discussion of evidence from cyto-, myelo-, and chemoarchitectural analyses (even though, as reviewed above, histological criteria have played a major part in the evolution of our concepts about the third tier cortex). Despite ongoing progress toward development of objective criteria (e.g., Schleicher et al., 1999), the assessment of histological patterns has remained largely subjective, and thus open to different interpretations. In particular, objective/quantitative analyses have not yet been applied to solving the problems we are trying to address.
Model predictions
V3-only model (Fig. 1A)
According to this model, a continuous and elongated area V3 occupies most, if not all, of the mediolateral extent of the cortex immediately rostral to dorsal V2. This V3d forms a single retinotopic map of the contralateral lower quadrant of the visual field, mirroring the retinotopy of dorsal V2. Thus, V3d shares with V2d the representation of the horizontal meridian at its caudal border, and represents the lower half of the vertical meridian at its rostral border, in a continuous and ordered manner (foveal representation laterally, and increasing eccentricities in progressively more medial locations). In this model, V3d separates V2 entirely from other areas of dorsal extrastriate cortex. These areas, including, for example, a revised version of DM (Lyon & Kaas, 2001; Lyon & Connolly, 2012), thus have retinotopic maps in which the lower quadrant vertical meridian is represented at their caudal border.
Model predictions (Fig. 3A–3C)
At any mediolateral level in dorsal cortex, a sequence of recording sites starting near the rostral border of V2 and moving rostrally will reveal receptive fields that move from near the horizontal meridian toward the vertical meridian, in the lower half of the visual field (red arrow in Fig. 3A).
Tracer injections placed at any location immediately rostral to dorsal V2 should result in labeled neurons in the lower quadrant representations of other retinotopically organized visual areas [except for injections near the horizontal meridian representation, which are expected to produce label restricted to the upper horizontal meridian representation in ventral cortex, as previously shown by Jeffs et al. (2009)]. This prediction stems from the principle that neuronal connections primarily link cells with overlapping receptive fields (e.g., Angelucci et al., 2002). More specifically, according to this model, a series of tracer injections at progressively more rostral locations in the dorsal third tier cortex (Fig. 3B) should always result in patches of labeled neurons that progressively approach the lower quadrant vertical meridian representation in areas with well-characterized retinotopic maps (such as V1 and V2). Importantly, injections straddling the border between V3d and the upper field representation of the area rostral to it (e.g., DM or V3A, according to different investigators) should produce labeled neurons at the vertical meridian representations in V1 and V2 (arrows in Fig. 3B).
Conversely, single tracer injections in the lower quadrant representations of these areas (V1, V2), or a series of injections across the width of these areas (Fig. 3C), should produce a single patch of labeled neurons (with some allowance for discontinuities due to modular organization), or a single label sequence that is a mirror reversal of the injection series (number 1 in Fig. 3C), in the cortex immediately rostral to dorsal V2, representing topographic connections between these areas and V3d.
Given that the entire extent of the dorsal third tier cortex is formed by parts of a same area (V3d), laminar patterns of connections with well-characterized areas (e.g., V1, V2, MT), as well as the patterns of connections with other areas, should be similar irrespective of the location of tracer injection within this region.
Multiple-areas models (Fig. 1B and 1E)
Even though the original multiple-areas model (Fig. 1B) has, to our knowledge, no current proponents, it shares key predictions with the revised multiple-areas model (Fig. 1E). In both formulations, the dorsal half of the third tier visual cortex consists of multiple areas. Further, common to these variants is the idea that this region includes area DM, which has the representations of the upper and lower quadrants that are both directly adjacent to dorsal V2. The caudal border of DM, shared with V2, always represents the horizontal meridian. Common to these model variants is also the idea that, laterally, dorsal V2 is bordered by the lower quadrant representation of a different area. In the original multiple-areas model, this was identified as area DL (Fig. 1B), and in the revised model it is VLP/V3 (Fig. 1E).
Model predictions (Fig. 3D–3F)
A critical prediction of the multiple-areas models is that at some levels of the dorsal extrastriate cortex a sequence of recording sites starting near the rostral border of dorsal V2 and moving rostrally will reveal receptive fields that move from near the horizontal meridian toward the vertical meridian in the upper visual quadrant (red arrow in Fig. 3D). It is important to note that this topographic pattern is only expected at specific mediolateral levels of the third tier cortex. At other levels, the receptive fields are expected to revert back toward the vertical meridian in the lower field, much as in the V3-only, incomplete-V3, and pinched-V3 models (Fig. 3A). Thus, differentiation between the models shown in Fig. 1 requires data that cover the cortex rostral to dorsal V2 in a comprehensive manner.
Tracer injections placed at some mediolateral levels of the cortex immediately rostral to dorsal V2 (i.e., in upper field DM) will result in labeled neurons that are clearly within the upper quadrant representations of areas V1 and V2 and other visual areas. More specifically, a series of tracer injections at progressively more rostral locations in the dorsal third tier cortex, at mediolateral levels corresponding to the lateral part of DM, should result in patches of labeled neurons that progressively approach the upper quadrant vertical meridian representation in areas V1 and V2 (Fig. 3E). Importantly, injections straddling the border between dorsal V2 and the upper field representation of the area rostral to it (DM) should produce labeled neurons at the horizontal meridian representations in V1 and V2 (arrows in Fig. 3E).
Given the hypothesis that the dorsal component of the third tier cortex includes at least two different areas, single tracer injections (or a caudorostral series of injections) in the lower quadrant representations of areas, such as V1 or V2 (particularly within the representation of central vision; Fig. 3F), should typically produce two patches (or two mirror reversals) of label immediately rostral to dorsal V2: one in DM, the second in VLP/V3 (or DL) (numbers 1 and 2 in Fig. 3F).
Unlike in the V3-only model (Fig. 1A), tracer injections in different parts of the dorsal third tier cortex would likely reveal different laminar, modular, and areal connection patterns.
Incomplete-V3 and pinched-V3 models (Fig. 1C and 1D)
The incomplete and pinched-V3 models share a series of key predictions for the topographic organization of the dorsal third tier cortex. Together with the V3-only model (Fig. 1A), they advocate that a large portion of the cortex rostral to dorsal V2 is formed by a single, elongated representation of the lower quadrant, which represents the vertical meridian of the lower visual field at its rostral border. In both models, receptive field eccentricity in the lower quadrant increases systematically from lateral to medial. However, unlike the V3-only model, they allow for the fact that some regions of the third tier cortex may not show this pattern. They do so by proposing that V3d (or the “incomplete” V3) has narrower regions in which only the vicinity of the horizontal meridian is represented, or that this area is broken into multiple islands. In the latter case, the regions in between islands belong to different areas, which could, in theory, show a different topographical organization. In addition, both models recognize the existence of at least one additional area in the medial part of the dorsal third tier cortex (area PO or V6).
Model predictions
In both models, sequences of recording sites starting near the rostral border of dorsal V2, and moving rostrally, should typically (if not always) reveal receptive fields located in the lower visual quadrant. In many cases, these receptive fields will move from near the horizontal meridian toward the lower vertical meridian (as in Fig. 3A). However, in some regions, the receptive fields will not reach this meridian, instead remaining near the horizontal meridian. Proponents of these models have not, to our knowledge, formally discussed the possibility of receptive fields moving into the upper quadrant. However, as suggested by the diagram shown in Fig. 2, published illustrations do not necessarily rule this out (see also Fig. 12).
Given the possibility of variable configurations of width and islands in V3d, these models make no strong predictions regarding topography in the results of tracer injections, at least in the absence of detailed knowledge about the configuration of V3 in a specific case. The results of injections in cortex immediately rostral to dorsal V2 could be highly dependent on the exact location relative to the borders of V3 in an individual animal. However, in most if not all cases, the locations of labeled cells in other areas should be within the lower visual quadrant representations.
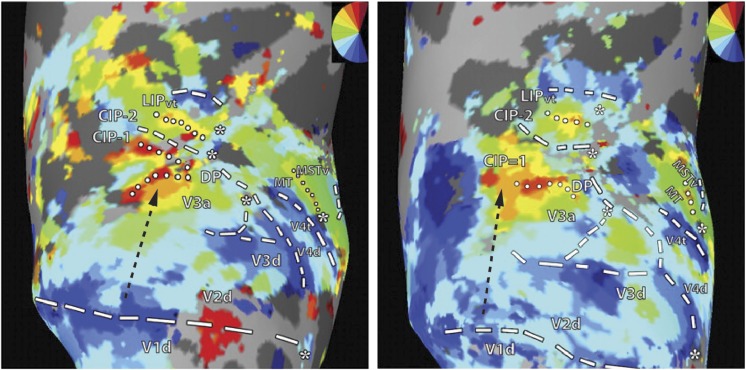
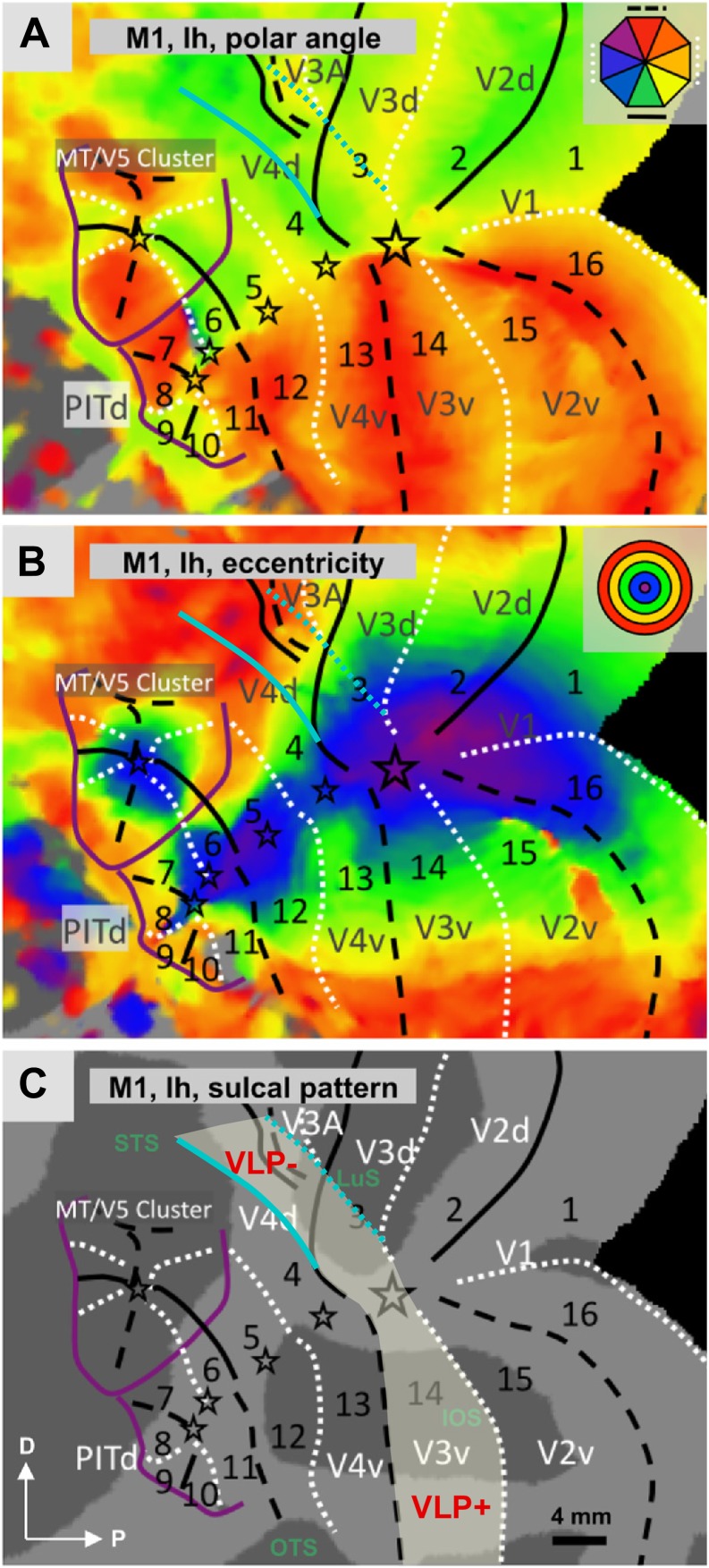
Fig. 12.
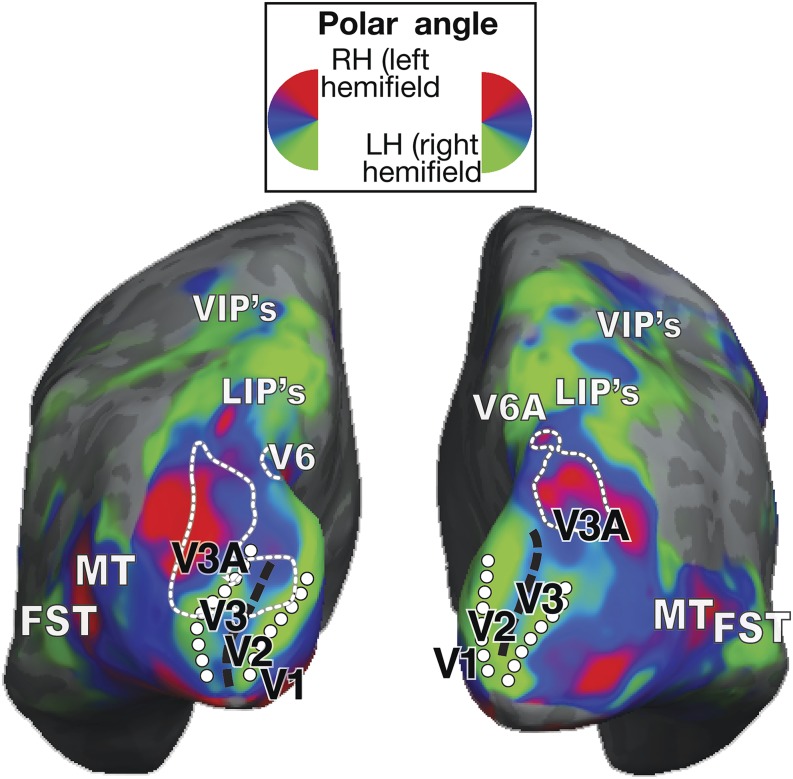
Macaque functional imaging data in dorsal extrastriate cortex. Reproduction of fMRI data obtained in the right hemispheres of two macaque monkeys by Arcaro et al. (2011; their Figures 1 and 2), in a study focused on the organization of the caudal intraparietal sulcus. The color code (top right inserts) represents the relationship between hemodynamic activation and the polar angle of stimuli presented in the visual field. The putative locations of dorsal visual areas (dashed contours indicate the representation of the lower vertical meridian, dotted contours the representation of the upper vertical meridian, and asterisks the representation of the central visual field), including V2 and V3, are shown in an inflated representation of the cortex, as indicated by the authors of the original study. Black dashed arrows point to a direct transition from V2 to an upper visual field representation, labeled V3A in the figure, i.e, however equally compatible with area DM directly abutting dorsal V2 (i.e., with the multiple-areas models, Fig. 1B and 1E), and the reinterpretation of the electrophysiological mapping studies in the macaque shown in Fig. 2.
The key differences between the incomplete-V3 and pinched-V3 models lie outside the realm of the topographic organization of the dorsal third tier cortex. While the pinched-V3 model (Fig. 1D) predicts that the connections, response properties, and histological characteristics will prove similar for corresponding locations in V3d (V3) and V3v (VP), the incomplete-V3 model (Fig. 1C) proposes that these differ markedly. As reviewed above, there is already substantial evidence in favor of differences in this respect (Burkhalter et al., 1986; Van Essen et al., 1986; Felleman et al., 1997), although these can also be accommodated by the multiple-areas models (Baker et al., 1981; Krubitzer & Kaas, 1990; see also Jeffs et al., 2015 in this special issue).
We now proceed to compare these model predictions with the experimental evidence obtained in New World monkeys. The evidence obtained in Old World monkeys and humans is discussed separately.
Receptive field mapping in the New World monkey third tier cortex
In this section, we review experimental observations in the marmoset (Callithrix jacchus), a small (300–400 g), diurnal New World monkey with a well-developed fovea (Wilder et al., 1996) and highly visual behavior (Mitchell et al., 2014). The technical advantages afforded by using the marmoset in anatomical and physiological studies have been reviewed recently (Solomon & Rosa, 2014; Mitchell & Leopold, 2015), as well as the similarities and potential differences relative to Old World monkeys. The most important points to emphasize here are that in this species the entire third tier visual cortex is exposed on the surface of the brain, allowing for dense sampling, and accurate anatomical reconstruction of recording sites over large expanses of cortex; moreover, robust protocols for in vivo electrophysiology have been developed for this species, allowing neurons in early visual cortical areas to respond vigorously to visual stimulation under anesthesia (Lui et al., 2006; Yu & Rosa, 2010; Yu et al., 2013).
The dorsal third tier cortex includes an upper visual quadrant representation adjacent to V2
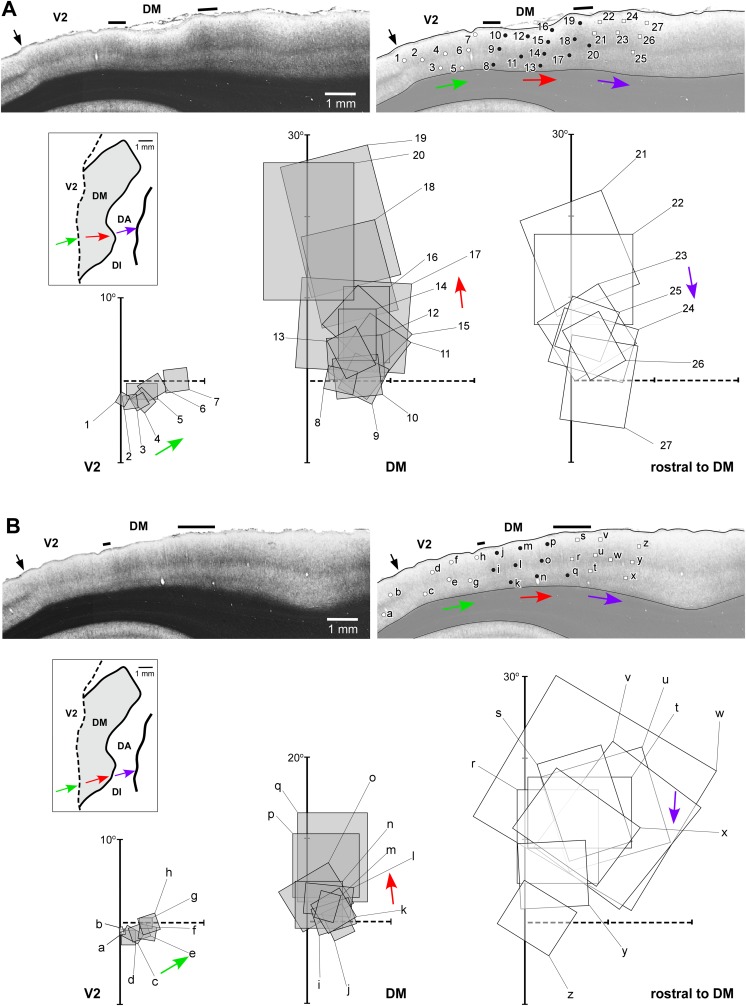
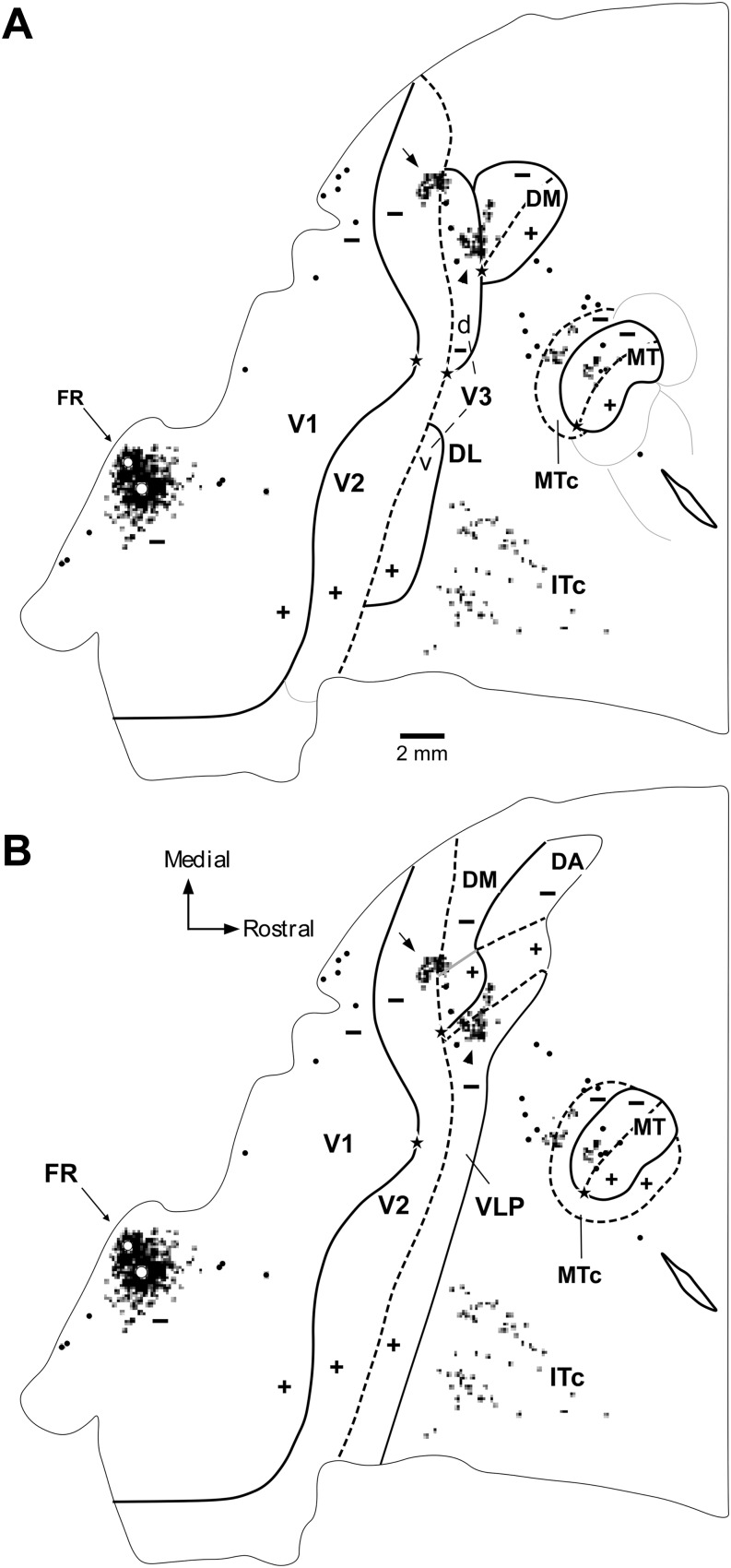
As reviewed above, one key test of the hypotheses linked to the different models shown in Fig. 1 is the location of receptive fields recorded in the strip of cortex immediately rostral to dorsal V2, relative to the horizontal meridian (Fig. 3A, 3D). Fig. 4 illustrates the receptive fields mapped across sequences of recording sites that move from dorsal V2, caudally, into the third tier cortex (Rosa & Schmid, 1995; Rosa et al., 2005). A critical finding is that, just as predicted in Fig. 3D, in a defined sector of approximately 3 mm length parallel to the V2 border, as the recording sites enter the area adjacent to V2, receptive fields move from the lower field horizontal meridian directly into the upper visual quadrant (red arrows in Fig. 4), without an intervening reversal of the receptive field sequence toward the lower visual quadrant vertical meridian. This observation cannot be attributed to an error in estimation of the horizontal meridian, since the marmoset has a well-defined fovea, which can be visualized and plotted with a precision of 1° or less. In addition, we have used recordings in V1 and V2 in the same animals to confirm the accuracy of our estimates: small receptive fields including the estimated center of the fovea were obtained in recording sites verified histologically to coincide with the apogee of the curvature of V1 (Fritsches & Rosa, 1996), and receptive fields including the horizontal meridian were obtained in recording sites throughout the extent of the rostral border of V2 (Rosa et al., 1997b). It is also the case that, contrary to the argument advanced by Lyon (2013), these findings also cannot be explained by inaccurate reconstruction of electrode tracks. In all cases, recordings from DM and areas in adjacent dorsal cortex were obtained as part of a systematic survey using electrode penetrations in the vertical stereotaxic plane, which reached the tentorial surface of the brain (thus leaving long and unambiguous tracks in coronal and parasagittal histological sections). In particular, as shown in Fig. 4, it is hard to imagine how evidence of a “narrow V3d”, which “forms a mirror reversal of the lower field representation in V2 and is approximately half the width” (i.e., 1.5–2 mm) could have been missed between recording sites in all our experiments until now. The example illustrated in Fig. 4A, where the recording sites are separated by 300–500 µm when projected to the surface of the brain, is typical of our materials (see also Figs. 5 and 6). Finally, it is important to reassert the fact that these findings cannot be regarded as inconclusive on the basis that they could represent “also the pattern one would expect for a narrow V3d” (Lyon, 2013). As discussed above, even though in all models the first receptive fields recorded just rostral to V2 are predicted to include the visual field's horizontal meridian, support for the V3-only hypothesis would entail a very different progression of receptive field locations beyond this point.
Fig. 4.
A representation of the upper visual quadrant directly adjacent to V2. Topographic and architectural transitions at the boundaries of DM in one marmoset. (A, B). Data obtained from two different parasagittal levels across the dorsal extrastriate cortex (level A is more medial than B). These levels are indicated by the colored arrows in the inserts, which illustrate bidimensional reconstructions of the “third tier” densely myelinated field (DM) and immediately adjacent areas. The following conventions apply to both (A) and (B). Top left panel: Myelin-stained parasagittal section illustrating myeloarchitectural transitions near the site of the penetrations. These sections are within 320 µm of the nearest Nissl-stained section containing electrode tracks; they were chosen for the purpose of illustration as they demonstrate the myeloarchitectural patterns without much interference from artifacts due to the electrode tracks, while still allowing an accurate plotting of the nearby recording sites. The border between V2 and DM is clearly defined by an increase in myelination (indicated by the left black bar above the cortex). The rostral border of DM (right black bar) is subtler, as the adjoining fields are also rich in myelin. As detailed elsewhere (Rosa & Schmid, 1995), the primary criterion for defining the rostral border of DM in myelin stain is an increase in the separation between the inner and outer bands of Baillarger. The V1/V2 border is indicated by a black arrow and the zones of uncertainty between other areas are indicated by the black bars above the cortex. Top right: The same section as in (A), with overlaid locations of recording sites from nearby sections. Recording sites deemed to belong to V1 or V2 are indicated by white circles, those deemed to belong to area DM in black circles, and those deemed to be rostral to DM in white squares. Bottom: The receptive fields corresponding to the recording sites; note that these data (from Rosa et al., 2005) have been re-plotted in the appropriate orientation to follow the same convention used in other figures of this manuscript. The main trends in receptive field topography are indicated by colored arrows (green, V2; red, DM; purple, rostral to DM). In both (A) and (B), recording sites crossing V2 from caudal to rostral result in receptive fields that move away from the vertical meridian and toward the horizontal meridian (left receptive field map). At the border of the densely myelinated zone the receptive fields become larger and move into the upper visual field, approaching higher elevations near the vertical meridian as more rostral sites are sampled (middle receptive field map). At the rostral border of DM, the receptive fields move back away from the vertical meridian and toward the central visual field (right receptive field map). While different observers may place the borders at slightly different points, these trends remain robust indicators of the limits of area DM. Scale bars = 1 mm.
Fig. 5.
Visuotopy of the part of DM exposed on the dorsal surface of the brain. (A–F) Receptive fields recorded from neurons at different mediolateral levels of DM. The location of each receptive field is shown relative to the vertical and the horizontal meridians. Note that only a portion of the visual field (up to 30°) is represented in the dorsal portion of DM. In each sequence, the receptive field recorded from neurons in the most caudal site is numbered 1 and highlighted in yellow, and the receptive field recorded from neurons in the most rostral site is highlighted in blue. The red line and arrow connect the centers of the receptive fields, indicating the trend in visual topography as DM is crossed from caudal to rostral. Left: Flat reconstruction of the location of recording sequences A–F in DM. In this representation, gray shading delineates the regions of the upper quadrant representation. Note the similar receptive field sizes in the upper and lower quadrant representations, at comparable eccentricities. To facilitate comparisons, this diagram (from Rosa & Schmid, 1995) has been re-plotted following the same conventions as in other figures (right hemisphere, with receptive fields as seen by the experimenter on the surface of a hemispheric screen where stimuli were projected).
Fig. 6.
The part of area DM located on the midline surface contains the representation of the peripheral visual field. Receptive fields obtained from sites on the medial surface of the occipital lobe, in long tangential electrode penetrations (from Rosa et al., 2005). (A) Diagram of an unfolded map of the cortex, showing the locations of recording sites relative to the borders of V2, DM and another third tier area (POm) that is located adjacent to the representation of the lower far peripheral visual field in V2. The dotted purple line indicates the medial convexity of the occipital lobe. (B–D) Receptive fields in area DM. In each diagram, the region of visual field that is represented on the dorsal surface of the brain of the same animal is shaded in pink. Recording sites and receptive fields in DM are numbered 1–33, and those in V2 and POm are designated by letters. Recording sites and receptive fields in POm are indicated in green, those in V2 in white.
The observation of an upper quadrant representation adjacent to dorsal V2 is not unique to the marmoset, since earlier similar findings were obtained in another species of New World primate, the owl monkey (Aotus trivirgatus). Indeed, the original observation dates back to Allman and Kaas (1975), who used it as the cornerstone of the original multiple-areas model (Fig. 1B); this observation was later confirmed in a subsequent study by Krubitzer and Kaas (Krubitzer & Kaas, 1993), in a brief report by Sereno and Allman (1991), and in a study by Sereno et al. (2015) published in this special issue. Receptive fields in the upper visual quadrant near the V2 border have also been observed in the larger, gyrencephalic Cebus monkey (Rosa, unpublished observations), but have been explained by invoking a version of the “pinched-V3” model, whereby area DM separates two islands of dorsal V3 (Rosa et al., 1993). However, subsequent work highlighted that the data in the Cebus are also compatible with the revised multiple-areas model (Rosa et al., 2000; Rosa & Manger, 2005).
The upper quadrant representation in the dorsal third tier cortex is complemented by a lower quadrant representation
Medial to the upper quadrant representation in dorsal third tier cortex, the pattern of topographic representation changes: rather than moving toward the upper visual quadrant, the receptive field sequences crossing the V2 border into the third tier cortex revert toward the lower visual field (Fig. 5). This medial region forms a complete representation of the lower visual quadrant. Importantly, the receptive fields recorded in this region have sizes that are identical to those observed in the adjacent upper quadrant representation discussed above (Rosa & Schmid, 1995).
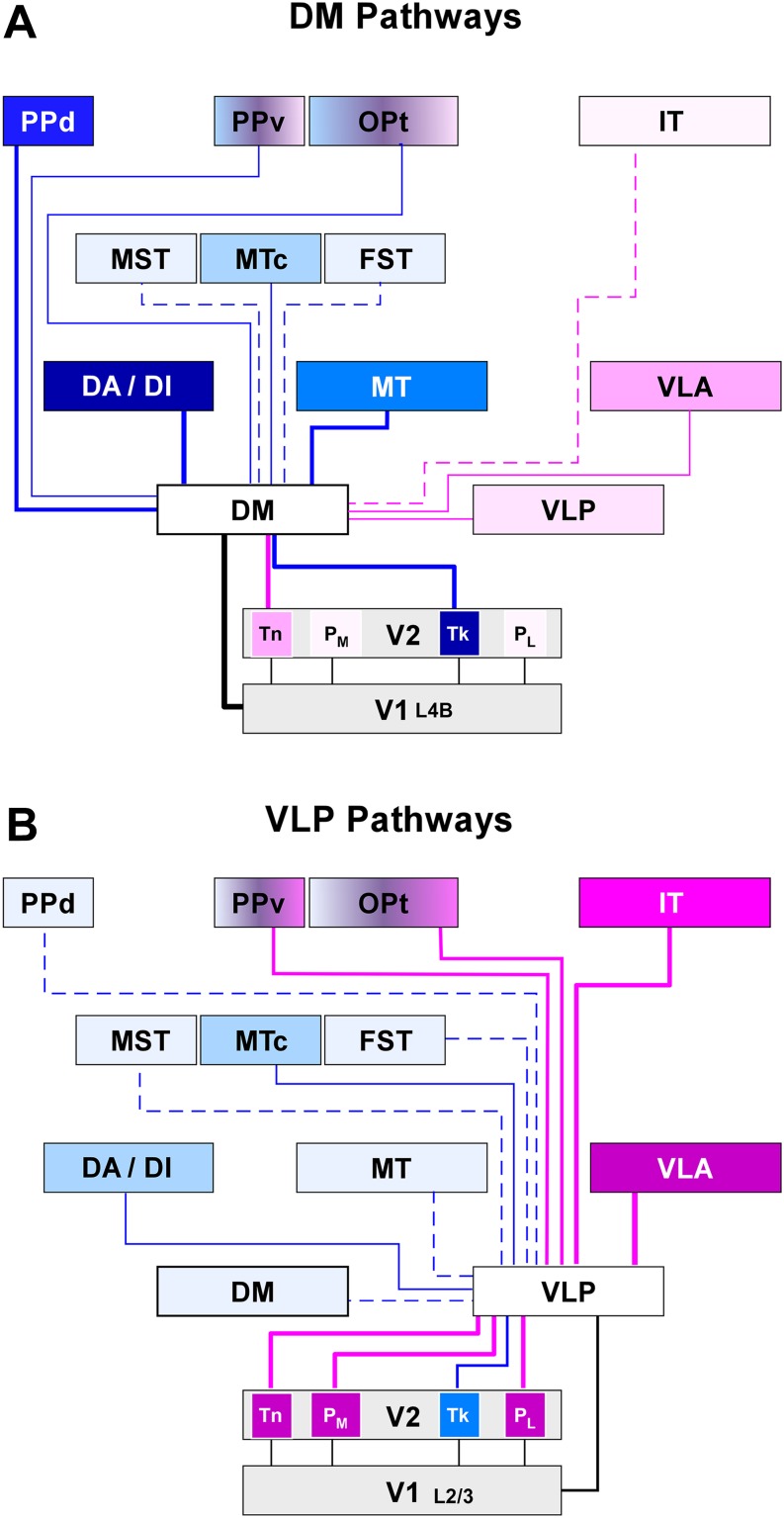
These upper and lower quadrant representations show similar architectural appearance, as evidenced by myelin and SMI-32 stains (Rosa et al., 2005), as well as similar patterns of connections with other brain areas (Rosa et al., 2009; see also Jeffs et al., 2015 in this special issue), including a major input from V1 layer 4B (see also Vogt Weisenhorn et al., 1995, and further evidence discussed below). In summary, according to physiological and anatomical criteria, the upper and lower quadrant representations can justifiably be interpreted as part of the same visual area, even though they differ according to the “visual field sign” criterion used to parse retinotopic representations in some studies (Sereno et al., 1995). Based on location, topographic organization and connections (see below), as well as myeloarchitectural appearance, it was concluded that they represent the marmoset homologue of area DM, first identified in the owl monkey.
Representation of the visual field periphery
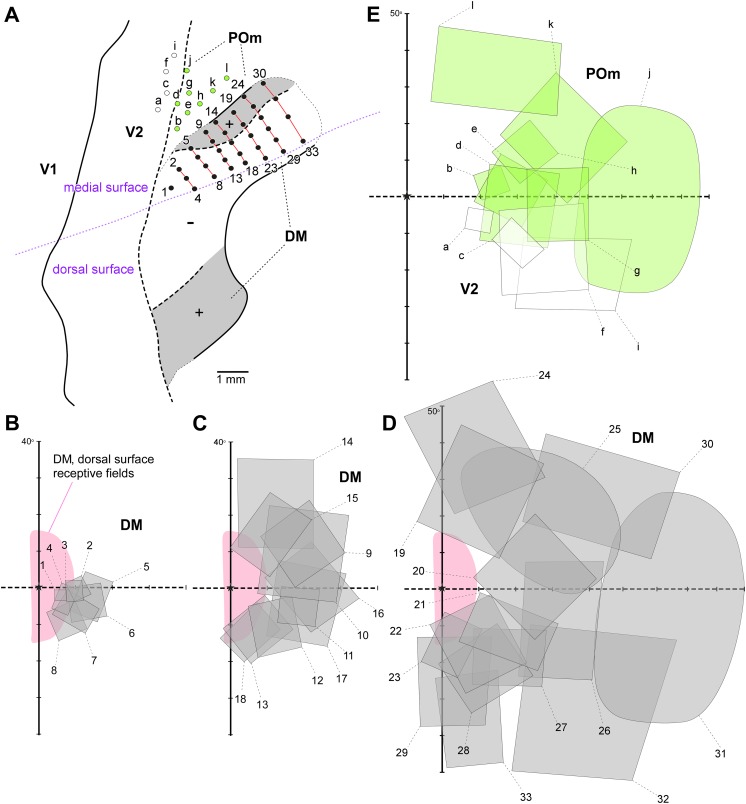
In the revised multiple-areas model (Fig. 1E), the visual topography of area DM, as defined in the marmoset, differs from the one suggested for the owl monkey in the original multiple-areas model (Fig. 1B), in which the representation of the upper quadrant is not continuous. Extensive receptive field mapping in various animals has consistently shown that the lateral sector of the upper quadrant representation only includes receptive fields within 20–30° of the fovea (sector indicated in pink, in Fig. 6). One proposal to circumvent this problem is that the remainder of the upper visual field is represented along the midline, medial to the peripheral lower quadrant representation (Fig. 6). Consistent with this interpretation, the lateral and medial upper quadrant sectors are topographically complementary (Fig. 6), both lie within the architecturally defined DM, and both share the main input from V1 layer 4B (Rosa et al., 2009). Although this unusual visuotopic map is a point that requires further studies, in particular, to determine whether the lateral and medial sectors of the upper quadrant representation indeed belong to the same area, as argued below, this is relevant for assessing the homology of marmoset DM with other species of primate.
Here, it is useful to remind the reader that DM and VLP/V3 are not the only third tier visual areas. As shown in Fig. 6, at least one additional visual field representation, named the parietooccipital media area (POm) (Neuenschwander et al., 1994; Rosa & Schmid, 1995), adjoins the representation of the far peripheral representation of V2 in the ventral aspect of the midline cortex. Area POm lies outside the densely myelinated zone, thus probably corresponding to the original definition of medial visual area (M), proposed by Allman and Kaas (1976) as part of the multiple-areas model (Fig. 1B). However, some of the territory likely assigned to area M in owl monkeys can be justifiably seen as an extension of DM (Rosa and Schmid, 1995; Sereno et al., this special issue), leaving the full retinotopy of POm/M as a subject to be tackled by future studies.
Evidence from owl monkey data
Importantly, careful comparison of Fig. 1B and 1E reveals that the original and revised multiple-areas models show a very similar pattern of visual field representation in the dorsal part of the third tier cortex; this is despite the fact that the primary evidence has been obtained in different species (owl monkey vs. marmoset), more than two decades apart (Allman & Kaas, 1975; Rosa & Schmid, 1995). Specifically, in both models, there are three representations of the horizontal meridian running obliquely to the one present at the V2 border, with alternation of representations of the lower and upper quadrants between them. Thus, the primary evidence from receptive field mapping is remarkably similar across studies, the only difference between them being the delineation of areal borders. In the revised multiple-areas model (Fig. 1E), part of what was originally considered area M in the owl monkey (Fig. 1B) has been deemed part of area DM, given similarities in myeloarchitecture and connections of this region with DM, and complementarity in the extent of visual field representation (see above).
This complexity in topographic organization has been deemed a “possible aberration from microelectrode mapping” (Lyon, 2013), since it was not found in experiments using intrinsic signal imaging in owl monkeys (Lyon et al., 2002). However, we argue that single-unit mapping offers a higher resolution view of the topographic organization of the cortex, by providing direct evidence of the locations of receptive fields, and their changes over small distances. The consistency across species and different research groups that used this technique is in our view a strong argument in favor of the latter interpretation. We add here that the results of those optical imaging experiments, in fact, demonstrated a significant gap of up to 2 mm in the lower vertical meridian representation that forms part of the rostral border of the dorsal component of the third visual complex, and found no evidence of an upper quadrant representation anywhere in the dorsal cortex (including regions just rostral to V3d, where an upper quadrant representation is supposed to exist according to the V3-only model, Fig. 3B, and has been observed even in macaque, Fig. 2). As argued previously (Rosa et al., 2005), this absence of evidence should not be taken as evidence of an absence of an upper quadrant representation, particularly in view of the fact that the upper quadrant representation in owl monkey DM is typically located within, and around the tip of the lateral sulcus, where vascular artifacts are most likely to hamper optical imaging and other blood flow-based techniques (Spitzer et al., 2001; Harrison et al., 2002).
Connections of the New World monkey third tier cortex
Feedforward and feedback connections between early visual areas are topographically organized (Angelucci et al., 2002). Therefore, mapping connections with areas that have well-characterized visuotopic maps can be a powerful method for revealing the topographic organization of a cortical region. Before we review the connectional data obtained in marmosets, it is worth considering some general issues related to the interpretation of this type of information, in the context of testing hypotheses on the topographic organization of visual cortex.
Studies involving sparsely-distributed tracer injections cannot provide conclusive tests of models of the third tier cortex organization
Most connectional studies which have addressed the organization of the third tier cortex have mapped the distribution of labeled neurons arising from single or sparsely distributed tracer injections made in one or two areas, such as V1, V2, MT or the third tier cortex (e.g., Weller et al., 1991; Krubitzer & Kaas, 1993; Beck & Kaas, 1998a; Lyon & Kaas, 2001, 2002b). Here, we argue that the results from these studies either allow for multiple interpretations (therefore being consistent with more than one model of third tier cortex organization; Fig. 1A–1E), or are difficult to interpret without additional information. This is because the topography of label arising from single tracer injections is most often insufficient to constrain the location of areal boundaries, and, with rare exceptions, the latter cannot be reliably identified solely on the basis of subjective myelo-, cyto-, and/or chemoarchitectural criteria (for a discussion, see Jeffs et al., 2009, 2013).
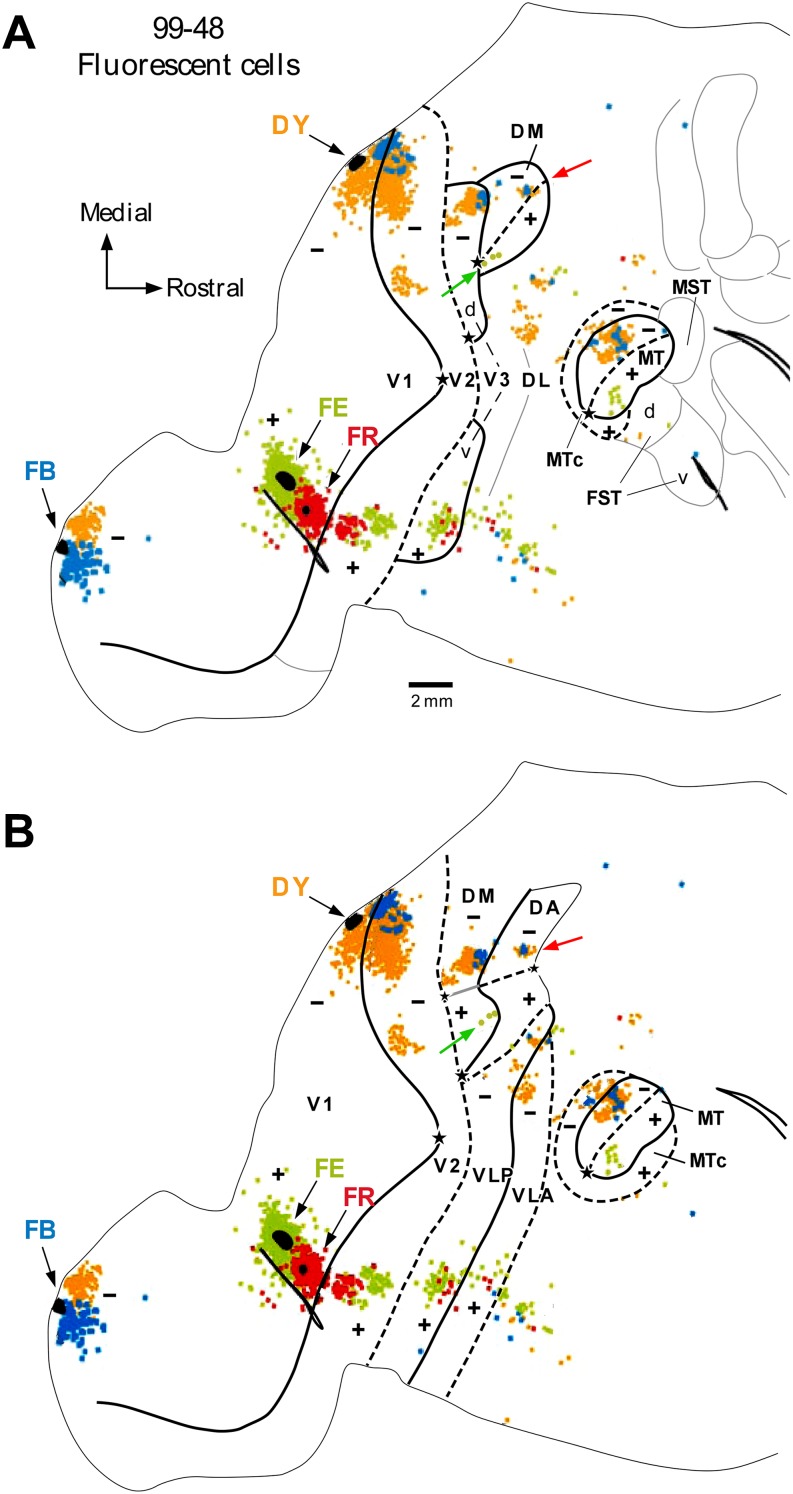
For example, the connectional studies of Lyon and Kaas have provided evidence for the existence of reciprocal projections between V1 and both ventral and dorsal third tier cortex in both New World monkeys (Lyon & Kaas, 2001, 2002b) and macaque (Lyon & Kaas, 2002a). Since the absence of connections from V1 to the ventral half of the third tier cortex was one of the main arguments upon which the incomplete-V3 model (Fig. 1C) was found, Lyon and Kaas took their finding as evidence against this model, but as support for the V3-only model (Fig. 1A). In reality, all that can be inferred from these findings, or any study using sparsely distributed tracer injections in V1 or V2, is that these areas make topographic connections with dorsal and ventral halves of extrastriate cortex near the rostral border of V2. However, whether the targeted regions are part of the same cortical area (V3 according to these authors' interpretation) or of different areas (e.g., DM and ventral VLP/VP according to the multiple-areas models, or V3 and VP according to the incomplete-V3 model) cannot be resolved with this experimental approach. Moreover, with this approach it is also difficult to determine whether a labeled connectional patch in dorsal cortex abuts V2 or V3, given the difficulty of accurately determining areal borders using architectural criteria alone. To illustrate this problem, in Fig. 7A, we reproduce data from Fig. 6 of Lyon and Kaas (2001), showing the distribution of labeled neurons resulting from the four different tracer injections placed in marmoset V1, two at/near the upper vertical meridian representation, and two at/near the lower vertical meridian representation. The two dorsal injections (blue and yellow) produced labeled connections in a mirror reversal sequence in dorsal cortex rostral to V2, while the two ventral injections (green and red) produced a similar mirror reversal pattern of labeled connections in ventral cortex rostral to V2. These results were interpreted as evidence in support of a single elongated area V3 bordering V2 (the V3-only model of Fig. 1A), with the labeled connections in dorsal cortex residing in V3d, and those in ventral cortex in V3v. However, in Fig. 7B, we demonstrate that the same data are arguably more consistent with the revised multiple-areas model (Fig. 1E). According to this interpretation, the connections labeled by the dorsal V1 injections reside in the lower quadrant representations of DM and VLP, while the green cells labeled by the ventral V1 injection of fluoroemerald reside in the upper quadrant representations of DM and VLP (see also legend of Fig. 7).
Fig. 7.
Ambiguity in the interpretation of connectional data from sparse tracer injections. (A) Original interpretation, according to the V3-only model, of data shown in Figure 6 of Lyon and Kaas (2001), showing the distribution of labeled neurons in unfolded and flattened marmoset visual cortex resulting from two tracer injections (fast blue, FB, and diamidino yellow, DY) placed in dorsal V1 near the V1/V2 border (site of vertical meridian representation), and two injections (fluoroemerald, FE, and fluororuby, FR) placed in ventral V1 at the V1/V2 border. The black ovals represent the estimated size of the injection sites. Other conventions are as in Fig. 1. The presence of two patches of DY label in V2, presumptive V3d and MT suggests to us that the DY injection slightly straddled into V2. The FR injection failed to produce consistent long-range transport (note lack of label in area MT or in dorsal cortex rostral to V2). (B) The same data are shown with the areal boundaries re-interpreted, according to the revised multiple-areas model. The topography revealed by this interpretation is more consistent with the visual field location of the injection sites than the interpretation shown in panel (A). Specifically, the FE (green) label is located at the upper vertical meridian representation in DM at about 8° eccentricity (according to the maps of Rosa et al., 2005) (green arrow in B), in agreement with the location of the injection site in V1, which is far from the foveal representation (star). In contrast, in panel (A), the same label (green arrow) is located at the confluence of the horizontal and vertical meridian representations, near the foveal representation of DM (according to Lyon & Kaas, 2001), which is inconsistent with the topographic location of the FE injection site. Similarly, in (B), the label resulting from the FB and DY injections is appropriately located at the lower vertical meridian representation of both areas DM and DA (red arrow), whereas according to the interpretation in (A) these cells would be located at the horizontal meridian representation in the far visual field periphery of DM (red arrow in A).
Note that the data in Fig. 7A are also consistent with the incomplete-V3 model, and that disproving this model would require additional studies of neuronal response properties, architectonics, and interareal connections in different mediolateral portions of the third tier cortex. While prior studies by Van Essen and colleagues (reviewed above), indeed, have shown that the dorsal and ventral halves of the third tier cortex in macaque have different anatomical and physiological properties, these properties have not been characterized throughout their mediolateral extent. Therefore, the observed differences in dorsal and ventral third tier cortex remain compatible with other models, including the multiple-areas model.
Here, it is worth discussing the potential basis for the conflicting data regarding the existence, or lack thereof, of feedforward projections from the upper quadrant representation of V1 to the ventral third tier cortex (VLP/V3v/VP). Until the early 21st century, injections of retrograde tracers in macaque VP/V3v or of anterograde tracers in ventral V1 had consistently failed to reveal these projections (Weller & Kaas, 1983; Burkhalter et al., 1986; Newsome et al., 1986; Van Essen et al., 1986; Felleman et al., 1997; Nakamura et al., 2004). Up to that point, no study had investigated these projections in New World primates, although there was evidence for the existence of the reverse feedback projections from ventral VLP/V3v to V1 in New World Cebus monkeys (Sousa et al., 1991), and for weak projections from dorsal V1 to dorsal VLP in marmosets (Rosa & Tweedale, 2000). Using large injections of more sensitive anterograde and retrograde neuronal tracers in ventral V1, Lyon and Kaas (2001, 2002a,b) have since demonstrated both feedforward and feedback projections between ventral V1 and V3v/ventral VLP in macaque and New World primates. In a study reported in this special issue (Jeffs et al., 2015), injections of retrograde tracers in dorsal VLP at parafoveal eccentricities revealed only weak projections from V1, but stronger projections from V1 were observed after injections at near-foveal eccentricities in dorsal VLP. Lyon and Kaas observed very dense V1-to-V3v connections in Old and New World primates after very large tracer injections into ventral V1, but weaker connections after smaller injections. Therefore, the discrepant results among groups on the existence of V1 projections to the ventral third tier cortex could be attributed to differences in tracer sensitivity (with less sensitive tracers failing to label weak connections), and/or visual field eccentricity of the injection site location (with nonfoveal injections failing to reveal significant V1 connections). Others (Nakamura et al., 2004), however, have attributed this discrepancy to technical issues with the data from Lyon and Kaas, particularly in macaque, suggesting, for example, that the large injection sites near the V1/V2 border may have spilled into adjacent V2, revealing otherwise nonexistent or weak V1 projections. While this criticism may apply to some injections (e.g., in Fig. 7B of Lyon and Kaas, 2001, labeled connections that are described to arise from injections near the V1 upper vertical meridian representation appear, inexplicably, to fill most of area MT), it cannot be used to entirely dismiss these authors' evidence for projections between ventral V1 and ventral V3/VLP.
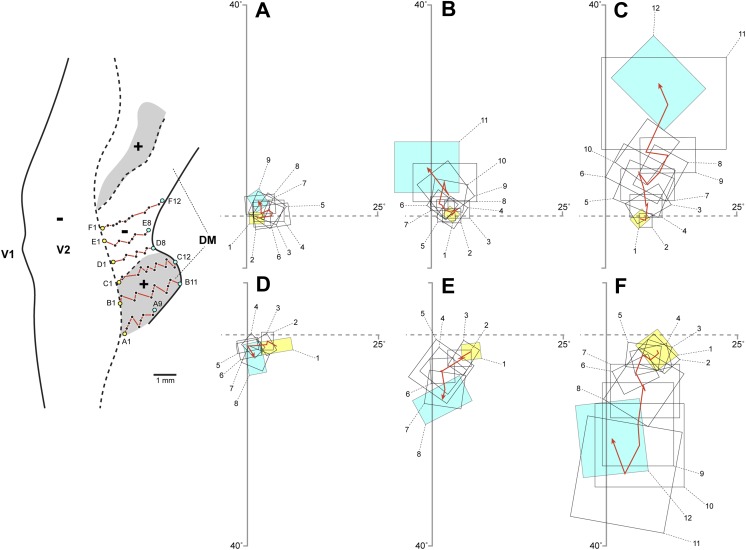
Although data from sparsely distributed tracer injections have been in most cases inadequate to disprove any one model unambiguously, there are a few examples of previously published studies in which single tracer injections produced data that seem incompatible with the V3-only model. One such example is illustrated in Fig. 8. In panel A, we reproduce data from Figure 7D of Lyon and Kaas (2001), showing the distribution of labeled neurons resulting from two adjacent tracer injections placed in marmoset V1 near the horizontal meridian at near-parafoveal eccentricities. These injections produced two patches of labeled connections in dorsal cortex rostral to V2 (arrow and arrowhead), just as predicted by the multiple-areas models. This result is inconsistent with the prediction of the V3-only model, according to which only a single patch of labeled connections should be observed rostral to dorsal V2 after injections in dorsal V1. Nonetheless, as illustrated in Fig. 8A, these results were interpreted by these investigators as evidence for the V3-only model, with one patch of label residing at the horizontal meridian representation in dorsal V2 (arrow), and the second patch residing, incongruently, at the vertical meridian representation in V3d and adjacent DM (arrowhead). In Fig. 8B, we offer a re-interpretation of the same data that is more consistent with the topographic location of the tracer injection sites: one patch of label is at the horizontal meridian representation between V2 and the lower quadrant representation in DM (arrow), while the second patch resides at the horizontal meridian representation between dorsal VLP and adjacent area DI (arrowhead). The multiple-areas models can, therefore, accommodate these data, as well as other, similar, published data which have been presented as evidence for the V3-only model, from various species of New World primates [e.g., Fig. 8A–8B in Lyon and Kaas (2001), Figs. 6B and 8B in Lyon and Kaas (2002b)] and macaques [e.g., Figure 4 in Lyon and Kaas (2002a)].
Fig. 8.
Connectional data used in support of the V3-only model, which we argue are, instead, inconsistent with this model, but consistent with the multiple-areas models. (A) Original interpretation, according to the V3-only model, of data shown in Figure 7D of Lyon and Kaas (2001), showing the distribution of labeled neurons in unfolded and flattened marmoset visual cortex resulting from two tracer injections (fluororuby, FR) in dorsal V1 near the horizontal meridian representation at parafoveal eccentricities. The white circles represent the estimated size of the injection sites. Other conventions are as in Figs. 1 and 7. (B) The same data are shown with the areal boundaries re-interpreted, according to the revised multiple-areas model. Notice that the topography revealed by this interpretation is consistent with the visual field location of the injection sites, unlike the interpretation shown in panel (A) (see text).
The published literature includes additional examples of results from single tracer injections in cortex rostral to dorsal V2 that are inconsistent with the predictions of the V3-only model. For example, Krubitzer and Kaas (1993) reported that a tracer injection straddling the border between upper field DM and adjacent posterior cortex produced labeled connections at and near the horizontal meridian representation in both upper and lower field V1 and V2 (e.g., their Fig. 5), just as predicted by the multiple-areas model (light blue injection site in Fig. 3E). In contrast, the V3-only model predicts that such an injection would produce label at the vertical meridian representations of V1 and V2 (darkest blue injection site in Fig. 3B).
Studies involving sequences of closely spaced tracer injections in the third tier cortex: Evidence for an upper quadrant representation bordering dorsal V2
A more rigorous approach to test unambiguously the specific model predictions depicted in Fig. 3B and 3E is to make sequences of multiple closely spaced injections of distinguishable anatomical tracers across the full width of the third visual complex, and analyze the resulting topography of label in areas V1 and V2 (areas for which the retinotopic organization is well known). As shown in Fig. 3B and 3E, different models predict different outcomes with respect to this experimental paradigm.
Recently, Jeffs et al. (2013) used this approach in a study of the marmoset third tier visual cortex. Caudorostral sequences of 4 closely spaced tracer injections across the full width of presumptive upper field DM resulted in the fields of cell label in upper field V1 and V2 that progressed from the horizontal meridian representation in these areas, to their vertical meridian representation, indicating the injection sequence involved a region of upper quadrant representation (Fig. 9A). The fact that this region directly abutted dorsal V2, rather than V3, was demonstrated by the location of label resulting from the most caudal injection (blue), which resided near the horizontal meridian representation in both dorsal and ventral V1 and V2. The presence of label in both upper and lower field V1 and V2 indicated that the injection site straddled the border between a region of lower field representation and one of upper field representation, while the location of this label at the horizontal meridian indicated that the border between these regions represents the horizontal meridian. This result is in agreement with the predictions of the multiple-areas models (Fig. 3E), but is inconsistent with the predictions of the V3-only model. According to the latter (Fig. 3B), had the caudal (blue) injection instead resided at the border between lower field V3 and upper field DM, the resulting label would be located at the vertical meridian representations (i.e., at the location indicated by the blue arrows in Fig. 9A). While injections straddling the border between dorsal V2 and V3 would also be expected to produce label at the horizontal meridian representations in both dorsal and ventral V1 and V2 [as previously argued by Lyon and Connolly (2012), and as demonstrated by Jeffs et al., (2009)], injections located progressively more rostral in third tier cortex (e.g., the yellow, red, and green injections in Fig. 9A) would be expected to produce label in the lower, rather than the upper field representations of V1 and V2. Instead, Jeffs et al. (2013) found that the more rostral injections formed connections with a topographical sequence of V1 and V2 sites in the upper quadrant representation of these areas (Fig. 9A). These data demonstrate unambiguously that the upper quadrant representation of area DM directly abuts dorsal V2 and shares with V2 the horizontal meridian representation, confirming the predictions of the multiple-areas models (Figs. 1B, 1E, and 3E), as well as the conclusions of the electrophysiological mapping results discussed above.
Fig. 9.
Anatomical evidence for an upper quadrant and two lower quadrant representations directly bordering dorsal V2. (A, C) Schematic representations of data from Jeffs et al. (2013) rendered on a diagram of unfolded and flattened marmoset V1, V2 and third tier cortex showing the location of injection sites and transported label (intra-areal label is omitted). Insets in (A, C): visual field maps of the location of injection sites (small circles outlined in black) and transported label in V1 (shaded colored regions). Other conventions are as in Fig. 3. (A) Evidence for an upper quadrant representation abutting dorsal V2 provided by an experimental paradigm in which a caudorostral series of 4 different tracer injections was made across the width of upper field DM. The actual data are illustrated in panel (B) for the region inside the red box in panel (A). (B) Original data reproduced from Fig. 5 of Jeffs et al. (2013), showing, for the blue and yellow injections in panel (A), the actual location of injection sites (encircled in black) and plots of resulting cell label (blue, CTB-alexa-647; yellow, CTB-alexa-555) over a CO-stained section of marmoset dorsal visual cortex. Solid and dashed white contours: vertical and horizontal meridian representations, respectively, at areal borders. Shaded gray area indicates a CO-transition zone at the V2 rostral border. Dashed black contours delineate the hypothetical borders of putative V3d assuming a 1- or 2-mm-wide V3d, respectively. The rostral border of this V3d is constrained by the location of the blue injection, as the topography of label resulting from it (see panel A) indicates the injection straddled a region of upper field representation. A 2-mm-wide V3d, would thus reduce the width of dorsal V2 to an improbable 1.3 mm (the latter varies between 2.5 and 4 mm across studies). The 1-mm-wide V3d, would lack a representation of the vertical meridian, as its rostral border (site of the blue injection site) represents the horizontal meridian (based on the topography of blue label in V1 and V2), and its caudal border with V2 is known to also represent the horizontal meridian. Black arrows point at the vertical meridian representations in V1 and MT, where blue label is expected to occur after an injection straddling the caudal border of upper field DM with V3d. Clearly, there is no blue label in these regions. White arrowhead points at the blue label located at the horizontal meridian representation of dorsal VLP. (C) Evidence for two regions of lower quadrant representation abutting dorsal V2 rostrally, and upper field DM medially and laterally. Numbers 1 and 2 indicate two mirror reversals of the injection site sequence rostral to V2.
The results illustrated in Fig. 9 cannot be accommodated by the V3-only model (Figs. 1A and 3B), but are potentially compatible with the incomplete-V3 model (Fig. 1C), if one assumes that an upper field representation is interposed between the islands of V3. Moreover, it is difficult to disprove the pinched-V3 model solely on the basis of the data in Fig. 9A, given that a very narrow strip of V3d may not easily be revealed by these studies (especially if this narrow V3 strip only represents visual field regions near the horizontal meridian). In this respect, it is important to mention that Jeffs et al. (2013) have reported that tracer injections straddling the caudal border of the upper field representation of DM sometimes produced a narrow strip of dense label interposed between dorsal V2 and the caudal DM border (red arrow in Fig. 9B). Lyon (2013) has interpreted this label as “exhibiting a V3d-like characteristic pattern of connections”. However, as previously discussed in Jeffs et al. (2013), this “V3-like” label pattern cannot be accounted for by the V3-only model, because there is no representation of the vertical meridian within this strip of cortex between dorsal V2 and DM. At best, the data shown in Fig. 9B (Figs. 3 and 5 of the original publication) could be seen as consistent with the existence of a very narrow (<1 mm) strip of cortex representing a portion of the visual field exclusively near the lower quadrant horizontal meridian, interposed between V2 and DM. Although it is possible that this region represents portions of a highly pinched V3d, the lack of a vertical meridian representation at this mediolateral level makes this region an unlikely area (as discussed in detail by Rosa et al., 2005). One alternative hypothesis is that this region may simply contain an expanded representation of the horizontal meridian.
In summary, the data by Jeffs et al. (2013) are consistent with the multiple-areas models, and cannot disprove the incomplete-V3 or the pinched-V3 model, but disprove the V3-only model. Additional studies in the next section, however, provide evidence that is difficult to reconcile with the organization of the dorsal third tier cortex proposed in the incomplete-V3 and pinched-V3 models.
Connectional evidence for two distinct visual areas bordering dorsal V2
To test the predictions of different models illustrated in Fig. 3C and 3F, Jeffs et al. (2013) made sequences of multiple closely spaced tracer injections across the full width of dorsal V2 and analyzed the resulting topography of labeled connections in extrastriate cortex rostral to V2 (Fig. 9C). This approach allowed them to constrain the location of areal boundaries in the third visual complex and other extrastriate regions, using topography as one objective criterion. Seven closely spaced injections of different tracers (only three are represented for clarity of illustration in Fig. 9C) were made across the full width of dorsal V2. The fact that the injections were confined to V2, and did not involve V3, was demonstrated by the topography of transported label in V1, which showed an orderly progression from the lower vertical meridian representation at the V1/V2 border, for the more caudal injection, to the horizontal meridian representation, for the more rostral injection, with no label reversal back toward the vertical meridian. This injection sequence produced two rows of neuronal label in the third tier cortex, each mirroring the injection site sequence, consistent with the existence of two lower quadrant representations abutting dorsal V2 (as predicted in Fig. 3F). There was no evidence for a label reversal posterior to these two reversals that may have indicated the existence of an area V3 bordering dorsal V2. These data are consistent with the multiple-areas models (Figs. 1B,1E, and 3F), but inconsistent with the V3-only (Figs. 1A and 3C), the incomplete-V3 (Fig. 1C) and pinched-V3 (Fig. 1D) models, unless one assumes a modular organization of dorsal V3.
To further determine whether the two islands of lower field representation bordering dorsal V2 rostrally are part of a single area V3 or of two different areas, Jeffs et al. (2015, in this special issue) made multiple closely spaced tracer injections in a mediolateral sequence along the dorsal third tier cortex. All injection sequences involved upper field DM, and either the region of lower quadrant representation just medial to it or both the region medial and the region lateral to it. Quantitative and laminar analyses of the labeled interareal connections resulting from these tracer injections suggested that the region medial to upper field DM is, in fact, the lower field representation of DM (as previously suggested by the electrophysiological mapping studies of Rosa and colleagues, see above), while the region lateral to upper field DM is part of a different area, likely VLP/V3. This is because the pattern of corticocortical connections arising from tracer injections in upper field DM and the region just medial to it was highly similar, albeit located in complementary quadrant representations of the same cortical areas, but they differed significantly from the corticocortical connection patterns arising from injections in the lower field region located just lateral to it.
The upper and lower field representations of DM, both received dominant V1 projections from layer 4B of V1, and from V2 neurons located preferentially in the dark cytochrome oxidase (CO) stripes, and were preferentially connected with areas of the dorsal visual stream, including MT, the dorso-anterior (DA) and dorso-intermediate (DI) areas (DA/DI), and the dorsal subdivisions of the posterior parietal cortex (Fig. 10A). Similar results were obtained in a previous study of DM connections in marmosets (Rosa et al., 2009), owl monkeys, and squirrel monkeys (Krubitzer & Kaas, 1993; Beck & Kaas, 1998a). Consistent with these anatomical results, functional studies have shown that DM in marmoset emphasizes peripheral vision, and neurons respond preferentially to large moving patterns and low spatial frequency stimuli; these functional properties suggest that DM may play a role in the analysis of optic flow patterns experienced during locomotion (Lui et al., 2006).
Fig. 10.
Major corticocortical connections of area DM and VLP. Connections of (A) DM and (B) VLP with areas of the dorsal (blue) and ventral (pink/purple) streams. Cortical areas are arranged in approximate hierarchical fashion. Color gradients indicate an area's contribution to both streams. Darkest colors indicate strongest connections with DM (in A) or VLP (in B), with lighter shades of color indicating progressively weaker connections. Line thickness also indicates the relative strength of connections. DM is more strongly connected with the dorsal stream (dark blue and lighter pink in A), whereas VLP is more strongly connected with the ventral stream (dark purple and lighter blue in B). Abbreviations: FST: fundus of the superior temporal sulcus area; IT: inferotemporal cortex; MTc: middle temporal crescent area; MST: medial superior temporal area; OPt: occipitoparietotemporal subfield of the ventral posterior parietal cortex (PPv); PPd: dorsal subdivision of the posterior parietal cortex; PPv: ventral subdivision of the posterior parietal cortex; VLA: ventrolateral anterior area. Tn, Tk, PM, PL: thin, thick, pale-medial, pale-lateral CO stripes, respectively, of V2. L4B and L2/3 denote layers 4B and 2/3, respectively, of V1. Reproduced from Jeffs et al. (2015) in this special issue.
In contrast, injections in dorsal cortex lateral to DM, i.e., in dorsal VLP/V3, revealed connections primarily from layer 2/3 of V1 and all CO stripe types of V2, as well as preferential connections with areas of the ventral processing stream, such as the New World primate homologue of area V4 (i.e., rostral DL – Figs. 7A and 8A – or the ventrolateral anterior area, VLA – Fig. 7B – of a different nomenclature), and the inferotemporal cortex (IT) (Fig. 10B). These patterns of dorsal VLP/V3 interareal connections are qualitatively similar to the connection patterns of macaque VP/V3v (Felleman et al., 1997) in terms of topological distribution, despite uncertainties about area homologies and different nomenclatures. These similarities are compatible with the proposal that dorsal VLP corresponds to the lower quadrant representation of ventral VLP/VP/V3v (Fig. 1E). Functional studies of VLP/V3 in marmosets have shown that this area emphasizes central vision, and that its neurons have small, orientation-selective, but direction-insensitive, receptive fields (Rosa & Tweedale, 2000). These properties are consistent with this area belonging to the ventral stream of visual processing, and with a similarly proposed role for area VP/V3v in macaque (Burkhalter & Van Essen, 1986) and area 19 in cat (Tanaka et al., 1987; Dinse & Kruger, 1990). Rosa and Manger (2005) have, indeed, proposed that VLP/V3 is the homologue of the originally defined area 19 of non primate species, and of human V3.
Overall the results presented above indicate that the dorsal third tier cortex contains two distinct areas, a full area DM, representing the upper and lower quadrants, and the lower visual quadrant representation of area VLP. These results are, therefore, consistent with the multiple-areas models (Fig. 1B and1E), but are inconsistent with the V3-only model (Fig. 1A), and less consistent with the incomplete-V3 (Fig. 1C) and pinched-V3 (Fig. 1E) models of third tier cortex organization, all of which view most of the dorsal third tier cortex as occupied by a single area V3.
Third tier areas in Old World primates: Solved and unresolved issues
How similar is the third tier visual cortex likely to be in different primate species?
In this section, we consider the currently available evidence for the organization of third tier areas in macaques and humans. Before we proceed, it is important to point out that we do not believe that all primates are necessarily identical in this respect. Indeed, variations in cortical organization have long been known to exist. The cerebral cortex does not scale isometrically with brain volume (Deacon, 1990; Orban et al., 2004; Hill et al., 2010; Preuss, 2011; Chaplin et al., 2013; Kaas, 2013; Amlien et al., 2015), and thus rearrangements in the number, size, and spatial layout of areas, and their interconnections, are expected (Changizi & Shimojo, 2005), even if they result from common developmental mechanisms, conserved in evolution (Rosa, 2002). Thus, our results in marmoset monkeys (one of the smallest primates), reviewed in previous sections of this paper, are likely to represent one end of a continuum of likely variations on a theme.
One relevant point in this discussion is the well-established fact that visual cortical areas scale disproportionally as a function of brain size (Hill et al., 2010; Chaplin et al., 2013). For example, area V1 occupies approximately 20% of the volume of the cerebral cortex in the marmoset brain, 10% in the macaque brain, and less than 5% in the human brain (Rosa & Tweedale, 2005), whereas the human homologue of V3 appears disproportionally large in humans, compared to macaques (Sereno et al., 1995; Schira et al., 2009). This observation has been related to the finding that late-developing brain structures tend to become disproportionally large in larger species (Finlay et al., 2001; Rosa & Tweedale, 2005; Hill et al., 2010). Indeed, the marmoset homologue of V3 (VLP/V3), which is primarily affiliated with the ventral stream of processing (Jeffs et al., 2015), undergoes postnatal maturation at a later stage in comparison with V1 and the entire set of dorsal stream areas (Bourne & Rosa, 2006). Therefore, the expectation of significant changes in the relative size of the two main components of the third tier cortex discussed in this review, areas DM and VLP/V3, and the areas they adjoin, need to be factored in. This in turn may have implications for the exact retinotopic configuration of specific areas, considering that the development of these maps appears to be constrained by a developmental mechanism that maximizes topographic continuity not only within, but also between areas (thus leading to congruent borders; Rosa, 2002).
The above considerations can be distilled into two key points, which should guide our analyses of homologies in the third tier visual cortex.
First, there is reasonable expectation that the third tier cortex will contain homologous areas across species of primates. Brain evolution tends to happen by adding new layers of processing to existing systems, but rarely, if ever, there has been evidence that “old” structures disappear (Allman, 1999; Striedter, 2006). In addition, preferential expansion and subdivision of the cortex into new areas occur primarily in regions corresponding to higher hierarchical levels of processing (Hill et al., 2010; Chaplin et al., 2013). Thus, there is a reasonable expectation that early processing stages, such as the third tier visual areas, will be conserved.
Second, there is also a reasonable expectation that one will find changes in the spatial configuration and internal details of the topographic map of homologous areas, in different species. In other words, “homologous” should not be interpreted as “identical”. Given this, ascertaining homologies between cortical areas in different species needs to be based on a range of criteria beyond location and visual topography, which include (but are not restricted to) anatomical connections, histological appearance, and, when applicable, highly distinctive functional properties (e.g., an overwhelming proportion of direction selective neurons was instrumental in establishing the homology of area MT across primates).
Multiple areas in the third tier cortex of Old World primates
It has been suggested that the evidence from imaging of retinotopic maps in monkeys and humans is overwhelmingly in favor of an elongated dorsal V3 directly adjoining V2 for most of its rostral border, in a manner that excludes area DM from the third tier visual cortex (Lyon, 2013). Although we believe that much still has to be learned about the dorsal cortex in Old World primates, a careful evaluation of the existing data in all studied simian primate species leads to the conclusion that there are at least two areas in the dorsal third tier cortex: a smaller area that is restricted to the dorsal component of the third visual complex, and a larger area that occupies the ventral component of this region and a substantial fraction of its dorsal component. We argue that the first corresponds to the New World monkey area DM, and Old World primate areas PO and V6 (according to different nomenclatures) (Fig. 2). Like DM, PO/V6 is characterized by heavy myelination, relative emphasis on peripheral vision, and strong projections from layer 4B of V1. The second area corresponds to New World monkey area VLP, and to Old World monkey V3; this is more lightly myelinated, has strong emphasis on central vision representation, and receives much weaker projections from V1 (Rosa & Tweedale, 2000; see also Jeffs et al., 2015 in this special issue). As argued below, this conclusion stands even in the face of different opinions on where to best place the boundaries of these areas.
As shown in Fig. 2, the retinotopic organization of the macaque dorsal extrastriate cortex revealed by the most comprehensive electrophysiological study so far (Gattass et al., 1988) can be reinterpreted in a manner that is compatible with the existence of DM, occupying parts of the annectant gyrus and parietooccipital sulcus, together with the adjacent precuneus (Rosa & Tweedale, 2001). According to this interpretation, the macaque homologue of DM not only overlaps extensively with area PO, but also encompasses a part of what is traditionally considered the most medial part of V3d. We have already pointed out above that these regions share the heavy myelination and projections from V1 layer 4B, and show some complementarity in the extent of their visual field representations (e.g., Colby et al., 1988; Felleman et al., 1997). Indeed, Galletti et al. (1999) have subsequently demonstrated that PO, as originally defined, is unlikely to correspond to the full extent of a visual area. In terms of visual topography and connections (Galletti et al., 2001), these authors have argued that PO is best described as part of area V6, which also has a central vision representation which is located more laterally, in the annectant gyrus.
Fig. 11 reproduces summary diagrams from a recent publication (Pitzalis et al., 2013), which depicts the extent of V6 in both macaques and humans, based on extensive comparative studies using neural recordings in macaques and functional imaging in humans (Fattori et al., 2009). Relative to the situation in macaques, human V6 is relatively small in comparison with the dorsal component of V3, and adjoins a smaller portion of the V2 border. As argued in the previous section, this is not unexpected, according to the “late equals large” principle of brain evolution (Finlay et al., 2001; Striedter, 2006): V3 develops relatively late in comparison with dorsal stream areas (Bourne & Rosa, 2006), and therefore should become relatively larger in larger (or longer-developing) brains. Importantly, the configuration of areas proposed for marmoset third tier cortex according to our studies reviewed above (Fig. 11, left) can be seen as a straightforward extension of this trend: area V3 (VLP) is relatively smaller, and thus does not reach as far as toward the midline as in the macaque or human brain.
Fig. 11.
Comparison of the relative size and extent of two third tier visual areas in dorsal cortex. Left: Extents of areas VLP/V3 and DM in the marmoset relative to the dorsal halves (lower quadrant representations) of V1 and V2, based on the study of Rosa and Tweedale (2000). Middle and right: Extents of areas V3 and V6 in the macaque and human, based on Pitzalis et al. (2013); note that the diagrams are represented in right hemisphere convention, to facilitate comparison. Putative homologous areas are indicated by similar colors.
In summary, we believe that there is no fundamental incompatibility in the experimental observations gathered in marmosets, owl monkeys, macaques, and humans, once the data are interpreted within an evolutionary-developmental context. Importantly, the data from all of these species can be brought together without the need to reinterpreting the results of experiments in New World monkeys in a manner that displaces area DM from the third tier cortex (Lyon & Kaas, 2001; Lyon & Connolly, 2012).
Once this is recognized, the outstanding discrepancies in the literature can be reduced to two specific issues: nomenclature, and the accurate placement of the border between DM (or V6/PO) and VLP/V3. With respect to the first point, even though the different names and definitions of cortical areas constitute a significant source of confusion (particularly to those being initiated in this field of research), at this point in time it appears sufficient to recognize that the homologies exist, and, when relevant, apply labels that recognize this (e.g., Paxinos et al., 2012).
Resolution of the second point hinges, to some extent, on data that would require new experiments combining high-resolution retinotopic mapping with anatomical tracing, quantitative histological analyses, and study of response properties (see below). For example, the pinched-V3 model (Fig. 1D) proposed by Gattass et al. (1988) has become the predominant description of the topographic organization of the macaque third tier dorsal cortex, and many of the experimental observations that formed the basis of this proposal have been subsequently replicated in experiments using neuroimaging techniques; this includes, in particular, the discontinuous nature of the representation of the vertical meridian at the rostral border of putative V3 (Arcaro et al., 2011). However, many (if not all) of the available mapping results are equally compatible with the incomplete-V3 model (Fig. 1C), and, indeed, the revised multiple-areas model (Figs. 2, 12, and 13). For example, in Fig. 12, we highlight (black dashed arrows) a region of dorsal cortex where the topographic pattern revealed by fMRI in two macaque monkeys (Arcaro et al., 2011) appears to be compatible with a direct transition from V2 to an upper visual field representation, similar to the electrophysiological results obtained in New World monkeys. While this representation has been traditionally attributed to area V3A, this is also the pattern expected from the existence of area DM, according to the multiple-areas models (Fig. 1B and 1E), and the reinterpretation of the electrophysiological mapping studies in the macaque shown in Fig. 2. Importantly, in Fig. 12, note that the representation of the vertical meridian at the rostral border of V3d is limited to the lateral portion of the dorsal third tier cortex, rather than being continuous along the rostral border of V3d. Likewise, in Fig. 13, we indicate how the most recent high-resolution fMRI data in the lateral extrastriate cortex of macaques (Kolster et al., 2014) can also be reinterpreted, in an equally parsimonious manner, in light of the revised multiple-areas model (compare with Fig. 2B). In this reinterpretation, the lower quadrant representation that complements ventral V3 (VP) projects rostrally, across the rostral bank of the lunate sulcus and prelunate gyrus (cyan borders), rather than following the V2 border (Fig. 13A), thus forming a representation like that proposed for the marmoset area VLP. In the macaque, as in the marmoset, this would result in a dorsal “wing” of VLP which maps increasing eccentricities in a smooth and predictable manner (Fig. 13B).
Fig. 13.
Macaque functional imaging data in lateral extrastriate cortex. Reanalysis of fMRI data obtained in the left hemisphere of a macaque monkey by Kolster et al. (2014; their Fig. 5), in a study focused on the organization of the cortex between areas V1 and MT. The color codes (top right in panels A and B) represent the relationship between hemodynamic activation and the polar angle (A) or eccentricity (B) of the visual stimuli. The locations of the visual field meridian representations that mark putative borders between visual areas, as originally assessed by the authors (based on the classical interpretation by Gattass et al. 1988) are indicated as follows: solid black lines – lower vertical meridian, dashed black lines – upper vertical meridian, white dotted lines – horizontal meridian. The stars indicate representations of the center of the fovea. In panel (A), we have indicated a reinterpretation of the same data, whereby a continuous representation of the lower vertical meridian arches rostrally across the prelunate gyrus (solid cyan line), and a continuous representation of the horizontal meridian arches rostrally across the rostral bank of the lunate sulcus and dorsal prelunate gyrus (dotted cyan line). When the data are reinterpreted in this way, a continuous area that resembles the proposed area VLP of marmoset monkeys becomes apparent (area shaded in lighter gray in panel C), which includes an ordered representation of eccentricity (B). The key point here is that the existing data on retinotopic organization, when taken in isolation, are equally compatible with multiple interpretations of the location of the lower quadrant representation that complements V3v/VP. Thus, resolution of this type of ambiguity must necessarily rely on the use of additional criteria, such as anatomical connections, cyto-and myeloarchitecture, or functional response properties. It is unclear why no representations of the upper quadrant are apparent in the dorsal region (which would be expected given earlier electrophysiological studies, see for example Figs. 2 and 12); this could be due to exclusion of more medial regions of the lunate/intraparietal transition from the regions that could be imaged at high resolution (given the location of imaging coils for the purpose of the experiment).
Finally, it is useful to indicate that, even though the evidence in humans supports the notion that V3 shares most of the rostral border of dorsal V2, the possibility of a DM-like configuration in the medial part of the third tier complex cannot be ruled out. For example, in Fig. 14, we reproduce data from a recent study (Sereno et al., 2013) which combined fMRI-based retinotopic mapping with MRI signals related to density of myelination (quantitative T1 maps). This figure suggests a representation of the upper quadrant immediately adjacent to the horizontal meridian representation that forms the rostral border of V2. This upper quadrant representation coincides with a patch of high myelination (delineated by the white dotted line), and if combined with the currently recognized human V6 (Fig. 11), would form a DM-like representation that is very similar to that proposed for the marmoset monkey, including a split representation of the upper quadrant.
Fig. 14.
Relationship between retinotopy and densely myelinated zones in the human brain. Inflated representations of the left and right hemispheres of the human brain, seen from a dorsocaudal viewpoint, reproduced from Figure 3 of Sereno et al. (2013). The representation of polar angle in the cortex is depicted according to the color key. The white dotted lines indicate highly myelinated regions, estimated from the longitudinal relaxation rate (R1) in the MRI signal. In both hemispheres, the expected lower vertical meridian representation in dorsal V3 (green shading, and white circles) only reaches part way toward dorsomedial extrastriate cortex. Closer to the midline, one finds representations of the upper quadrant immediately rostral to the representation of the horizontal meridian that marks the border of V2 (blue shading, and black dashed line). This region is also characterized by dense myelination. These diagrams represent the average of six individuals, inflated to a common brain template.
Our point here is not to propose that these re-analyses of published materials demonstrate an identical organization across marmoset, macaque, and human; for reasons explained above, we do not believe that this is likely, even with homologous areas. Rather, we hope to convince the reader that much more needs to be learned about the macaque and human brains, before we can fully assess the extent to which they differ from New World monkeys with respect to the organization of areas in the dorsal third tier cortex. If anything, Figs 12–14 demonstrate the point that the same data on visuotopic organization can be compatible with multiple models, and that a parsimonious resolution of the issue of boundaries will likely depend on the application of additional (functional and anatomical) criteria.
Remaining issues and future studies
Based on the evidence discussed above, we conclude that there are currently no published data that are inconsistent with the multiple-areas models of third tier cortex organization. In contrast, there are many examples of electrophysiological and anatomical data, especially in New World primates, that cannot be explained by the V3-only model.
The incomplete-V3 and pinched-V3 models are more difficult to reject based on the available data. Evidence that the two cortical territories in dorsal third tier cortex located medial and lateral to the upper quadrant representation of DM belong to two distinct cortical areas is difficult to reconcile with either model. However, the variable configuration of V3d across cases that is advocated by these models, and the fragmentary organization of this putative area may require more extensive physiological and connectional data in a large number of animals than currently available. Such studies are especially lacking in macaque, although a careful analysis of published neuroimaging data provides tantalizing glimpses of an upper quadrant representation that could belong to area DM (Fig. 12). Unfortunately, given the limited spatial resolution of noninvasive imaging techniques, such as functional (f)MRI, and the inability of performing optical imaging in deep brain area, the only feasible approach to investigate the organization of the third tier cortex in macaque may require laborious microelectrode receptive field characterization combined with tracer injections at various mediolateral levels of the macaque third tier cortex. At a minimum, the use of high-resolution fMRI techniques needs to be combined with an experimental design that includes explicit hypotheses based on different models, and confirmation using electrophysiological recordings as a gold standard.
On the other hand, the more extensive studies available in New World primates, especially marmosets, do not support the notion of large individual variability in the organization of the third visual complex in these species.
A second remaining question regards the location of the peripheral upper quadrant representation of area DM, in particular to test the proposal that the upper field of this area has a discontinuous representation (Fig. 6). This would require more extensive characterization of the response properties of neurons in this medial region of the dorsal third tier cortex to determine whether they share similar properties with neurons located more centrally in upper field DM. Recordings in awake-behaving marmosets (Mitchell et al., 2014) would be particularly useful in providing another criterion for comparison with the putative homologue of DM in the macaque, area V6, for which response properties have been thoroughly characterized, including a specific population of “real position” cells, which are rarely observed in other visual areas (Galletti et al., 1995).
A third issue that needs further studies regards the location of the peripheral lower quadrant representation of area VLP/V3. The proposal that this extends rostromedially in dorsal cortex (Fig. 1E) requires additional connectional data from peripheral VLP, and characterization of neuronal response properties throughout the proposed extent of this area. Large field optical imaging combined with tangential electrode array recordings (e.g., Utah arrays) could be used to map receptive field locations and response properties across large expanses of this cortical region in New World primates. As indicated in Fig. 2, early work in awake-behaving macaques (Maguire & Baizer, 1984) has provided evidence that is compatible with a similar organization of this area in the macaque, but the proposal of two subdivisions within V4 (the most caudal of which would correspond to dorsal VLP/V3) has not gained much traction since (note however that this proposal is compatible with the reanalysis shown in Fig. 13). Further testing of this hypothesis would be fruitful, using combinations of functional imaging techniques, single-unit recordings, and tracer injections for the purpose of comparing the posteromedial and anteromedial subregions of the prelunate gyrus.
Finally, the existence and significance of a narrow strip of V3/VLP between dorsal V2 and DM in both New and Old World primates remain to be addressed. Our observations of what could be interpreted as evidence for this strip in some marmosets (Fig. 9B) indicate that, if present, it only represents the horizontal meridian of the visual field. In the pinched-V3 model, this region bridges two islands of V3d. However, we know that this is not the case in marmosets, as connectional data indicate the existence of two distinct areas in the dorsal third tier cortex (Jeffs et al., 2015); therefore, in marmosets it is unclear whether this strip region shares the properties of area DM, VLP/V3 or simply represents an expanded representation of the V2 horizontal meridian. Similar experiments are needed in macaque to compare the connections and response properties of the third tier cortical regions lateral and medial to the putative connecting bridge, to confirm or dispute the finding in marmoset that these regions are part of two distinct visual areas.
Determining the areal identity of this cortical strip is a very difficult task, given this can be so narrow (<1 mm) and contains only neurons that overlap with the horizontal meridian. Although it is difficult to imagine an experiment that could yield conclusive evidence in favor of such a narrow bridge or strip of V3 cortex, in theory it would be possible to use high-resolution two-photon imaging, or high-density electrode array recordings at various locations along the rostral border of V2 in a lissencephalic New World primate to seek such evidence. Specifically, one would expect a distinctive set of similar functional properties in islands of third tier cortex lateral and medial to the upper quadrant representation of area DM (which are distinct from those found in DM itself, according to the macaque pinched-V3 model), combined with evidence that this set of response properties is the same along a narrow strip that separates V2 from DM. The difficulty in obtaining such evidence highlights, in our view, the main weakness of the pinched-V3 model: it fitted the expectation that a continuous area V3 exists, based on the history of investigation of the third tier visual cortex in macaques, at the time when the results were reported; however, it was not based on strong experimental evidence that disproved competing models. Instead, as discussed elsewhere (Rosa et al., 2005), we regard this model, and more in general any idea of a pinched V3 (Lyon, 2013), as not parsimonious in light of other lines of evidence. Nevertheless, based on the present review and that by Kaas and colleagues in this same special issue, it is clear that, while the simple V3-only model is dead, current data cannot refute two of the competing models, the “pinched-V3” and the “multiple-areas” model.
As a general recommendation for future studies, an effort needs to be made to include formal assessment of the margin of error involved in the different types of measurements that can be used to define a specific border, using different methods (architectural analysis, single-unit recordings, neuroimaging or transitions in connectional patterns). The habit of depicting borders of areas as lines may mask important aspects of the uncertainty involved in such assignments, which become relevant when understanding the origin of controversies such as the ones we highlight in this review.
We would like to conclude by pointing out that, ultimately, resolving the controversy regarding the organization of the third tier cortex requires a rigorous model-testing approach: experiments must be designed with the goal of disproving a model, by testing its predictions, and converting these into hypotheses that can be conclusively tested.
Acknowledgments
Alessandra Angelucci's work reported in this review was supported by the US National Science Foundation (grant IOS-1355075 to A.A.), the US National Institute of Health (grants R21 EY022757 and R01 EY019743 to A.A.), and a grant from Research to Prevent Blindness, Inc., to the Department of Ophthalmology, University of Utah. Marcello Rosa's work reported in this review was supported by the National Health and Medical Research Council (grants 237009, 237012, 384115, 1003906, 1020839) and Australian Research Council (DP110101200, DP140101968).
References
- Albus K. & Beckmann R. (1980). Second and third visual areas of the cat: Interindividual variability in retinotopic arrangement and cortical location. The Journal of Physiology 299, 247–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allman J.M. (1999). Evolving Brains. NY: W.H. Freeman. [Google Scholar]
- Allman J.M. & Kaas J.H. (1975). The dorsomedial cortical visual area: A third tier area in the occipital lobe of the owl monkey (Aotus trivirgatus). Brain Research 100, 473–487. [DOI] [PubMed] [Google Scholar]
- Allman J.M. & Kaas J.H. (1976). Representation of the visual field on the medial wall of occipital-parietal cortex in the owl monkey. Science 191, 572–575. [DOI] [PubMed] [Google Scholar]
- Amlien I.K., Fjell A.M., Tamnes C.K., Grydeland H., Krogsrud S.K., Chaplin T.A., Rosa M.G. & Walhovd K.B. (2015). Organizing principles of human cortical development-thickness and area from 4 to 30 years: Insights from comparative primate neuroanatomy. Cerebral Cortex (in press). [DOI] [PubMed] [Google Scholar]
- Angelucci A., Levitt J.B., Walton E.J., Hupe J.M., Bullier J. & Lund J.S. (2002). Circuits for local and global signal integration in primary visual cortex. The Journal of Neuroscience 22, 8633–8646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcaro M.J., Pinsk M.A., Li X. & Kastner S. (2011). Visuotopic organization of macaque posterior parietal cortex: A functional magnetic resonance imaging study. The Journal of Neuroscience 31, 2064–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J.F., Petersen S.E., Newsome W.T. & Allman J.M. (1981). Visual response properties of neurons in four extrastriate visual areas of the owl monkey (Aotus trivirgatus): A quantitative comparison of medial, dorsomedial, dorsolateral, and middle temporal areas. Journal of Neurophysiology 45, 397–416. [DOI] [PubMed] [Google Scholar]
- Beck P.D. & Kaas J.H. (1998a). Cortical connections of the dorsomedial visual area in new world owl monkeys (Aotus trivirgatus) and squirrel monkeys (Saimiri sciureus). The Journal of Comparative Neurology 400, 18–34. [DOI] [PubMed] [Google Scholar]
- Beck P.D. & Kaas J.H. (1998b). Cortical connections of the dorsomedial visual area in prosimian primates. The Journal of Comparative Neurology 398, 162–178. [PubMed] [Google Scholar]
- Beck P.D. & Kaas J.H. (1999). Cortical connections of the dorsomedial visual area in old world macaque monkeys. The Journal of Comparative Neurology 406, 487–502. [PubMed] [Google Scholar]
- Bourne J.A. & Rosa M.G. (2006). Hierarchical development of the primate visual cortex, as revealed by neurofilament immunoreactivity: Early maturation of the middle temporal area (MT). Cerebral Cortex 16, 405–414. [DOI] [PubMed] [Google Scholar]
- Boussaoud D., Desimone R. & Ungerleider L.G. (1991). Visual topography of area TEO in the macaque. The Journal of Comparative Neurology 306, 554–575. [DOI] [PubMed] [Google Scholar]
- Brodmann K. (1994). Brodmann's Localisation in the Cerebral Cortex. London, UK: Smith-Gordon. [Google Scholar]
- Burkhalter A., Felleman D.J., Newsome W.T. & Van Essen D.C. (1986). Anatomical and physiological asymmetries related to visual areas V3 and VP in macaque extrastriate cortex. Vision Research 26, 63–80. [DOI] [PubMed] [Google Scholar]
- Burkhalter A. & Van Essen D.C. (1986). Processing of color, form and disparity information in visual areas VP and V2 of ventral extrastriate cortex in the macaque monkey. The Journal of Neuroscience 6, 2327–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changizi M.A. & Shimojo S. (2005). Parcellation and area-area connectivity as a function of neocortex size. Brain, Behavior and Evolution 66, 88–98. [DOI] [PubMed] [Google Scholar]
- Chaplin T.A., Yu H.H., Soares J.G., Gattass R. & Rosa M.G. (2013). A conserved pattern of differential expansion of cortical areas in simian primates. The Journal of Neuroscience 33, 15120–15125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby C.L., Gattass R., Olson C.R. & Gross C.G. (1988). Topographical organization of cortical afferents to extrastriate visual area PO in the macaque: A dual tracer study. The Journal of Comparative Neurology 269, 392–413. [DOI] [PubMed] [Google Scholar]
- Cragg B.G. (1969). The topography of the afferent projections in the circumstriate visual cortex of the monkey studied by the Nauta method. Vision Research 9, 733–747. [DOI] [PubMed] [Google Scholar]
- Cusick C.G., Gould H.J. & Kaas J.H. (1984). Interhemispheric connections of visual cortex of owl monkeys (Aotus trivirgatus), marmosets (Callithrix jacchus), and galagos (Galago crassicaudatus). The Journal of Comparative Neurology 230, 311–336. [DOI] [PubMed] [Google Scholar]
- Deacon T.W. (1990). Problems of ontogeny and phylogeny in brain-size evolution. International Journal of Primatology 11, 237–282. [Google Scholar]
- Dinse H.R. & Kruger K. (1990). Contribution of area 19 to the foreground-background-interaction of the cat: An analysis based on single cell recordings and behavioural experiments. Experimental Brain Research 82, 107–122. [DOI] [PubMed] [Google Scholar]
- Fan R.H., Baldwin M.K., Jermakowicz W.J., Casagrande V.A., Kaas J.H. & Roe A.W. (2012). Intrinsic signal optical imaging evidence for dorsal V3 in the prosimian galago (Otolemur garnettii). The Journal of Comparative Neurology 520, 4254–4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattori P., Pitzalis S. & Galletti C. (2009). The cortical visual area V6 in macaque and human brains. Journal of physiology, Paris 103, 88–97. [DOI] [PubMed] [Google Scholar]
- Felleman D.J., Burkhalter A. & Van Essen D.C. (1997). Cortical connections of areas V3 and VP of macaque monkey extrastriate visual cortex. The Journal of Comparative Neurology 379, 21–47. [DOI] [PubMed] [Google Scholar]
- Felleman D.J. & Van Essen D.C. (1987). Receptive field properties of neurons in area V3 of macaque monkey extrastriate cortex. Journal of Neurophysiology 57, 889–920. [DOI] [PubMed] [Google Scholar]
- Finlay B.L., Darlington R.B. & Nicastro N. (2001). Developmental structure in brain evolution. The Behavioral and Brain Sciences 24, 263–278; discussion 278–308. [PubMed] [Google Scholar]
- Fritsches K.A. & Rosa M.G. (1996). Visuotopic organisation of striate cortex in the marmoset monkey (Callithrix jacchus). The Journal of Comparative Neurology 372, 264–282. [DOI] [PubMed] [Google Scholar]
- Galletti C., Battaglini P.P. & Fattori P. (1995). Eye position influence on the parieto-occipital area PO (V6) of the macaque monkey. The European Journal of Neuroscience 7, 2486–2501. [DOI] [PubMed] [Google Scholar]
- Galletti C., Fattori P., Gamberini M. & Kutz D.F. (1999). The cortical visual area V6: Brain location and visual topography. The European Journal of Neuroscience 11, 3922–3936. [DOI] [PubMed] [Google Scholar]
- Galletti C., Gamberini M., Kutz D.F., Fattori P., Luppino G. & Matelli M. (2001). The cortical connections of area V6: An occipito-parietal network processing visual information. The European Journal of Neuroscience 13, 1572–1588. [DOI] [PubMed] [Google Scholar]
- Gattass R., Sousa A.P. & Gross C.G. (1988). Visuotopic organization and extent of V3 and V4 of the macaque. The Journal of Neuroscience 8, 1831–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattass R., Sousa A.P., Mishkin M. & Ungerleider L.G. (1997). Cortical projections of area V2 in the macaque. Cerebral Cortex 7, 110–129. [DOI] [PubMed] [Google Scholar]
- Harrison R.V., Harel N., Panesar J. & Mount R.J. (2002). Blood capillary distribution correlates with hemodynamic-based functional imaging in cerebral cortex. Cerebral Cortex 12, 225–233. [DOI] [PubMed] [Google Scholar]
- Hassler R. (1996). Comparative anatomy of the central visual system in day- and night-active primates In Evolution of the Forebrain, ed. Hassler R. & Stephan H., pp. 419–434. Stuttgart: Thieme. [Google Scholar]
- Hill J., Inder T., Neil J., Dierker D., Harwell J. & Van Essen D. (2010). Similar patterns of cortical expansion during human development and evolution. Proceedings of the National Academy of Sciences of the United States of America 107, 13135–13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D.H. & Wiesel T.N. (1965). Receptive fields and functional architecture in two nonstriate visual areas (18 and 19) of the cat. Journal of Neurophysiology 28, 229–289. [DOI] [PubMed] [Google Scholar]
- Jeffs J., Federer F. & Angelucci A. (2015). Corticocortical connection patterns reveal two distinct visual cortical areas bordering dorsal V2 in marmoset monkey. Visual Neuroscience (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffs J., Federer F., Ichida J.M. & Angelucci A. (2013). High-resolution mapping mapping of anatomical connections in marmoset extrastriate cortex reveals a complete representation of the visual field bordering dorsal V2. Cerebral Cortex 23, 1126–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffs J., Ichida J.M., Federer F. & Angelucci A. (2009). Anatomical evidence for classical and extra-classical receptive field completion across the discontinuous horizontal meridian representation of primate area V2. Cerebral Cortex 19, 963–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas J.H. (1996). Theories of visual cortex organization in primates: Areas of the third level. Progress in Brain Research 112, 213–221. [DOI] [PubMed] [Google Scholar]
- Kaas J.H. (2013). The evolution of brains from early mammals to humans. Wiley Interdisciplinary Reviews. Cognitive Science 4, 33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas J.H. & Morel A. (1993). Connections of visual areas of the upper temporal lobe of owl monkeys: The MT crescent and dorsal and ventral subdivisions of FST. The Journal of Neuroscience 13, 534–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolster H., Janssens T., Orban G.A. & Vanduffel W. (2014). The retinotopic organization of macaque occipitotemporal cortex anterior to V4 and caudoventral to the middle temporal (MT) cluster. The Journal of Neuroscience 34, 10168–10191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krubitzer L.A. & Kaas J.H. (1990). Cortical connections of MT in four species of primates: Areal, modular, and retinotopic patterns. Visual Neuroscience 5, 165–204. [DOI] [PubMed] [Google Scholar]
- Krubitzer L.A. & Kaas J.H. (1993). The dorsomedial visual area of owl monkeys: Connections, myeloarchitecture, and homologies in other primates. The Journal of Comparative Neurology 334, 497–528. [DOI] [PubMed] [Google Scholar]
- Lewis J.W. & Van Essen D.C. (2000). Mapping of architectonic subdivisions in the macaque monkey, with emphasis on parieto-occipital cortex. The Journal of Comparative Neurology 428, 79–111. [DOI] [PubMed] [Google Scholar]
- Lin C.S., Weller R.E. & Kaas J.H. (1982). Cortical connections of striate cortex in the owl monkey. The Journal of Comparative Neurology 211, 165–176. [DOI] [PubMed] [Google Scholar]
- Lui L.L., Bourne J.A. & Rosa M.G. (2006). Functional response properties of neurons in the dorsomedial visual area of New World monkeys (Callithrix jacchus). Cerebral Cortex 16, 162–177. [DOI] [PubMed] [Google Scholar]
- Lyon D.C. (2013). The case for a dorsal V3 in the "third-tier" of primate visual cortex: A reply to "the case for a dorsomedial area in the primate "third-tier" visual cortex". Proceedings of the Royal Society of London. Series B, Biological Sciences 280, 20121994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon D.C. & Connolly J.D. (2012). The case for primate V3. Proceedings. Biological Sciences / The Royal Society 279, 625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon D.C. & Kaas J.H. (2001). Connectional and architectonic evidence for dorsal and ventral V3, and dorsomedial area in marmoset monkeys. The Journal of Neuroscience 21, 249–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon D.C. & Kaas J.H. (2002a). Evidence for a modified V3 with dorsal and ventral halves in macaque monkeys. Neuron 33, 453–461. [DOI] [PubMed] [Google Scholar]
- Lyon D.C. & Kaas J.H. (2002b). Evidence from V1 connections for both dorsal and ventral subdivisions of V3 in three species of New World monkeys. The Journal of Comparative Neurology 449, 281–297. [DOI] [PubMed] [Google Scholar]
- Lyon D.C., Xu X., Casagrande V.A., Stefansic J.D., Shima D. & Kaas J.H. (2002). Optical imaging reveals retinotopic organization of dorsal V3 in New World owl monkeys. Proceedings of the National Academy of Sciences of the United States of America 99, 15735–15742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire W.M. & Baizer J.S. (1984). Visuotopic organization of the prelunate gyrus in rhesus monkey. The Journal of Neuroscience 4, 1690–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J.F. & Leopold D.A. (2015). The marmoset monkey as a model for visual neuroscience. Neuroscience research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J.F., Reynolds J.H. & Miller C.T. (2014). Active vision in marmosets: A model system for visual neuroscience. The Journal of Neuroscience 34, 1183–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H., Le W.R., Wakita M., Mikami A. & Itoh K. (2004). Projections from the cytochrome oxidase modules of visual area V2 to the ventral posterior area in the macaque. Experimental Brain Research 155, 102–110. [DOI] [PubMed] [Google Scholar]
- Neuenschwander S., Gattass R., Sousa A.P. & Pinon M.C. (1994). Identification and visuotopic organization of areas PO and POd in Cebus monkey. The Journal of Comparative Neurology 340, 65–86. [DOI] [PubMed] [Google Scholar]
- Newsome W.T. & Allman J.M. (1980). Interhemispheric connections of visual cortex in the owl monkey, Aotus trivirgatus, and the bushbaby, Galago senegalensis. The Journal of Comparative Neurology 194, 209–233. [DOI] [PubMed] [Google Scholar]
- Newsome W.T., Maunsell J.H. & Van Essen D.C. (1986). Ventral posterior visual area of the macaque: Visual topography and areal boundaries. The Journal of Comparative Neurology 252, 139–153. [DOI] [PubMed] [Google Scholar]
- Orban G.A., Van Essen D. & Vanduffel W. (2004). Comparative mapping of higher visual areas in monkeys and humans. Trends in Cognitive Sciences 8, 315–324. [DOI] [PubMed] [Google Scholar]
- Paxinos G., Watson C., Petrides M., Rosa M.G.P. & Tokuno H. (2012). The Marmoset Brain in Stereotaxic Coordinates. San Diego: Academic. [Google Scholar]
- Pitzalis S., Sereno M.I., Committeri G., Fattori P., Galati G., Tosoni A. & Galletti C. (2013). The human homologue of macaque area V6A. Neuroimage 82, 517–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss T.M. (2011). The human brain: Rewired and running hot. Annals of the New York Academy of Sciences 1225, 182–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa M. (2002). Visual maps in the adult primate cerebral cortex: Some implications for brain development and evolution. Brazilian Journal of Medical and Biological Research 35, 1485–1498. [DOI] [PubMed] [Google Scholar]
- Rosa M., Palmer S.M., Gamberini M., Burman K.J., Yu H., Reser D.H., Bourne J.A., Tweedale R. & Galletti C. (2009). Connections of the dorsomedial visual area: Pathways for early integration of dorsal and ventral streams in extrastriate cortex. The Journal of Neuroscience 29, 4548–4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa M.G., Casagrande V.A., Preuss T. & Kaas J.H. (1997a). Visual field representation in striate and prestriate cortices of a prosimian primate (Galago garnetti). Journal of Neurophysiology 77, 3193–3217. [DOI] [PubMed] [Google Scholar]
- Rosa M.G., Fritsches K.A. & Elston G.N. (1997b). The second visual area in the marmoset monkey: Visuotopic organisation, magnification factors, architectonical boundaries, and modularity. The Journal of Comparative Neurology 387, 547–567. [DOI] [PubMed] [Google Scholar]
- Rosa M.G. & Manger P.R. (2005). Clarifying homologies in the mammalian cerebral cortex: The case of the third visual area (V3). Clinical and Experimental Pharmacology & Physiology 32, 327–339. [DOI] [PubMed] [Google Scholar]
- Rosa M.G., Palmer S.M., Gamberini M., Tweedale R., Pinon M.C. & Bourne J.A. (2005). Resolving the organization of the new world monkey third visual complex: The dorsal extrastriate cortex of the marmoset (Callithrix jacchus). The Journal of Comparative Neurology 483, 164–191. [DOI] [PubMed] [Google Scholar]
- Rosa M.G., Pinon M.C., Gattas R. & Sousa A.P. (2000). "Third tier" ventral extrastriate cortex in the New World monkey, Cebus apella. Experimental Brain Research 132, 287–305. [DOI] [PubMed] [Google Scholar]
- Rosa M.G. & Schmid L.M. (1995). Visual areas in the dorsal and medial extrastriate cortices of the marmoset. The Journal of Comparative Neurology 359, 272–299. [DOI] [PubMed] [Google Scholar]
- Rosa M.G., Soares J.G., Fiorani M. Jr. & Gattass R. (1993). Cortical afferents of visual area MT in the Cebus monkey: Possible homologies between New and Old World monkeys. Visual Neuroscience 10, 827–855. [DOI] [PubMed] [Google Scholar]
- Rosa M.G. & Tweedale R. (2000). Visual areas in lateral and ventral extrastriate cortices of the marmoset monkey. The Journal of Comparative Neurology 422, 621–651. [DOI] [PubMed] [Google Scholar]
- Rosa M.G. & Tweedale R. (2001). The dorsomedial visual areas in new world and old world monkeys: Homology and function. The European Journal of Neuroscience 13, 421–427. [DOI] [PubMed] [Google Scholar]
- Rosa M.G. & Tweedale R. (2005). Brain maps, great and small: Lessons from comparative studies of primate visual cortical organization. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 360, 665–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa M.G.P., Angelucci A., Jeffs J. & Pettigrew J.D. (2013). The case for a dorsomedial area (DM) in the primate third tier visual cortex. Proceedings of the Royal Society B: Biological Sciences 280, 20121372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schira M.M., Tyler C.W., Breakspear M. & Spehar B. (2009). The foveal confluence in human visual cortex. The Journal of Neuroscience 29, 9050–9058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleicher A., Amunts K., Geyer S., Morosan P. & Zilles K. (1999). Observer-independent method for microstructural parcellation of cerebral cortex: A quantitative approach to cytoarchitectonics. Neuroimage 9, 165–177. [DOI] [PubMed] [Google Scholar]
- Sereno M.I. & Allman J.M. (1991). Cortical visual areas in mammals In Vision and Visual Dysfunction, ed. Leventhal A.G., pp. 160–172. London: Maacmillian. [Google Scholar]
- Sereno M.I., Dale A.M., Reppas J.B., Kwong K.K., Belliveau J.W., Brady T.J., Rosen B.R. & Tootell R.B. (1995). Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science 268, 889–893. [DOI] [PubMed] [Google Scholar]
- Sereno M.I., Lutti A., Weiskopf N. & Dick F. (2013). Mapping the human cortical surface by combining quantitative T(1) with retinotopy. Cerebral Cortex 23, 2261–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereno M.I., McDonald C.T. & Allman J.M. (2015). Retinotopic organization of extrastriate cortex in the owl monkey: Dorsal and lateral areas. Visual Neuroscience (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon S.G. & Rosa M.G. (2014). A simpler primate brain: The visual system of the marmoset monkey. Front Neural Circuits 8, 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa A.P., Pinon M.C., Gattass R. & Rosa M.G. (1991). Topographic organization of cortical input to striate cortex in the Cebus monkey: A fluorescent tracer study. The Journal of Comparative Neurology 308, 665–682. [DOI] [PubMed] [Google Scholar]
- Spitzer M.W., Calford M.B., Clarey J.C., Pettigrew J.D. & Roe A.W. (2001). Spontaneous and stimulus-evoked intrinsic optical signals in primary auditory cortex of the cat. Journal of Neurophysiology 85, 1283–1298. [DOI] [PubMed] [Google Scholar]
- Stepniewska I. & Kaas J.H. (1996). Topographic patterns of V2 cortical connections in macaque monkeys. The Journal of Comparative Neurology 371, 129–152. [DOI] [PubMed] [Google Scholar]
- Striedter G.F. (2006). Précis of principles of brain evolution. The Behavioral and Brain Sciences 29, 1–12; discussion 12–36. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Ohzawa I., Ramoa A.S. & Freeman R.D. (1987). Receptive field properties of cells in area 19 of the cat. Experimental Brain Research 65, 549–558. [DOI] [PubMed] [Google Scholar]
- Tusa R.J., Rosenquist A.C. & Palmer L.A. (1979). Retinotopic organization of areas 18 and 19 in the cat. The Journal of Comparative Neurology 185, 657–678. [DOI] [PubMed] [Google Scholar]
- Ungerleider L.G. & Desimone R. (1986). Cortical connections of visual area MT in the macaque. The Journal of Comparative Neurology 248, 190–222. [DOI] [PubMed] [Google Scholar]
- Ungerleider L.G. & Mishkin M. (1982). Two cortical visual systems In Advances in the Analysis of Visual Behavior, ed. Ingle D.J., Mansfield J.W. & Goodale M.A., pp. 549–586. Cambridge: MIT Press. [Google Scholar]
- Van Essen D.C., Newsome W.T. & Bixby J.L. (1982). The pattern of interhemispheric connections and its relationship to extrastriate visual areas in the macaque monkey. The Journal of Neuroscience 2, 265–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen D.C., Newsome W.T., Maunsell J.H. & Bixby J.L. (1986). The projections from striate cortex (V1) to areas V2 and V3 in the macaque monkey: Asymmetries, areal boundaries, and patchy connections. The Journal of Comparative Neurology 244, 451–480. [DOI] [PubMed] [Google Scholar]
- Van Essen D.C. & Zeki S.M. (1978). The topographic organization of rhesus monkey prestriate cortex. The Journal of Physiology 277, 193–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vogt Weisenhorn D.M., Illing R.B. & Spatz W.B. (1995). Morphology and connections of neurons in area 17 projecting to the extrastriate areas MT and 19DM and to the superior colliculus in the monkey Callithrix jacchus. The Journal of Comparative Neurology 362, 233–255. [DOI] [PubMed] [Google Scholar]
- Weller R.E. & Kaas J.H. (1983). Retinotopic patterns of connections of area 17 with visual areas V-II and MT in macaque monkeys. The Journal of Comparative Neurology 220, 253–279. [DOI] [PubMed] [Google Scholar]
- Weller R.E., Steele G.E. & Cusick C.G. (1991). Cortical connections of dorsal cortex rostral to V II in squirrel monkeys. The Journal of Comparative Neurology 306, 521–537. [DOI] [PubMed] [Google Scholar]
- Wilder H.D., Grunert U., Lee B.B. & Martin P.R. (1996). Topography of ganglion cells and photoreceptors in the retina of a new world monkey: The marmoset Callithrix jacchus. Visual Neuroscience 13, 335–352. [DOI] [PubMed] [Google Scholar]
- Yu H.H., Chaplin T.A., Egan G.W., Reser D.H., Worthy K.H. & Rosa M.G. (2013). Visually evoked responses in extrastriate area MT after lesions of striate cortex in early life. The Journal of Neuroscience 33, 12479–12489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H.H. & Rosa M.G. (2010). A simple method for creating wide-field visual stimulus for electrophysiology: Mapping and analyzing receptive fields using a hemispheric display. Journal of Vision 10, 15. [DOI] [PubMed] [Google Scholar]
- Zeki S.M. (1969). Representation of central visual fields in prestriate cortex of monkey. Brain Research 14, 271–291. [DOI] [PubMed] [Google Scholar]
- Zeki S.M. (1971). Cortical projections from two prestriate areas in the monkey. Brain Research 34, 19–35. [DOI] [PubMed] [Google Scholar]
- Zeki S.M. (1977). Simultaneous anatomical demonstration of the representation of the vertical and horizontal meridians in areas V2 and V3 of rhesus monkey visual cortex. Proceedings of the Royal Society of London. Series B, Biological Sciences 195, 517–523. [DOI] [PubMed] [Google Scholar]
- Zeki S.M. (1978a). The third visual complex of rhesus monkey prestriate cortex. The Journal of Physiology 277, 245–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki S.M. (1978b). Uniformity and diversity of structure and function in rhesus monkey prestriate visual cortex. The Journal of Physiology 277, 273–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki S.M. & Sandeman D.R. (1976). Combined anatomical and electrophysiological studies on the boundary between the second and third visual areas of rhesus monkey cortex. Proceedings of the Royal Society of London. Series B, Biological Sciences 194, 555–562. [DOI] [PubMed] [Google Scholar]