Abstract
Aims
Ventricular arrhythmias (VA) originating from a papillary muscle (PM) have recently been described as a distinct clinical entity with peculiar features that make its treatment with catheter ablation challenging. Here, we report our experience using an intracardiac echo-facilitated 3D electroanatomical mapping approach in a case series of patients undergoing ablation for PM VA.
Methods and results
Sixteen patients who underwent catheter ablation for ventricular tachycardia (VT) or symptomatic premature ventricular contractions originating from left ventricular PMs were included in the study. A total of 24 procedures (mean 1.5 per patient) were performed: 15 using a retrograde aortic approach and 9 using a transseptal approach. Integrated intracardiac ultrasound for 3D electroanatomical mapping was used in 15 of the 24 procedures. The posteromedial PM was the most frequent culprit for the clinical arrhythmia, and the body was the part of the PM most likely to be the successful site for ablation. The site of ablation was identified based on the best pace map matching the clinical arrhythmia and the site of earliest the activation. At a mean follow-up of 10.5 ± 7 months, only two patients had recurrent arrhythmias following a repeat ablation procedure.
Conclusion
An echo-facilitated 3D electroanatomical mapping allows for real-time creation of precise geometries of cardiac chambers and endocavitary structures. This is useful during procedures such as catheter ablation of VAs originating from PMs, which require detailed representation of anatomical landmarks. Routine adoption of this technique should be considered to improve outcomes of PM VA ablation.
Keywords: Papillary muscles, Ventricular tachycardia, Premature ventricular contraction, Catheter ablation, 3D electroanatomical mapping, Intracardiac ultrasound
What's new?
This is the first report describing an echo-facilitated 3D electroanatomical mapping for papillary muscle premature ventricular contraction (PVC)/ventricular tachycardia (VT) ablation. Insights into the utility of this approach to overcome the challenges of targeting arrhythmias originating from these complex anatomical structures are discussed in detail.
Outcomes of papillary muscle PVC/VT ablation are presented in a case series of patients with mixed ischaemic and not ischaemic cardiomyopathies and preserved or low ejection fraction.
The study also aims to increase awareness about the spatial geometry of anatomical structures present within ventricular chambers, which should be considered during PVC/VT ablation.
Introduction
Left ventricular (LV) papillary muscles (PMs) are increasingly recognized as potential sites of origin for ventricular arrhythmias (VAs) in patients with or without structural heart disease.1–4 In patients with ischaemic heart disease, scarring inside the PMs can cause a mismatch of source sink activation and circuitous propagation of electrical impulse, thus favouring the onset of re-entry.1 Conversely, in patients with a normal heart, the mechanism of VAs appears to be focal, due to either triggered activity or enhanced automaticity.2
Doppalapudi et al.2 described the electrocardiographic and electrophysiological characteristics of VAs originating from the posteromedial PM as a distinct clinical entity; they identified four major challenges during ablation: (i) a limited efficacy of pace mapping; (ii) repeated changes in the morphology of the arrhythmias; (iii) difficulty in obtaining good contact between the catheter tip and the PM; and (iv) frequent need for ablation on both sides of the PM in order to terminate the arrhythmia.2
The above-mentioned issues are related to the anatomical complexities of the PMs that represent myocardial growths inside the LV cavity, consisting of myocardial cells and an abundant network of specialized conductive tissue.5 Recently, the term ‘fourth dimension’ has been proposed to describe the endocavitary structures such as the moderator band, false tendons, and PMs.6 These structures are all potential arrhythmogenic foci and are difficult to target during ablation due to their complex 3D geometry.6
In the past few years, ultrasound imaging has been introduced in cardiac electrophysiology as a tool that allows reproduction of the anatomy in real time during catheter navigation through the cardiac chambers.7 Notably, the ultrasound imaging can be integrated with electroanatomical mapping and has been used in catheter ablation of atrial fibrillation and scar-related ventricular tachycardia (VT).8 The use of an intracardiac echo-facilitated 3D mapping system may offer a considerable advantage for the ablation of arrhythmia foci localized to the PMs although this has been scantily reported in the literature.9–11
The aim of this report is to describe our experience using an integrated electroanatomical and intracardiac echocardiographic approach in a case series of patients undergoing ablation for VAs arising from the left anterolateral and posteromedial PMs.
Methods
Patients undergoing VT or premature ventricular contraction (PVC) ablation originating from LV PMs at the Centre Hospitalier Universitaire de Sherbrooke and the McGill University Health Centre between April 2011 and January 2015 were retrospectively included in the study. The same operators performed the procedures at both study sites.
A detailed medical history was collected at baseline and careful analysis of the electrocardiogram was performed before the procedure.
Three-dimensional intracardiac echocardiography (ICE) imaging was performed when available, using the SOUNDSTAR® Catheter with the CARTOSOUND® Module in the CARTO® 3 System (Biosense-Webster, Diamond Bar, CA, USA). Reasons for not using 3D ICE in some of the procedures included ablations preformed prior to acquisition of the CARTOSOUND® Module at respective institutions, unavailability of onsite technician to run the CARTOSOUND® Module on the day of the procedure, unavailability of the SOUNDSTAR® Catheter on the day of the procedure, and choice of the operator not to use 3D ICE.
When a PM origin of the arrhythmia was confirmed, demographics and procedural data were collected and patients underwent regular follow-up visits. To use the collected data, patients' information was de-identified.
Electrophysiological study
All patients underwent electrophysiological study and catheter ablation. A standard quadripolar catheter was placed at the right ventricular (RV) apex through the femoral vein. A 3.5-mm externally irrigated ablation catheter was advanced into the LV using a retrograde aortic (Ao) or transseptal (TS) approach depending on the preference of the operator. When a TS approach was chosen, a steerable sheath was used (Agilis Large Curl, St Jude Medical). Isoprotenerol infusion and/or ventricular burst pacing were used if necessary for PVC or VT induction. Unfractionated IV heparin was administered to maintain an activated clotting time of 300 or higher.
Intracardiac echocardiography
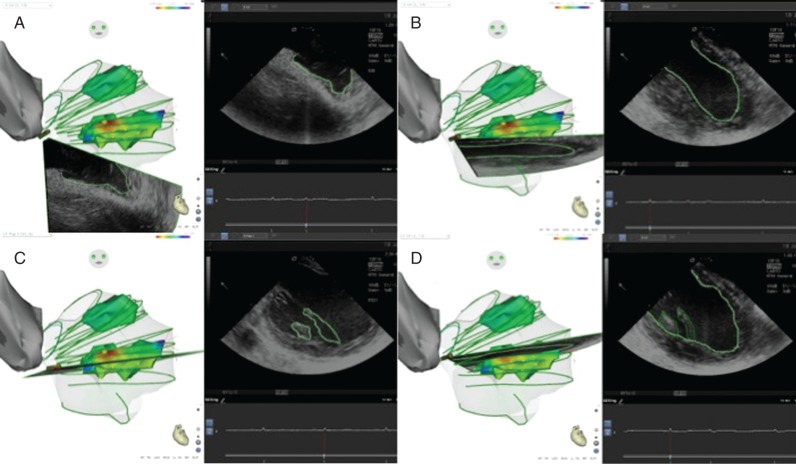
An ICE catheter (SOUNDSTAR® Catheter, Biosense-Webster) was advanced through the femoral vein towards the RV, where 2D clips of the LV were obtained in different planes. The SOUNDSTAR® ICE mapping catheter was initially positioned towards the RV outflow tract and was then rotated clockwise to define the LV anatomy. The following structures were identified and outlined while performing clockwise rotation: posteromedial and anterolateral PMs, LV outflow tract, RV outflow tract, aortic valve, left main and right coronary arteries, pulmonic valve, and finally the pulmonary artery (Figure 1).
Figure 1.
(A) ICE 3D catheter orientated towards the LV apex. (B) Clockwise rotation of the ICE 3D catheter showing an LV long axis. (C) Posteromedial papillary muscle body and chordae. (D) Posteromedial papillary muscle base. Contours are drawn in the ICE 2D clip and then transpolated to the 3D geometry.
Using the CARTO® 3 electroanatomical mapping system (Biosense-Webster), anatomical structures were visualized in each 2D clip, including the LV endocardium, and delineated to create a detailed 3D geometry of the LV. After the geometry was obtained, activation maps were created and projected over the involved PMs (Figures 2 and 3). For the procedures performed without the ICE SOUNDSTAR® Catheter, an approximate geometry of the target PM was created with CARTO and an activation map was performed.
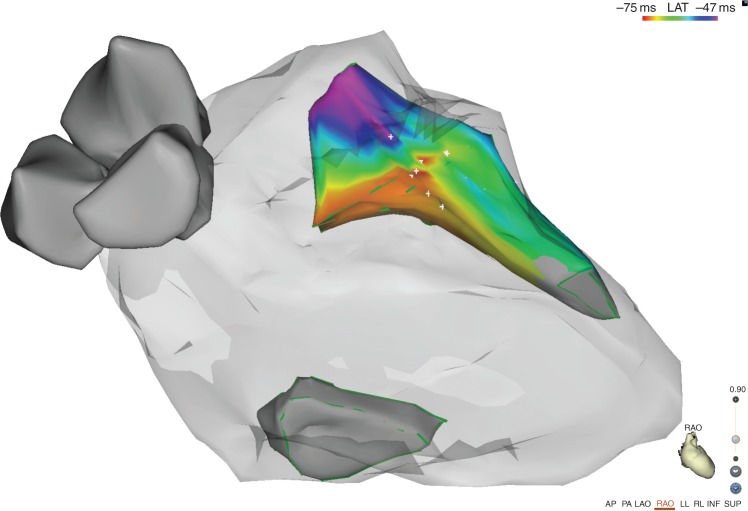
Figure 2.
Right anterior oblique projection of the final geometry obtained with CARTO Sound 3D. The activation map shows the earliest activation site on the septal side the anterolateral PM.
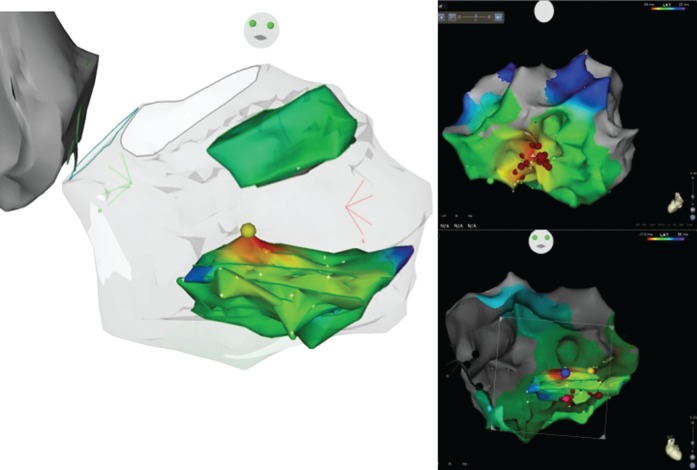
Figure 3.
Left image: final CARTO Sound 3D geometry, showing an activation map of the posteromedial PM. The yellow dot represents the earliest activation site. Upper right image: LV activation map showing the earliest activation site at the mid-ventricular inferior septal area. Radiofrequency (RF) delivery at this site fails to eliminate the clinical PVC. Lower right image: CARTO Sound 3D geometry and CARTO system LV activation map merge. The pink dot represents the initial unsuccessful site of RF delivery. The blue dot shows the earliest activation site on the PM and the successful ablation site. Note that both dots are at the same LV region, but on different sides of the papillary muscle body.
Mapping and ablation
An activation map of the PM was obtained in all cases to identify the site of origin of the arrhythmia. Pace mapping was routinely performed at 500 ms and stimulus amplitude 1 mA higher than the late diastolic threshold in order to identify the site with best pace map. During mapping and ablation over the PMs, catheter position and stability were continuously assessed by ICE, electrogram monitoring, and visualization by the 3D mapping system. In addition, Contact Force (THERMOCOOL SMARTTOUCH® Catheter, Biosense-Webster) was systematically used after it became commercially available in June 2012. The target contact force during radiofrequency (RF) delivery was 10 g.
Radiofrequency current was delivered at myocardial sites exhibiting the earliest bipolar activity preceding the QRS onset during arrhythmia and best pace map. Irrigated RF current was delivered in power control mode at 30 W with an irrigation flow rate of 30 mL/min using a 3.5-mm tip externally irrigated ablation catheter (THERMOCOOL® or THERMOCOOL SMARTTOUCH® Catheter, Biosense-Webster). The goal was to achieve a decrease in impedance of 5–10 ohms, with a temperature limit of 48°C. When an acceleration or reduction of the incidence of PVCs or VT was observed, RF delivery was continued for 60 s and a second application of RF was delivered; otherwise, the RF delivery was discontinued and the ablation catheter repositioned.
The acute procedural endpoint of the ablation procedure was the suppression and non-inducibility of the clinical arrhythmia during isoproterenol infusion and burst pacing from the RV or LV.
Post-procedural follow-up was based on symptom recurrence and Holter monitoring. Transthoracic echocardiography was routinely performed before the first clinical visit to assess for mitral insufficiency onset or worsening.
Statistical analysis
Continuous variables are reported as mean and standard deviation (SD). Fisher's exact test was used to compare the occurrence of events between groups, with a two-sided P-value significant at 0.05 to reject the null hypothesis. The coefficient of Cramer was used to provide an estimation of the size of association between two variables, with values ranging between 0 and 1.
Results
Sixteen patients underwent catheter ablation for VAs originating from the anterolateral and/or posteromedial PMs. Five patients had PVCs originating from both PMs. In four of them, ablation of arrhythmias from both PMs was performed during the same procedure. The baseline characteristics of these patients are summarized in Table 1.
Table 1.
Baseline characteristics of the patients
| N= | Age | Sex | EF (%) | Aetiology | Clinical |
|---|---|---|---|---|---|
| Patient 1 | 73 | F | 27 | Ischaemic | PVC |
| Patient 2 | 74 | M | 34 | Ischaemic | PVC |
| Patient 3 | 54 | F | 55 | Idiopathic | PVC and VT |
| Patient 4 | 70 | M | 30 | Ischaemic | PVC |
| Patient 5 | 65 | F | 40 | Ischaemic | PVC |
| Patient 6 | 76 | M | 40 | Idiopathic | PVC and VT |
| Patient 7 | 56 | F | 50 | Idiopathic | PVC |
| Patient 8 | 74 | M | 34 | Ischaemic | PVC and VT |
| Patient 9 | 72 | M | 52 | Ischaemic | PVC |
| Patient 10 | 74 | M | 32 | Ischaemic | PVC and VT |
| Patient 11 | 63 | M | 38 | Ischaemic | PVC and VT |
| Patient 12 | 54 | M | 55 | Idiopathic | VT |
| Patient 13 | 68 | M | 40 | Ischaemic | PVC and VT |
| Patient 14 | 80 | M | 30 | Idiopathic | PVC |
| Patient 15 | 54 | F | 60 | Idiopathic | PVC |
| Patient 16 | 66 | M | 25 | Ischaemic | PVC |
EF, ejection fraction; Aetiology, ischaemic or idiopathic cardiomyopathies; Clinical, clinical arrhythmia; Device, type of defibrillator (Def.); TTE, relevant findings in transthoracic echocardiography; PVC, premature ventricular contraction; VT, ventricular tachycardia.
The age range was 54–80 years (mean age 67 ± 6); 11 males and 5 females were included. Most patients had a history of ischaemic cardiomyopathy (10 of 16) and the mean ejection fraction was 40.1 ± 11%. All patients presented PM PVCs, and in 7 cases, VT refractory to pharmacological treatment.
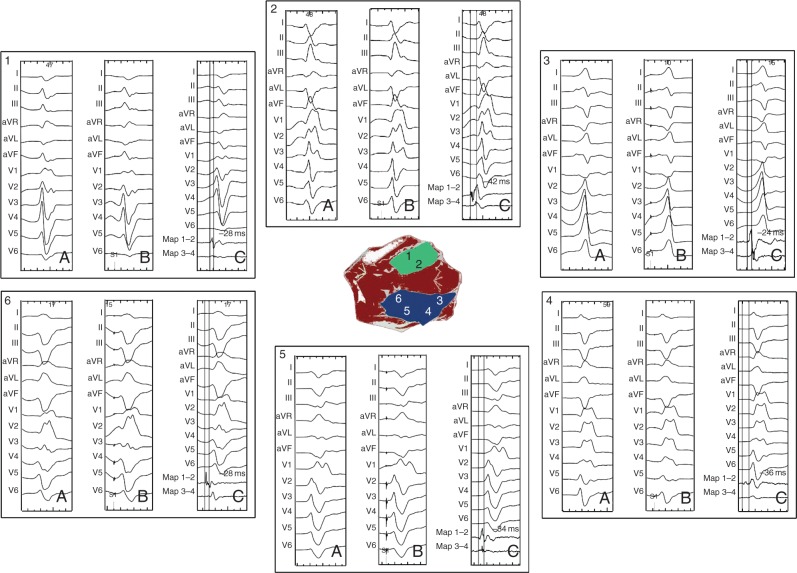
The PVC morphology was either right bundle branch block with left superior axis, if the origin was the posteromedial PM, or right bundle branch block with right inferior axis for those originating from the anterolateral PM (Figure 4).
Figure 4.
PVC originating at different location from the posteromedial PM (in blue) and anterolateral PM (green). (A) PVC QRS morphology; (B) best pace map at the ablation site; and (C) earliest bipolar signal at the successful ablation site.
Overall, 24 procedures were performed: 15 using a retrograde Ao approach and 9 using a TS approach. All procedures were performed using CARTO® 3. Three-dimensional ICE with SOUNDSTAR® was additionally used in 15 of 24 interventions. Detailed procedural data are reported in Table 2.
Table 2.
Procedural data
| N= | Procedure | RFT | PT | FT | LV approach | EAM | ICE 3D | Acute success | Recurrence |
|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | First | 407 | 180 | 16 | Retroaortic | Yes | Yes | Yes | No |
| Patient 2 | First | 871 | 305 | 18 | Retroaortic | Yes | Yes | Yes | No |
| Patient 3 | First | 601 | 198 | 27 | Transseptal | Yes | No | Yes | Yes |
| Patient 3 | Second | 301 | 280 | 16 | Retroaortic | Yes | No | Yes | Yes |
| Patient 4 | First | 780 | 240 | 10 | Retroaortic | Yes | No | Yes | Yes |
| Patient 4 | Second | 416 | 240 | 30 | Retroaortic | Yes | Yes | Yes | No |
| Patient 5 | First | 266 | 285 | 42 | Retroaortic | Yes | No | Yes | Yes |
| Patient 5 | Second | 266 | 135 | 19 | Transseptal | Yes | No | Yes | Yes |
| Patient 6 | First | 300 | 230 | 17 | Transseptal | Yes | Yes | Yes | No |
| Patient 7 | First | 750 | 195 | 30 | Transseptal | Yes | No | No | Yes |
| Patient 7 | Second | 321 | 115 | 10 | Transseptal | Yes | Yes | Yes | No |
| Patient 8 | First | 260 | 195 | 22 | Transseptal | Yes | No | Yes | No |
| Patient 9 | First | 340 | 205 | 23 | Retroaortic | Yes | Yes | Yes | No |
| Patient 10 | First | 615 | 405 | 57 | Retroaortic | Yes | Yes | Yes | Yes |
| Patient 10 | Second | 420 | 135 | 15 | Retroaortic | Yes | Yes | Yes | No |
| Patient 11 | First | 346 | 210 | 20 | Retroaortic | Yes | Yes | Yes | No |
| Patient 12 | First | 315 | 195 | 33 | Retroaortic | Yes | Yes | Yes | No |
| Patient 13 | First | 420 | 140 | 9 | Retroaortic | Yes | Yes | Yes | No |
| Patient 14 | First | 350 | 250 | 67 | Transseptal | Yes | No | Yes | No |
| Patient 14 | Second | 320 | 240 | 48 | Retroaortic | Yes | No | Yes | No |
| Patient 15 | First | 659 | 150 | 14 | Transseptal | Yes | No | Yes | Yes |
| Patient 15 | Second | 530 | 230 | 25 | Transseptal | Yes | Yes | Yes | Yes |
| Patient 15 | Third | 511 | 215 | 21 | Retroaortic | Yes | Yes | Yes | No |
| Patient 16 | First | 1055 | 150 | 12 | Retroaortic | Yes | Yes | Yes | No |
N, patients 1–16; Procedure, first or second procedure; Success, complete suppression of the arrhythmia; Recurrence, reappearance of the same clinical arrhythmia during follow-up; FU, follow-up (months); RFT, duration of radiofrequency delivery during the ablation in seconds; PT, duration of the procedure in minutes; FT, fluoroscopy time in minutes; EAM, use of 3D electroanatomical mapping systems (CARTO); ICE 3D, use of 3D intracardiac echocardiography (CATO Sound).
The posteromedial PM had a higher prevalence rate of clinical arrhythmias, and the PM body was the most frequent site of effective lesions (Table 3). The long-term success rate after a first procedure was 10/16 (62.5%); with a tendency for a higher success rate in patients whose first procedure was performed with vs. without 3D ICE (8/9 88.8% vs. 2/7 28.5%, P-value 0.0035). A second procedure was performed in 7 of 16 patients and one case necessitated a third procedure because of PVC recurrence after awakening from general anaesthesia. Notably, one patient (Patient 14) underwent a second procedure for a different arrhythmia originating from the posteromedial PM, after successful ablation of a VT from the anterolateral PM during the first procedure (Table 3).
Table 3.
Characteristic of the PVC/VT
| PM | Origin | Early site (ms) | Procedure |
|---|---|---|---|
| Patient 1 AL | Body | 40 | First |
| Patient 1 PM | Base | 36 | First |
| Patient 2 PM | Base | 31 | First |
| Patient 3 AL | Base | 28 | First |
| Patient 3 AL | Base | 34 | Second |
| Patient 3 PM | Base | 32 | Second |
| Patient 4 PM | Tip | 26 | First |
| Patient 4 PM | Body | 41 | Second |
| Patient 5 PM | Base | 34 | First |
| Patient 5 PM | Body | 40 | Second |
| Patient 6 PM | Tip | 38 | First |
| Patient 7 PM | Body | 29 | First |
| Patient 7 PM | Body | 33 | Second |
| Patient 8 AL | Base | 37 | First |
| Patient 9 PM | Body | 41 | First |
| Patient 10 PM | Base | 25 | First |
| Patient 10 PM | Base | 34 | Second |
| Patient 11 AL | Body | 32 | First |
| Patient 11 PM | Body | 39 | First |
| Patient 12 PM | Body | 42 | First |
| Patient 13 PM | Body | 35 | First |
| Patient 14 AL | Body | 36 | First |
| Patient 14 PM | Base | 36 | Second |
| Patient 15 PM | Base | 36 | First |
| Patient 15 PM | Base | 39 | Second |
| Patient 15 PM | Base | 43 | Third |
| Patient 16 PM | Body | 30 | First |
| Patient 16 AL | Body | 30 | First |
Origin, site showing the earliest activation in the PM; Pace map, 12 leads out of 12 leads paced QRS matches the clinical arrhythmia; Early site, early activity registered before the QRS onset; Match, early site correlates with the best pace map region; Procedure, first procedures or second procedures in case of recurrence; Success, complete abolition of the arrhythmia; PVC, premature ventricular contraction; VT, ventricular tachycardia.
Among the seven patients who had a repeat procedure, four were arrhythmia-free at follow-up. Three of these four had their repeat procedure performed using 3D ICE. In two patients, both the first and the repeat procedure were performed without 3D ICE because of an accessibility issue. Successful ablation was only achieved in one of these two patients (Figure 5).
Figure 5.
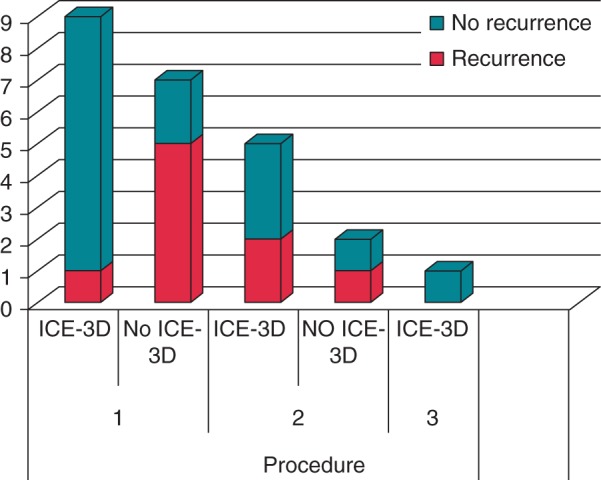
Bar graph illustrating recurrence among 16 patients, according to the presence or absence of ICE 3D, in first and repeat procedures.
There was no difference in the outcome of the procedure whether TS or retro-Ao approach was used, either on the acute success rate (P = 0.375) or long-term recurrence of arrhythmias (P = 0.212).
Overall, 87.5% of patients were arrhythmia-free at a mean follow-up of 10.5 ± 7 months as a result of the interventions, whereas two patients continued to experience frequent PVCs despite pharmacological treatment with β-blockers. At long-term follow-up, we did not detect any worsening of mitral valve insufficiency.
Discussion
To our knowledge, this is the first report to describe the use of 3D ICE-facilitated electroanatomical imaging in a case series of patients undergoing ablation for VAs originating from the PMs. Our experience suggests that the integration of echocardiography may improve feasibility of this type of procedure for several reasons: (i) the direct visualization of endocavitary structures, which is currently not provided by any other real-time mapping system; (ii) the provision of detailed anatomical landmarks to improve the navigation of the ablation catheter; (iii) the monitoring of contact between the catheter tip and the PM during pace mapping, activation mapping, and ablation; (iv) the creation of a definite activation map limited to the targeted PMs; and (v) the potential to mark the ablation points on both sides of the PM.
Although PM arrhythmias have been described as a novel clinical entity, they were likely underdiagnosed in the past.2 This may be due to the difficulty in recognizing the precise LV endocardial anatomy by fluoroscopic or electroanatomical approaches. The advent of ICE has allowed us to recognize and differentiate this arrhythmia as a distinct entity.
The high prevalence of ischaemic heart disease with low ejection fraction in our cohort of patients suggests that a progressive dilation of the left ventricle can potentially stretch and overload the PMs, making them a likely arrhythmia culprit.
While performing PM PVC/VT catheter ablation, some obstacles can arise. First and foremost, there is commonly a beat-to-beat variation in QRS morphology during pace mapping from the PMs. As a result, it has been reported2 that there is poor correlation between pace mapping and local ventricular activation time relative to QRS onset, and that ablation at sites with excellent pace maps is often unsuccessful.3 Various explanations can be provided for this finding, including the capture of adjacent myocardial tissue due to the instability of the catheter tip during pace mapping and the presence of a common exit from different pacing/arrhythmia sites on the PMs. In our experience, better visualization of the tip of the catheter on the PM reconstructed by 3D ICE during pace mapping might partially overcome the aforementioned limitations. Nevertheless, a perfect pace map was usually not obtained; consequently, ablation was performed at the site of best pace map and earliest activation site.
A further challenge of PM VA ablation is that almost half of the patients present multiple PVC morphologies,4,12 which may be due to the presence of multiple exit sites.4,12 Additionally, the 12 lead ECG for VT originating from the posteromedial PMs is similar to that of left posterior fascicular VT, and the fluoroscopic ablation catheter position is also similar.2 Some even wonder if PM VT could be a variant of fascicular VT.13
Electroanatomical mapping can also be misleading, as early diffuse activation sites along the base of the PM can be observed. This can occur either when the early activation site is in the endocardial LV free wall or is mid-myocardial or epicardial. A 3D ICE-facilitated electroanatomical mapping approach overcomes this caveat by specifically visualizing the contours of the PM.
One concern while delivering RF current over the PM is mitral valve dysfunction. This may be more frequent when applying energy at the tip of the PM rather than at its base; hence, defining the anatomy plays a key role in avoiding complications.6
The high rate of recurrence after the first procedure detected in our cohort of patients points out to the fact that PM PVC/VT ablation remains a challenging procedure. Larger studies are required to determine whether the systematic use of 3D ICE electroanatomical mapping actually increases the success rate of PM PVC/VT ablation.
Limitations
The small number of patients in our report does not allow for an exhaustive statistical assessment. This is due, in part, to the fact that patients with PM arrhythmias represent a small subset of subjects referred for ablation, making them less suitable for larger studies. It is difficult to definitively comment on the recurrence of arrhythmias in patients whose ablations were performed with vs. without 3D ICE since the ultrasound strategy was not randomized. However, the OR calculated on the outcomes after the first procedure shows a risk of recurrence of VT 20 times higher in patients who underwent ablation without 3D ICE. In addition, a strong association was detected between the use of 3D ICE and success of the procedure (Cramer V 0.68).
Additionally, it is difficult to compare our results with those of prior experiences1,2 since our population includes patients with both ischaemic and non-ischaemic cardiomyopathy and normal or low ejection fraction.
Finally, although unipolar electograms can be very useful in identifying the site of earliest activation during PVC/VT ablation, our approach has been to rely mainly on bipolar electrograms for the procedures described in this report.
Conclusion
Three-dimensional ICE-facilitated electroanatomical imaging allows for the real-time creation of precise geometries of the cardiac chambers. Moreover, it provides an accurate visualization of endocavitary structures. This is of critical importance when targeting arrhythmias originating from the PMs, which requires a detailed visualization of anatomical landmarks. Adoption of a 3D ICE-guided electroanatomical approach should be considered to improve the safety and feasibility of this procedure. Furthermore, an integrated approach using ICE and electroanatomical mapping may increase the success rate of PM VT catheter ablation.
Funding
V.E. is the recipient of a Clinician Scientist award from the Canadian Institutes of Health Research (CIHR).
Conflict of interest: V.E. and J.-F.R. have received honoraria from Biosense-Webster, Medtronic, and St Jude Medical.
References
- 1. Bogun F, Desjardins B, Crawford T, Good E, Jongnarangsin K, Oral H et al. Post-infarction ventricular arrhythmias originating in papillary muscles. J Am Coll Cardiol 2008;51:1794–802. [DOI] [PubMed] [Google Scholar]
- 2. Doppalapudi H, Yamada T, McElderry HT, Plumb VJ, Epstein AE, Kay GN. Ventricular tachycardia originating from the posterior papillary muscle in the left ventricle: a distinct clinical syndrome. Circ Arrhythm Electrophysiol 2008;1:23–9. [DOI] [PubMed] [Google Scholar]
- 3. Good E, Desjardins B, Jongnarangsin K, Oral H, Chugh A, Ebinger M et al. Ventricular arrhythmias originating from a papillary muscle in patients without prior infarction: a comparison with fascicular arrhythmias. Heart Rhythm 2008;5:1530–7. [DOI] [PubMed] [Google Scholar]
- 4. Yamada T, Doppalapudi H, McElderry HT, Okada T, Murakami Y, Inden Y et al. Electrocardiographic and electrophysiological characteristics in idiopathic ventricular arrhythmias originating from the papillary muscles in the left ventricle: relevance for catheter ablation. Circ Arrhythm Electrophysiol 2010;3:324–31. [DOI] [PubMed] [Google Scholar]
- 5. Partridge JB, Anderson RH. Left ventricular anatomy: its nomenclature, segmentation, and planes of imaging. Clin Anat 2009;22:77–84. [DOI] [PubMed] [Google Scholar]
- 6. Madhavan M, Asirvatham SJ. The fourth dimension: endocavitary ventricular tachycardia. Circ Arrhythm Electrophysiol 2010;3:302–4. [DOI] [PubMed] [Google Scholar]
- 7. Packer DL, Johnson SB, Kolasa MW, Bunch TJ, Henz BD, Okumura Y. New generation of electro-anatomic mapping: full intracardiac ultrasound image integration. Europace 2008;10(Suppl 3):iii35–41. [DOI] [PubMed] [Google Scholar]
- 8. Khaykin Y, Skanes A, Whaley B, Hill C, Beardsall M, Seabrook C et al. Real-time integration of 2D intracardiac echocardiography and 3D electroanatomical mapping to guide ventricular tachycardia ablation. Heart Rhythm 2008;5:1396–402. [DOI] [PubMed] [Google Scholar]
- 9. Seiler J, Lee JC, Roberts-Thomson KC, Stevenson WG. Intracardiac echocardiography guided catheter ablation of incessant ventricular tachycardia from the posterior papillary muscle causing tachycardia—mediated cardiomyopathy. Heart Rhythm 2009;6:389–92. [DOI] [PubMed] [Google Scholar]
- 10. Yamada T, McElderry HT, Doppalapudi H, Kay GN. Real-time integration of intracardiac echocardiography and electroanatomic mapping in PVCs arising from the LV anterior papillary muscle. Pacing Clin Electrophysiol 2009;32:1240–3. [DOI] [PubMed] [Google Scholar]
- 11. Duggirala S, Gerstenfeld EP. Ventricular tachycardia originating from unusual sites. Card Electrophysiol Clin 2014;6:581–93. [Google Scholar]
- 12. Yokokawa M, Good E, Desjardins B, Crawford T, Jongnarangsin K, Chugh A et al. Predictors of successful catheter ablation of ventricular arrhythmias arising from the papillary muscles. Heart Rhythm 2010;7:1654–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen PS, Karagueuzian HS, Kim YH. Papillary muscle hypothesis of idiopathic left ventricular tachycardia. J Am Coll Cardiol 2001;37:1475–6. [DOI] [PubMed] [Google Scholar]