Abstract
Abstract. In order to investigate the effects of color stimuli of the Rorschach inkblot method (RIM), the cerebral activity of 40 participants with no history of neurological or psychiatric illness was scanned while they engaged in the Rorschach task. A scanned image of the ten RIM inkblots was projected onto a screen in the MRI scanner. Cerebral activation in response to five achromatic color cards and five chromatic cards were compared. As a result, a significant increase in brain activity was observed in bilateral visual areas V2 and V3, parietooccipital junctions, pulvinars, right superior temporal gyrus, and left premotor cortex for achromatic color cards (p < .001). For the cards with chromatic color, significant increase in brain activity was observed in left visual area V4 and left orbitofrontal cortex (p < .001). Furthermore, a conjoint analysis revealed various regions were activated in responding to the RIM. The neuropsychological underpinnings of the response process, as described by Acklin and Wu-Holt (1996), were largely confirmed.
Keywords: achromatic and chromatic colors, brain hemodynamics, functional magnetic resonance imaging (fMRI), Rorschach
The Rorschach Inkblot Method (RIM), developed by the Swiss psychiatrist Hermann Rorschach in 1921, consists of 10 inkblot plates. These plates include five achromatic color cards and five chromatic color cards. Responses in which clients verbalize or imply the use of chromatic color stimuli are coded as chromatic color responses.
Based on his research, Rorschach (1921/1951) considered color responses to be “the representative of affectivity” (p. 76). He described that the “absolute number of color responses is a good measure of affective lability” (p. 98). At a later date, Schachtel (1966/2009) pointed out that emotional experiences and color perception on the RIM share characteristics of passivity, immediacy, and directness, although “color responses are not identical to color perception” (p. 163). Furthermore, in the Comprehensive System (CS; Exner, 2003), different codes for color responses and their proportions are used (FC; CF + C) as an index of affective modulation.
Numerous psychometric studies and clinical observations have been conducted to examine the effects of color stimuli and the characteristics of chromatic color responses, but there are some inconsistencies between studies. For example, Malone and colleagues (Malone et al., 2013) indicated a significant correlation between scales measuring emotional regulation and integration and the number of color responses (CF and C) in clinical groups. On the other hand, Stevens and colleagues (Stevens, Edwards, Hunter, & Bridgman, 1993) showed that, although the sample size was small, there was no significant correlation between indices such as FC; CF + C in the CS and emotional indices obtained from their experimental procedure in college students. However, in a recent meta-analysis of the validity literature, indices relating to color responses (e.g., Weighted Sum of Color and Form-Color Ratio) were listed as variables with good validity (Mihura, Meyer, Dumitrascu, & Bombel, 2013).
An increasing number of studies have focused on examining the characteristics of the RIM from a neuropsychological perspective. Belyi (1983) showed that patients with tumors in the right hemisphere produced more CF + C and a lower F+% than those with tumors in the left hemisphere. More recently, the correlation between the RIM and the laterality of the Thematic Apperception Test (Hiraishi, Haida, Matsumoto, Hayakawa, Inomata, & Matsumoto, 2012) and the association between brain activity and thought disorders, or syntax structures, indicated by the RIM (Kircher et al., 2001; Kircher, Tomasina, Brammer, & McGuire, 2005) have been investigated. Jimura and colleagues (Jimura, Konishi, Asari, & Miyashita, 2009) found a positive correlation between the SumC’ of the RIM performed in a regular setting and medial frontal lobe activity when negative feedback was given. Also, Acklin and Wu-Holt (1996) discussed functional localization of brain activity during the RIM response process (Exner, 2003), from encoding the stimulus field to articulation of the selected responses. However, this general perceptual process involved in the RIM has been insufficiently studied. Furthermore, the question remains as to whether the presence of chromatic color stimuli can produce differences in perceptual processes in response to the inkblots, and, if so, what is the nature of these differences?
The goal of the present study was to confirm the response process involved in the RIM as described by Acklin and Wu-Holt (1996). We measured brain hemodynamics of healthy participants using fMRI (Cohen & Bookheimer, 1994; Kim & Ugurbil, 1997) while they engaged in the RIM, and examined response characteristics by comparing brain activation patterns between achromatic and chromatic cards.
Method
Participants
Forty volunteers (20 men, 20 women; age M = 24.7 years, SD = 4.8) were recruited from an inter-university community by advertisement. We verified each subject had no history of neurological or psychiatric illness by a self-report questionnaire and an interview. All participants reported being right-handed and the Edinburgh Handedness Inventory (Oldfield, 1971) supported their reports (Laterality Quotient: M = .85, SD = .14, minimum = .27, maximum = 1.00). They also reported they were unfamiliar with the Rorschach inkblot stimuli.
Ethical Consideration
The protocol was approved by the ethical committee of Osaka Kyoiku University (Osaka, Japan), and the experiments were undertaken in compliance with national legislation and the Code of Ethical Principles for Medical Research Involving Human Subjects of the World Medical Association (the Declaration of Helsinki). All participants gave their written informed consent to participate in the study.
Magnetic Resonance Imaging
A time-course series of 88 volumes was acquired using T2*-weighted, gradient echo, echo planar imaging (EPI) sequences with a 3.0 Tesla MR imager (Discovery MR750; General Electric Medical Systems, Milwaukee, WI, USA) and a 32 channel head coil. Each volume consisted of 40 slices, each 3.0 mm thick, with a 0.5 mm gap to cover the entire cerebral and cerebellar cortex. Oblique scanning was used to exclude the eyeballs from the images. The time interval between two successive acquisitions of the same slice (TR: Repetition time) was 3000 ms with a flip angle (FA) of 90 degrees and 25 ms echo time (TE). The field of view (FOV) was 192 mm and the in-plane matrix size was 64 × 64 pixels. For anatomical reference, T1-weighted FSPGR [TR = 6.38 ms, TE = 1.996 ms, FA = 11 degrees, FOV = 256 mm, matrix size = 256 × 256 mm, slice thickness = 1 mm, a total of 172 trans axial images] was obtained for each subject.
Experimental Design and Task Procedure
The ten inkblot plates of the Rorschach were scanned and used as stimuli. We also produced a series of figures drawn only by the outline of the 10 inkblots for the line task. The visual stimuli were projected using a MRI compatible liquid crystal display (LCD) projector (SV-6011, AVOTEC, FL, USA) connected to a personal computer, which generated visual stimuli using Presentation (Neurobehavioral Systems, CA, USA) onto a half transparent screen, and were presented at a visual angle of 14.7° × 18.3°.
During the fMRI experiment, participants performed a total of four 4 min 24 sec sessions. The experimental session consisted of 10 blocks for each of the two conditions (RIM and line task blocks) and two rest blocks. During the RIM task block, participants were asked to keep thinking what the inkblots might be and make as many responses as possible throughout the trial, and were asked to press the button when they came up with a response. During a line task block, instead, participants were asked to press the button when a part of the outline of a figure turned gray (about three times per a trial, on average; intended to counterbalance the number of button presses on the RIM task). This task block involved detecting the luminance change of a part of the outline (about 1 degree of visual angle), requiring participants to attend to figure shape and figure outlines. Moreover, the line task was designed to subtract the effect of the motions of button press from the RIM task. For each block, Cards I to X were presented in the standard order and in the upright position. Then, all the cards were presented in the inverted position (see Figure 1). We did not ask participants to respond to each task verbally in this study in order to control the influence of verbalization on brain hemodynamic change.
Figure 1. Experimental task and design. Five RIM task blocks and five line task blocks, each lasting 21 s, were presented alternately in one fMRI session. During a RIM task block, participants were asked to press the button when they came up with an answer about what the RIM figures looks like. During a line task block, participants were asked to press the button when a part of the outline of a figure turned gray. Inkblots were presented from Card I to Card X in order in the upright position, then repeated in the inverted position. Note that the pictures here were created for illustrative purposes alone.
After completing the scanning session, participants were asked to write down as many responses as they remembered for each Rorschach card. We did not conduct the inquiry in the present study.
Imaging Data Processing
The first 4 volumes of each fMRI session were discarded because of unsteady magnetization, and the remaining 84 volumes per session were used for analysis. Image and statistical analyses were performed using Statistical Parametric Mapping (SPM8; Wellcome Department of Cognitive Neurology, London, UK) implemented in Matlab R2010a (Mathworks, Sherborn, MA) (Friston, Ashburner et al., 1995; Friston, Holmes et al., 1995).
Head motion was corrected with the realignment program of SPM8. There was no trend of head motion correlated with the task. Following realignment, the volumes were normalized to the Montreal Neurological Institute (MNI) space (Evans, Kamber, Collins, & MacDonald, 1994) using a transformation matrix obtained from the normalization process of the first EPI image of each individual subject to the EPI template. Finally, the normalized fMRI data were spatially smoothed with a Gaussian kernel of 8 mm (full-width at half-maximum) in the x, y, and z axes.
Statistical Analysis
Statistical analyses were conducted at two levels. First, the individual task-related activation was evaluated. Second, the summary data for each individual were incorporated into a second-level analysis using a random-effect model (Friston, Holmes, & Worsley, 1999) to make inferences at a population level. The signal time course for each subject was modeled with a general linear model (Friston, Holmes et al., 1995). The design matrix for the single-subject analyses contained two task-related regressors (achromatic and chromatic cards). Regressors that were of no interest, such as the session effect and high-pass filtering (128 s), were also included to eliminate the low-frequency trend. The weighted sum of the parameters estimated in the individual analysis consisted of ‘‘contrast’’ images, which were used for the group analyses (Friston et al., 1999). The contrast images obtained by individual analysis represented the normalized increment of the fMRI signal for each subject. Significant signal changes for each contrast were assessed by means of t statistics on a voxel-by-voxel basis. The threshold for the SPM{t} of group analyses was set at p < .001 (uncorrected for multiple comparisons) for height and p < .05 for cluster size (corrected for multiple comparisons) (Friston, Holmes, Poline, Price, & Frith, 1996).
For each inkblot response, brain activity during the line task was subtracted from that of the RIM task. In addition, to reveal the effects of color stimuli on the RIM, we compared brain activity for five achromatic (I, IV, V, VI and VII) and five chromatic cards (II, III, VIII, IX, and X) for each voxel. A paired t test was conducted to investigate differences between these two types of cards.
Results
Number of Responses
The average number of times the participants pressed the button (R) in response to the 10 RIM inkblots was 69.88 ± 16.81, maximum = 114, median = 67, and minimum = 38. The average R for the five achromatic cards was 33.63 ± 8.91, and for the five chromatic cards 36.25 ± 8.74, resulting in significantly more R for the chromatic cards (t(39) = 3.553, p = .001, d = .562). Cards with relatively more R produced were X, I, and III; and those with relatively less R were IV, VII and VIII (see Table 1). Using the obtained R, the value corresponding to Afr (Exner, 2003) was calculated as .455, and (VIII+IX+X)% (Kataguchi, 1988) yielded 31.1.
Table 1. Mean and SD of number of responses (R) for each card of the RIM.
| Card | Mean | SD |
|---|---|---|
| I | 8.05 | 2.39 |
| IV | 6.08 | 2.18 |
| V | 6.23 | 1.93 |
| VI | 7.08 | 1.95 |
| VII | 6.20 | 1.96 |
| All achromatic cards | 33.63 | 8.91 |
| II | 6.83 | 2.18 |
| III | 7.80 | 2.56 |
| VIII | 6.43 | 1.62 |
| IX | 7.08 | 1.87 |
| X | 8.13 | 2.42 |
| All chromatic cards | 36.25 | 8.74 |
Comparisons of Brain Activation Between Achromatic and Chromatic Cards
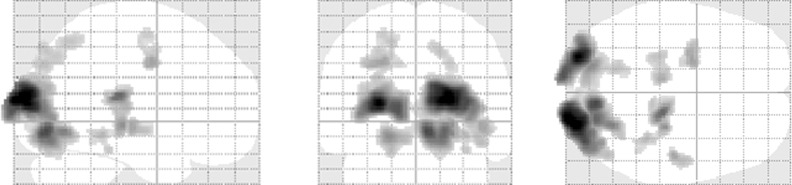
Significant increases in brain activity were observed in the bilateral medial occipital gyrus (BA; Brodmann area 18, 19), the bilateral pulvinar, the left middle frontal gyrus (BA 6), and the right superior lateral gyrus (BA 22) for the achromatic cards. On the other hand, brain activities significantly increased in the left lingual gyrus (BA 18) and the left orbitofrontal area (BA 11) for responses to the chromatic cards (Table 2; Figure 2 and Figure 3).
Table 2. Regions with significant differences between achromatic and chromatic color response.
| Region |
BA |
Laterality |
MNI Coodinates (mm) |
t | d | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Note. BA, Brodmann area; L, left; R, right. The threshold for the SPM{t} of group analyses was set at p < .001 (uncorrected for multiple comparisons) for height, and cluster p < .05 (corrected for multiple comparisons). ***p < .001, uncorrected. Effect size for paired t test (d) was calculated as t / square root of N (Toyoda, 2009). | |||||||
| Achromatic > chromatic | |||||||
| Middle occipital gyrus | 18 | R | 24 | –94 | 16 | 8.86*** | 1.40 |
| Precuneus | 7 | R | 16 | –78 | 48 | 4.00*** | 0.63 |
| Middle occipital gyrus | 18 | L | –12 | –102 | 8 | 6.60*** | 1.04 |
| Precuneus | 7 | L | –22 | –78 | 38 | 4.12*** | 0.65 |
| Pulvinar | R | 14 | –26 | 1 | 6.08*** | 0.96 | |
| Parahippocampal gyrus | 37 | R | 34 | –38 | –8 | 4.29*** | 0.68 |
| Pulvinar | L | –10 | –26 | 18 | 4.64*** | 0.73 | |
| Middle frontal gyrus | 6 | L | –24 | –4 | 40 | 4.72*** | 0.75 |
| Superior temporal gyrus | 22 | R | 46 | –18 | –4 | 4.72*** | 0.75 |
| Chromatic > achromatic | |||||||
| Lingual gyrus | 18 | L | –26 | –104 | –8 | 7.33*** | 1.16 |
| Middle frontal gyrus | 11 | L | –24 | 30 | –16 | 4.66*** | 0.74 |
| Middle orbital gyrus | 10 | L | –32 | 50 | –6 | 4.49*** | 0.71 |
Figure 2. Maximum intensity projection (MIP) images of the brain regions with significantly increased activity when the achromatic cards are shown.
Figure 3. MIP images of the brain regions with significantly increased activity when the chromatic cards are shown.
Regions Significantly Activated in Both Sets of Cards: A Conjoint Analysis
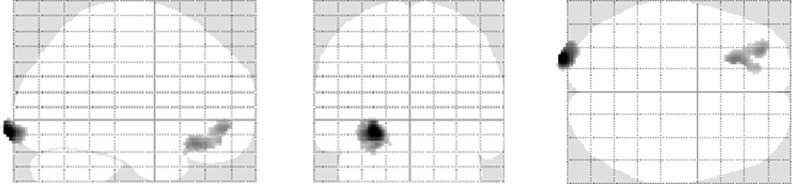
To test whether there were common areas that were significantly activated in both contrasts for chromatic and achromatic RIM cards (compared to the line task), a conjunction analysis was calculated. Results showed significant activation in the following regions (see Table 3): the bilateral superior and middle occipital gyri (BA 17 and 18), the fusiform gyri (BA 19), the inferior parietal lobules (BA 40), and the inferior temporal gyri (BA 20 in left and BA 37 in right). A significant increase in activation was also observed bilaterally in the hippocampus and parahippocampal gyri (BA 36 in left and BA35 in right). Moreover, significant activation was shown in the left amygdala, the bilateral anterior cingulate cortices (ACC, BA 32), the orbitofrontal cortices (OFC, BA 47), and the dorsal and ventral lateral prefrontal cortices (BA 9, 45).
Table 3. Regions commonly activated in achromatic and chromatic cards, calculated by a conjoint analysis.
| Legions | BA | Laterality | MNI coodinates (mm) |
t | d | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Note. BA: Brodmann area, L: left, R: right. The threshold for the SPM{t} of group analyses was set at p < .001 (uncorrected for multiple comparisons) for height, and cluster p < .05 (corrected for multiple comparisons). ***p < .001, uncorrected. Effect size for paired t test (d) was calculated as t / square root of N (Toyoda, 2009). | |||||||
| Middle occipital gyrus | 17 | L | –14 | –98 | –8 | 16.77*** | 2.65 |
| Middle occipital gyrus | 18 | R | 28 | –88 | –8 | 16.96*** | 2.68 |
| Superior pariatal lobule | 7 | L | –24 | –60 | 44 | 17.15*** | 2.71 |
| Superior pariatal lobule | 7 | R | 26 | –64 | 46 | 13.74*** | 2.17 |
| Inferior parietal lobule | 40 | L | –46 | –42 | 50 | 12.39*** | 1.96 |
| Inferior parietal lobule | 40 | R | 38 | –42 | 44 | 9.18*** | 1.45 |
| Fusiform gyrus | 19 | L | –36 | –68 | –12 | 18.06*** | 2.86 |
| Fusiform gyrus | 19 | R | 40 | –68 | –14 | 19.48*** | 3.08 |
| Inferior temporal gyrus | 20 | L | –52 | –40 | –18 | 8.24*** | 1.30 |
| Inferior temporal gyrus | 37 | R | 48 | –42 | –20 | 9.41*** | 1.49 |
| Hippocampus | L | –28 | –22 | –10 | 7.14*** | 1.13 | |
| Hippocampus | R | 26 | –20 | –12 | 6.85*** | 1.08 | |
| Parahippocampal gyrus | 36 | L | –28 | –28 | –26 | 6.16*** | 0.97 |
| Parahippocampal gyrus | 35 | R | 26 | –32 | –20 | 5.12*** | 0.81 |
| Amygdala | L | –28 | –6 | –20 | 3.50*** | 0.55 | |
| Anterior cingulate gyrus | 32 | L | –6 | 32 | 30 | 7.07*** | 1.12 |
| Anterior cingulate gyrus | 32 | R | 8 | 26 | 32 | 6.86*** | 1.08 |
| Orbitofrontal cortex | 47 | L | –38 | 28 | –18 | 7.95*** | 1.26 |
| Orbitofrontal cortex | 47 | R | 36 | 28 | –14 | 4.69*** | 0.74 |
| Dorsolateral prefrontal cortex | 9 | L | –46 | 8 | 26 | 15.99*** | 2.53 |
| Dorsolateral prefrontal cortex | 9 | R | 46 | 14 | 28 | 11.30*** | 1.79 |
| Ventrolateral preftontal cortex | 45 | L | –50 | 36 | 10 | 8.10*** | 1.28 |
| Ventrolateral preftontal cortex | 45 | R | 56 | 40 | 8 | 9.62*** | 1.52 |
Discussion
Regions Significantly Activated in Response to Achromatic Cards
In response to achromatic cards, significant activation was observed in the large area of the bilateral medial occipital gyri (BA 18, 19). These areas are also called prestriate visual cortex and include secondary visual areas (V2) and visual area V3. V2 is known to be responsible for perceiving subjective contour (Peterhans & von der Heydt, 2003), and one study has suggested that the lateral area of the occipital lobe is related to processing contour of a shape (Humphrey et al., 1997). Tsuji (1997) pointed out, citing Shachtel (1966/2009), that form recognition requires more activity and indirectness compared to color recognition, which is characterized by passivity and directness. Attention to the contour of a form response might be more focused in response to achromatic cards.
Significantly increased activation was also observed for the achromatic cards in the bilateral pulvinars. Studies have indicated that the pulvinar mediates maintaining visual attention (Fischer & Whitney, 2012) and making decisions about the precision of integrated visual information (Komura, Nikkuni, Hirashima, Uetake, & Miyamoto, 2013). Moreover, significant increase in right superior temporal gyrus (BA22) activation was also observed in response to achromatic cards. This region shows significant increase in activation when selective attention is paid to shapes (Corbetta, Miezin, Dobmeyer, Shulman, & Peterson, 1990). These results also suggest that participants directed more attention to shapes when responding to achromatic cards.
The bilateral precunei (parieto-occipital junction, POJ; BA7) were also activated significantly in responding to achromatic cards. POJ is related to scene processing (Sato et al., 1999) or mental rotation (Mourao-Miranda, Ecker, Sato, & Brammer, 2009). In addition, various functions such as visuo-spatial imagery, episodic memory retrieva,l and self-processing might activate the POJ (see Cavanna & Trimble, 2006, for a review). The right hippocampal gyrus (BA 37) was also activated. This area involves memory retrieval (Greicius, Krasnow, Boyett-Anderson, Eliez, Schatzberg, Reiss, & Menon, 2003), and is related to processing visual information regarding both formal and spatial characteristics (Sato, 2000).
Regions Significantly Activated in Response to Chromatic Cards
In response to chromatic cards, a significant increase in left linguial gyrus (visual area V4, BA18) activity was observed. A study suggests that V4 is closely related to color perception and its consistency (Mullen, Dumoulin, McMahon, de Zubicaray, & Hess, 2007). Chromatic color stimuli could promote significant V4 activation during the responses to chromatic cards.
Significant activation was also shown in the left orbitofrontal cortex (OFC). The OFC is considered to be a region of processing rewards, and is widely involved in emotion regulation, attention and executive functions (see Ono & Tabuchi, 1993, for a review). The lateral OFC is specifically related to paying selective attention to specific information (Corbetta, Miezin, Dobmeyer, Shulman, & Petersen, 1991) or to regulating task-irrelevant emotional information (Vuilleumier, Armony, Driver, & Dolan, 2001). For the RIM task, form is the fundamental factor for a response (Tsuji, 1997), and chromatic color stimuli may interfere with the task in some cases (Shapiro, 1960). One study showed that high impulsivity on the NEO-PI-R was related to an increase in CF and C, as high impulsivity might interfere with the capacity to produce a relevant response (Yasuda, 2012). Significant increase in OFC activation when responding to chromatic cards implies that chromatic color stimuli might interfere with focusing on form processing as “noise,” and that OFC might be activated to regulate the influence of such stimuli.
Another discussion about the significant increase in OFC activation in response to chromatic cards might be considered. Chromatic color stimuli could be a cue to a RIM response, in a setting where the instruction was to give as many responses as possible. Greater OFC activation could have emerged because the stimuli were operated as a reward. However, regions involved in processing rewards and emotional control such as the amygdala, the ACC, and the DLPFC did not show significant activation in response to chromatic cards. Significant increase in R and V4 activation suggests that subjects perceived chromatic color stimuli, and that such stimuli increased the number of responses produced.
Regions Significantly Activated in Both Card Groups
Results from a conjoint analysis revealed that various regions are related to responding to the RIM. These regions could be grouped into three clusters. First, regions which involve processing visual information were significantly activated, including the visual cortices, the dorsal and ventral visual streams, and the fusiform gyri. The lateral occipital gyrus is related to processing the outline of an object, and the fusiform gyrus and parahippocampal gyrus involve the processing of texture (Koyama & Kawamura, 2007). Second, regions involving various functions of memory were significantly activated, such as the hippocampus and the parahippocampal gyri. Both episodic and semantic memory appear to be involved in the RIM task. These regions are critical for episodic memory (Tulving & Markowitsch, 1998). Regarding semantic memory, it has been hypothesized that repeated parts of episodic memories might be fixed as semantic memories (Yamadori, 2003). Third, regions including the ACC, the OFC, the LPFC, and the amygdala are involved in emotional control during a cognitive task (Salzman & Fusi, 2010). Further investigation is required to better understand why the OFC showed significant activation on chromatic cards.
This study experimentally investigated brain hemodynamics during the RIM task, and confirmed that the results are generally consistent with the RIM response process as described by Exner (2003) and by Acklin and Wu-Holt (1996). We also found differences in brain activation between achromatic and chromatic cards. In accordance with Hermann Rorschach, who named his test a “form interpretation test” (Rorschach, 1921/1951), we confirmed that several cerebral regions involving the perceptual processing of form were activated in response to the RIM. Furthermore, regions involving emotional expression and processing also showed significant activation throughout the task. This seems to suggest that the RIM is not merely a “form interpretation test,” but also a task which involves emotional processing, as proposed by Muzio (2004) in his review.
However, our study presents several limitations. First, Rorschach data were not collected in a standard setting. Subjects were instructed to produce as many responses as possible in a trial, which resulted in a substantial increase in R. The size of the projected images of RIM stimuli might also have affected the responses, as the images were smaller than their standard size. Above all, we did not collect verbal responses directly, which limited our capacity to investigate the relation between brain activity as measured by fMRI and various RIM scores, especially scores such as Form Quality and Form-Color Ratio. Second, the validity of the empirical design of the analysis comparing the data between five achromatic and five chromatic cards could have been taken into account. Chromatic cards could have been divided into two black-and-red cards and three cards with various colors. We could also have compared the activation to each of the achromatic cards. Kataguchi (1988) suggested the “A-B-C series,” a set of parallel cards including an achromatic series (all chromatic colors of blots were converted to grayscale), a black series (all blots were colored black without shading) and a contour series (only outlines of the blots were presented). These series are available to explore the influence or meaning of chromatic color and shading in the RIM response process.
Numerous studies have shed light on the neurological correlates of various mental health problems, such as depression or posttraumatic stress disorder. Muzio (2004) discussed the potential of the RIM as an instrument capable of transcending trends in neuropsychology and personality psychology. We believe that further investigation of the neurological correlates of the RIM and other projective methods should lead us to confirm their validity as psychological assessment tools with neuropsychological underpinnings.
Acknowledgments
The main results of this paper were originally presented at XXI International Congress of Rorschach and Projective Methods in Istanbul, Turkey (July, 2014). This study was conducted with a grant from JSPS KAKENHI (23530897).
Summary
The color responses in the Rorschach inkblot method (RIM) has been interpreted in relation to emotion; however, how color stimuli are processed during the response process is as yet unclear. The present study was conducted to obtain basic information about brain activities during the RIM task by measuring the brain activities and comparing them to the responses made to the chromatic and achromatic color cards. Forty right-handed adults (20 females and 20 males) without a history of neurological or psychiatric problems participated in the study. Scanned figures were prepared of the 10 RIM inkblot plates (in upright and inverted positions) and the cards comprised of only the contour of the inkblot for the concurrent stimuli, and these cards were projected onto the screen in the MRI scanner (GE MR750) for 21 s each. The participants were required to think about what they saw in the inkblots as much as possible during the card presentation and to press the button when they came up with a response. For the cards with only black contour, the color of a small part of contour changed gray during the presentation and the subjects were asked to press the button when they noticed when the color changed. The data obtained in the concurrent task was subtracted from the data obtained in the RIM task and the responses in chromatic and achromatic cards were compared for each voxel. As a result, for achromatic color cards, significant increases in cerebral activity were observed in the bilateral visual areas V2 and V3, parietoccipital juncions, pulvinars, right superior temporal gyrus, and left premotor cortex (p < .001). For the chromatic color cards, significant increases were observed in the left visual area V4 and orbitofrontal cortex (p < .001). A conjoint analysis to seek commonly activated regions between the two conditions revealed that various regions associated with visual, memory, and emotion regulation were significantly activated during the RIM task. The response process described by Acklin and Wu-Holt (1996) was largely confirmed. Limitations and future challenges were also discussed.
要 約
ロールシャッハ法(Rorschach Inkblot Method; RIM)における色彩反応は情動性との関連を中心に解釈されてきたが,反応の過程において色彩刺激がどのように処理されているかはわかっていないところが多い。本研究ではRIM実施時の脳活動を測定し無彩色図版と彩色図版との比較をおこない,RIMにおける色彩刺激の特徴を検討することで,RIM実施時の脳活動に関する基礎的資料を得ることを目的とした。研究協力者として,精神科既往歴のない右利きの成人40名(男性20名,女性20名)が参加した。10枚のRIM図版(正位置,逆位置)のスキャン画像と,並行課題の刺激としてRIM図版の輪郭だけをとりだした図版を準備し,MRI装置(GE MR750)内のスクリーンにプロジェクターで投影した(それぞれ21秒)。RIM図版提示時は,インクブロットが何に見えるかできるだけ多く考え,思いついたらボタンを押下するよう求めた。輪郭だけの図版は,提示時間内にランダムに輪郭の一部の色を変え,変わったらボタンを押下するよう求めた。RIM課題実施時のデータから平行課題のデータを引き,voxelごとに彩色図版と無彩色図版との間の差を比較した。結果として,無彩色図版においては両側2次および3次視覚野,両側視床枕,右側上側頭回,左側運動前野での有意な活動の上昇が観察された。一方で彩色図版においては左の4次視覚野と眼窩前頭野での有意な活動の上昇が示された(いずれもp<.001)。またconjoint分析より,両図版において共通に活動の上昇を見せる領域として,視覚情報の処理に関する領域,記憶に関する領域,そして感情の統制に関する領域などが示された。これはAcklin & Wu-Holt (1996)が考察したRIMの反応過程に関与する諸領域と多くの一致を示した。今後の課題についても考察をおこなった。
Résumé
Les réponses chromatiques dans la Méthode à tache d’encre de Rorschach (RIM) ont été interprétées par rapport à l’émotion. Néanmoins, on ne sait toujours pas comment les stimuli de couleur sont traités lors du processus de réponse. Le but de la présente étude était d’obtenir des informations de base sur les activités du cerveau lors de l’emploi de la RIM en mesurant les activités du cerveau et en les comparant aux réponses obtenues pour les cartes chromatiques et achromatiques. Quarante adultes droitiers (20 femmes et 20 hommes) sans antécédents de problèmes neurologiques ou psychiatriques ont participé à l’étude. Les chiffres numérisés des 10 planches de tache d’encre de RIM (dans les positions droite et inversée) et les cartes comportant uniquement le contour de la tache d’encre pour les stimuli concomitants ont été préparés, et chacune de ces cartes a été projetée sur un écran dans le scanner IRM (GE MR750) pendant 21 secondes. Il a été demandé aux participants de penser autant que possible à ce qu’ils voyaient dans les taches d’encre lors de la présentation des cartes, et d’appuyer sur le bouton quand ils y pensaient. Pour les cartes ayant uniquement un contour noir, la couleur d’une petite partie du contour est devenue grise pendant la présentation et il a été demandé aux participants d’appuyer sur le bouton dès qu’ils remarqueraient ce changement de couleur. Les données obtenues dans la tache concomitante ont été déduites des données obtenues dans la tache RIM et les réponses dans les cartes chromatiques et achromatiques ont été comparées pour chaque voxel. On a alors constaté que pour les cartes achromatiques, des augmentations significatives de l’activité cérébrale dans les aires visuelles bilatérales V2 et V3, les jonctions pariéto-occipitales, les pulvinars, le gyrus temporal supérieur droit, le cortex prémoteur gauche (p < .001). Les cartes chromatiques, des augmentations significatives ont été observés dans l’aire visuelle gauche V4 et dans le cortex orbitofrontal (p < .001). Une analyse conjointe destiné à identifier des régions généralement actives entre deux états a révélé que diverses régions ont été considérablement activées lors de l’emploi de RIM impliquant une régulation visuelle, mémorielle et émotionnelle. Le processus de réponse décrit par Acklin et Wu-Holt (1996) a été largement confirmé. Les limites et défis à venir ont également été abordés.
Resumen
Las respuestas al color en el método de manchas de tinta de Rorschach (RIM) han sido interpretadas con relación a la emoción; sin embargo, cómo se procesan los estímulos de color durante el proceso de respuesta aún es incierto. El presente estudio se realizó para obtener información básica sobre las actividades que ocurren en el cerebro mientras se realiza la tarea RIM, esta información se obtiene mediante la medición de las actividades cerebrales y se la compara con las respuestas dadas a las tarjetas cromáticas y acromáticas. Cuarenta adultos diestros (20 mujeres y 20 hombres) sin antecedentes de problemas neurológicos o psiquiátricos participaron en el estudio. Se prepararon figuras escaneadas de las 10 láminas de manchas de tinta del método RIM (en posición vertical y posición invertida) y se prepararon tarjetas que incluían solamente el contorno de la mancha de tinta para los estímulos concurrentes, y estas tarjetas se proyectaron en la pantalla en el escáner de resonancia magnética (GE MR750), cada una durante 21 de segundos. Se pidió a los participantes que piensen sobre qué veían en las manchas de tinta tanto como les sea posible durante la presentación de la tarjeta y que pulsen el botón cuando piensen sobre ello. En el caso de las tarjetas con solamente un contorno negro, el color de una pequeña parte del contorno cambió a gris durante la presentación y se pidió a los sujetos participantes que pulsen el botón cuando se den cuenta del cambio de color. Los datos obtenidos en la tarea concurrente se restaron de los datos obtenidos en la tarea RIM y las respuestas obtenidas para las tarjetas cromáticas y acromáticas se compararon según cada vóxel. Como resultado, en el caso de las tarjetas acromáticas, se observaron aumentos significativos en la actividad cerebral en las áreas visuales bilaterales V2 y V3, articulaciones parieto-occipitales, núcleos pulvinares, el giro temporal superior derecho, y la corteza pre-motora izquierda (p < 0,001). En el caso de las tarjetas cromáticas, se observaron aumentos significativos en el área visual izquierda V4 y la corteza orbito-frontal (p < 0,001). Un análisis conjunto para buscar regiones comúnmente activadas entre las dos condiciones reveló que varias regiones se activaron de manera significativa durante la tarea RIM, que son regiones que involucran la regulación visual, de la memoria y de las emociones. El proceso de respuesta descrito por Acklin y Wu-Holt (1996) se confirmó en gran medida. También se examinaron limitaciones y futuros desafíos.
References
- Acklin M. W. & Wu-Holt P. (1996). Contributions of cognitive science to the Rorschach technique: Cognitive and neuropsychological correlates of the response process. Journal of Personality Assessment, (1), 169–178. [DOI] [PubMed] [Google Scholar]
- Belyi B. I. (1983). Reflection of functional hemispheric asymmetry in some phenomena of visual perception. Human Physiology, (6), 410–417. [PubMed] [Google Scholar]
- Cavanna A. E. & Trimble M. R. (2006). The precuneus: A review of its functional anatomy and behavioural correlates. Brain, (3), 564–583. [DOI] [PubMed] [Google Scholar]
- Cohen M. S. & Bookheimer S. Y. (1994). Localization of brain function using magnetic resonance imaging. Trends in Neuroscience, (7), 268–277. [DOI] [PubMed] [Google Scholar]
- Corbetta M., Miezin F. M., Dobmeyer S., Shulman G. L. & Petersen S. E. (1990). Attentional modulation of neural processing of shape, color, and velocity in humans. Science, (4962), 1556–1559. [DOI] [PubMed] [Google Scholar]
- Corbetta M., Miezin F. M., Dobmeyer S., Shulman G. L. & Petersen S. E. (1991). Selective and divided attention during visual discriminations of shape, color, and speed: Functional anatomy by positron emission tomography. The Journal of Neuroscience, (8), 2383–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exner J. E. (2003). The Rorschach: A Comprehensive System. Vol. 1 Basic Foundations (4th ed.). New York, NY: Wiley. [Google Scholar]
- Evans A. C., Kamber M., Collins D. L. & MacDonald D. (1994). An MRI based probalistic atlas of neuroanatomy In Shorvon D., Fish D. R., Andermann F., Byddered G. M. & Stefan H. (Eds.), Magnetic resonance scanning and epilepsy (pp. 263–274). New York, NY: Plenum Press. [Google Scholar]
- Fischer J. & Whitney D. (2012). Attention gates visual coding in the human pulvinar. Nature Communications, , 1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K. J., Ashburner J., Frith C. D., Poline J. B., Heather J. D. & Frackowiak R. S. J. (1995). Spatial registration and normalization of images. Human Brain Mapping, , 165–189. [Google Scholar]
- Friston K. J., Holmes A. P., Worsley K. J., Poline J. P., Frith C. D. & Frackowiak R. S. J. (1995). Statistical parametric maps in functional imaging: A general linear approach. Human Brain Mapping, , 189–210. [Google Scholar]
- Friston K. J., Holmes A., Poline J. B., Price C. J. & Frith C. D. (1996). Detecting activations in PET and fMRI: levels of inference and power. NeuroImage, (3), 223–235. [DOI] [PubMed] [Google Scholar]
- Friston K. J., Holmes A. P. & Worsley K. J. (1999). How many subjects constitute a study? NeuroImage, (1), 1–5. [DOI] [PubMed] [Google Scholar]
- Greicius M. D., Krasnow B., Boyett-Anderson J. M., Eliez S., Schatzberg A. F., Reiss A. L. & Menon V. (2003). Regional analysis of hippocampal activation during memory encoding and retrieval: fMRI Study. Hippocampus, , 164–174. [DOI] [PubMed] [Google Scholar]
- Hiraishi H., Haida M., Matsumoto M., Hayakawa N., Inomata S. & Matsumoto H. (2012). Differences of prefrontal cortex activity between picture-based personality tests: A near-infrared spectroscopy study. Journal of Personality Assessment, (4), 366–371. [DOI] [PubMed] [Google Scholar]
- Humphrey G. K., Goodale M. A., Bowen C. V, Gati J. S., Vilis T., Rutt B. K. & Menon R. S. (1997). Differences in perceived shape from shading correlate with activity in early visual areas. Current Biology, (2), 144–147. [DOI] [PubMed] [Google Scholar]
- Jimura K., Konishi S., Asari T. & Miyashita Y. (2009). Involvement of medial prefrontal cortex in emotion during feedback presentation. Neuroreport, (9), 886–890. [DOI] [PubMed] [Google Scholar]
- Kataguchi Y. (1988). Kaitei Shin Shinri Shindan Hou [Revised edition of psychodiagnostics] Tokyo, Japan: Kaneko Shobo. [Google Scholar]
- Kim S.-G. & Ugurbil K. (1997). Functional magnetic resonance imaging of the human brain. Journal of Neuroscience Methods, , 229–243. [DOI] [PubMed] [Google Scholar]
- Kircher T. T. J., Liddle P. F., Brammer M. J., Williams S. C. R., Murray R. M. & McGuire P. K. (2001). Neural correlates of formal thought disorder in schizophrenia: Preliminary findings from a functional magnetic resonance imaging study. Archives of General Psychiatry, , 769–774. [DOI] [PubMed] [Google Scholar]
- Kircher T. T. J., Tomasina M. O., Brammer M. J. & McGuire P. K. (2005). Neural correlates of syntax production in schizophrenia. The British Journal of Psychiatry: The Journal of Mental Science, , 209–214. [DOI] [PubMed] [Google Scholar]
- Komura Y., Nikkuni A., Hirashima N., Uetake T. & Miyamoto A. (2013). Responses of pulvinar neurons reflect a subject’s confidence in visual categorization. Nature Neuroscience, (6), 749–755. [DOI] [PubMed] [Google Scholar]
- Koyama S. & Kawamura M. (2007). Katachi to iro wo ninshiki suru shikumi [Neural mechanisms for object and color recognition]. Brain and Nerve, (1), 31–36. [PubMed] [Google Scholar]
- Malone J. C., Stein M. B., Slavin-Mulford J., Bello I., Sinclair S. J. & Blais M. A. (2013). Seeing red: Affect modulation and chromatic color responses on the Rorschach. Bulletin of the Menninger Clinic, (1), 70–93. [DOI] [PubMed] [Google Scholar]
- Mihura J. L., Meyer G. J., Dumitrascu N. & Bombel G. (2013). The validity of individual Rorschach variables: Systematic reviews and meta-analyses of the comprehensive system. Psychological Bulletin, (3), 548–605. [DOI] [PubMed] [Google Scholar]
- Morey L. C. (2007). Personality Assessment Inventory: Professional manual (2nd ed.). Odessa, FL: Psychological Assessment Resources. [Google Scholar]
- Mourao-Miranda J., Ecker C., Sato J. R. & Brammer M. (2009). Dynamic changes in the mental rotation network revealed by pattern recognition analysis of fMRI data. Journal of Cognitive Neuroscience, (5), 890–904. [DOI] [PubMed] [Google Scholar]
- Mullen K. T., Dumoulin S. O., McMahon K. L., de Zubicaray G. I. & Hess R. F. (2007). Selectivity of human retinotopic visual cortex to S-cone-opponent, L⁄M-cone-opponent and achromatic stimulation. European Journal of Neuroscience, , 491–502. [DOI] [PubMed] [Google Scholar]
- Muzio E. (2004). Le Rorschach Système Intégré en Neuropsychologie: articulation du cognitif et de l’affectif [The Rorschach Comprehensive System in neuropsychology: Integrating cognition and affect] Psychologie Française, (1), 33–49. [Google Scholar]
- Oldfield R. C. (1971). The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia, , 97–113. [DOI] [PubMed] [Google Scholar]
- Ono T. & Tabuchi E. (1997). Ganka zentou ya no kinou [Functions of the orbitofrontal cortex] Advances in Neurological Science, (1), 56–71. [Google Scholar]
- Peterhans E. & von der Heydt R. (2003). Subjective contours – bridging the gap between psychophysics and physiology. Trends in Neurosciences, (3), 112–119. [DOI] [PubMed] [Google Scholar]
- Rorschach H. (1951). Psychodiagnostics Remkau P. & Kronenberg B. (Eds., Trans.). New York, NY: Grune & Stratton; (Original work published 1921). [Google Scholar]
- Salzman C. D. & Fusi S. (2010). Emotion, cognition, and mental state representation in amygdala and prefrontal cortex. Annual Review of Neuroscience, , 173–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N. (2000). Reichourui ni okeru kaibaboukai no shikaku kinou ni kansuru kenkyu [Visual function of the parahippocampal cortex in primates] Unpublished doctoral dissertation, Hiroshima University, Japan. [Google Scholar]
- Sato N., Nakamura K., Nakamura A., Sugiura M., Ito K., Fukuda H. & Kawashima R. (1999). Different time course between scene processing and face processing: A MEG study. NeuroReport, , 3633–3637. [DOI] [PubMed] [Google Scholar]
- Schachtel E. G. (2009). Experiential foundations of Rorschach’s Test. New York, nY: Routledge. [Google Scholar]
- Shapiro D. (1960). A perceptual understanding of color response In Rickers-Ovsiankina M. A. (Ed.), Rorschach psychology (pp. 154–201). New York, NY: John Wiley & Sons. [Google Scholar]
- Stevens D. T., Edwards K. J., Hunter W. F. & Bridgman L. (1993). An investigation of the color-affect hypothesis in Exner’s Comprehensive System. Perceptual and Motor Skills, (3f), 1347–1360. [DOI] [PubMed] [Google Scholar]
- Tsuji S. (1997). Rorschach Kensa Hou [The Rorschach Test] Tokyo, Japan: Kaneko Shobo. [Google Scholar]
- Tulving E. & Markowitsch H. J. (1998). Episodic and declarative memory: Role of the hippocampus. Hippocampus, (3), 198–204. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P., Armony J. L., Driver J. & Dolan ; R. J. (2001). Effects of attention and emotion on face processing in the human brain. Neuron, (3), 829–841. [DOI] [PubMed] [Google Scholar]
- Yamadori A. (2003). Hito no kioku kinou ni okeru kaiba kaibakboukai no yakuwari [Roles of hippocampus and hippocampal gyrus for human memory functions] No To Hattatsu, , 105–112.12661090 [Google Scholar]
- Yasuda M. (2012). Rorschach shikisai hannou no kisoteki kenkyu, [Fundamental study on the Rorschach color responses] Unpublished doctoral dissertation, Kwansei Gakuin University, Hyogo, Japan [Google Scholar]