Abstract
Background
The aim of this study was to evaluate the clinical efficacy of modified posterior vertebral column resection (MPVCR) in treating osteoporotic Kummell disease.
Material/Methods
Between January 2013 and January 2015, 10 patients who were diagnosed with Kummell disease underwent MPVCR treatment, and their medical records were retrospectively collected. Every patient had follow-up for at least one year, with an average of 15 months. Clinical efficacy of MPVCR treatment was evaluated by kyphotic Cobb’s angle, Oswestry disability index (ODI) and visual analogue scale (VAS) score.
Results
Data analyses showed that operation time was 188.39±30.8 minutes, and blood loss was 860±130 mL with 600±200 mL of blood transfusions. VAS score decreased significantly after MPVCR surgery (p<0.001, Mann-Whitney U test). In addition, data analyses showed that postoperative ODI was less than preoperative ODI, which was a statistically significant difference (p<0.001, Mann-Whitney U test). X-ray radiograph showed that kyphotic Cobb’s angle was 45°±12° preoperatively, 10°±4° two weeks after surgery, and 15°±6° at last follow-up, indicating that Cobb’s angle after MPVCR surgery was significantly improved, compared to the preoperative scores (p<0.05, SNK-q test).
Conclusions
MPVCR surgery was an effective and safe surgical method to treat Kummell disease, especially for patients with kyphotic deformity and obvious nerve-oppressed symptoms. However, the long-term clinical effect still needs further studies.
MeSH Keywords: Follow-Up Studies; Osteoporotic Fractures; Spine; Surgical Procedures, Operative
Background
Kummell disease was first reported in 1895 by Kummell. Since then, Kummell disease has been studied in-depth with the development of medical image technology [1–4]. Kummell disease involves vertebral collapse and is a delayed complication of osteoporotic compression fractures. It is not common and most often encountered in patients with severe osteoporosis that have taken long-term courses of corticosteroids or sustained a spinal injury [5]. In particular, spine fractures are associated with poor outcomes, and they also significantly increase the risk of disability, morbidity, and mortality [6].
With severe symptoms, Kummell disease often needs surgical intervention, since general conservative treatment is usually less effective. Percutaneous vertebroplasty (PVP) or kyphoplasty (PKP) is often used in the treatment of these patients due to the desire for minimally invasion procedures, and it can achieve the purpose of stable vertebral body and pain relief, but has a higher bone cement leakage rate [7–10]. However, those surgical interventions were not applicable for Kummell disease accompanied with symptoms caused by spinal cord compression.
In this study, we used a modified posterior vertebral column resection (MPVCR) [11] to treatment 10 patients diagnosed with Kummell disease accompanied with spinal cord compression; satisfactory treatment effect was achieved in all patients.
Material and Methods
Ethics statement
This retrospective study was approved by the Ethics Committee of the Third Hospital of Hebei Medical University. The approval number is K2016-011-01.
Patients
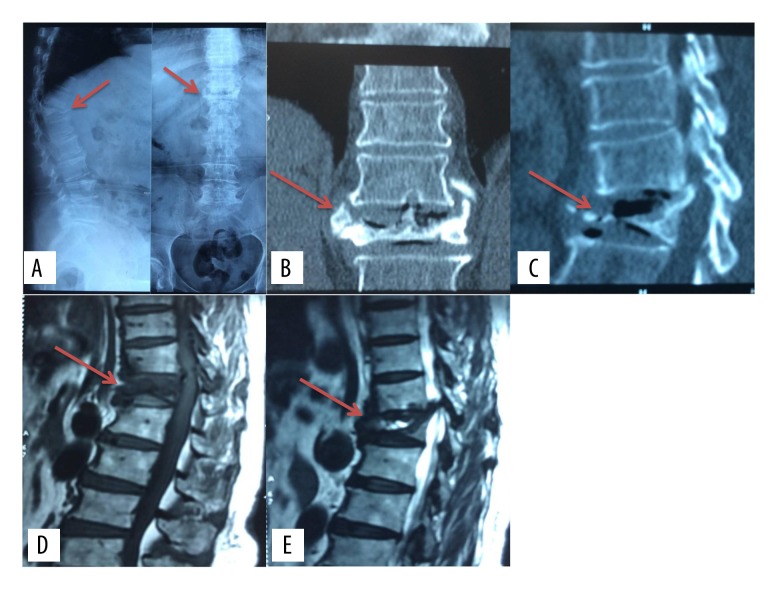
Between January 2013 and January 2015, 10 patients with Kummell disease underwent MPVCR treatment, and their medical records were retrospectively collected. In all, there were two male patients and 8 female patients; ages ranged from 58 to 79 years old (69.9 years on average). All patients had severe backache; four patients had concurrent other symptoms resulting from spinal cord or nerve root compression. Two patients had a history of minor trauma; the rest of the patients did not. Back pain lasted for from three months to two years, with an average of 4.8 months. For all cases, there was no effect from a stay in bed, and internal medicine medications did not relieve pain. There were no tumors and inflammation in the vertebral body lesions. Preoperatively, all patients were checked by x-ray images, CT scan, and MRI. As shown in Figure 1, pathologic vertebral bodies were obviously compressed on x-ray film, and an x-ray transparent area occurred in the compressed vertebral body, like a “cleft change”. CT scan showed “cleft change” again in fractured vertebral bodies. Low signals in T1-weighted MRI image were found, and high signals both in T2-weighted and fat-suppressed sequence images were found. All patients suffered from single-segment fracture, including one patient with a fracture in T8, two patients with a fracture in T11, three patients with a fracture in T12, three patients with a fracture in L1, and one patient with a fracture in L2. All patients were diagnosed with osteoporotic Kummell disease confirmed by bone mineral density (BMD) examination with T-value less than −2.5.
Figure 1.
Pathologic vertebral bodies are obviously compressed in x-ray film, and an x-ray transparent area appears in the compressed vertebral body, which seems like an intra-vertebral vacuum cleft, as marked by the arrows.
Surgical procedures
MPVCR is often used as a standard surgical strategy [11] and was used in this study. Our patients received general anesthesia in a prone position. After sterilization and draping, a longitudinal incision was performed in the center of operating zone. With the compressed vertebrae as the center segment, the incision ranged from two upper segments to two lower vertebral segments. Then a total of eight pedicle screws were implanted into vertebrae bodies with four on each side. Spinous process, lamina process, and articular process were removed. A temporary fixed rod was installed on one side. Then the transverse process of the fractured vertebral body on the opposite side was cut off. Decompression was conducted along the side, leading to heavier symptoms for patients with spinal cord or nerve compression symptoms. Subperiosteal dissection of pedicle and vertebra wall was performed to the front of vertebral body, but reserving anterior longitudinal ligaments. The compressed fractured vertebral body was the target vertebrae in which an osteotomy was performed. Briefly, the majority of cancellous bone and two adjacent intervertebral discs were all resected via the pedicle using a homemade trephine, osteotome, ball mill drill, and rongeur. At the same time, a thin layer of sclerotin in the posterior of the target vertebrae was reserved in order to protect the dural sac. It should be noted that it is important to protect the nerve root on the bone-cutting side during the intraoperative period.
The temporarily fixed rod was then replaced with a permanent orthopedic rod in accordance with sagittal physiological radian. Opening the bone-cutting clearance appropriately, the titanium mesh with autologous bone particles was placed into the front of bone-cutting clearance. A large amount of physiological saline was flushed into the clearance, and autologous bone particles were implanted into the clearance. Then a permanent orthopedic rod was fixed in the other side with appropriate compression given to the bone-cutting clearance (the fold of the dural sac should be avoided). Thorough hemostasis was achieved with the help of bipolar electrocoagulation. Negative pressure drainage was applied and the suturing was layer by layer. Postoperative management was as follows. The volume of drainage was recorded daily and the drainage tube was pulled out when the volume of drainage was less than 50 mL in 24 hours. The patients could start to walk, wearing a rigid lumbar orthopedic instrument after two weeks, which contended up to three months. At the same time, postoperative anti-osteoporotic therapy was regularly conducted.
Herein, it must be noted that appropriate use of titanium mesh intraoperatively can complete the reconstruction of the anterior and middle column, avoiding excessive shortening of the spinal cord. Surgeons should try to avoid damage to the segmental blood vessels so as not to affect the blood supply to the spinal cord. It should not be routine to lower blood pressure intraoperatively in order to avoid spinal cord ischemia. Surgeons should perform the operation carefully, and may selectively resect thoracic segmental nerve roots to avoid them becoming obstructed in the process of implantation of the titanium mesh. But the lumbar nerve roots should be carefully protected without excessive traction.
Effectiveness evaluation
In order to evaluate treatment effectiveness of MPVCR in Kummell disease patients, the operation time, intraoperative blood loss, kyphotic Cobb’s angle, Oswestry disability index (ODI), and visual analogue scale (VAS) score were analyzed in this study. Preoperatively, two weeks after surgery, and at the last follow-up, the measurement of ODI and the VAS score were used to evaluate changes. In addition, thoracolumbar x-ray films were taken from a standing position to measure Cobb’s angle for assessment of kyphosis. Also, complications due to internal fixation, bone graft fusion, and heterotopic ossification were evaluated in this study.
Statistical analyses
SPSS18.0 statistical software (SPSS Inc., USA) was used for data analyses in this study. All data were presented as mean ±SD (standard deviation) or median (IQR, interquartile range) depending on the normality test of the data. Regarding comparisons of ODI and VAS score, Mann-Whitney U tests were applied. Cobb’s angle preoperatively and that at follow-ups after operation were compared using repeated measurement ANOVA (analysis of variance), with SNK-q test used for pairwise comparison. A p-value less than 0.05 was considered statistically significant.
Results
Data analyses show that operation time was 188.39±30.8 minutes, and blood loss was 860±130 mL with 600±200 mL of blood transfusions. All patients had follow-up for at least 12 months, with an average of 15 months. VAS score was 5 points (IQR=4) preoperatively; 3 points (IQR=1) two weeks after surgery; and 2 points (IQR=0) at last follow-up. Obviously, VAS score decreased significantly after MPVCR surgery (p<0.001, Mann-Whitney U test).
Data analyses showed that ODI was 57.6% (IQR=30.2%) preoperatively, 15.8% (IQR=15%) at two weeks after surgery, and 10.4% (IQR=5%) at last follow-up. There was a statistically significant difference comparing preoperative ODI with postoperative ODI (p<0.001, Mann-Whitney U test).
X-ray radiographs showed that kyphotic Cobb’s angle was 45°±12° preoperatively, 10°±4° at two weeks after surgery, and 15°±6° at last follow-up. Data analyses indicated that Cobb’s angle after MPVCR surgery was significantly improved, compared to preoperative (p<0.05, SNK-q test), as shown in Figure 2.
Figure 2.
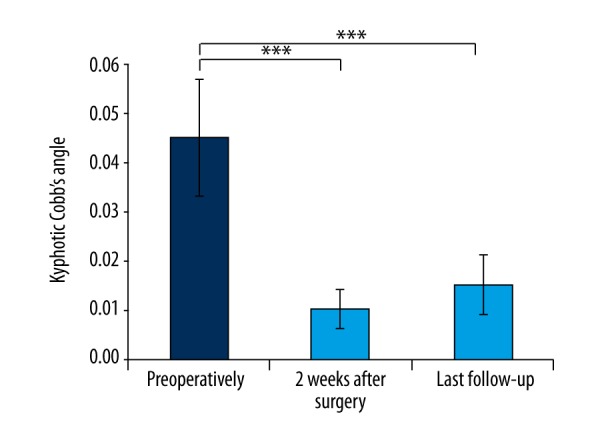
The changes of kyphotic Cobb’s angle. *** p<0.05.
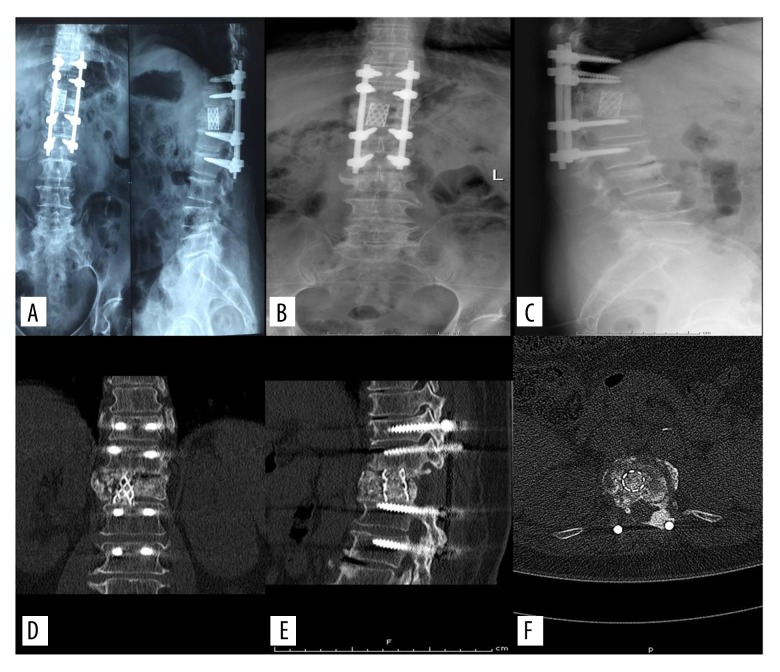
As shown in Figure 3, the last follow-up review revealed that the bony particles in titanium mesh were well fused with adjacent vertebral bodies and bone trabecula had formed. During the period of patient follow-up, no false joints were found and no complications occurred related to internal fixation.
Figure 3.
The last follow-up review reveals that the bony particles in the titanium mesh have well fused with adjacent vertebral bodies and bone trabecula has formed.
Discussion
Diagnosis of Kummell disease
Usually, Kummell disease occurs in the thoracolumbar spine. Patients often have a history of minor trauma, or even a no trauma history. After a few weeks or months of an asymptomatic period, activity-related back pain gradually develops [12]. Deformity of kyphosis may be caused after continuous progression. The development of Kummell disease mainly has three stages. First, spinal vertebral bodies are slightly injured by minor trauma, sometimes even without any trauma. Second, vertebral bodies experience dynamic instability in the cleft area and then a fracture and bone collapse occurs. At the last stage, compressed fractured vertebral body develops to compress the posterior spinal cord, leading to continuous back pain and other neural symptoms [13].
Intrabony cleft is the most important characteristics used to diagnose Kummell disease. The unenhanced area presented in contrast enhancement MRI is the shape of the intrabony cleft [14]. The reason leading to the signal could be that gas is released from the subchondral cleft after a vertebral fracture and then a “gas phenomenon” forms. Then the intrabony cleft is filled with liquid, to further form the characteristics seen in the MRI image [15].
Kummell disease is different from common old osteoporotic vertebral compression fractures. Old osteoporotic vertebral compression fractures, if already healed, only present wedge-shaped or double-concave change, with no signal changes like edema of vertebral body observed in MRI images. If unhealed, it usually shows disseminated signal changes in MRI imaging, but no observed “intrabony cleft” like changes on x-ray or CT-scanned images. However, Kummell disease always presents a signal-changed area with clear boundaries, and “intrabony cleft” like changes appear on x-ray, CT-scan, and MRI.
Treatment of Kummell disease
Usually, Kummell disease undergoes progressive development. The effect of conservative treatment tends to be limited to bed rest, narcotic analgesics, and braces fixation, due to intervertebral instability caused by false joints. With a better understanding of Kummell disease, less invasive percutaneous vertebroplasty (PVP) and percutaneous kyphoplasty (PKP) are often applied as regular surgical procedures to treat these patients. PVP has an important effect on bone nonunion caused by thoracolumbar osteoporotic fracture, especially in terms of pain relief and kyphotic correction, but this treatment has been reported to have higher risk of bone cement leakage [16]. Peh et al. [7] reported that bone cement leakage using PVP to treat Kummell disease was up to 79%, which was believed to be closely related to vertebral rupture. Applying PVP to treat bone nonunion caused by vertebral compression fracture, Ha et al. [8] reported a rate of 75% bone cement leakage, while Jung et al. [9] reported 55.5%. According to the leakage sites, leakage to intervertebral disc was 65%, vertebral peripheral veins was 25%, epidural was 5%, and neuroforamen was 5%.
PKP has been proven to have a better effect on kyphosis correction and prevention of bone cement leakage [17–19] because more viscous bone cement can be injected into the vertebral body using PKP after the balloon expands the cleft, thereby blocking the export to the vertebral body wall. In a previous study, Wang et al. [10] reported only 25% bone cement leakage related to PKP use. In addition, Yang et al. [20] reported that modified PKP was used to treat bone nonunion caused by thoracolumbar osteoporotic fracture in 21 cases. As a result, back pain was relieved, loss of vertebral height recovered, and kyphosis was also corrected.
For Kummell disease, vertebral compression is often above 70%, and most cases have anterior vertebral wall ruptures. Thus, bone cement leakage is more likely to occur when PVP or PKP is performed as a surgical procedure. So, how to prevent leakage of bone cement from the ruptured gap of anterior vertebral wall and how to improve operation safety, is a problem that clinicians must face and try to solve. Li et al. [21] reported that short-segmental fixation had a good effect on 21 patients diagnosed with Kummell disease with spinal cord compression. Nevertheless, intervertebral instability may still exist, and fixation may fail in the long run. Ito et al. [13] adopted the anterior-posterior approach surgery to treat Kummell disease, and achieved good clinical efficacy. However, with this approach, operative duration is long and the surgical procedures are complicated. Therefore, some elderly patients cannot go through this surgery, which is also often relatively contraindicated. Moreover, traditional anterior surgery has an impact on the function of the heart and lung, increasing surgical risk.
The merits of MPVCR surgery are as follows. First, operative duration is shortened because the unilateral bone-cutting method is used to resect most fractured vertebral bodies and adjacent intervertebral discs, and reserves the contralateral vertebral pedicle and a part of vertebral body. Second, MPVCR can reduce the disturbance to the spinal cord, effectively avoiding complications resulting from injury of the spinal cord and nerves. Third, reserving the contralateral vertebral pedicle and a part of vertebral body may contribute to the stability of the spinal column and increase the bone fusion rate. And last, it can avoid some complications caused by anterior surgical procedures using a single posterior approach to complete the reconstruction of the anterior column.
Techniques in MPVCR
Intraoperative bleeding occurs mainly during the process of removing the ventral and dorsal wall of the vertebral canal, because there is rich venous plexus therein. It can be difficult to stop bleeding once the rupture of venous plexus happens. Therefore, the venous plexus should be blocked using a bipolar electrocoagulation. But once the rupture of venous plexus occurs and if bleeding cannot be easily stopped by bipolar electrocoagulation, a gelatin sponge should be used for hemostasis via a pressing method with the help of thrombin powder. However, control of the bone-cutting duration is the key to reducing blood loss. Because patients with Kummell disease suffer from different degrees of osteoporosis, it would be better to select relatively long and thick pedicle screws, in order to increase the ability of resistance to pulling out from the vertebral bodies. For reoperation due to loose screw or screw breakage, implantation of autologous cancellous bone into the screw path in advance is required. Generally, it is not recommended to use bone cement for reinforcement of the screw path [22]. It is essential to implant two couples of pedicle screws respectively in the two upper vertebras and two lower vertebras next to the resected vertebrae.
How to avoid damage to the spinal cord and nerves is an important matter that should be paid attention to in any operation of bone resection. Gertzbein et al. [23] found that posterior deformity correction by bone resection could result in shortening, circuity, and accumulation of the spinal cord if the correction was beyond 40 degrees. And it would be safest when the shortening of spinal cord was less than two centimeters.
Conclusions
In summary, MPVCR surgery is an effective and safe surgical method to treat Kummell disease, especially for patients with kyphotic deformity and obvious nerve-oppressed symptoms. However, the long-term clinical effect still needs further studies.
Footnotes
Source of support: Departmental sources
Conflict of interest
None of the authors of this paper have any financial or personal relationships with other people or organizations that could inappropriately influence or bias the content of the paper. The authors indicate no potential conflicts of interest.
References
- 1.Stäbler A, Schneider P, Link TM, et al. Intravertebral vacuum phenomenon following fractures: CT study on frequency and etiology. J Comput Assist Tomogr. 1999;23(6):976–80. doi: 10.1097/00004728-199911000-00030. [DOI] [PubMed] [Google Scholar]
- 2.Chen GD, Lu Q, Wang GL, et al. Percutaneous kyphoplasty for Kummell disease with severe spinal canal stenosis. Pain Physician. 2015;18(6):E1021–28. [PubMed] [Google Scholar]
- 3.Huang Y, Peng M, He S, et al. Clinical efficacy of percutaneous kyphoplasty at the hyperextension position for the treatment of osteoporotic Kümmell disease. Clin Spine Surg. 2016;29(4):161–66. doi: 10.1097/BSD.0000000000000259. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X, Hu W, Yu J, et al. An effective treatment option for Kümmell disease with neurological deficits: Modified transpedicular subtraction and disc osteotomy combined with long-segment fixation. Spine (Phila Pa 1976) 2016;41(15):E923–30. doi: 10.1097/BRS.0000000000001467. [DOI] [PubMed] [Google Scholar]
- 5.Kim P, Kim SW. Balloon kyphoplasty: An effective treatment for Kummell disease. Korean J Spine. 2016;13(3):102–6. doi: 10.14245/kjs.2016.13.3.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruan J, Gong X, Kong J, et al. Effect of B vitamin (folate, B6, and B12) supplementation on osteoporotic fracture and bone turnover markers: A meta-analysis. Med Sci Monit. 2015;21:875–81. doi: 10.12659/MSM.893310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peh WC, Gelbart MS, Gilula LA, Peck DD. Percutaneous vertebroplasty: Treatment of painful vertebral compression fractures with intraosseous vacuum phenomena. Am J Roentgenol. 2003;180(5):1411–17. doi: 10.2214/ajr.180.5.1801411. [DOI] [PubMed] [Google Scholar]
- 8.Ha KY, Lee JS, Kim KW, Chon JS. Percutaneous vertebroplasty for vertebral compression fractures with and without intravertebral clefts. J Bone Joint Surg Br. 2006;88(5):629–33. doi: 10.1302/0301-620X.88B5.17345. [DOI] [PubMed] [Google Scholar]
- 9.Jung JY, Lee MH, Ahn JM. Leakage of polymethylmethacrylate in percutaneous vertebroplasty: Comparison of osteoporotic vertebral compression fractures with and without an intravertebral vacuum cleft. J Comput Assist Tomogr. 2006;30(3):501–6. doi: 10.1097/00004728-200605000-00025. [DOI] [PubMed] [Google Scholar]
- 10.Wang GL, Yang HL, Meng B. Kyphoplasty for osteoporotic Kümmell’s disease. Chinese Journal of Spine and Spinal Cord. 2011;21(1):46–49. [Google Scholar]
- 11.Wang H, Zhang D, Sun YP, et al. Unilateral posterior vertebral column resection for severe thoracolumbar kyphotic deformity caused by old compressive vertebrae fracture: A technical improvement. Int J Clin Exp Med. 2015;8(3):3579–84. [PMC free article] [PubMed] [Google Scholar]
- 12.Kim DY, Lee SH, Jang JS, et al. Intravertebral vacuum phenomenon in osteoporotic compression fracture: Report of 67 cases with quantitative evaluation of intravertebral instability. J Neurosurg. 2004;100(1 Suppl. Spine):24–31. doi: 10.3171/spi.2004.100.1.0024. [DOI] [PubMed] [Google Scholar]
- 13.Ito Y, Hasegawa Y, Toda K, Nakahara S. Pathogenesis and diagnosis of delayed vertebral collapse resulting from osteoporotic spinal fracture. Spine J. 2002;2(2):101–6. doi: 10.1016/s1529-9430(01)00165-6. [DOI] [PubMed] [Google Scholar]
- 14.Oka M, Matsusako M, Kobayashi N, et al. Intravertebral cleft sign on fat-suppressed contrast-enhanced MR: Correlation with cement distribution pattern on percutaneous vertebroplasty. Acad Radiol. 2005;12(8):992–99. doi: 10.1016/j.acra.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Baur A, Stäbler A, Arbogast S, et al. Acute osteoporotic and neoplastic vertebral compression fractures: fluid sign at MR imaging. Radiology. 2002;225(3):730–35. doi: 10.1148/radiol.2253011413. [DOI] [PubMed] [Google Scholar]
- 16.Grohs JG, Matzner M, Trieb K, Krepler P. Treatment of intravertebral pseudarthroses by balloon kyphoplasty. J Spinal Disord Tech. 2006;19(8):560–65. doi: 10.1097/01.bsd.0000211232.91340.6b. [DOI] [PubMed] [Google Scholar]
- 17.Chen L, Yang H, Tang T. Unilateral versus bilateral balloon kyphoplasty for multilevel osteoporotic vertebral compression fractures: A prospective study. Spine (Phila Pa 1976) 2011;36(7):534–40. doi: 10.1097/BRS.0b013e3181f99d70. [DOI] [PubMed] [Google Scholar]
- 18.Zhang HT, Sun ZY, Zhu XY, et al. Kyphoplasty for the treatment of very severe osteoporotic vertebral compression fracture. J Int Med Res. 2012;40(6):2394–400. doi: 10.1177/030006051204000638. [DOI] [PubMed] [Google Scholar]
- 19.Shen M, Wang H, Chen G, et al. Factors affecting kyphotic angle reduction in osteoporotic vertebral compression fractures with kyphoplasty. Orthopedics. 2013;36(4):e509–14. doi: 10.3928/01477447-20130327-31. [DOI] [PubMed] [Google Scholar]
- 20.Yang H, Gan M, Zou J, et al. Kyphoplasty for the treatment of Kümmell’s disease. Orthopedics. 2010;33(7):479. doi: 10.3928/01477447-20100526-07. [DOI] [PubMed] [Google Scholar]
- 21.Li KC, Li AF, Hsieh CH, et al. Another option to treat Kümmell’s disease with cord compression. Eur Spine J. 2007;16(9):1479–87. doi: 10.1007/s00586-006-0094-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu GH, Cui ZM, Li WD. [Posterior osteotomy way for thoracolumbar kyphosis due to delayed osteoporotic vertebral fracture in elderly]. Zhongguo Gu Shang. 2015;28(8):749–53. [in Chinese] [PubMed] [Google Scholar]
- 23.Gertzbein SD, Harris MB. Wedge osteotomy for the correction of post-traumatic kyphosis. A new technique and a report of three cases. Spine (Phila Pa 1976) 1992;17(3):374–79. doi: 10.1097/00007632-199203000-00025. [DOI] [PubMed] [Google Scholar]