Abstract
In an effort to elucidate differences in functioning brain networks between youth with obsessive-compulsive disorder and controls, we used fMRI signals to analyze brain network interactions of the dorsal anterior cingulate cortex (dACC) during visually coordinated motor responses. Subjects made a uni-manual response to briefly presented probes, at periodic (allowing participants to maintain a “motor set”) or random intervals (demanding reactive responses). Network interactions were assessed using psycho-physiological interaction (PPI), a basic model of functional connectivity evaluating modulatory effects of the dACC in the context of each task condition. Across conditions, OCD were characterized by hyper-modulation by the dACC, with loci alternatively observed as both condition-general and condition-specific. Thus, dynamically driven task demands during simple uni-manual motor control induce compensatory network interactions in cortical-thalamic regions in OCD. These findings support previous research in OCD showing compensatory network interactions during complex memory tasks, but establish that these network effects are observed during basic sensorimotor processing. Thus, these patterns of network dysfunction may in fact be independent of the complexity of tasks used to induce brain network activity. Hypothesis-driven approaches coupled with sophisticated network analyses are a highly valuable approach in using fMRI to uncover mechanisms in disorders like OCD.
1. Introduction
Obsessive-compulsive disorder (OCD) is a common neuropsychiatric disorder typically diagnosed during childhood or adolescence that is characterized by persistent, intrusive thoughts and urges (obsessions) and repetitive, intentional behaviors (compulsions). These compulsive behaviors are often, but not always, aimed at decreasing anxiety caused by obsessions. OCD is highly prevalent in youth: 1-year incidence rates of sub-clinical and clinical OCD in adolescents are 8.4% and 0.7%, respectively (Valleni-Basile et al., 1996), and obsessive-compulsive traits are common and continuously distributed in the general population (Park et al., 2016). Yet, relatively little is known about the underlying brain network interactions that characterize and mediate OCD symptoms. Here we focus on network interactions of the dorsal anterior cingulate cortex (dACC) during simple uni-manual behavior in youth with OCD. These functional investigations are underpinned by a) the relevance of the dACC in OCD-related pathology (Diwadkar et al., 2015; Rosenberg et al., 2004; Szeszko et al., 2004) and b) the structure's importance in control-related mechanisms during simple uni-manual motor responses (Asemi et al., 2015). We employed a simple visuo-motor integration paradigm with varying chronometric and attention demands to induce network dynamics modeling network interactions from the acquired fMRI signals using psychophysiological interaction (PPI), a well-established method for inferring functional connectivity in brain networks (Friston et al., 1997; O'Reilly et al., 2012; Silverstein et al., 2016).
OCD often manifests during childhood and adolescence, an aspect of significance, given that brain networks remain dynamic in this critical phase of the life span (Paus et al., 2008), thus motivating our focus on youth. By implication, neurodevelopmental mechanisms are salient in the disorder (Rosenberg and Keshavan, 1998), and indeed the emergence in youth leads to persistent dysfunctional neural organization and longstanding behavioral symptoms of OCD into adulthood (Tottenham and Sheridan, 2009). These patterns of dysfunction in OCD are characterized by an inability to disengage persistent thoughts from behavior, suggestive of impaired functioning, and dysfunctional network profiles of control regions of the brain, including the dACC (Diwadkar et al., 2015), such that disordered brain network interactions may lead to dysfunctional (i.e. plausibly exaggerated) inhibitory control (Bari and Robbins, 2013).
1.1 The dACC and motor control
The dACC occupies a highly specific role in the functional economy of the brain, reflecting both its evolutionary history as well as its anatomical connectivity (Paus, 2001). The structure has significant projections to the motor cortex, priming it to be an influential modulator of core and extended motor networks (Asemi et al., 2015) as well as an interface between sensorimotor and cognitive processing. Moreover, and as previously noted, the structure is of particular relevance in OCD. Previous evidence suggests impairments in both neurochemistry characterized by decreased anterior cingulate glutamate (Rosenberg et al., 2004), and brain network function during complex tasks such as working memory (Diwadkar et al., 2015). Notably, motor control is a more elementary psychological process than working memory or executive control, yet behavioral and electrophysiological studies point to impairments in OCD. During basic flanker tasks characterized by motor response choice, individuals with OCD show increased error-related negativity (ERN) on medial leads, suggestive of performance hyper-monitoring. This hyper-monitoring may in turn impair mechanisms of motor control primarily by impairing behavioral inhibition (Grützmann et al., 2014; van den Heuvel et al., 2015; van Velzen et al., 2014). fMRI evidence of brain activation profiles (i.e., how brain regions are differentially activated under different task conditions) has been slightly equivocal. For example, even when subjects evince increased ERN, they do not necessarily show differential activity in the dorsal and mid-cingulate cortices (Grützmann et al., 2014). These effects suggest that network as opposed to activation profiles may be a more sensitive marker for OCD. Indeed, recent graph theoretic analyses (albeit of resting state fMRI signals) suggest disorganization in “network structure” in OCD, such that more dense local connectivity, but more sparse inter-regional connectivity, may underpin less efficient network profiles (Armstrong et al., 2016).
Given strong evidence of the involvement of the dACC in motor control and the relevant behavioral symptoms of OCD, we investigated brain network profiles (Friston et al., 1997; O'Reilly et al., 2012) of the dACC in OCD during a simple visuo-motor task. The task induces network interactions by requiring uni-manual finger responses to a stimulus flashing at different periodicities (periodic or random). Previous evidence has suggested that variations in task demands induce differential changes in network interactions in healthy adolescents (Asemi et al., 2015). Specifically, as evidenced from behavioral and connectivity analyses, rhythmic tapping in response to a periodically presented stimulus resulted in systematic functional connectivity from the dACC to the supplementary motor area (SMA). These previous results motivate the value of simple manipulations of this visuo-motor paradigm in inducing sophisticated and differential variations in network interactions in adolescence. Our goal was to extend this rationale to the study of network interactions in OCD. Previous evidence has established that the task induces strong directed functional connectivity from the dACC (Asemi et al., 2015), rendering it both a) a suitable behavioral representation for studying motor control mechanisms in OCD and b) sensitive for inducing brain network interactions originating in the dACC.
Increased demands on cortical-striatal-thalamic (CST) circuitry during complex tasks may undermine cortical processing in OCD (Diwadkar et al., 2015; Li and Modi, 2016). It is plausible that the increased demands may express themselves through the dACC, given the structure's sensitivity to task difficulty, error monitoring and cognitive control (Bakshi et al., 2011; Carter et al., 1999). Therefore, we hypothesized increased dACC modulation of CST targets in OCD, particularly during visuo-motor conditions with increased demand.
2. Methods
2.1 Subjects
Thirty-six participants with a diagnosis of OCD and 18 healthy controls (HC), all right-handed, participated in fMRI studies (see Table 1). All participants and their parents were interviewed with the Schedule for Obsessive-Compulsive and Other Behavioral Syndromes (Hanna, 2014) and Schedule for Schizophrenia and Affective Disorders for School-Aged Children-Present and Lifetime Version (Kaufman et al., 1997). The lifetime (maximum) and current severity of OCD were assessed in patients with a modified version of the Children's Yale-Brown Obsessive-Compulsive Disorder Scale (CY-BOCS)(Goodman et al., 1989; Scahill et al., 1997). Lifetime and current Axis I diagnoses were independently confirmed by two clinicians (DRR, GLH) according to DSM-5 criteria. Exclusion criteria for patients and controls included lifetime history of schizophrenia, other psychotic disorder, bipolar disorder, substance abuse or dependence, anorexia nervosa, bulimia nervosa, epilepsy, head injury with sustained loss of consciousness, Huntington's disease, dyskinesia, autism, mental retardation, or a score >15 on the lifetime version of the Social Communication Questionnaire (Rutter et al., 2003). The OCD sample included comorbidities such as general anxiety disorder, attention deficit hyperactivity disorder, depression, oppositional defiant disorder and autism spectrum disorder (Table 1). Additionally, some OCD subjects were currently receiving pharmacotherapy and/or cognitive behavioral therapy at the time the study was performed. This is also outlined in Table 1. Controls were free of psychopathology. Parents or legal guardians provided written informed consent and children gave written assent prior to participating in the study. The Human Subjects Investigative committees at Wayne State University and the University of Michigan approved the protocol and all methods.
Table 1.
Demographics for healthy control (HC) and OCD participants.
| M/F | Mean age | Range | Height (in.) | Weight (lbs) | CY-BOCS | Comorbidities | Current Treatment | |
|---|---|---|---|---|---|---|---|---|
| Healthy controls (n = 18) | 13/5 | 17.3 (2.88) | 12-21 | 67.3 (4.8) | 154.0 (55.5) | Grooming disorder - 1 | None | |
| OCD (n = 36) | 16/20 | 16.8 (3.14) | 9-21 | 65.1 (4.4) | 139.3 (49.3) |
T: 29/18 (6.8/8.7) O: 15/9 (3.7/4.2) C: 14/9 (1.0/4.9) |
GAD – 11 Separation anxiety – 11 Grooming disorder – 11 Motor/vocaltic – 11 ADHD – 9 Social phobia – 8 Specific phobia – 6 Depression – 6 Enuress – 4 Tourette's – 2 Panic attacks/disorder – 2 Encopresis – 1 ODD – 1 Agoraphobia – 1 ASD – 1 Dysthymia – 1 |
SSRI – 18 Buspirone – 2 |
Groups did not differ in terms of age (t = 0.65, p = 0.671), height (t = 1.499, p = 0.375), weight (t = 0.918, p > 0.05), or gender (χ2 = 3.724, p > 0.05). Values in parentheses represent standard deviation. For lifetime CY-BOCS (lifetime/current): T = Total symptoms, O = Obsessive symptoms, C = Compulsive symptoms. The numbers of subjects with diagnosed comorbidities are listed. GAD: General anxiety disorder; ADHD: attention deficit hyperactivity disorder; Grooming disorder includes nail biting, skin picking; Depression includes major depressive disorder and depression not otherwise specified; ODD: oppositional defiant disorder; ASD: autism spectrum disorder. Number of subjects receiving listed treatments is also noted. SSRI: selective serotonin reuptake inhibitors, includes citalopram, sertraline, fluoxetine.
2.2 fMRI
Gradient echo EPI fMRI data acquisition was conducted at the Vaitkevicius Magnetic Resonance Centre on a 3T Siemens Verio system using a 12-channel volume head coil (TR: 2.6s, TE: 29ms, FOV: 256×256mm2, acquisition matrix: 128×128, 36 axial slices, voxel dimensions: 2 × 2 × 3mm3). In addition, a 3D T1-weighted anatomical MRI image was acquired (TR: 2200ms, TI: 778ms, TE: 3ms, flip-angle=13°, FOV: 256×256mm2, 256 axial slices of thickness = 1.0mm, matrix=256×256). A neuroradiologist reviewed all scans to rule out clinically significant abnormalities.
2.3 Uni-manual Motor Control Task
During the uni-manual motor task, participants were instructed to tap the forefinger of their right hand in response to a flashing white probe (Asemi et al., 2015). Because we were primarily interested in uni-manual responses associated with the periodicity of the motor response, two classes of epochs were assessed (each 30 s duration): a) During Periodic epochs, probes flashed with fixed stimulus onset asynchrony (SOA; .5 s or 1 s) allowing participants to maintain a motor set over the course of the epoch; b) During Random epochs, SOA was randomly perturbed, resulting in reactive responses from participants (see behavioral effects for validation). Across the epochs, SOAs were created by pseudo-randomly sampling values from Gaussian distributions (μ = 1.0/2.0 s; σ = 0.5/1.0 s). The lower bound on the SOA was 300 ms (to exceed typical lower limits in response latency). The number of elicited responses during each of the Periodic and Random conditions was held constant. During the resting control condition between epochs, participants were instructed to fixate on a cross hair in the center of the field of vision. Finger responses were collected from the receptive surface (extent: 33 × 33 mm) of a fiber-optic response touchpad (Current Design Systems, Inc.) interfaced with the Presentation software package (Neurobehavioral Systems, Inc.; Technical issues with the response collection equipment permitted analyses of a limited sample: OCD: 15, HC: 6). During the course of the scan, participants alternated between task and control (rest) epochs (30 s each, four epochs for each of the Periodic and Random conditions and two rest epochs) interspersed with short (10s) rest intervals. The task conditions are schematically depicted in Figure 1.
Figure 1.
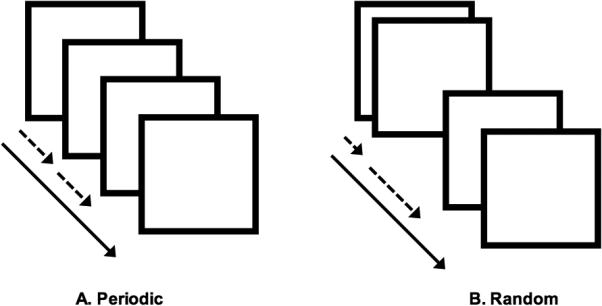
The experimental design is depicted for the (A) periodic and (B) random tasks. The stimulus onset asycnchrony (SOA) was held constant for the periodic task at either .5 s or 1 s, as shown by the equal spacing between the squares representing the flashing white probe. During the random task, the SOA was randomly perturbed, illustrated by the variable spacing in time of the probe represented by the squares. The subjects were instructed to tap their right forefinger in response to each stimulus, and the responses were collected for analysis.
2.4 fMRI Processing
MR images were preprocessed and analyzed using SPM 8 (Statistical Parametric Mapping, Wellcome Department of Imaging and Neuroscience, London, UK) using established methods for temporal (slice timing correction) followed by spatial preprocessing. For spatial pre-processing, the EPI images were manually oriented to the AC-PC line with the reorientation vector applied across the EPI image set, realigned to a reference image to correct for head movement, and co-registered to the anatomical high resolution T1 image. This high-resolution T1 image was normalized to the MNI template, with the resultant deformations subsequently applied to the co-registered EPI images for normalization. Low frequency components were removed using a low-pass filter (128 s) and images were spatially smoothed using a Gaussian filter (8 mm full-width half maximum; FWHM). An autoregressive AR (1) model was used to account for serial correlation, and regressors modeled as box-car vectors (for each of the task-related conditions: Periodic, Random and Rest) were convolved with a canonical hemodynamic reference wave form, with the six motion parameters included as effects of no interest.
Network interactions were modeled using psycho-physiological interaction (PPI) (Friston et al., 1997), a basic model of functional connectivity that characterizes modulatory effects of an a priori defined seed region (e.g., dACC) on target regions during the specific psychological condition of interest (e.g., Periodic or Random). The anatomy of the dACC was motivated by previously published theoretical motivations (Paus, 2001) and empirical evidence (Carter et al., 1998; Hoffstaedter et al., 2014; Woodcock et al., 2016)(See Supplementary Figure 1 for definition) including the supragenual and rostral elements of Brodmann Areas 24 and 32. For each subject, time series from the dACC (effects of interest, p < 0.05; see Supplementary Figure 1 for anatomical definition) were extracted, subsequently convolved with the contrasts of interest (Periodic > Rest; Random > Rest), with the resultant interaction positively weighted to assess the facilitative effects of the dACC in each task context. Each participant contributed two first-level PPI maps for subsequent group-level random effects analyses, modeled using independent sample t-tests. These separate models allowed us to quantify differences in dACC modulation between OCD and HC during Periodic and Random conditions. Significant clusters in regions of interest signifying differences in PPI modulation between HC and OCD were identified using 104 Monte Carlo simulations of the data. These allowed us to identify minimum cluster extents (p<0.05, cluster-level) (Ward, 2000), by estimating the minimum cluster extent in order for activated clusters to be rejected as false positive (noise-only) clusters. The Monte Carlo alpha probability simulation computes the probability of a random field of noise (after accounting for spatial correlations of voxels based on the image smoothness within each region of interest estimated directly from the data set). The resultant cluster size reflects a minimum cluster extent, after the noise is thresholded at a given level. Thus, instead of using the individual voxel probability threshold alone in achieving the desired overall significance level, the method uses a combination of both probability thresholding and minimum cluster size thresholding. The underlying assumption is that true regions of activation will occur over contiguous voxels whereas noise has much less of a tendency to form clusters of activated voxels.
3. Results
3.1 Behavior
Available response data were analyzed to separately assess effects of condition and group on the frequencies of missed responses, and the latencies for made responses. The percentage of missed responses and latencies for made responses were analyzed in separate repeated measures analyses of variance with task condition (Periodic vs. Random) as the within-participant factor and group (OCD vs. HC) as the between-participant factor. There was no significant effect of task condition on the percentage of missed responses [F(1,19) = 2.67, p > 0.05], suggesting that participants performed with similar accuracy during both task conditions. There was a significant effect of task condition for response latencies [F(1,19) = 30.995, p < 0.001, two-tailed], with the latencies during the Random condition averaging 51 ms longer than the Periodic condition. There was no significant main effect of group for response latencies [F(1,19) = 0.382, p > 0.05] or for percentage of missed responses [F(1,19) = 0.044, p > 0.05], suggesting that both OCD and HC performed similarly.
3.2 PPI Results
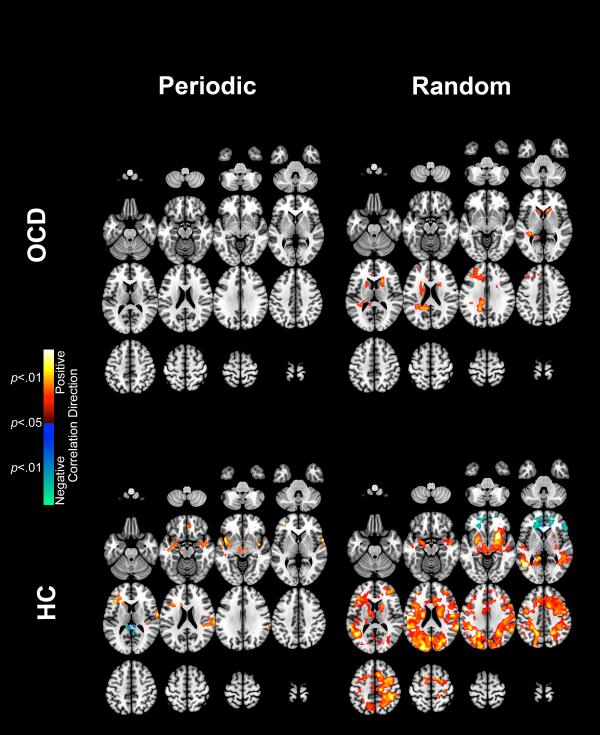
We present the PPI results in the following order. First, we present evidence of hyper-modulation by the dACC in OCD compared to HC during the Periodic condition (Figure 2). Next, we present evidence of hyper-modulation by the dACC in OCD compared to HC during the Random condition (Figure 3). In foreshadowing the results, we note that across both conditions, OCD participants were characterized by hyper-modulation by the dACC, though patterns of overlap and condition-specific effects were observed (explored in Figures 5-7, Discussion). Cluster relevant information is displayed in Table 2.
Figure 2.
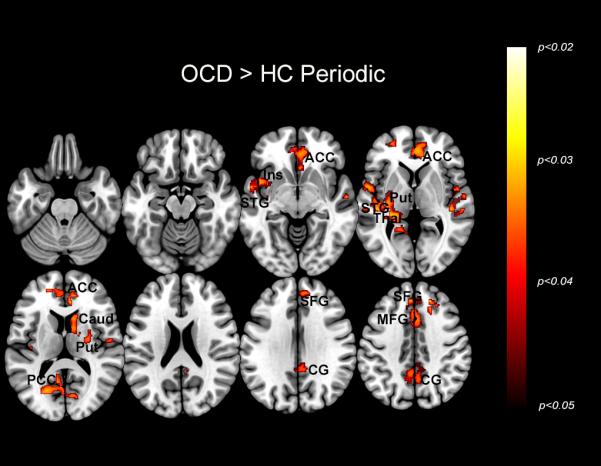
The maps depict regions of exaggerated dACC modulation in OCD during the Periodic condition. The significant clusters (p<0.05, cluster level) are depicted across a mosaic of axial slices. This hyper-modulation by the dACC is seen in the anterior cingulate cortex (ACC), insula (Ins), superior temporal gyrus (STG), putamen (Put), thalamus (Thal), caudate (Caud), posterior cingulate cortex (PCC), superior frontal gyrus (SFG), medial frontal gyrus (MFG), and cingulate gyrus (CG). The inset in the upper left of the figure depicts the task condition, with the squares representing the presentation of visual stimuli across time.
Figure 3.
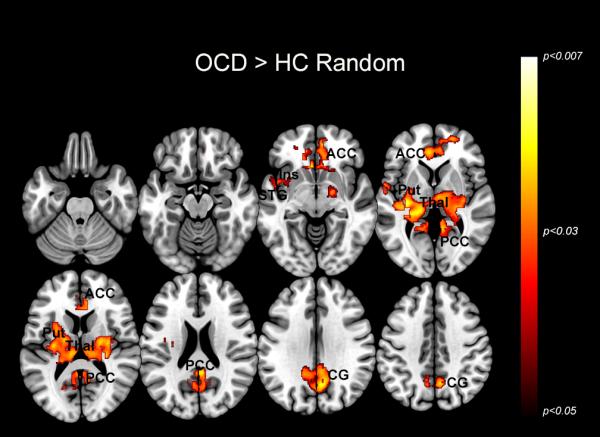
The maps depict regions of exaggerated dACC modulation in OCD during the Random condition. The significant clusters (p<0.05, cluster level) are depicted across a mosaic of axial slices. This hyper-modulation by the dACC is seen in the anterior cingulate cortex (ACC), insula (Ins), superior temporal gyrus (STG), putamen (Put), thalamus (thal), posterior cingulate cortex (PCC), and cingulate gyrus (CG). The inset in the upper left of the figure depicts the task condition, with the squares representing the presentation of visual stimuli across time.
Figure 5.
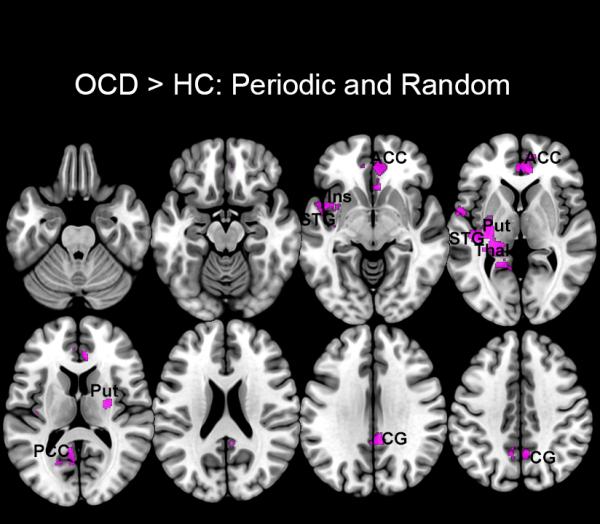
Regions characterized by dACC hyper-modulation during both Periodic and Random conditions are shown. These clusters are based on a union of the clusters sets in Figures 2 and 3 and depict common patterns of hyper-modulation in the anterior cingulate cortex (ACC), insula (Ins), superior temporal gyrus (STG), putamen (Put), thalamus (Thal), posterior cingulate cortex (PCC), and cingulate gyrus (CG).
Figure 7.
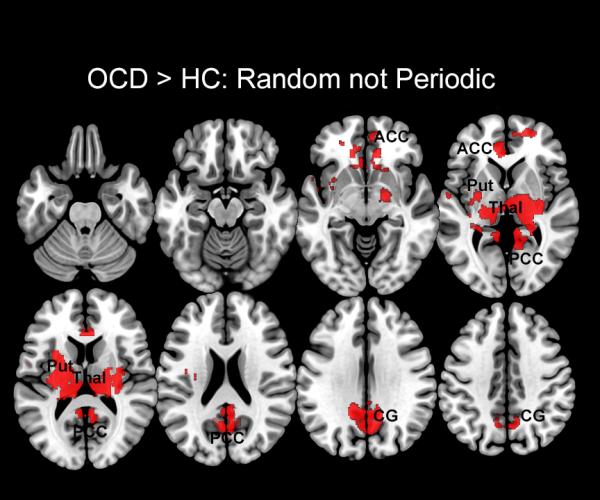
Regions characterized by dACC hyper-modulation during the Random but not Periodic conditions are shown. These clusters depict the difference between the clusters sets in Figure 2 from Figure 3, and are evident in the anterior cingulate cortex (ACC), putamen (Put), thalamus (Thal), posterior cingulate cortex (PCC), and cingulate gyrus (CG).
Table 2.
Information on clusters of significance and peaks showing increased modulation by the dACC in OCD compared to HC (Figures 2 and 3).
| Region (nearest gray matter) | MNI coordinates (x, y, z) | Z score | Cluster Extent | p (peak) | ||
|---|---|---|---|---|---|---|
| Periodic | ||||||
| Superior frontal gyrus | 20 | 38 | 37 | 3.27 | 1247 | 0.001 |
| Posterior cingulate cortex | −12 | −54 | 6 | 3.24 | 826 | 0.001 |
| Anterior cingulate cortex | 10 | 42 | 9 | 3.20 | 1758 | 0.001 |
| Superior temporal gyrus | −56 | 0 | −3 | 3.14 | 2109 | 0.001 |
| Caudate | 12 | 15 | 15 | 2.76 | 770 | 0.003 |
| Thalamus | −27 | −31 | 1 | 2.69 | 2109 | 0.004 |
| Putamen | 27 | −9 | 15 | 2.68 | 770 | 0.004 |
| Superior temporal gyrus | 44 | −25 | 3 | 2.58 | 574 | 0.005 |
| Medial frontal gyrus | −6 | 38 | 40 | 2.47 | 1247 | 0.007 |
| Insula | −40 | −19 | 6 | 2.42 | 2109 | 0.007 |
| Cingulate gyrus | −4 | −51 | 42 | 2.23 | 837 | 0.013 |
| Random | ||||||
| Thalamus | −14 | −27 | 6 | 3.47 | 8213 | 0.000 |
| Posterior cingulate cortex | −14 | −52 | 6 | 3.27 | 4117 | 0.001 |
| Putamen | 20 | −7 | −3 | 3.13 | 8213 | 0.001 |
| Insula | 30 | −28 | 10 | 2.98 | 8213 | 0.001 |
| Anterior cingulate cortex | −14 | 42 | −3 | 2.97 | 1966 | 0.001 |
| Cingulate gyrus | 8 | −49 | 31 | 2.53 | 4117 | 0.006 |
| Superior temporal gyrus | −54 | −3 | 4 | 2.20 | 8213 | 0.01 |
3.2.1 dACC modulation: Periodic Condition
Figure 2 depicts significant clusters (p < 0.05, cluster level) showing dACC hyper-modulation in OCD compared to HC during the Periodic condition. This hyper-modulation by the dACC is seen in the insula, superior temporal gyrus, putamen, thalamus, caudate, cingulate cortex, superior frontal gyrus, and medial frontal gyrus.
3.2.2 dACC modulation: Random Condition
Figure 3 depicts clusters (p < 0.05, cluster level) showing dACC hyper-modulation in OCD compared to HC during the Random condition. This hyper-modulation by the dACC is seen in the insula, superior temporal gyrus, putamen, cingulate cortex and thalamus. The extent of these effects is exaggerated with respect to the Periodic condition, prominently in regions such as the thalamus.
Notably for both conditions, effects were not observed in core regions of the motor system including the primary-, pre- and supplementary motor cortices. Moreover, the regions with exaggerated modulation in OCD are recognized for psychological functions associated with attention and attention gating, and externally guided motor responses.
3.2.3 Developmental effects on network profiles
To characterize age-related effects across the study groups, for each condition and group, the first level PPI maps were submitted to regression modeling, with age as the single regressor of interest. Figure 4 depicts clusters (p < 0.05, cluster level) where age exerted significant statistical effects (both positive and negative) in each group and condition (Cluster relevant information is displayed in Table 3). In general, HC were characterized by a more developmentally articulated relationship between age and patterns of dACC modulation, whereas these effects are moderate to absent in OCD. Moreover, in HC age-related effects were differentially mediated by condition, with a greater developmental effect observed in the random condition in HC.
Figure 4.
The maps depict regions of dACC modulation in OCD and HC regressed positively and negatively by age during both the Periodic and Random conditions. The significant clusters (p<0.05, cluster level) are depicted across a mosaic of axial slices. The peak clusters are listed in Table 3.
Table 3.
Information on clusters of significance and peaks showing positive and negative age regressions for OCD and HC during Periodic and Random tasks (Figure 4).
| Region (nearest gray matter) | MNI coordinates (x, y, z) | Z score | Cluster Extent | p (peak) | ||
|---|---|---|---|---|---|---|
| OCD: Periodic – no clusters of significance | ||||||
| OCD: Random – positive regression | ||||||
| Precuneus | −16 | −49 | 39 | 3.24 | 4148 | 0.001 |
| Caudate | 21 | 20 | 10 | 2.96 | 1585 | 0.002 |
| Extra-nuclear | −21 | 5 | 15 | 2.57 | 2833 | 0.005 |
| OCD: Random – negative regression – no clusters of significance | ||||||
| HC: Periodic – positive regression | ||||||
| Precentral gyrus | 63 | 6 | 3 | 3.67 | 2439 | 0.001 |
| Midbrain | −12 | −27 | −14 | 3.37 | 719 | 0.001 |
| Insula | −42 | 5 | −9 | 3.24 | 2068 | 0.001 |
| Inferior frontal gyrus | −40 | 26 | 12 | 2.97 | 1486 | 0.001 |
| Insula | 39 | −3 | −9 | 2.97 | 790 | 0.002 |
| Sub-gyral | 15 | 42 | −14 | 2.06 | 632 | 0.020 |
| HC: Periodic – negative regression | ||||||
| Posterior cingulate | 4 | −52 | 10 | 2.87 | 764 | 0.002 |
| HC: Random – positive regression | ||||||
| Putamen | 24 | 12 | −6 | 4.75 | 81822 | 0.001 |
| Postcentral gyrus | −57 | −16 | 21 | 4.42 | 19076 | 0.001 |
| Middle temporal gyrus | −45 | −57 | 3 | 4.23 | 6419 | 0.001 |
| Insula | −42 | −19 | 19 | 3.92 | 1839 | 0.001 |
| Caudate | 16 | 9 | 15 | 3.91 | 296 | 0.001 |
| Middle frontal gyrus | 36 | 44 | 16 | 3.75 | 21528 | 0.001 |
| Supplementary motor area | 9 | 12 | 48 | 3.74 | 21528 | 0.001 |
| Thalamus | −16 | −12 | 18 | 3.05 | 374 | 0.001 |
| HC: Random – negative regression | ||||||
| Medial frontal gyrus | −14 | 52 | −5 | 3.60 | 2780 | 0.001 |
| Inferior frontal gyrus | 52 | 28 | 3 | 2.93 | 394 | 0.002 |
4. Discussion
Previous studies have used more complex working memory tasks to study OCD (Diwadkar et al., 2015; Li and Modi, 2016), and have shown complex compensatory increases in modulation of cortical-striatal activity by the dACC. Here we extend these concepts in two ways: 1) First, we use more basic sensorimotor paradigms that do not rely on complex behavioral processing and can be more tractably performed across adolescence and 2) We demonstrate that network effects generalize, suggesting a “trait-related” pattern of network dysfunction in OCD that may be relatively independent of task complexity.
In this study, we studied the modulation of sensorimotor networks by the dACC during a simple uni-manual visuo-motor task with varying demands. The effects of these demands were revealed in the behavioral data, such that the random condition resulted in longer response latencies, and in more missed responses than the periodic condition. Moreover, the absence of main effects of group are important in suggesting that task compliance and performance was similar for OCD and HC. This null effect permits interpretation of the fMRI results without the confounds of generalized performance deficits that can affect clinical fMRI studies (Carter et al., 2008), and allow us to interpret exaggerated modulation effects from the perspective of compensatory network responses in OCD.
The differences in network profiles were clear: 1) OCD participants were characterized by increased dACC modulation of cortical regions, regardless of the demands of the task. Notably, increased modulation was evident in core regions of the executive attention network including the middle frontal and cingulate gyri, the basal ganglia and the thalamic nuclei (Visintin et al., 2015; Xuan et al., 2016), but not in regions that lie in core motor sub-networks (including regions such as the primary-, pre- and supplementary motor cortices); 2) The degree of “hyper”-modulation varied as a function of the sensorimotor demands of the task. During random epochs, where the task demands induced greater attention processing by virtue of the unpredictable demands of the task, dACC hyper-modulation was more pronounced.
The study was not optimized to assess neurodevelopmental differences, yet our cross-sectional explorations revealed interesting developmental patterns across study groups (Figure 4). Most generally, the results suggested that age predicted dACC network profiles differently in OCD and HC. Specifically, during random epochs, participant age in HC was a strong positive predictor of dACC profiles, more so than in OCD (Figure 4, right panels). In HC, these effects were observed in bilateral striatal, thalamic, frontal, insular, parietal and visual regions; In OCD, the effects were circumscribed, restricted to the pre-cuneus and bilateral caudate nuclei. By comparison, in HC negative correlations with age were observed in the inferior frontal cortex. Age was less of a predictor of dACC profiles for the periodic condition (Figure 4, left panels) with no effects observed in OCD, and relatively circumscribed clusters in HC. Because OCD is considered to be neurodevelopmental in origin, age should be less predictive of fMRI-estimated changes in brain function through the adolescent developmental window. Indeed, these general effects are consistent with previous evidence of neurodevelopmental deviations in OCD (Boedhoe et al., 2016; Fitzgerald et al., 2011). The specific nature of these developmental differences will need more detailed modeling of developmental trends (Stanley et al., 2008), but the results support the notion that age is generally a poorer predictor of brain profiles in illness than in health (Keshavan et al., 2005; Shaw et al., 2011). These statistical effects may reflect a lack of age-related synchrony in network interactions that may result from or drive clinical disorders.
The absence of effects in motor regions, and the pronounced presence of effects in attention regions, highlights the effectiveness of this simple task in discovering dysfunction in “higher order” networks in OCD. The effects suggest that the patterns of dysfunction are directed towards brain sub-networks that are most germane to the psychological sub-processes of interest, in this case attention. Below, we highlight the relevance of extant studies of brain networks in OCD, and place our result in an overall framework that relates to the biological bases of the disorder.
In analyzing the effects of the task condition on dACC related network profiles, three classes of brain regions could be identified: a) regions that were characterized by hyper-modulation during both periodic and random conditions, and therefore suggestive of task-independent network impairment in OCD (shown in Figure 5); b) regions of specific hyper-modulation during periodic (but not random epochs)(shown in Figure 6), and most extensively c) regions of specific hyper-modulation during random (but not periodic epochs)(shown in Figure 7) that highlight demand related effects on network dysfunction.
Figure 6.
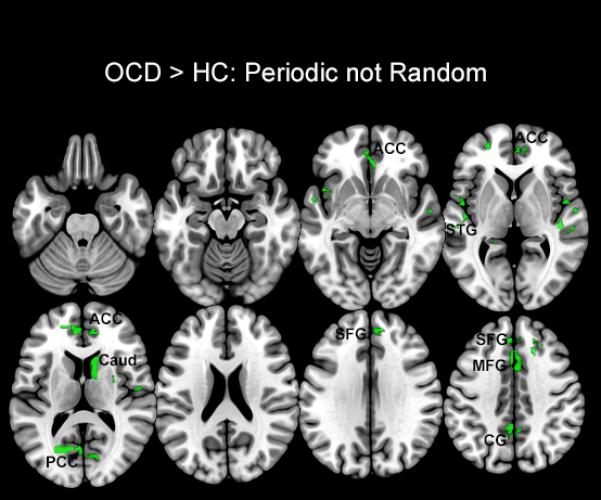
Regions characterized by dACC hyper-modulation during the Periodic but not Random conditions are shown. These clusters depict the difference between the clusters sets in Figure 3 from Figure 2, and are evident in regions of the anterior cingulate cortex (ACC), superior temporal gyrus (STG), caudate (Caud), posterior cingulate cortex (PCC), superior frontal gyrus (SFG), medial frontal gyrus (MFG), and cingulate gyrus (CG).
4.1 Common substrates of network dysfunction in OCD
During both conditions, OCD participants were characterized by dACC hyper-modulation of a network of regions, including the superior temporal gyrus, putamen, insula, anterior and posterior cingulate cortices, cingulate gyrus, and thalamus (Figure 5). The putative functions of these regions reinforce the idea that generalized network dysfunction in OCD may be sensitive to attention-related effects associated with the paradigm.
Both periodic and random conditions required the integration of visual stimuli and the output of motor function, demanding extended periods of attention commitment to sequences of briefly presented visual stimuli. The thalamus is an important center that gates sensory inputs and projects outputs to cortical and subcortical targets (Haber and Calzavara, 2009), and studies suggest that the activity of thalamic nuclei are highly susceptible to descending frontal inputs (Haber and McFarland, 2001), and that descending network effects are detectable with network modeling (Jagtap and Diwadkar, 2016). Thus, the hyper-modulation of the thalamus across conditions suggests plausibly exaggerated mechanisms of “top-down” control of frontal-thalamic processing units in OCD. This may reflect a compensatory attention mechanism in brain networks in OCD regardless of the extent of the attention demands of the task. The superior temporal gyrus is activated during externally guided finger tapping tasks (Adhikari et al., 2013; Witt et al., 2008), suggesting its fundamental role in finger movements. Moreover, studies demonstrate that the structure is also engaged during obligatory attention tasks (Alho et al., 2014), suggesting that the common bases of hyper-modulation reflects an interface between exaggerated motor and attention control in OCD. The basal ganglia have been previously implicated in both finger-tapping tasks (Witt et al., 2008) and in the executive attention network (Diwadkar et al., 2011). Studies indicate that the basal ganglia are activated during rhythmic finger movements to externally guided stimuli (Bednark et al., 2015), suggestive of complimentary functionality to the core motor system. Moreover, the basal ganglia and the putamen are implicated in OCD: The size and shape of the putamen has been correlated with obsessive and compulsive symptoms, with marked bilateral hypertrophy accompanying traits of OCD (Kubota et al., 2016). Our results show dACC hyper-modulation of the putamen in OCD, suggesting dysfunctional network interactions involving extended motor regions.
Previous evidence has also supported abnormalities of the anterior and posterior cingulate cortices in OCD, including lower gray matter density (Tang et al., 2013) and lower glutathione levels suggestive of increased oxidative stress (Brennan et al., 2016). From a functional perspective, aspects of the cingulate cortex have been implicated in the pacing of auditory temporal predictions in finger tapping tasks (Hoffstaedter et al., 2013; Pecenka et al., 2013), suggesting a functional relevance for its hyper-modulation regardless of task condition. Moreover, the anterior cingulate cortex plays a key role in attention and performance monitoring during finger tapping tasks (Wesley and Bickel, 2014), and in the context of OCD, previous studies have shown greater activity of the anterior cingulate cortex in OCD in relation to error processing correlated with disease severity (Fitzgerald and Taylor, 2015) and during working memory (Diwadkar et al., 2015).
Finally, across conditions, OCD were characterized by hyper-modulation of the insula. The insula is part of the extended motor circuit, known to play a role in the sensorimotor synchronization of visually-paced finger tapping, as well as movement of the hands (Witt et al., 2008). The structure has also been implicated in OCD, with anatomical (Pujol et al., 2004; Valente et al., 2005) and functional abnormalities (van den Heuvel et al., 2005; Velikova et al., 2010). These patterns of condition-independent hyper-modulation may reflect network impairment that is central to the attention and motor demands that are common across both periodic and random conditions.
4.2 Network dysfunction during specific task conditions
During the Periodic condition, OCD were characterized by dACC hyper-modulation of sub-areas in the superior temporal gyrus, caudate, cingulate gyrus and superior and medial frontal gyri (Figure 6). Previous evidence suggests that motor responses to periodically presented external stimuli assist in the development of a motor set, facilitated by the establishment of stable control processes within core motor sub-networks (Asemi et al., 2015). The dACC may play a particularly salient role in mediating this automated control of motor movements in an efficient and autonomous manner (Paus, 2001), and as confirmed in an analysis of behavioral latencies, responses during the periodic condition were more efficient (on average 51 ms faster than the random condition). We expect that the hyper-modulatory effects of the dACC may reflect a compensatory network signature on visual motor and attention sub-networks to maintain efficient motor control.
The Random condition is noted for the presentation of unpredictable visual onsets, that pre-empt the establishment of motor sets in responding, lead to correspondingly increased attention demand, and subsequent decrement in performance (51 ms slower than the periodic condition). We expect these behavioral effects to be reflected in network profiles in OCD, and indeed as depicted in Figure 7, the random condition induced highly exaggerated dACC modulation in multiple brain regions including aspects of the cingulate cortices, the putamen, and most notably the thalamus (Figure 7).
The highly exaggerated pattern of bilateral thalamic modulation extends evidence from Figure 5, with these extensive effects, reiterating the importance of the thalamus in attention gating (McAlonan et al., 2008), and suggesting that in OCD, compensatory descending hyper-modulation serves to underpin task performance. These implications generalize to both the cingulate gyrus and the putamen, each of which have been implicated in attention processing, finger tapping, or both (Diwadkar et al., 2011; Fan et al., 2005; Ullen et al., 2003).
The behavioral data provide partial support for the speculation that attention demands may drive many of the observed network effects. It is evident that OCD did not differ from HC in terms of response times. While this lack of a difference may be attributed to a relatively small sample size, the literature is equivocal in this regard. Studies using tasks with substantial attention demands have reported both a slowing in OCD youth (Hirschtritt et al., 2009), but also no differences between OCD and HC (Cocchi et al., 2012). In general however, the latency data from the available behavioral responses unequivocally suggest that the random condition was more challenging (though the data on miss rates did not). This notion that our observed effects relate to attention remain speculative as does the possibility that our results might be a result of impairments in OCD related to predicting error likelihood or in predictive coding.
While the experiment (and our analysis) is not specifically designed to address issues of predicting error likelihood (Brown and Braver, 2005) or predictive coding (Friston et al., 2014), it is highly plausible that the exaggerated network profiles of the dACC do indeed reflect aberrations in either or both of those mechanisms in OCD. Because behavioral errors and the realization of errors are highly salient, expectedly the dACC and its network signatures are highly sensitive to errors particularly on tasks involving choice between competing responses (Ham et al., 2013). Indeed impaired error monitoring is associated with OC dimensions (O'Toole et al., 2012). However, in our paradigm, a finger response was simply evoked by a binary response cue (the presentation of the visual probe), suggesting more likely that the task evoked principles of predictive coding in the motor system (Adams et al., 2013). In our case, it is plausible that descending predictions from the motor system are adjusted to the incoming sensory inputs that generate an ascending prediction error (from the visual system), that themselves are sensitive to the descending predictions. The reiterative adjustment of motor prediction to the visual inputs may be a mechanism that optimizes neural responses to sensory inputs (Friston, 2009). Impaired prediction error is an emerging framework for understanding dysfunction in illnesses such as schizophrenia (Friston et al., 2016), and it is plausible that clinical obsessions distort neural mechanisms for optimizing prediction error in OCD. Because the uncertainty of sensory onsets in the random condition is certainly greater than those in the periodic condition, it is certainly true that participants are operating under a higher degree of uncertainty (reflected in the response profiles for the periodic and random conditions). However, it is also plausible that the relatively brief condition blocks are too short for the system to learn that the onsets in the periodic condition have certainty. In sum, it is wholly plausible that the exaggerated network profiles of the dACC reflect an aberrant or compensatory network signature of dysfunctional error prediction mechanisms in OCD, though we note the challenge in conclusively stating this. Therefore, these considerations remain speculative and must await more detailed experimental designs that permit more powerful and appropriately tailored analyses of the fMRI signals.
4.3 Conclusions and Limitations
Understanding brain network dysfunction is of particular interest in clinical neuroscience. As suggested by the National Institute of Mental Health (Insel et al., 2010), understanding of network profiles and patterns of dysfunction can better describe mechanisms underlying the behavioral phenotypes seen in clinical psychiatric illness. Additionally, brain network profiles may be indicative of neurochemical dysfunction which characterizes psychiatric disease (Almeida et al., 2009; Diwadkar et al., 2012; Schmidt et al., 2013). From this perspective, fMRI is a useful tool for probing task-induced brain network dynamics assessed at the macroscopic scale (Passingham et al., 2013)(Silverstein et al., 2016). The spatial and temporal resolutions of the technique are blind to multiple and specific neuronal signals (Logothetis, 2008), and the fMRI or BOLD (Blood Oxygen Level Dependent) response has been characterized as a “sink” into which a multiplicity of neuronal mechanisms contribute signals (Singh, 2012). Having participants perform well-specified paradigms that induce variations in the temporal signals of brain regions can dynamically perturb the agglomerated BOLD signals. When modeled with appropriate time-domain related connectivity techniques (Friston, 2011), it is possible to discover patterns of task-induced brain network interactions that distinguish clinical (e.g., OCD) from healthy populations.
Inferring brain function (and dysfunction) from fMRI data presents a non-trivial challenge, largely because fMRI signals are overt manifestations of hidden brain states that must be recovered through the quantitative modeling (or “reverse engineering”) of those signals (Friston, 2009)(Silverstein et al., 2016). From that perspective, the current work was explicitly guided by the value of using uni-manual motor control tasks to assess the network profiles of the dACC, a region that has previously been theoretically and experimentally linked to motor control. These strengths allow us to interpret our results from the perspective of network dysfunction in OCD, and how this dysfunction is manifest under basic uni-manual tasks. In that sense, the task, the analyses and the results provide a simple, yet logically (and perhaps clinically) useful framework for assessing network dysfunction in OCD, complementing previous assessments using more complex paradigms (Diwadkar et al., 2015). Although these more complex paradigms have previously demonstrated compensatory cortico-striato-thalamo-cortical circuitry in OCD (Diwadkar et al., 2015; Li and Mody, 2016) and inefficient neural recruitment in frontoparietal networks (de Vries et al., 2013), the present study is able to extend our understanding of more general network profiles in OCD.
PPIs constitute a limited framework for modeling simple network interactions based on those very signals. In part, PPIs depend on the use of well-motivated tasks that in turn dovetail with well-motivated choices for seed regions the modulatory effects of which must be assessed (O'Reilly et al., 2012). It is not possible to ascertain precise neuronal correlates of these effects in OCD, because the analytic method's basis is statistical (Friston et al., 1997), in effect simply characterizing contributions of activity in one region to that in another. Therefore, the effects observed do not disambiguate whether OCD participants are characterized by hyper-“signaling” by the dACC, or the effects are amplified at the target by intermediate (and un-modeled effects). Moreover, issues of causality are somewhat orthogonal to these analyses given the emphasis on purely statistical effects, and the hidden (and potentially common) mediation of these effects by other regions cannot be ascertained.
Nevertheless, within a clinical framework, these methods allow a quantitatively and objectively characterized glimpse into brain network dysfunction in OCD. In our ongoing analyses, we are attempting stronger connectivity analyses employing fuller measures of effective connectivity (Friston et al., 2003; Jagtap and Diwadkar, 2016), and are exploring relationships between the degree of network dysfunction in OCD and clinical dimensions. We also note that we are limited in addressing the correspondence between the observed behavioral effects (see Results) and the compelling fMRI network effects as we are compromised by the smaller sample size of the behavioral analyses. The observed behavioral effects do constitute a partial replication of previously published results in an independent sample (Asemi et al., 2015), but more extensive replication in OCD will be valuable. Finally, these analyses are an initial step in a more general program of research that will seek to systematically relate network analyses of fMRI signals to clinical dimensions in OCD. As such (see Table 1), our OCD sample was characterized by comorbidities including general anxiety disorder, attention deficit hyperactivity disorder, depression, oppositional defiant disorder and autism spectrum disorder, and we are not yet in a position to systematically assess the effects of symptom dimensions on the assessed network profiles. Moreover, the absence of effects within core motor circuitry is notable, given that OCD appear to be characterized by reduced resting and active motor thresholds (Khedr et al., 2016). Recent work in larger samples has suggested that OCD subjects show altered network profiles in supplementary motor and primary motor networks (Armstrong et al., 2016) though other studies have not reported similar effects (Harrison et al., 2009). It is likely that the specific effects in motor regions may well be mediated by the degree of obsessive and compulsive symptoms, and understanding these relationships is an ongoing focus of our work.
It has been argued that network-based and a priori approaches must necessarily be applied with fMRI data if mechanisms of brain function and dysfunction are to be divined (Stephan and Roebroeck, 2012). We place our efforts as consistent with these prescriptions.
Supplementary Material
We studied youth with OCD compared to healthy controls using fMRI data acquired during a simple uni-manual motor response paradigm.
fMRI data were modeled using functional connectivity techniques to assess network profiles of the dorsal anterior cingulate cortex (dACC) across task conditions.
OCD were characterized by exaggerated network modulation by the dACC, particularly in brain regions associated with attention and executive processing.
These results imply that basic visuo-motor paradigms can serve as powerful modulators of underlying brain network function, and by corollary, successfully define aberrant brain network profiles in OCD.
Acknowledgements
This work was supported by the National Institute of Mental Health (MH059299), the Children's Hospital of Michigan Foundation, the Prechter World Bipolar Foundation, the Lyckaki-Young Fund from the State of Michigan, the Miriam Hamburger Endowed Chair of Child Psychiatry, the Paul and Anita Strauss Endowment, the Donald and Mary Kosch Foundation, a Charles H. Gershenshon Distinguished Faculty Fellowship, the Cohen Neuroscience Endowment, the Detroit Wayne County Mental Health Authority and Gateway Community Health. The funding agencies played no role in the analyses or reporting of the data. We thank three anonymous reviewers for helpful comments on prior versions of the manuscript.
Footnotes
Conflicts of Interest
The authors report no conflicts of interest.
REFERENCES
- Adams RA, Shipp S, Friston KJ. Predictions not commands: active inference in the motor system. Brain Struct Funct. 2013;218:611–643. doi: 10.1007/s00429-012-0475-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhikari BM, Quinn KM, Dhamala M. Is the brain's inertia for motor movements different for acceleration and deceleration? PLoS ONE. 2013;8:e78055. doi: 10.1371/journal.pone.0078055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alho K, Rinne T, Herron TJ, Woods DL. Stimulus-dependent activations and attention-related modulations in the auditory cortex: a meta-analysis of fMRI studies. Hear Res. 2014;307:29–41. doi: 10.1016/j.heares.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Almeida JR, Versace A, Mechelli A, Hassel S, Quevedo K, Kupfer DJ, Phillips ML. Abnormal amygdala-prefrontal effective connectivity to happy faces differentiates bipolar from major depression. Biological Psychiatry. 2009;66:451–459. doi: 10.1016/j.biopsych.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong CC, Moody TD, Feusner JD, McCracken JT, Chang S, Levitt JG, Piacentini JC, O'Neill J. Graph-theoretical analysis of resting-state fMRI in pediatric obsessive-compulsive disorder. Journal of Affective Disorders. 2016;193:175–184. doi: 10.1016/j.jad.2015.12.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asemi A, Ramaseshan K, Burgess A, Diwadkar VA, Bressler SL. Dorsal anterior cingulate cortex modulates supplementary motor area in coordinated unimanual motor behavior. Front Hum Neurosci. 2015;9:309. doi: 10.3389/fnhum.2015.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakshi N, Pruitt P, Radwan J, Keshavan MS, Rajan U, Zajac-Benitez C, Diwadkar VA. Inefficiently increased anterior cingulate modulation of cortical systems during working memory in young offspring of schizophrenia patients. J Psychiatr Res. 2011;45:1067–1076. doi: 10.1016/j.jpsychires.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Bari A, Robbins TW. Inhibition and impulsivity: behavioral and neural basis of response control. Progress in neurobiology. 2013;108:44–79. doi: 10.1016/j.pneurobio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Bednark JG, Campbell ME, Cunnington R. Basal ganglia and cortical networks for sequential ordering and rhythm of complex movements. Front Hum Neurosci. 2015;9:421. doi: 10.3389/fnhum.2015.00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boedhoe PS, Schmaal L, Abe Y, Ameis SH, Arnold PD, Batistuzzo MC, Benedetti F, Beucke JC, Bollettini I, Bose A, Brem S, Calvo A, Cheng Y, Cho KI, Dallaspezia S, Denys D, Fitzgerald KD, Fouche JP, Gimenez M, Gruner P, Hanna GL, Hibar DP, Hoexter MQ, Hu H, Huyser C, Ikari K, Jahanshad N, Kathmann N, Kaufmann C, Koch K, Kwon JS, Lazaro L, Liu Y, Lochner C, Marsh R, Martinez-Zalacain I, Mataix-Cols D, Menchon JM, Minuzzi L, Nakamae T, Nakao T, Narayanaswamy JC, Piras F, Piras F, Pittenger C, Reddy YC, Sato JR, Simpson HB, Soreni N, Soriano-Mas C, Spalletta G, Stevens MC, Szeszko PR, Tolin DF, Venkatasubramanian G, Walitza S, Wang Z, van Wingen GA, Xu J, Xu X, Yun JY, Zhao Q, Group EOW, Thompson PM, Stein DJ, van den Heuvel OA. Distinct Subcortical Volume Alterations in Pediatric and Adult OCD: A Worldwide Meta- and Mega-Analysis. The American Journal of Psychiatry. 2016:appiajp201616020201. doi: 10.1176/appi.ajp.2016.16020201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan BP, Jensen JE, Perriello C, Pope HG, Jr., Jenike MA, Hudson JI, Rauch SL, Kaufman MJ. Lower posterior cingulate cortex glutathione levels in obsessive-compulsive disorder. Biological Psychiatry : Cognitive Neuroscience and Neuroimaging. 2016;1:116–124. doi: 10.1016/j.bpsc.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JW, Braver TS. Learned predictions of error likelihood in the anterior cingulate cortex. Science. 2005;307:1118–1121. doi: 10.1126/science.1105783. [DOI] [PubMed] [Google Scholar]
- Carter CS, Botvinick MM, Cohen JD. The contribution of the anterior cingulate cortex to executive processes in cognition. Reviews in the Neurosciences. 1999;10:49–57. doi: 10.1515/revneuro.1999.10.1.49. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Carter CS, Heckers S, Nichols T, Pine DS, Strother S. Optimizing the design and analysis of clinical functional magnetic resonance imaging research studies. Biological Psychiatry. 2008;64:842–849. doi: 10.1016/j.biopsych.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Cocchi L, Harrison BJ, Pujol J, Harding IH, Fornito A, Pantelis C, Yucel M. Functional alterations of large-scale brain networks related to cognitive control in obsessive-compulsive disorder. Human Brain Mapping. 2012;33:1089–1106. doi: 10.1002/hbm.21270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries FE, de Wit SJ, Cath DC, van der Werf YD, van der Borden V, van Rossum TB, van Balkom AJLM, van der Wee NJA, Veltman DJ, van den Heuvel OA. Compensatory frontoparietal activity during working memory: An endophenotype of obsessive-compulsive disorder. Biol Psychiatry. 2013;76:878–887. doi: 10.1016/j.biopsych.2013.11.021. [DOI] [PubMed] [Google Scholar]
- Diwadkar VA, Burgess A, Hong E, Rix C, Arnold PD, Hanna GL, Rosenberg DR. Dysfunctional activation and brain network profiles in youth with obsessive-compulsive disorder: A focus on the dorsal anterior cingulate during working memory. Front Hum Neurosci. 2015;9:149. doi: 10.3389/fnhum.2015.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwadkar VA, Pruitt P, Goradia D, Murphy E, Bakshi N, Keshavan MS, Rajan U, Reid A, Zajac-Benitez C. Fronto-parietal hypo-activation during working memory independent of structural abnormalities: conjoint fMRI and sMRI analyses in adolescent offspring of schizophrenia patients. NeuroImage. 2011;58:234–241. doi: 10.1016/j.neuroimage.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwadkar VA, Wadehra S, Pruitt P, Keshavan MS, Rajan U, Zajac-Benitez C, Eickhoff SB. Disordered cortico-limbic interactions during affective processing in children and adolescents at risk for schizophrenia revealed by fMRI and Dynamic Causal Modeling. Archives of General Psychiatry. 2012;69:231–242. doi: 10.1001/archgenpsychiatry.2011.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. NeuroImage. 2005;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KD, Taylor SF. Error-processing abnormalities in pediatric anxiety and obsessive compulsive disorders. CNS Spectr. 2015;20:346–354. doi: 10.1017/S1092852915000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald KD, Welsh RC, Stern ER, Angstadt M, Hanna GL, Abelson JL, Taylor SF. Developmental alterations of frontal-striatal-thalamic connectivity in obsessive-compulsive disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50:938–948. e933. doi: 10.1016/j.jaac.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K. The free-energy principle: a rough guide to the brain? Trends in Cognitive Sciences. 2009;13:293–301. doi: 10.1016/j.tics.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Friston KJ. Functional and effective connectivity: a review. Brain Connect. 2011;1:13–36. doi: 10.1089/brain.2011.0008. [DOI] [PubMed] [Google Scholar]
- Friston K, Brown HR, Siemerkus J, Stephan KE. The dysconnection hypothesis (2016). Schizophrenia Research. 2016;176:83–94. doi: 10.1016/j.schres.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W. Dynamic causal modelling. NeuroImage. 2003;19:1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Stephan KE, Montague R, Dolan RJ. Computational psychiatry: the brain as a phantastic organ. The Lancet. Psychiatry. 2014;1:148–158. doi: 10.1016/S2215-0366(14)70275-5. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Delgado P, Heninger GR, Charney DS. The Yale-Brown Obsessive Compulsive Scale. II. Validity. Arch Gen Psychiatry. 1989;46:1012–1016. doi: 10.1001/archpsyc.1989.01810110054008. [DOI] [PubMed] [Google Scholar]
- Grützmann R, Endrass T, Kaufmann C, Allen E, Eichele T, Kathmann N. Presupplementary motor area contributes to altered error monitoring in obsessive- compulsive disorder. Biol Psychiatry. 2014:1–10. doi: 10.1016/j.biopsych.2014.12.010. [DOI] [PubMed] [Google Scholar]
- Haber S, McFarland NR. The place of the thalamus in frontal cortical-basal ganglia circuits. Neuroscientist. 2001;7:315–324. doi: 10.1177/107385840100700408. [DOI] [PubMed] [Google Scholar]
- Ham T, Leff A, de Boissezon X, Joffe A, Sharp DJ. Cognitive control and the salience network: an investigation of error processing and effective connectivity. J Neurosci. 2013;33:7091–7098. doi: 10.1523/JNEUROSCI.4692-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna GL. Schedule for Obsessive-Compulsive and Other Behavioral Syndromes (SOCOBS) University of Michigan; Ann Arbor, MI.: 2014. [Google Scholar]
- Harrison BJ, Soriano-Mas C, Pujol J, Ortiz H, Lopez-Sola M, Hernandez-Ribas R, Deus J, Alonso P, Yucel M, Pantelis C, Menchon JM, Cardoner N. Altered corticostriatal functional connectivity in obsessive-compulsive disorder. Archives of General Psychiatry. 2009;66:1189–1200. doi: 10.1001/archgenpsychiatry.2009.152. [DOI] [PubMed] [Google Scholar]
- Hirschtritt ME, Hammond CJ, Luckenbaugh D, Buhle J, Thurm AE, Casey BJ, Swedo SE. Executive and attention functioning among children in the PANDAS subgroup. Child Neuropsychol. 2009;15:179–194. doi: 10.1080/09297040802186899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffstaedter F, Grefkes C, Caspers S, Roski C, Palomero-Gallagher N, Laird AR, Fox PT, Eickhoff SB. The role of anterior midcingulate cortex in cognitive motor control: Evidence from functional connectivity analyses. Hum Brain Mapp. 2014;36:2741–53. doi: 10.1002/hbm.22363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P. Research Domain Criteria (RDoC): Toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Jagtap P, Diwadkar VA. Effective connectivity of ascending and descending frontalthalamic pathways during sustained attention: Complex brain network interactions in adolescence. Human Brain Mapping. 2016;37:2557–70. doi: 10.1002/hbm.23196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–8. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Diwadkar VA, Rosenberg DR. Developmental biomarkers in schizophrenia and other psychiatric disorders: common origins, different trajectories? Epidemiologia e Psichiatria Sociale. 2005;14:188–193. doi: 10.1017/s1121189x00007934. [DOI] [PubMed] [Google Scholar]
- Khedr EM, Elbeh KA, Elserogy Y, Khalifa HE, Ahmed MA, Hafez MH, Ali AM, Elfetoh NA. Motor cortical excitability in obsessive-compulsive disorder: Transcranial magnetic stimulation study. Neurophysiol Clin. 2016;46:135–143. doi: 10.1016/j.neucli.2016.02.003. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Sato W, Kochiyama T, Uono S, Yoshimura S, Sawada R, Sakihama M, Toichi M. Putamen volume correlates with obsessive compulsive characteristics in healthy population. Psychiatry Res Neuroimaging. 2016:1–8. doi: 10.1016/j.pscychresns.2016.01.014. [DOI] [PubMed] [Google Scholar]
- Li B, Mody M. Cortico-striato-thalamo-cortical circuitry, working memory, and obsessive-compulsive disorder. Frontiers in Psychiatry. 2016;7:1–3. doi: 10.3389/fpsyt.2016.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008;453:869–878. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- McAlonan K, Cavanaugh J, Wurtz RH. Guarding the gateway to cortex with attention in visual thalamus. Nature. 2008;456:391–4. doi: 10.1038/nature07382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly JX, Woolrich MW, Behrens TE, Smith SM, Johansen-Berg H. Tools of the trade: psychophysiological interactions and functional connectivity. Soc Cogn Affect Neurosci. 2012;7:604–609. doi: 10.1093/scan/nss055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Toole SA, Weinborn M, Fox AM. Performance monitoring among non- patients with obsessive-compulsive symptoms: ERP evidence of aberrant feedback monitoring. Biological Psychology. 2012;91:221–228. doi: 10.1016/j.biopsycho.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Park LS, Burton CL, Dupuis A, Shan J, Storch EA, Crosbie J, Schachar RJ, Arnold PD. The Toronto Obsessive-Compulsive Scale: psychometrics of a dimensional measure of obsessive-compulsive traits. Journal of the American Academy of Child and Adolescent Psychiatry. 2016;55:310–318. e314. doi: 10.1016/j.jaac.2016.01.008. [DOI] [PubMed] [Google Scholar]
- Passingham RE, Rowe JB, Sakai K. Has brain imaging discovered anything new about how the brain works? NeuroImage. 2013;66:142–150. doi: 10.1016/j.neuroimage.2012.10.079. [DOI] [PubMed] [Google Scholar]
- Paus T. Primate anterior cingulate cortex: Where motor control, drive and cognition interface. Nat Rev Neurosci. 2001;2:417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecenka N, Engel A, Keller PE. Neural correlates of auditory temporal predictions during sensorimotor synchronization. Front Hum Neurosci. 2013;7:1–16. doi: 10.3389/fnhum.2013.00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol J, Soriano-Mas C, Alonso P, Cardoner N, Menchon JM, Deus J, Vallejo J. Mapping structural brain alterations in obsessive-compulsive disorder. Archives of General Psychiatry. 2004;61:720–730. doi: 10.1001/archpsyc.61.7.720. [DOI] [PubMed] [Google Scholar]
- Rosenberg D, Keshavan M. A. E. Bennett Research Award. Toward a neurodevelopmental model of obsessive-compulsive disorder. Biological Psychiatry. 1998;43:623–640. doi: 10.1016/s0006-3223(97)00443-5. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Mirza Y, Russell A, Tang J, Smith JM, Banerjee SP, Bhandari R, Rose M, Ivey J, Boyd C, Moore GJ. Reduced anterior cingulate glutamatergic concentrations in childhood OCD and major depression versus healthy controls. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43:1146–1153. doi: 10.1097/01.chi.0000132812.44664.2d. [DOI] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C. The Social Communication Questionnaire. Western Psychological Services; Los Angeles: 2003. [Google Scholar]
- Scahill L, Riddle MA, McSwiggin-Hardin M, Ort SI, King RA, Goodman WK, Cicchetti D, Leckman JF. Children's Yale-Brown Obsessive Compulsive Scale: reliability and validity. J Am Acad Child Adolesc Psychiatry. 1997;36:844–852. doi: 10.1097/00004583-199706000-00023. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Smieskova R, Aston J, Simon A, Allen P, Fusar-Poli P, McGuire PK, Riecher-Rossler A, Stephan KE, Borgwardt S. Brain connectivity abnormalities predating the onset of psychosis: correlation with the effect of medication. JAMA Psychiatry. 2013;70:903–912. doi: 10.1001/jamapsychiatry.2013.117. [DOI] [PubMed] [Google Scholar]
- Shaw P, Gogtay N, Rapoport J. Childhood psychiatric disorders as anomalies in neurodevelopmental trajectories. Human Brain Mapping. 2011;31:917–925. doi: 10.1002/hbm.21028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein B, Bressler S, Diwadkar VA. Inferring the dysconnection syndrome in schizophrenia: Interpretational considerations on methods for the network analyses of fMRI data. Frontiers in Psychiatry. 2016;7:132. doi: 10.3389/fpsyt.2016.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh KD. Which “neural activity” do you mean? fMRI, MEG, oscillations and neurotransmitters. NeuroImage. 2012;62:1121–1130. doi: 10.1016/j.neuroimage.2012.01.028. [DOI] [PubMed] [Google Scholar]
- Stanley JA, Kipp H, Greisenegger E, Macmaster FP, Panchalingam K, Keshavan MS, Bukstein OG, Pettegrew JW. Evidence of Developmental Alterations in Cortical and Subcortical Regions of Children with ADHD: A Multi-voxel In Vivo 31P Spectroscopy Study. Archives of General Psychiatry. 2008;65:1419–1428. doi: 10.1001/archgenpsychiatry.2008.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Roebroeck A. A short history of causal modeling of fMRI data. NeuroImage. 2012;62:856–863. doi: 10.1016/j.neuroimage.2012.01.034. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, MacMillan S, McMeniman M, Chen S, Baribault K, Lim KO, Ivey J, Rose M, Banerjee SP, Bhandari R, Moore GJ, Rosenberg DR. Brain structural abnormalities in psychotropic drug-naive pediatric patients with obsessive-compulsive disorder. The American Journal of Psychiatry. 2004;161:1049–1056. doi: 10.1176/appi.ajp.161.6.1049. [DOI] [PubMed] [Google Scholar]
- Tang W, Li B, Huang X, Jiang X, Li F, Wang L, Chen T, Wang J, Gong Q, Yang Y. Morphometric brain characterization of refractory obsessive- compulsive disorder: Diffeomorphic anatomic registration using exponentiated Lie algebra. Prog Neuro-Psychopharmacology Biol Psychiatry. 2013;46:126–131. doi: 10.1016/j.pnpbp.2013.07.011. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Sheridan MA. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Front Hum Neurosci. 2009;3:68. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullen F, Forssberg H, Ehrsson HH. Neural networks for the coordination of the hands in time. Journal of Neurophysiology. 2003;89:1126–1135. doi: 10.1152/jn.00775.2002. [DOI] [PubMed] [Google Scholar]
- Valente AA, Jr., Miguel EC, Castro CC, Amaro E, Jr., Duran FL, Buchpiguel CA, Chitnis X, McGuire PK, Busatto GF. Regional gray matter abnormalities in obsessive-compulsive disorder: a voxel-based morphometry study. Biological Psychiatry. 2005;58:479–487. doi: 10.1016/j.biopsych.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Valleni-Basile LA, Garrison CZ, Waller JL, Addy CL, McKeown RE, Jackson KL, Cuffe SP. Incidence of obsessive-compulsive disorder in a community sample of young adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35:898–906. doi: 10.1097/00004583-199607000-00015. [DOI] [PubMed] [Google Scholar]
- van den Heuvel OA, van Wingen G, Soriano-Mas C, Alonso P, Chamberlain SR, Nakamae T, Denys D, Goudraiaan AE, Veltman DJ. Brain circuitry of compulsivity. Eur Neuropsychopharmacol. 2016;26:810–827. doi: 10.1016/j.euroneuro.2015.12.005. [DOI] [PubMed] [Google Scholar]
- van den Heuvel OA, Veltman DJ, Groenewegen HJ, Witter MP, Merkelbach J, Cath DC, van Balkom AJ, van Oppen P, van Dyck R. Disorder-specific neuroanatomical correlates of attentional bias in obsessive-compulsive disorder, panic disorder, and hypochondriasis. Archives of General Psychiatry. 2005;62:922–933. doi: 10.1001/archpsyc.62.8.922. [DOI] [PubMed] [Google Scholar]
- van Velzen LS, Vriend C, de Wit SJ, van den Heuvel OA. Response inhibition and interference control in obsessive-compulsive spectrum disorders. Front Hum Neurosci. 2014;8:419. doi: 10.3389/fnhum.2014.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velikova S, Locatelli M, Insacco C, Smeraldi E, Comi G, Leocani L. Dysfunctional brain circuitry in obsessive-compulsive disorder: source and coherence analysis of EEG rhythms. NeuroImage. 2010;49:977–983. doi: 10.1016/j.neuroimage.2009.08.015. [DOI] [PubMed] [Google Scholar]
- Visintin E, De Panfilis C, Antonucci C, Capecci C, Marchesi C, Sambataro F. Parsing the intrinsic networks underlying attention: a resting state study. Behavioural Brain Research. 2015;278:315–322. doi: 10.1016/j.bbr.2014.10.002. [DOI] [PubMed] [Google Scholar]
- Ward BD. Simultaneous inference for fMRI data. Milwaukee, WI Med Coll Wisconsin. 2000 [Google Scholar]
- Wesley MJ, Bickel WK. Remember the future II: meta-analyses and functional overlap of working memory and delay discounting. Biol Psychiatry. 2014;75:435–448. doi: 10.1016/j.biopsych.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt ST, Laird AR, Meyerand ME. Functional neuroimaging correlates of finger-tapping task variations: an ALE meta-analysis. NeuroImage. 2008;42:343–356. doi: 10.1016/j.neuroimage.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock EA, Wadehra S, Diwadkar VA. Network profiles of the dorsal anterior cingulate and dorsal prefrontal cortex in schizophrenia during hippocampal- based associative memory. Front Systems Neurosci. 2016;10:32. doi: 10.3389/fnsys.2016.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan B, Mackie MA, Spagna A, Wu T, Tian Y, Hof PR, Fan J. The activation of interactive attentional networks. NeuroImage. 2016;129:308–319. doi: 10.1016/j.neuroimage.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.