Abstract
Background
Rates of drug-resistant tuberculosis (TB) and TB associated with human immunodeficiency virus (HIV) infection have increased dramatically, intensifying challenges in TB control. New formulations of TB treatment drugs that control drug release and increase local drug concentrations will have a significant impact on mitigating the toxic side effects and increasing the clinical efficacy of anti-TB drugs.
Objectives
The aim was to observe the sustained release characteristics of rifapentine polylactic acid sustained-release microspheres in vivo and the accumulation of rifapentine in other tissues following paravertebral implantation.
Methods
This study is a basic animal experimental study that began on July 17, 2014 in the Fifth Affiliated hospital of Xinjiang Medical University. One hundred and eight New Zealand white rabbits (weighing 2.8 - 3.0 kg, male and female, China) were randomly divided into three groups of 36 rabbits each. Blood and tissue samples from the liver, lungs, kidneys, vertebrae, and paravertebral muscle were collected at different time points post-surgery. High performance liquid chromatography (HPLC) analysis with a biological internal standard was used to determine the drug concentrations in samples.
Results
In group A, no significant differences in rifapentine concentrations in the liver were detected between any two time points (P > 0.05). However, the differences in rifapentine concentrations between day 10 and day 21 were statistically significant (P < 0.05); for days 21, 35, 46, and 60, the differences in rifapentine concentrations between two sequential time points were not statistically significant (P > 0.05). In group B, the differences in rifapentine concentration between days 3 and 10 in vertebral bone and in paravertebral muscles were statistically significant (P < 0.05). Rifapentine was detected in the vertebral bone tissue in the group C animals. The rifapentine concentrations between two sequential time points were statistically significant (P < 0.05). Rifapentine could not be detected in the paravertebral muscles 46 days after the operation. The differences in rifapentine concentrations between two sequential time points among days 3, 10, 21, and 35 were statistically significant (P < 0.05).
Conclusions
After paravertebral implantation of rifapentine polylactic acid sustained-release microspheres, the concentration of rifapentine in local vertebral bone tissues was maintained above the TB minimum inhibitory concentration for up to 60 days with no apparent accumulation of the drug in other tissues.
Keywords: Rifapentine Polylactic Acid, Sustained-Release Microsphere, Spinal Tuberculosis, Paravertebral Implantation
1. Background
Spinal tuberculosis is the most common type of extrapulmonary tuberculosis (TB), accounting for 3% - 5% of the total incidence of TB and 35% of extrapulmonary tuberculosis cases (1-3). In recent years, the rates of drug resistant TB and TB associated with HIV infection have increased dramatically, intensifying the challenges in TB control (4). Current anti-TB drugs require long treatment periods, cause toxic side effects, and are difficult to concentrate locally, which prevents clinicians from adequately meeting treatment goals. New formulations that control drug release and increase local drug concentration will have a significant impact on mitigating the toxic side effects and increasing the clinical efficacy of these drugs.
Rifapentine is a new semi-synthetic antibiotic of the rifamycin class with high efficiency, low toxicity, and long-lasting properties that can reduce the number of times the drug is administered. In this study, 15 mg of rifapentine polylactic acid sustained-release microspheres were implanted in the paravertebral muscles of New Zealand rabbits. Rifapentine concentrations in plasma and other tissues (liver, kidneys, lungs, paravertebral muscles, and vertebral bones) were continuously measured for two months to study the in vivo drug release and tissue accumulation properties of the rifapentine polylactic acid sustained-release microspheres.
2. Objectives
The aim of this study was to observe the sustained release characteristics of rifapentine polylactic acid sustained-release microspheres in vivo and the accumulation of rifapentine in other tissues following paravertebral implantation.
3. Methods
The study is a basic animal experimental study.
3.1. Ethics Statement
The research protocol was approved by the ethics committee of the Fifth Affiliated hospital of Xin Jiang Medical University (protocol NO: 20140216-12) in 2014. All the patients were provided with appropriate informed consent.
3.2. Apparatus, Drugs, and Reagents
For these studies, we utilized the following equipment: a high performance liquid phase chromatography apparatus (Shimadzu, Japan), a high speed refrigerated centrifuge HC-3018R (USTC Chuangxin Co. Ltd., China), a vibration ball mill GT100X (Beijing Grinder instrument Co., Ltd), and a nitrogen and Termovap sample concentrator HGC-12A (Tianjin Hengao technology development Co., Ltd). We obtained the crude rifapentine from Mingxin pharmaceutical Co. (Sichuan, lot number: 20100609, content: 93.8%) and synthesized the rifapentine polylactic acid sustained-release microspheres in our laboratory (patent number: 201210281222.6). The methanol, ultra-pure water, and p-nitrophenol were from SIGMA-ALDRICH.
3.3. Animal Grouping and Surgery
Healthy purebred New Zealand white rabbits (weighing 2.8 - 3.0 kg, male and female, China) were randomly divided into three groups of 36 rabbits each according to body weight, for a total of 108 animals. All rabbits were purchased at the First Affiliated hospital of Xinjiang Medical University. The animals were of first class quality (permission number: SCXK (Xin) 2003-001). Group A was administered crude rifapentine by gavage, group B was administered crude rifapentine by paravertebral implantation, and group C was administered rifapentine polylactic acid sustained-release microspheres by paravertebral implantation. The rabbits were weighed, then injected with 3% pentobarbital via an ear vein for anesthesia (1 - 1.5 mL/Kg). The abdominal and back fur was then removed, the skin was sterilized, and the rabbit was fixed on the operation board. A cut was made from the left side of the peritoneum behind the psoas major muscle to expose lumbar vertebrae L4 and L5. A rectangular bone groove measuring 1.0 cm × 0.3 cm × 0.5 cm was cut on the left side of the L4. The drugs given to the animals in groups B and C were placed in the bone groove, and any excess drug was covered with the paravertebral muscle (Figure 3). Group A rabbits were gavaged with 70 mg/d crude rifapentine, group B rabbits were each implanted with 6 mg crude rifapentine, and group C rabbits were each implanted with 15 mg of rifapentine polylactic acid sustained-release microspheres. The animals were housed in a standard-condition room at constant temperature and humidity (23°C, relative humidity: 55%) on a 12 hours light/dark cycle. Sample size formula:
Figure 3. Group C (Paravertebral Microsphere Implantation Group).
Rifapentine concentration in serum and tissues at each time point.
n = Ψ2 (∑ (Si2) / K)/ [∑ (Xi - (X1 + X2 + X3)/K)2 / (K-1)]
α = 0.05, β = 0.10, K: number of groups, Ψα, β, K-1, ∞ = 2.52, Xi, Si: mean (X1 =, X2 =…) and standard deviation (S1 =, S2 =…).
3.4. Concentration of Rifapentine in Biological Samples by HPLC Chromatographic Conditions
The size of the Shim-pack VP-ODS chromatographic column was 150 mm × 4.6 mm, 5 μm; flow speed was 1.0 mL/minute; detection wavelength was 336 nm; column temperature was 25°C; sample size was 10 μL; mobile phase A was methanol; mobile phase B was ultra-pure water; and the sample was eluted by gradient elution.
3.5. Biological Sample Processing
Blood from the blank controls and drug-treated rabbits was collected and centrifuged. The internal control (200 μL) was added to 500 μL of plasma and then vortexed. Methanol (1.5 mL) was then added, and the sample was vortexed again for 1 minute, then centrifuged at 12,000 rpm for 10 minutes. The supernatant (1.7 mL) was concentrated using the Termonvap Sample Concentrator, re-dissolved in 100 μL of methanol, and filtered through a 0.22 μm microporous membrane. The samples were then ready for analysis.
Tissue samples were prepared for rifapentine detection as follows: a proper quantity of tissue from the right lobe of the liver, the right lower lobe of the lung, the right upper pole of the kidney, the L4 vertebral body, and the lumbar muscle tissue 1 cm from the L4 vertebra were collected from rabbits in the blank control and drug treatment groups. Blood was wiped from the collected tissue samples with filter paper prior to weighing the samples. Saline solution was added to the samples (l mL of saline for every 1 g of tissue), which were then grinded in the vibration ball mill. Ground samples were centrifuged at 12,000 rpm for 10 minutes, and a 500 μL aliquot of the supernatant was taken from each sample for analysis.
3.6. Linearity and Range
Different concentrations of a rifapentine reference solution (1.25 μg/mL, 10 μg/mL, 20 μg/mL, 40 μg/mL, 80 μg/mL, and 160 μg/mL) were prepared using methanol. A 20 μg/mL solution of p-nitrophenol was prepared using methanol and used as an internal standard. Plasma from blank controls (500 μL) was added to rifapentine reference solutions that had been concentrated using the Termonvap sample concentrator to reach final concentrations of 0.25, 2, 4, 8, 16, and 32 μg/mL rifapentine. Then, 200 μL of the internal standard solution was added and mixed well. Methanol (1.5 mL) was added to remove proteins, and the mixture was vortexed for 1 minute and then centrifuged at 12,000 rpm at 4°C for 10 minutes. The supernatant (1.7 mL) was then concentrated using the Termonvap Sample Concentrator, re-dissolved in 100 μL of methanol, and filtered using a 0.22 μm microporous membrane. Samples were analyzed by HPLC, and the chromatographic peak areas were recorded. Using the ratio of the peak area of the rifapentine reference solution to the peak area of the internal standard as coordinates (Y) and the drug concentration in the plasma samples as abscissa (X), a linear regression was calculated as follows: Y = 0.051X + 0.022 (r = 0.999, n = 6). Plasma concentrations of rifapentine between 0.25 and 32.0 μg/mL showed a good linear relationship (r = 0.999). The lowest detection value of the standard curve was 0.25 μg/mL.
3.7. Postoperative Observation and Examination
3.7.1. General Observations
Animal activities, eating habits, and wound healing were observed on a regular basis.
3.7.2. Specimen Collection
Blood samples were collected from groups A, B, and C on days 3, 10, 21, 35, 46, and 60 following drug administration. Six animals from each group at each time point were sacrificed, and tissues from the right lobe of the liver, the right lower lobe of the lung, the right upper pole of the kidney, the L4 vertebral body, and the lumbar muscle tissue 1 cm from the L4 vertebra were collected, and the concentration of rifapentine in each tissue type was measured.
3.8. Statistical Analysis
The Statistical package for social sciences (SPSS) software package v.10.0 was used for statistical analysis. The results are expressed as. Repeated measurement analysis was used for the comparison between multiple groups. P values < 0.05 were considered to be statistically significant.
4. Results
4.1. General Animal Conditions
Surgeries were successful in all animals, with no postoperative deaths. Wounds healed well without infection.
4.2. Precision Test
Precise amounts of rifapentine reference standard stock solutions were concentrated and diluted to three concentrations low, medium, and high using plasma from blank controls and then analyzed by HPLC as described above. Measurements were taken five times to obtain the intraday precision value; the RSD values of the ratio between the peak areas of low, medium, and high drug concentrations and the peak areas of the internal standard were 3.64%, 2.24%, and 0.87%, respectively. Measurements of the precision value were conducted for five consecutive days to determine the interday precision value; the RSD values of the ratio between the peak areas of low, medium, and high drug concentrations and the peak areas of the internal standard were 2.99%, 1.94%, and 0.49% respectively. The RSD values were all less than 5% (Tables 1 and 2).
Table 1. Intraday Precision Values.
| Concentration, g/mL | 1 | 2 | 3 | 4 | 5 | RSD, % |
|---|---|---|---|---|---|---|
| 2.00 | 0.10 | 0.11 | 0.12 | 0.11 | 0.11 | 3.64 |
| 4.00 | 0.22 | 0.21 | 0.21 | 0.22 | 0.21 | 2.24 |
| 16.00 | 0.81 | 0.83 | 0.83 | 0.82 | 0.83 | 0.87 |
Table 2. . Interday Precision Values.
| Concentration, g/mL | 1 | 2 | 3 | 4 | 5 | RSD, % |
|---|---|---|---|---|---|---|
| 2.00 | 2.10 | 2.13 | 2.11 | 1.98 | 1.99 | 2.99 |
| 4.00 | 4.12 | 3.99 | 3.98 | 4.19 | 4.14 | 1.94 |
| 16.00 | 16.21 | 16.12 | 16.10 | 15.97 | 15.99 | 0.49 |
4.3. Reproducibility
As described above, precise amounts of rifapentine reference standard stock solution were concentrated and diluted to three concentrations low, medium and high-using plasma from blank controls and then analyzed using HPLC. Each drug concentration was measured five times in parallel to determine reproducibility; the RSD values of the ratio between the peak areas of low, medium, and high drug concentrations and the peak areas of the internal standard were 7.08%, 2.96%, and 4.50%, respectively. The RSD values were all less than 10%, which is in line with the requirements for the analysis of biological samples (Table 3).
Table 3. Reproducibility.
| Concentration, g/mL | 1 | 2 | 3 | 4 | 5 | RSD, % |
|---|---|---|---|---|---|---|
| 2.00 | 0.10 | 0.10 | 0.12 | 0.10 | 0.11 | 7.08 |
| 4.00 | 0.23 | 0.21 | 0.22 | 0.22 | 0.21 | 2.96 |
| 16.00 | 0.81 | 0.90 | 0.83 | 0.82 | 0.91 | 4.50 |
4.4. Results of Biological Sample Processing
The concentration of rifapentine in each sample was determined using the HPLC conditions as described. Rifapentine concentrations in plasma and tissues from animals in different groups were compared (see Figures 1 - 3).
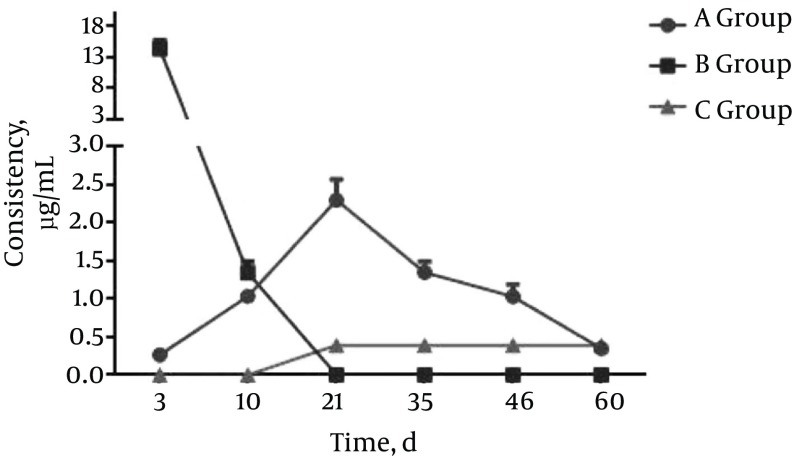
Figure 1. Group A (Gavage Group). Rifapentine Concentration in Serum and Tissues at Each Time Point.
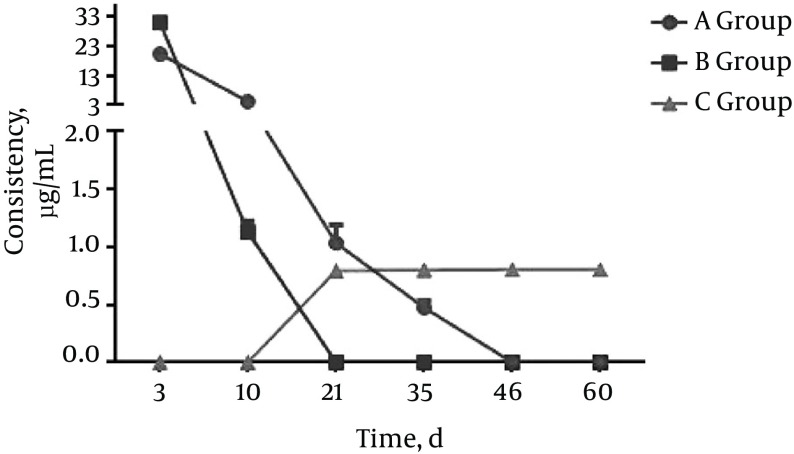
Figure 2. Group B (Crude Drug Paravertebral Implantation Group).
Rifapentine concentration in plasma and tissues at each time point.
In group A, no significant differences in rifapentine concentrations in the liver were detected between any two time points (P > 0.05). No significant differences in rifapentine concentrations among blood, lung, kidney, vertebral bone, and paravertebral muscle samples within the same sample between day 3 and 10 were detected (P > 0.05); however, the differences in rifapentine concentrations between day 10 and day 21 were statistically significant (P < 0.05); for days 21, 35, 46, and 60, the differences in rifapentine concentrations between two sequential time points were not statistically significant (P > 0.05).
In group B: the differences in rifapentine concentration between days 3 and 10 in vertebral bone and in paravertebral muscles were statistically significant (P < 0.05).
Rifapentine was detected in the vertebral bone tissue of group C animals. The rifapentine concentrations between two sequential time points were statistically significant in this group (P < 0.05). Rifapentine could not be detected in the paravertebral muscles 46 days after the operation. The differences in rifapentine concentrations from two sequential time points between days 3, 10, 21, and 35 were statistically significant (P < 0.05); however, no significant differences were detected between days 46 and 60 (P > 0.05).
5. Discussion
The efficacy of anti-TB drugs largely depends on the concentration of the drug at the location of the lesion. Most cases of osteoarticular tuberculosis form local necrotic foci at the lesion. Because blood supply is poor within the necrotic tissues in the lesions and the surrounding bone tissues, it is difficult to achieve an effective concentration of oral or intravenous drug formulations at the focal necrotic lesions (5). Treatment measures such as systemic oral anti-TB drug treatments, lesion removal, and partial movement restraint are only partially effective for treating osteoarticular TB. Therefore, implantation of anti-TB drugs in the focal lesion is necessary for maintaining a local effective drug concentration; this extra treatment measure is necessary to kill or inhibit the growth and reproduction of tubercle bacillus and is an important method for treating osteoarticular tuberculosis.
The clinical application of anti-TB drugs to local areas has been shown to be effective after the removal of the bone lesion. Streptomycin or isoniazid has primarily been directly administered topically or after absorption by gelatin sponge (6). However, because these drugs are highly water soluble, they are dissolved and absorbed quickly after being implanted during surgery. Thus, it is difficult to maintain a local effective concentration of these anti-TB drugs. Changing the existing forms of anti-TB drugs to achieve controlled release and increase local drug concentration will greatly reduce the toxic side effects of these drugs and increase the scope of their clinical application.
Preparing a sustained-release form of any drug requires a good scaffold. Poly-lactic-co-glycolic acid (PLGA) is an ideal material for making microspheres. It can be degraded in the body into glycolic acid and lactic acid, then metabolized into water and carbon dioxide and released from the body (7). PLGA is a nontoxic, non-irritating, non-immunogenic, non-carcinogenic material with good biocompatibility that can be completely degraded in the body. The United States food and drug administration has approved the use of PLGA for implantation in vivo (8). PLGA-encapsulated drugs are released to the local tissue area slowly through diffusion and dissolution, and the length of the releasing cycles can range from four to 24 weeks (9). Rifapentine, or cyclopentyl piperazine rifamycin, has adequate fat solubility and antibacterial properties identical to rifampin. However, the anti-tubercle bacillus properties of rifapentine are two to 10 times better than those of rifampin, with an MIC of 0.12 - 0.25 μg/mL (10, 11). Based on the solubility and stability of rifapentine, we used the double-emulsion solvent evaporation method for preparing rifampine isoniazid polylactic acid microspheres to prepare a sustained-release form with a similar average diameter, drug loading rate, and package seal rate as those reported in the literature (12-14). After paravertebral implantation of the microspheres into New Zealand rabbits, sustained-release characteristics and drug accumulation in other tissues were observed and compared with alternative drug delivery forms.
In this study, the data collected from group A animals were consistent with the reported drug dynamics of rifapentine; specifically, drugs in the gavage group were absorbed through enterohepatic circulation and mainly concentrated in tissues such as the liver, kidneys, lungs, and blood. Drug absorption in the vertebral body and the paravertebral muscle were slow, and the concentrations were relatively low. With prolonged gavage, rifapentine was detected in the vertebral bone and paravertebral muscle, with concentrations becoming stable over time. However, the concentration of rifapentine in the vertebral body did not reach the minimum bacteria inhibitory concentration until it was 10 times higher than the minimum bacteria inhibition concentration in blood and other tissues (liver, kidneys, and lungs). This phenomenon also explains the liver and kidney damage often observed with long-term anti-TB drug treatment for spinal tuberculosis, as well as the high relapse rate. When comparing group A and group C animals, the results indicated that rifapentine from the microspheres was released slowly in group C animals and continuously absorbed by the vertebral bone and paravertebral muscle and was then gradually eliminated from the body, with no obvious accumulation in other organs.
The data suggest that after paravertebral implantation of crude rifapentine in group B animals, the drug was quickly dissolved, absorbed, and excreted by the local vertebral bone and paravertebral muscles with no sustained release effect. In contrast, following paravertebral implantation of rifapentine polylactic acid sustained-release microspheres, group C animals showed an initial burst of drug release followed by a slow release that gradually declined over time. These data are consistent with the general release pattern of the polylactic acid sustained-release material (15). The rifapentine release cycle in the paravertebral muscle was shorter than in the vertebral bone in the group C animals. Compared to the vertebral bone, the paravertebral muscle has greater blood supply and is rich in blood vessels, resulting in faster drug absorption and metabolism. In the later stages, when the bone heals slowly, a bone callus is formed that wraps around the drug, limiting the relative drug distribution in bones, which is more beneficial for local bone tissue absorption of the drug. This explains the long-lasting effect of the drug observed in bone tissues, which lasted longer than the 60 days in these experiments. Comparing the results from group B and group C, rifapentine from the microspheres are released slowly in the group C animals, and there was no obvious accumulation in the body. The microspheres had obvious sustained release characteristics compared to crude rifapentine.
In recent years, the rapid development of microspheres as a drug delivery system has shown great potential for improving TB lesion targeting and reducing toxic side effects of anti-TB drugs (16). Analysis of biological samples by HPLC has also proven effective. By using the HPLC method, we repeatedly determined rifapentine concentrations in the plasma and other tissues (liver, lungs, kidneys, vertebral bone body, and paravertebral muscle) of animals from the crude rifapentine gavage group, the raw rifapentine paravertebral implantation group, and the rifapentine polylactic acid sustained-release microsphere paravertebral implantation group. The results indicate that after paravertebral implantation of rifapentine polylactic acid sustained-release microspheres, the local concentration of rifapentine was maintained above the MIC level for 60 days, and there was no accumulation of the drug in other tissues.
The objective of this study was to observe the sustained release characteristics of rifapentine polylactic acid sustained-release microspheres in vivo and the accumulation of rifapentine in other tissues following paravertebral implantation. Further studies are needed to determine the long-term outcome of spinal tuberculosis treatment in rabbits using this system and whether a combined administration of anti-TB drugs can shorten the treatment cycle. Further research is also needed to confirm the local effects of combining implanted rifapentine polylactic acid sustained-release microspheres and fat stem cells on cell growth and the sustained-release characteristics of the drug.
5.1. Conclusions
After paravertebral implantation of rifapentine polylactic acid sustained-release microspheres, the concentration of rifapentine in local vertebral bone tissues was maintained above the TB minimum inhibitory concentration for up to 60 days with no apparent accumulation of the drug in other tissues.
Footnotes
Authors’ Contribution:Zheng Zhang and Linbo Wu are Equal contributors. Xinghua Song developed the original study idea and the protocol; Zheng Zhang abstracted and analyzed data and wrote the manuscript; and Linbo Wu, Haijian Li, and Zhicheng Long contributed to the development of the protocol, abstracted data, and preparation of the manuscript.
Funding/Support:This work was supported by grant 81360283 from The national natural science foundation.
References
- 1.Wu J, Zuo Y, Hu Y, Wang J, Li J, Qiao B, et al. Development and in vitro characterization of drug delivery system of rifapentine for osteoarticular tuberculosis. Drug Des Devel Ther. 2015;9:1359–66. doi: 10.2147/DDDT.S78407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Golden MP, Vikram HR. Extrapulmonary tuberculosis: an overview. Am Fam Physician. 2005;72(9):1761–8. [PubMed] [Google Scholar]
- 3.Gupta R, Garg RK, Jain A, Malhotra HS, Verma R, Sharma PK. Spinal cord and spinal nerve root involvement (myeloradiculopathy) in tuberculous meningitis. Medicine (Baltimore). 2015;94(3):404. doi: 10.1097/MD.0000000000000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shukla M, Sharma A, Jaiswal S, Lal J. Insights into the pharmacokinetic properties of antitubercular drugs. Expert Opin Drug Metab Toxicol. 2016;12(7):765–78. doi: 10.1080/17425255.2016.1183643. [DOI] [PubMed] [Google Scholar]
- 5.Kurtz SM, Kocagoz S, Arnholt C, Huet R, Ueno M, Walter WL. Advances in zirconia toughened alumina biomaterials for total joint replacement. J Mech Behav Biomed Mater. 2014;31:107–16. doi: 10.1016/j.jmbbm.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LIU J, WANG Y, XIA K. Preparation of isoniazid polylactic acid microspheres and characteristics of the drug release in both vitro and vivo. Chin J Spine Spinal Cord . 2008;4:018. [Google Scholar]
- 7.Habraken WJ, Wolke JG, Mikos AG, Jansen JA. Injectable PLGA microsphere/calcium phosphate cements: physical properties and degradation characteristics. J Biomater Sci Polym Ed. 2006;17(9):1057–74. doi: 10.1163/156856206778366004. [DOI] [PubMed] [Google Scholar]
- 8.Chaubal M. Polylactides/glycolides-excipients for injectable drug delivery and beyond. Drug Deliv Technol. 2002;2:34–6. [Google Scholar]
- 9.Zhang L, Long C, Pan J, Qian Y. A Dissolution‐Diffusion Model and Quantitative Analysis of Drug Controlled Release from Biodegradable Polymer Microspheres. Can J Chem Eng. 2006;84(5):558–66. [Google Scholar]
- 10.Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 2012;21(14):2724–52. doi: 10.1089/scd.2011.0722. [DOI] [PubMed] [Google Scholar]
- 11.Sun XC, Gong F, Niu DS. Adipose mesenchymal stem cells combined with hyaluronic acid sodium in treatment of rabbit knee osteoarthritis. Orthop J China. 2013;21(19):1991–7. doi: 10.3977/j.issn.1005-8478.2013.19.18. [DOI] [Google Scholar]
- 12.Liu X, Jia W, Wang H, Wang Y, Ma J, Wang H, et al. Establishment of a rabbit model of spinal tuberculosis using Mycobacterium tuberculosis strain H37Rv. Jpn J Infect Dis. 2015;68(2):89–97. doi: 10.7883/yoken.JJID.2014.147. [DOI] [PubMed] [Google Scholar]
- 13.Dooley KE, Bliven-Sizemore EE, Weiner M, Lu Y, Nuermberger EL, Hubbard WC, et al. Safety and pharmacokinetics of escalating daily doses of the antituberculosis drug rifapentine in healthy volunteers. Clin Pharmacol Ther. 2012;91(5):881–8. doi: 10.1038/clpt.2011.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gajendiran M, Divakar S, Raaman N, Balasubramanian S. In vitro drug release behavior, mechanism and antimicrobial activity of rifampicin loaded low molecular weight PLGA-PEG-PLGA triblock copolymeric nanospheres. Curr Drug Deliv. 2013;10(6):722–31. doi: 10.2174/15672018113109990002. [DOI] [PubMed] [Google Scholar]
- 15.Jiang B, Akar B, Waller TM, Larson JC, Appel AA, Brey EM. Design of a composite biomaterial system for tissue engineering applications. Acta Biomater. 2014;10(3):1177–86. doi: 10.1016/j.actbio.2013.11.029. [DOI] [PubMed] [Google Scholar]
- 16.Ahmad Z, Pandey R, Sharma S, Khuller GK. Novel chemotherapy for tuberculosis: chemotherapeutic potential of econazole- and moxifloxacin-loaded PLG nanoparticles. Int J Antimicrob Agents. 2008;31(2):142–6. doi: 10.1016/j.ijantimicag.2007.10.017. [DOI] [PubMed] [Google Scholar]