Abstract
Do physiological and behavioral performance indicators of effortful cognitive self-regulation converge additively or interactively in their statistical prediction of individual differences in harsh parenting? To answer this question, we examined heart rate (HR) and EEG alpha (α) reactivity during executive function (EF) tasks, along with observed and self-reported indicators of harsh parenting. A socioeconomically diverse sample of 115 mothers with 3-to-7-year old children completed questionnaires and a laboratory visit. Three-quarters of the mothers showed typical patterns of task reactivity that were interpretable (i.e., increases in HR and decreases in α). Among them, we found no evidence to suggest that variance in harsh parenting was associated with magnitude of HR or α reactivity independently. Instead, the physiological variables interacted to enhance the EF statistical effect. EF explained one-third of the variance in harsh parenting among mothers showing the largest α decreases when accompanied by modest to moderate (rather than substantial) HR increases. Physiological indicators can clarify the role and estimation of the strength of the effect of direct behavioral measures of cognitive regulation, in the etiology of harsh parenting behaviors.
Keywords: parenting, executive function, heart rate, EEG alpha (α)
Individual differences in harsh reactive parenting (i.e., yelling, striking, shaming) arise in part from social cognitions attributing hostile intent to the child’s misbehaviors and physiological reactivity to stressors, that together with harsh behaviors represent a constellation of constructs linked with greater child abuse potential (Lorber & O’Leary, 2005; Rodriguez & Tucker, 2014). Evidence is emerging that parental cognitive self-regulation plays a key role in modulating harsh reactive parenting (Crandall, Deater-Deckard, & Riley, 2015). Although behavioral and psychophysiological (including electrocardiography [ECG] and electroencephalography [EEG]) indicators of effortful cognitive regulation have been implicated, it remains unknown whether and how these behavioral and physiological measures work together in conjunction with behavioral measures of cognitive regulation (such as executive function) to maximize explained variance in harsh reactive parenting. To address this gap in knowledge, our goal in the current study was to integrate behavioral, ECG and EEG indicators of effortful cognitive self-regulation to test whether these indicators converged to explain the most variance in harsh parenting, in a diverse sample of mothers of young children.
Cognitive Self-Regulation and Parenting
Cognitive self-regulation includes a constellation of indicators that can be measured at behavioral and physiological levels of analysis—a set of constructs reflecting “top down” regulatory processes in the central nervous system that develop and change over the lifespan and are transmitted between generations via gene-environment interaction (Bridgett, Burt, Edwards, & Deater-Deckard, 2015). Variance in these indicators represent the degree to which the individual engages effortful cognitive/affective resources to perceive and manipulate relevant information in order to respond appropriately during and following a reaction to a stressor (Finkenaur et al., 2015). Of particular relevance are an inter-related set of cognitive “executive” function indicators (EF) comprised of updating, set shifting and inhibiting internally processed information, in an effort to appraise the environment and enact responses in light of relevant features while disregarding irrelevant features (Friedman & Miyake, in press). EF is part of a broader set of cognitive regulatory constructs that have been implicated in many aspects of well-regulated versus poorly regulated reactive thoughts, emotions and behavior (Etkin, Büchel, & Gross, 2015; Schmeichel & Tang, 2015). This is just as true for well- versus poorly-regulated parenting behaviors.
Several decades of correlational and experimental animal model research have shown that variance in important aspects of caregiving behavior is associated with maternal cognitive self-regulation generally and EF specifically. For example, greater involvement and vigilance with rat pups (e.g., licking, grooming, arch-backed nursing) is observed in rat dams with better EF performance, and their female pups subsequently show higher quality of caregiving with their own offspring (Barrett & Fleming, 2011). As Barrett and Fleming note, some of these effects have been demonstrated experimentally using artificial and cross-fostered rearing, suggesting potential causal mechanisms. With regard to humans, foundational papers on maternal attention deficits and parenting by Wahler and Dumas (1989) and Dix (1991) paved the way for the recent growth in correlational and quasi-experimental studies of EF and supportive versus harsh parenting (see Crandall et al., 2015, for a review). As Crandall et al. indicated, harsh reactive parenting is operationalized as behaviors directed toward the child that are psychologically and sometimes physically controlling and punitive, such as striking, shouting, shaming, and expressing anger or humiliation. Also, the presence of these caregiving behaviors themselves, and the relative absence of warm, supportive, stimulating caregiving behaviors, has been implicated in poorer EF and self-regulation development in children (Cuevas et al., 2014; Hughes, 2011).
An important finding emerging from more recent studies of human maternal EF and parenting behavior is that the correlations between maternal cognitive regulation indicators and parenting indicators are not substantial in magnitude. For instance, maternal working memory and composite-EF deficits have been implicated in the etiology of harsh reactive parenting in the face of other risk factors (e.g., socioeconomic risks, hostile attribution bias), but the direct associations with harsh parenting in those same studies are modest in effect size (Deater-Deckard, Sewall, Petrill, & Thompson, 2010; Deater-Deckard, Wang, Chen, & Bell, 2012; Sturge-Apple, Suor, & Skibo, 2014). This may be because maternal cognitive regulation is not implicated as a direct risk factor for harsh reactive parenting, but instead serves as a modulator of a more basic reactive stress response. However, it could also be true that any direct association that exists is difficult to detect, because behavioral and questionnaire measures of cognitive regulatory capacity are relatively weak “signals”, due to measurement error that attenuates the effect. To address this possibility, we examined whether incorporating simultaneous psychophysiological indicators of effortful internal cognitive processing of information during EF tasks would better inform us about the direct association between maternal cognitive self-regulation and harsh parenting.
Effortful Cognitive Processing: Psychophysiological Indicators
Behavioral and questionnaire assessments of maternal cognitive regulation are important and useful, but there is a broader and theoretically coherent set of measures that can be brought to bear—specifically, indicators of central and peripheral nervous system processes (Bridgett et al., 2015) that represent recruitment of neurophysiological resources required for sustained, effortful regulation in the face of challenge. For instance, this can be seen in the role of glucose utilization and depletion (Hagger, Wood, Stiff, & Chatzisarantis, 2010) and cardiac vagal activation and withdrawal (e.g., heart rate and heart rate variability/respiratory sinus arrhythmia; Spangler, Deater-Deckard, & Bell, 2015) during effortful cognitive self-regulation, including EF task performance. It may be that the statistical effects of behavioral performance and questionnaire-based measures of maternal EF and cognitive regulation skills on harsh reactive parenting are more consistent and larger in magnitude, when considered in conjunction with more direct physiological indices of effort and engagement during the cognitive challenging tasks.
To that end, in the current study we examined two straightforward and commonly used psychophysiological indicators of effortful internal processing during tasks: heart rate (HR), and EEG [electroencephalograph] alpha power (α). Heart rate (HR) is measured by the number of heart contractions per minute, and is affected by the interaction of activity of acceleratory sympathetic nerves and inhibitory parasympathetic nerves via the sinoatrial node (Berntson, Quigley, & Lozano, 2007). Resting-state HR is a key indicator of cardiac function, stress, anxiety, and overall health, and reflects interaction between the autonomic nervous system with the central nervous system (Beauchaine, 2015). Specifically, the prefrontal cortex (PFC), cingulate cortex and insula are part of a network that influences amygdala, which in turn disinhibits sympathetic excitatory neurons (in rostral ventrolateral medulla) and inhibits parasympathetic excitatory neurons (in dorsal vagal motor nucleus) that has an effect on HR via the sinoatrial node (Berntson et al., 2007; Thayer, Hansen, Saus-Rose, & Johnsen, 2009).
When the individual experiences some kind of cognitive challenge (such as the very demanding EF tasks our participants completed), the prototypical pattern is to see a modest to moderate increase in HR (i.e., HR reactivity), as the individual shifts from a “baseline” resting state to a cognitively demanding, engaged state. This is due to increased activation of sympathetic neurons and inhibition of parasympathetic neurons, as part of a mild to moderate stress response involving increased glucose metabolization in the brain—a response to a challenge involving substantial cognitive load (Cacioppo & Sandman, 1978; Kennedy & Scholey, 2000). In the current study, we examined the variance in mothers’ HR reactivity (operationalized as magnitude of increase in HR from baseline during a resting state to the effortful EF task performance state), to investigate whether the magnitude of increasing HR with EF task performance was indicative of more or less harsh reactive parenting behavior.
The second physiological indicator we used was EEG α. EEG oscillations measured at the scalp are thought to be generated by the summation of excitatory and inhibitory post-synaptic potentials in the pyramidal neurons of the cortex (Pizzagalli, 2007). The EEG signal is composed of rhythmic activity oscillating at different frequencies. Although the genesis of this rhythmic activity is not well understood, there is evidence that the α rhythm, which cycles between 8 and 13 times per second in adults, emerges from interactions between the thalamus and the cortex (Lindgren et al., 1999; Schreckenberger et al., 2004). The EEG signal is spontaneous but context-related; thus, EEG generated during quiet rest is quantitatively different than generated during cognitive processing.
The EEG signal has temporal resolution on the order of milliseconds, resulting in postsynaptic changes being immediately reflected in the EEG and making this methodology outstanding for tracking rapid shifts in brain functioning. The spatial resolution of the EEG signal is not optimal, however. A particular scalp electrode detects electrical activity from groups of neurons across a relatively wide area making it inappropriate to highlight precise anatomical brain areas when discussing EEG findings. Thus, EEG researchers typically highlight cortical lobes or networks (Bell & Cuevas, 2012). We examined whole head EEG activity to take advantage of α oscillations that are apparent across multiple scalp locations. A whole head measurement was preferable to a narrower approach (e.g., frontal sites only), for at least two reasons. First, the relevant cortical networks for executive function and its required motor responses in our assessments, span frontal-parietal regions (Barbey et al., 2012); in addition, occipital locations involved in sustained visual perception of spatially dynamic stimuli also are relevant (Sauseng et al., 2005). Second, there is substantial inter-individual idiosyncratic variation in electrode site patterns of α power during cognitive performance, due to poor spatial resolution of EEG reflected in moderate to substantial correlations in power values between sites (Klimesch, 1999). Third, whole head α measurement maximizes reliability of measurement of thalamic-cortical networks’ metabolic activity during effortful cognitive processing (Lindgren et al., 1999).
EEG signals undergo quantitative processing to estimate the power (measured in mean square microvolts) of the EEG at particular frequency bands. Power is the quantitative measure that reflects the excitability of groups of neurons. When an individual confronts challenging cognitive tasks like the EF tasks in the current study, the prototypical pattern is a decrease in α power (i.e., α reactivity) from the baseline resting state to the cognitively demanding state; this is thought to reflect top-down widespread cortical control of lower-level internal processing of information (Benedek et al., 2011). Suppressed α oscillations (with accompanying increases in β and θ) reflect the degree of mobilization of neural resources in relevant brain regions, i.e., effort, for the cognitive tasks being performed (Klimesch, 1999; Wilson, Swain, & Ullsperger, 1999); accordingly, effortful and engaged processing corresponds with larger decreases in α power. In the current study, we examined the variance in mothers’ α reactivity (operationalized as magnitude of decrease in α from baseline resting state to effortful EF task performance state), to investigate whether the magnitude of decreasing α during EF task performance (together being indicative of the degree of cognitive regulatory capacity being exhibited) was associated with variance in harsh, reactive parenting behavior.
Aside from its potential relevance for understanding maternal EF and parenting, there is a more established literature examining more direct associations between maternal parenting and her psychophysiological indicators of reactivity and self-regulation, based on foundational work by Tronick, Feldman, Field and others (for a comprehensive review, see Butler & Randall, 2013). The most recent work converges with classic studies showing a consistent pattern in studies of observed mother-child interaction and maternal responses to infant/toddler crying. Specifically, less optimal or harsher parenting is more prevalent among mothers who show greater sympathetic nervous system reactive activity (i.e., skin conductance, cortisol, heart rate reactivity) and weaker parasympathetic regulation activity (i.e., heart rate variability, respiratory sinus arrhythmia). This pattern has been found primarily in studies of mothers with infants or toddlers (Joosen et al., 2013a; Joosen, Mesman, Bakermans-Kranenburg, & van IJzendoorn, 2013b; Leerkes et al., 2015; Lorber & O’Leary, 2005; Martorell & Bugental, 2006; Mills-Koonce et al., 2009), but there also are several studies with mothers of preschoolers (Giuliano, Skowron, & Berkman, 2015; Skowron et al., 2011). In addition to the simultaneous examination of maternal psychophysiology and EF, the current study addresses three gaps in the extant maternal psychophysiology literature. To our knowledge, it is the first such study to: 1) investigate mothers with 3–7 year olds; 2) examine reactivity in response to a cognitive challenge (i.e., EF tasks); and 3) consider additive and interactive effects of ECG heart rate, and EEG α power.
Hypotheses
We tested two hypotheses. Based on the literature reviewed above, our first hypothesis addressed anticipated additive effects—specifically, that better EF task performance, a larger increase in HR during task performance, and a larger decrease in EEG α during task performance each would contribute to the statistical prediction of lower levels of harsh parenting. Furthermore, any one of these three indicators is an imperfect and incomplete representation of degree of cognitive engagement and self-regulation, but in an interactive combination they might elucidate a clearer pattern of the role of cognitive regulatory deficits in harsh reactive parenting. To this end, our second hypothesis was that the statistical predictors would interact. We tested two competing mechanisms as part of this hypothesis. An “EF enhancement” mechanism would be present if the link between better maternal EF and less harsh parenting was maximized under conditions reflecting strong physiological engagement during the EF tasks (e.g., when HR increases or EEG α decreases). In contrast, a competing “EF attenuation” mechanism would be evident if the link between better maternal EF and less harsh parenting was weakened under conditions reflecting strong engagement during the EF tasks, perhaps because these changes reflect physiological reactions that can interfere with task performance and reduce the predictive validity of the EF task performance score as a result.
Method
Participants
The sample included 115 mothers (age, M = 32.77 yrs, SD = 5.96 yrs) and their 3 to 7 year-old children (age, M = 55.27 mos, SD = 15.29 mos; 54% female) with complete data on the composite measures used in the current study. The sample was diverse, with a demographic distribution that resembled those of families in the region; 74% Caucasian, 13% African American, 2% Asian, 6% multiple races, and 5% other. In addition, 4% reported being Hispanic. About one-third were single mothers, and maternal education varied widely: from 23% with high school diploma/GED or less, and 20% with a post-graduate degree.
Procedures
Two-thirds of the participants lived in or near a small city, and were enrolled through contacts through community agencies and advertisements (e.g., flyers in schools and common areas, university website, email). Interested individuals who were eligible, based on the age of the child, completed informed consent by telephone and then participated at our laboratory in the small city. One-third of the sample were part of an ongoing longitudinal community study; these families participated through a visit to our rural university laboratory located in the same geographic region as the small city.
Signed consent and child assent were obtained at the beginning of the visit to the laboratory. Mothers completed questionnaires prior to the visit. At the beginning of the visit, mother and child sat at a table and were recorded during three moderately challenging and potentially frustrating tasks (four to five minutes each) including drawing with an Etch-A-Sketch drawing toy, doing a puzzle, and building a model using Duplo blocks. Our goal in using these structured tasks was to elicit dyadic interaction behaviors in response to engaging but challenging toys that require communication and coordinated engagement and effort of both the child and the mother, so that variation in supportive and harsh caregiving behaviors could be observed. For the Etch-A-Sketch drawing task, the parent and child each was assigned a control knob and told not to touch each other’s knob, while they worked together to copy one simple line drawing of a square and then one complex line drawing of a smiling face. For the puzzle task, they were asked to put together a puzzle of animal pictures. For the Duplo blocks, the mother was asked to show the child a model castle and then to verbally instruct the child how to copy it. During the task, mothers were not allowed to point to or touch the Duplo blocks. An honorarium was provided.
Measures
Executive function (EF)
We counterbalanced four tasks to measure executive attention, inhibition, and memory that comprise a single underlying factor (Friedman & Miyake, in press). Performance distributions were typical for young-to-middle-age adults (see Deater-Deckard et al., 2012). The Stroop color-word task was administered on a computer (Stroop, 1935). Participants indicated the color of the ink of color words in which the actual color of the letters and the color being named are congruent (e.g., “red” written in red ink) or incongruent (e.g., “red” written in yellow ink), following an initial trial in which the participant simply reported the color of the ink of a series of Xs. We used a set of 20 words with mixed incongruent and congruent stimuli (which minimizes practice effects), and mothers’ scores on the task were calculated as the percentage of correct responses out of 20.
A computerized version of the Wisconsin Card Sorting Test (WCST) involved presentation of four stimulus cards with different colors, quantities, and shapes (Heaton & PAR Staff, 2003). Mothers attempted to match a stack of 64 (at the rural university lab) or 128 (at the urban lab) cards to the original stimulus cards according to a rule which they had to ascertain (i.e., either by color, quantity or shape). The matching rule changed several times and the participant had to infer the new rule based on feedback from the computer regarding correct vs. incorrect responses. We used the number of perseveration errors per 64 trials which represents mistakes made by continuously using the same incorrect matching rule (i.e., difficulty inhibiting the dominant practiced response) even after receiving feedback indicating that the rule was no longer correct.
A computerized version of the Tower of Hanoi was used to measure mothers’ problem solving abilities (Davis & Keller, 1998). The task involved moving three disks of different sizes to a target peg in the same order, using two rules: only one disk can be moved each turn, and larger disks cannot be placed on smaller disks. Time to completion (up to 60 secs) was used as the score for the task; those who did not finish received a score of 60 secs.
An experimenter also administered a backward digit span task. The experimenter read a seemingly random series of single-digit numbers (0–9) and the participant attempted to reproduce the sequence in reverse. Following a practice trial with two sets of two digits, the task began with a four-digit sequence and then added one more digit in each subsequent trial. Mothers had two chances to correctly reproduce the new digit sequence in reverse. The task ended when the mother provided incorrect responses on both chances. The last correct trial was used as the mother’s backward digit span score.
The four indicators positively covaried, with correlations ranging from .19, one-tailed p < .05 to .38, p < .001. There was no evidence of bivariate outliers based on visual inspection of scatterplots. We conducted CFA to test for a general EF construct, and model fit was acceptable: Χ2 (2) = 3.43, p = .18, CFI = .97, RMSEA = .07. Standardized factor loadings ranged from .40 [95% CI’s from .20 to .60] to .63 [95% CIs from .44 to .83]. Loadings on the first principal component in an EFA were .74 (digit span), .69 (Stroop), .65 (Tower of Hanoi), and .62 (Card sort). All four scores were standardized and averaged for every mother who had at least one task score. The average score was standardized again to yield a composite z-score that was widely and normally distributed.
Heart rate (HR)
Heart rate was measured using inter-beat interval. A research assistant instructed the mother on how to apply two disposable ECG electrodes using modified lead II alignment (right collarbone and lower left rib cage; Stern, Ray, & Guigley, 2001), grounded at the scalp near electrode site Fz. The cardiac electrical activity was amplified using a SA Instrumentation Bioamp (San Diego, CA) and bandpassed from 0.1 to 100 Hz. The QRS complex was displayed on the acquisition computer monitor and digitized at 512 samples per second. The acquisition software was Snapshot-Snapstream (HEM Data Corp.; Southfield, MI) and the raw data were stored for later analyses.
Baseline ECG was recorded for 2 minutes (one minute eyes opened and one minute eyes closed) while mothers were asked to clear their thoughts, sit quietly in a chair, and relax. ECG data were then examined and analyzed using IBI Analysis System software developed by James Long Company (Caroga Lake, NY). First, R waves were detected offline with a four-pass peak detection algorithm, resulting in a data file with onset times for each detected R-wave. Next, the ECG signal was viewed on a computer monitor along with tick marks representing the onset times of the IBI software detected R-waves. For undetected visible and obscured R-waves, the tick marks were inserted manually. Movement artifact was designated by the absence of at least three consecutive R-waves. These artifact-scored epochs were eliminated from all calculations.
Baseline HR was computed by averaging HR during eyes-open and eyes-closed conditions; between-condition r = .97, p < .001. Task HR was computed by averaging HR across the four EF tasks (including two HR measurements for digit span—one at encoding and one at retrieval). Correlations between tasks: r = .34 to .99, p < .001; in a PCA, the first component accounted for 66% for the variance with loadings from .78 to .85. HR reactivity was computed by subtracting baseline HR from task HR, so that positive values indicated the magnitude of the anticipated increase in HR when shifting from resting baseline state to executive function task state.
EEG alpha (α) power
Brain electrical activity was recorded with an Electro-Cap (Eaton, OH) from sixteen left and right scalp sites: frontal pole (F1, F2); medial frontal (F3, F4); lateral frontal (F7, F8); central (C3, C4); temporal (T7, T8); parietal (P3, P4, P7, P8); and occipital (O1, O2). The recording reference was Cz. Electrode impedance was measured and kept below 10K ohms. The electrical signals of each sites were amplified using separate SA Instrumentation Bioamps (San Diego, CA) and passed from 1 to 100 Hz. EEG Analysis System software (James Long Company; Caroga Lake, NY) was used to examine and analyze the EEG data. EEG data that reflected eye movement and gross motor were artifact scored and removed from all subsequent analyses. The artifact-free epochs were converted to 1 second Hamming windows with 50% overlap and subjected to a discrete Fourier transform. Power was computed for the 8–13 Hz frequency α band, expressed as mean square microvolts and transformed using the natural log to achieve a normal distribution.
Baseline α power was computed by averaging α across all scalp sites during eyes-open baseline and eyes-closed baseline. A PCA showed that the first component explained 75% of the variance, with loadings from .80 to .92. Task α power was computed by averaging across all scalp sites and across the four EF tasks. A PCA showed that the first component explained 58% of the variance, with loadings from .60 to .87. EEG α reactivity was computed by subtracting task from baseline α power, so that positive values indicated the magnitude of the anticipated decrease in α power when shifting from resting baseline state to EF task state.
Harsh negative parenting
We assessed maternal harsh negative parenting using three indicators: observed negativity with child, self-reported attribution biases, and self-reported use of harsh punitive discipline. For observers’ ratings, trained coders used the PARCHISY global ratings system (Deater-Deckard, 2000) to rate mothers’ behavior during the three structured tasks with the child, using the instrument’s 7-point Likert-type scales (1 = no occurrence of the behavior, to 7 = continual occurrence of the behavior). During training, two raters rated the sample video independently, then their scores were compared. For items with a rater difference score > 1 on the 7-point scale, the two raters would discuss the item and resolve the discrepancy. For actual data collection, every mother-child dyad was rated using consensus coding, whereby two coders watched and rated the interaction independently, then discussed their scores and resolved any discrepancies. Scores were averaged across the three tasks. We randomly selected 20% of families for reliability coding; these interactions were coded by all of the raters. Discrepancies of 1 point or less on the 7-point scale were treated as agreements, to mimic what we had done in the derivation of the consensus-based ratings used to compute the actual scores. Individual ratings were treated as items and used to calculate the reliability for each item across raters, based on their original ratings (i.e., pre-consensus scoring) to avoid artificially inflating reliability estimates. We applied generalizability theory by estimating coefficient alpha for each rating scale (i.e., covariance between raters while accounting for within-rater variance; Bakeman & Gottman, 1986, pp. 92–96). Inter-rater reliability was substantial for all scales that we used in the current study (α > .85).
We averaged four items from the PARCHISY to represent maternal harsh negativity: negative affect (i.e., expressed frustration or anger), negative control (i.e., physical manipulation of child and/or objects), lack of positive affect (i.e., reverse scored, expressions of happiness or pride), and lack of positive control (i.e., reverse scored, use of encouragement, praise, or elaborative speech during tasks). In a PCA, the first component explained 47% of the variance, with loadings from .53 to .76.
For self-reported social cognitive attribution biases, mothers completed the Parenting Possibilities Questionnaire (Nix et al., 1999). This measure includes 9 vignettes describing common events in everyday life in which a child misbehaves. When completing the questionnaire, mothers are asked to imagine that the child in the vignette is their own child. Mothers are given two possible explanations to account for each of the child behavior vignettes, and then asked to rate the degree to which both explanations account for the child’s misbehavior. One explanation is that the child’s misbehavior is intentional, and the other explanation is that the child’s misbehavior is situational or accidental. All items are rated on a 4-point Likert-type scale with 1 = not why to 4 = probably why. In accordance with Nix et al. (1999), the hostile attribution score was computed by subtracting mothers’ ratings of the likelihood of the situational attribution (α = .66) from their ratings of the likelihood of the intentional attribution (α = .75); correlation between situational and intentional attribution scores, r = −.57, p < .001. Higher scores represented more hostile attributions.
For self-reported harsh punitive discipline, we used the three-item harsh verbal scale (α = .73, M = 3.31, SD = 1.03, range = 1 to 5 on a 5-point Likert scale), the two-item verbal shaming scale (inter-item r = .50, M = 2.17, SD = .88, range = 1 to 5 on a 5-point Likert scale), and the two-item physical punishment scale (inter-item r = .39, M = 1.68, SD = .74, range = 1 to 5 on a 5-point Likert scale), from a brief discipline questionnaire developed by Lansford et al. (2010). Sample items include ‘raise your voice, yell, or scold your child’, ‘tell your child s/he should be ashamed’, and ‘spank, slap or hit your child’. The first component in a PCA explained 58% of the variance, with loadings from .60 to .88. The indicators were averaged so that a higher score corresponded with harsher discipline.
As a final step, we computed a composite score representing overall harsh negative parenting across the three indicators (observer rated negativity, self-reported attribution bias, and punitive discipline). Internal consistency reliability was substantial; the first component in a PCA explained 51% of the variance, with loadings of .76 (attribution bias), .70 (observed negativity), and .68 (punitive discipline).
Results
In preliminary analyses, as a manipulation check we examined the patterns of HR and α reactivity. We did this to ensure that we were seeing the expected prototypical increasing HR and decreasing α power in response to cognitive challenge (to disentangle potentially distinct processes that would obfuscate interpretable effects) and to determine that there was sufficient variance in the reactivity scores (to ensure adequate statistical power for detecting additive and interactive statistical effects). As anticipated based on the literature on HR reactivity (Cacioppo & Sandman, 1978; Kennedy & Scholey, 2000) and α power reactivity (Benedek et al., 2011), the vast majority of the participants (n = 84) showed the expected patterns, and we conducted our analyses using this sub-sample. Regarding unexpected contrary patterns, twenty-two women exhibited a decrease in HR from baseline to task, eight exhibited an increase in α from baseline to task, and one showed both patterns. These subsamples were too small to analyze, but we take into consideration the importance of considering such anomalies in psychophysiology research, in the Discussion section. There also were two participants who were extreme outliers on HR reactivity (+4 SD); they were removed from all subsequent analyses.
For the selected study sample of 84 participants, we computed descriptive statistics for ΔHR and Δ α (EF and harsh discipline were z-scores). On average, these mothers showed an average HR change [an increase] of 4.07 beats per minute (SD = 2.98), and an average α change [a decrease] of 0.77 (SD = 0.41). Bivariate correlations (see Table 1) showed that the only significant association was between better EF performance and less harsh parenting.
Table 1.
Descriptive Statistics and Bivariate Correlations for Selected Sub-Sample (n = 84)
| ΔHR | Δα | Exec | Harsh | |
|---|---|---|---|---|
| Function | Parenting | |||
| ΔHR (increase) | ----- | |||
| Δα (decrease) | .01 | ----- | ||
| Exec Function (z) | .08 | −.03 | ----- | |
| Harsh Parenting (z) | −.05 | .14 | −.23* | ----- |
| M | 4.07 | 0.77 | 0.11 | −0.19 |
| SD | 2.98 | 0.41 | 1.02 | 0.87 |
Note:
p < .05
Next, we estimated the following equation, regressing harsh parenting on the intercept, main effects and all higher-order interaction effects for EF, ΔHR and Δ α:
We used hierarchical decomposition, entering the intercept and main effects in step 1, the two-way interactions in step 2, and the three-way interaction in step 3.
The full equation was significant: F (7, 76) = 2.29, p < .01, R2 = .17. Significant predictors included the two-way interaction between Δ α and EF in Step 2 of the equation (β = −.27, p < .05), but this was subsumed by the three-way interaction between Δ α, ΔHR, and EF in Step 3 of the equation (β = −.28, p < .05); the main effect of EF was marginally significant (β = −.22, p < .06). Before interpreting interaction terms, we considered inclusion of covariates. The participants were assessed at two locations and were diverse in age and SES. Statistically controlling for site, maternal age or socioeconomic SES-risk level (a cumulative index of presence/absence of four risks using the same method as Deater-Deckard et al., 2012: mother high-school diploma or less education; father high-school or less; single mother; father unemployed) had virtually no impact on the effect size (β = −.28) of the three-way interaction term: β = −.27 (controlling for site), −.28 (controlling for maternal age), and −.24 (controlling for SES).
Thus, our first hypothesis regarding “additive effects”—that all three indicators (larger increase in HR, larger decrease in α, and higher EF scores) would be associated with less harsh parenting—was not supported. In contrast, there was evidence to support our second hypothesis of interactive effects, with more specific consideration of competing EF “enhancement versus attenuation” mechanisms in the statistical prediction of harsh parenting. We only interpreted the significant three-way interaction from Step 3 of the equation reported above, because it subsumed the only other significant predictor (the two-way interaction between Δ α and EF). There is no consensus regarding probing three-way interaction effects, because the resulting function is a three-dimensional quadratic surface; thus, there are many options available (Dawson & Richter, 2006). We used a parsimonious approach that estimates standard simple slopes estimation for two-way interactions (e.g., Aiken & West, 1991), within specified ranges on the third variable involved in the three-way interaction. Because the two-way interaction between EF and Δ α was significant, we conducted post-hoc probing of that two-way interaction term, examining Δ α as the moderator of the slope of harsh parenting regressed back on EF at low and high values of the third variable, ΔHR.
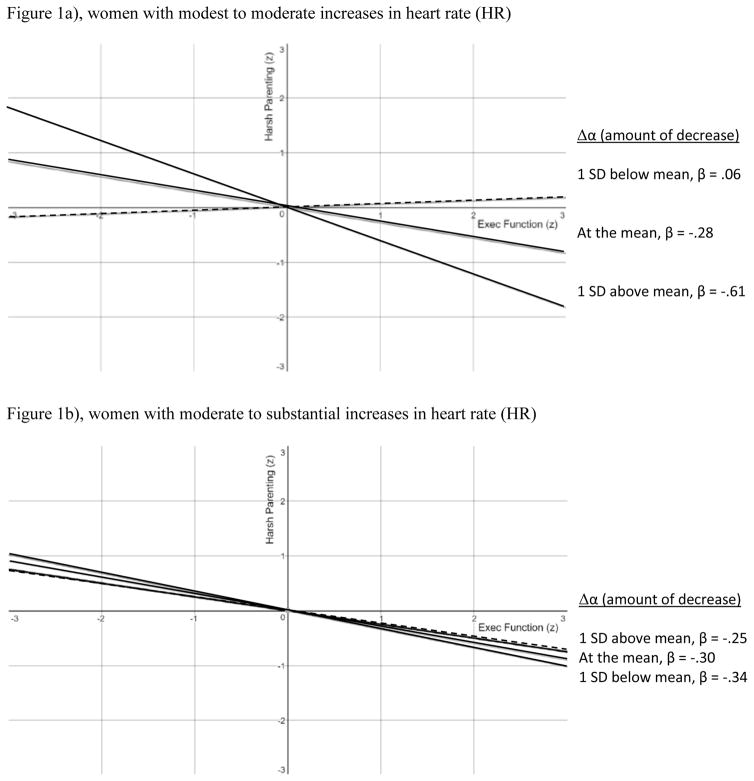
The two sets of simple slopes (mean centered) are shown in Figure 1a and 1b. In Figure 1b, we examined the two-thirds of the sample with the smallest HR increases; in Figure 1a, we examined the two-thirds of the sample with the largest HR increases. (We used two-thirds rather than one-half, i.e., median splitting, to maximize statistical power while selecting high and low sub-samples based on magnitude of HR increase). Nonsignificant slopes are shown as dashed lines, and significant slopes are shown as solid lines. Different slopes as a function of magnitude of change in α were seen only for those with modest to moderate increases in HR (see Fig. 1a); the largest slope estimate (.61, about one-third of the variance) was observed among those with smaller HR increases and larger α decreases.i In contrast, slopes were very similar regardless of change in α for those with the largest HR increases (see Fig. 1b). Overall, this pattern was most consistent with the “EF enhancement” hypothesis, among women showing the greatest decreases in α when accompanied by modest to moderate increases in HR.
Figure 1.
Simple slopes for prediction of harsh parenting from executive function as a function of magnitude of decrease in EEG α power. 1a shows results for mothers with the smallest HR increases from baseline resting state; 1b shows results for those with the largest HR increases. Significant slopes (p < .05) are solid lines, nonsignificant slopes are dashed lines.
Discussion
In a diverse community sample of mothers of 3–7 year olds, we examined whether co-occurring behavioral performance and psychophysiological indicators of effortful cognitive self-regulation accounted for variance in self-reported and observed indicators of harsh reactive parenting behavior. Our first hypothesis indicating “additive” effects was that women with the highest EF scores, the largest HR increases, and the largest α decreases during EF task performance (relative to baseline resting state), would show the lowest levels of harsh parenting. There was little evidence to support this additive effects hypothesis (i.e., in Step 1 of a hierarchical multiple regression equation, only EF was marginally significant as a main effect; β = −.22, p < .06).
To address potential nonadditive combined effects of EF with HR and EEG α changes, we tested a second hypothesis that the ECG and EEG indicators of cognitive engagement during the EF tasks would interact with EF performance in the prediction of variance in harsh parenting. The hypothesized interactive effect was present, regardless of whether we statistically controlled for covariates such as maternal age, family SES, and study site. Among the selected sub-sample of women showing typical α and HR reactivity (i.e., a decrease in α and an increase in HR), the link between EF deficits and harsher parenting was strongest among those with the largest decreases in α—but only among those with modest-to-moderate increases in HR. This pattern is indicative of a physiological “EF enhancement” process rather than an “EF depletion” effect, whereby the link between EF and parenting is strongest among those showing effortful, engaged internal processing (reflected in changes in α), accompanied by moderate (rather than extreme) cardiovascular arousal during task performance. Consistent with the EF and parenting literature, our interpretation is that a lower behavioral EF performance score captures deficits in the mother’s capacity to attend to and apply available relevant information and disregard irrelevant information, when regulating her thoughts, emotions and behaviors in the face of child behaviors that are sometimes challenging to manage (Barrett & Fleming, 2011; Crandall et al., 2015; Dix, 1991; Sturge-Apple et al., 2015; Wahler & Dumas, 1989). Our results indicate that the predictive validity of maternal EF task scores may be maximized for those showing clear physiological evidence of effortful cognitive processing during task performance, as long as it is not accompanied by more extreme cardiovascular arousal.
Although we could have ignored the presence of unexpected patterns of psychophysiological reactivity in our sample, that would have been ill advised for several reasons. Inadequate variance in changing HR or changing α would have reduced our statistical power for detecting higher-order interactive effects with EF by attenuating effect sizes due to range restriction (McClelland & Judd, 1993). Also, we observed wide variation in HR and α reactivity in this diverse community sample. Parenting young children is rewarding but also stressful, and the mothers in the sample face many persistent challenges arising from socioeconomic, childrearing, and personal stressors. Anecdotally, many of the participants had not participated in research before, and for nearly all of them it was almost certainly the first time they had conducted an EEG and ECG assessment.
Among participants showing unexpected, contrary patterns of psychophysiological reactivity (i.e., decreasing HR or increasing α when moving from baseline-resting to cognitive-challenge state), the individual differences in their HR and α reactivity scores very likely represent qualitatively different processes. For instance, it could reflect anticipatory anxiety during the “capping” procedure, in anticipation of the challenges to come (Davidson, Marshall, Tomarken, & Henriques, 2000; Deane, 1961)—that the participant was anxious during the preparation and resting-state phase, and became less distressed once the anticipatory preparation phase had passed. Alternatively, the unexpected opposing pattern of HR or α reactivity could indicate that the participant was not effortfully internally processing the challenging task stimuli, but instead was passively viewing the information (Benedek et al., 2011).
An analogous situation is seen in research on cortisol as a measure of stress reactivity and self-regulation. Although most adults show an expected 24-hour cyclical pattern, smaller subgroups with atypical (and sometimes even contrary) patterns show very distinct outcomes (Dmitrieva et al., 2013); lumping everyone together masks underlying effects for the majority who show an expected and interpretable pattern of within-person variation. Unfortunately, the small sub-samples of women showing unexpected atypical HR and α reactivity patterns were too small to analyze separately. However, we conducted a post-hoc analysis in which we combined those with theoretically anticipated and theoretically unexpected (and not easily interpreted) reactivity patterns; the three-way interaction was no longer significant, and the “main effects” also were not significant. Thus, there are likely to be varied patterns of responding to the study protocol, and those patterns should be ascertained and interpreted (based on theory and prior empirical literatures) carefully, whenever plausible. We would strongly advise researchers to be wary of aggregating distinct within-person change profiles, when attempting to identify interactive effects of physiological and behavioral indicators of cognitive self-regulation.
Limitations and Conclusion
There are three major caveats to bear in mind due to limitations of the study. First, as a cross-sectional correlational study, it was not possible to infer temporal patterns let alone causality. Based on the burgeoning empirical literature and theory regarding the role of cognitive regulation in parenting (Crandall et al., 2015), and animal model experiments that permit strong inferences of causality (Bartlett & Fleming, 2011), we have emphasized a likely causal role that EF and physiological indicators of reactivity and self-regulation play in harsh reactive parenting. However, experimental studies with humans have shown clear evidence of affective state (and in particular, variation in positive affect) on EF performance and its underlying functional neurochemistry (Mitchell & Phillips, 2007). Even more likely, then, is the idea that maternal cognitive self-regulation and harsh reactive parenting bidirectionally influence each other, through their joint contributions to the continuous stress and homeostasis system.
Second, although this was a socioeconomically and ethnically diverse community sample of mothers, the sample was not representative of the larger population of mothers; our findings may not generalize to other populations (e.g., clinically referred women with mood disorders; individuals with traumatic maltreatment histories). Both of these caveats have implications for translation of the findings to future research (i.e., the importance of also conducting quasi-experiments and experiments) and practice (i.e., whether directly targeting maternal cognitive regulation will influence reductions in harsh reactive parenting). To our knowledge, there are no published human experimental studies on EF training and resulting reductions in harsh reactive parenting. However, that work is a logical next step, given the explosion of experiments over the past two decades on training of EF and related cognitive regulation skills (see meta-analyses by Hagger et al., 2010; Melby-Lervåg & Hulme, 2013).
Third, there is a potential shortcoming with regard to the interpretation of the magnitude of the decrease in EEG α. Specifically, some research suggests that among individuals who are experienced performers of the tasks being completed (i.e., “experts” versus “novices”), small decreases actually reflect more efficient and accurate processing (e.g., Fairclough, Venables, & Tattersall, 2005). Although the four EF tasks we asked the mothers to perform were not typical tasks that they would have had much experience with, we did not assess their prior experience with the tasks, nor did we measure more broadly whether they enjoyed and played games that tend to challenge EF (e.g., Sudoku and other types of “brain training” games; Grabbe, 2011). It is possible that some of the variance observed in EEG α reactivity could be confounded with expertise or prior knowledge with the tasks.
Nevertheless, with these important limitations in mind, there are several key conclusions to be drawn from the current study. Multi-method assessment of effortful cognitive regulation that incorporates psychophysiological measurements provides “added value” when striving to estimate theorized effects on harsh reactive parenting. In our case, the effect size for the association between poorer EF and harsher parenting was −.23 (5% of the variance) when no other variables were considered (see Table 1), but substantially increased to −.61 (just over one-third of the variance) when we took into consideration the interactive combination of larger decreases in α accompanied by modest to moderate HR reactivity. Perhaps we should have anticipated this, given prior evidence from the psychophysiological literatures showing that inclusion of cardiovascular and cerebral stress reactivity and self-regulation measures can greatly enhance the internal (i.e., construct) and external (i.e., predictive) validity of self-reported and observed risk and resilience variables (Compton, Hofheimer, & Kazinka, 2013; Zanstra & Johnston, 2011). The current results also are consistent with prior stress physiology research on parenting, showing that harsher or less optimal parenting is linked with greater stress reactivity and poorer regulation capacity in mothers (Joosen et al., 2013a; Leerkes et al., 2015; Lorber & O’Leary, 2005; Martorell & Bugental, 2006; Mills-Koonce et al., 2009; Skowron et al., 2011). The take-home message is that scientists and practitioners would be wise to take into account the broader system of behavioral and psychophysiological factors pertaining to stress reactivity and regulation capacity, when studying or intervening to change maternal cognitive self-regulation in an effort to explain or reduce levels of harsh reactive parenting.
Acknowledgments
We thank the study participants and research staff. This research was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development grants HD57319 and HD60110, and National Institute of Mental Health grant MH99437. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD, NIMH, or National Institutes of Health. Major portions of this paper were reported in a symposium at the Biennial Meeting of the Society for Research in Child Development, March 19–21, 2015, in Philadelphia, PA.
Abbreviations
- HR
heart rate
- EF
executive function
- EEG
electroencephalograph
- ECG
electrocardiograph
Footnotes
To estimate indicator-specific results (given that composites were used for EF and harsh parenting), we computed the largest slope estimate of .61 (found for the lowest HR increase and largest α increase, shown in Fig. 1a). We iterated as a covariate each of the individual EF indicators. Adjusted simple slope estimates were, from lowest to highest: .38 (when covarying Stroop), .60 (Tower of Hanoi), .67 (Wisconsin Card Sort), and .83 (digit span). We did the same for the indicators of harsh parenting, with adjusted simple slope estimates: .15 (when covarying observed parenting), .44 (parenting attribution), and .47 (discipline).
References
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Newbury Park, CA: Sage; 1991. [Google Scholar]
- Bakeman R, Gottman J. Observing interaction: An introduction to sequential analysis. Cambridge, UK: Cambridge University Press; 1986. [Google Scholar]
- Barbey AK, Colom R, Solomon J, Krueger F, Forbes C, Grafman J. An integrative architecture for general intelligence and executive function revealed by lesion mapping. Brain. 2012;135(4):1154–1164. doi: 10.1093/brain/aws021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J, Fleming AS. All mothers are not created equal: Neural and psychobiological perspectives on mothering and the importance of individual differences. Journal of Child Psychology and Psychiatry. 2011;52:368–397. doi: 10.1111/j.1469-7610.2010.02306.x. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP. Future directions in emotion dysregulation and youth psychopathology. Journal of Clinical Child & Adolescent Psychology. 2015;44:875–896. doi: 10.1080/15374416.2015.1038827. [DOI] [PubMed] [Google Scholar]
- Bell MA, Cuevas K. Using EEG to study cognitive development: Issues and practices. Journal of Cognition and Development. 2012;13:281–294. doi: 10.1080/15248372.2012.691143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedek M, Bergner S, Könen T, Fink A, Neubauer AC. EEG alpha synchronization is related to top-down processing in convergent and divergent thinking. Neuropsychologia. 2011;49(12):3505–3511. doi: 10.1016/j.neuropsychologia.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson GG, Quigley KS, Lozano D. Cardiovascular psychophysiology. In: Cacioppo J, Tassinary LG, Berntson GG, editors. Handbook of psychophysiology. 3. New York: Cambridge University Press; 2007. pp. 182–210. [Google Scholar]
- Bridgett DJ, Burt NM, Edwards ES, Deater-Deckard K. Intergenerational transmission of self-regulation: A multidisciplinary review and integrative conceptual framework. Psychological Bulletin. 2015;141:602–654. doi: 10.1037/a0038662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler EA, Randall AK. Emotional coregulation in close relationships. Emotion Review. 2013;5(2):202–210. [Google Scholar]
- Cacioppo J, Sandman C. Physiological differentiation of sensory and cognitive tasks as a function of warning: Processing demands, and reported unpleasantness. Biological Psychology. 1978;6:181–192. doi: 10.1016/0301-0511(78)90020-0. [DOI] [PubMed] [Google Scholar]
- Compton RJ, Hofheimer J, Kazinka R. Stress regulation and cognitive control: evidence relating cortisol reactivity and neural responses to errors. Cognitive, Affective, & Behavioral Neuroscience. 2013;13(1):152–163. doi: 10.3758/s13415-012-0126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas K, Deater-Deckard K, Kim-Spoon J, Watson AJ, Morasch KC, Bell MA. What’s mom got to do with it? Contributions of maternal executive function and caregiving to the development of executive function across early childhood. Developmental science. 2014;17(2):224–238. doi: 10.1111/desc.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall AA, Deater-Deckard K, Riley AE. Maternal emotion and cognitive control capacities and parenting: A conceptual framework. Developmental Review. 2015;36:105–126. doi: 10.1016/j.dr.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ, Marshall JR, Tomarken AJ, Henriques JB. While a phobic waits: Regional brain electrical and autonomic activity in social phobics during anticipation of public speaking. Biological Psychiatry. 2000;47(2):85–95. doi: 10.1016/s0006-3223(99)00222-x. [DOI] [PubMed] [Google Scholar]
- Davis HP, Keller FR. Colorado Assessment Test Manual. Colorado Springs, CO: Colorado Assessment Tests; 1998. [Google Scholar]
- Dawson JF, Richter AW. Probing three-way interactions in moderated multiple regression: Development and application of a slope difference. Journal of Applied Psychology. 2006;91(4):917–926. doi: 10.1037/0021-9010.91.4.917. [DOI] [PubMed] [Google Scholar]
- Deane GE. Human heart rate responses during experimentally induced anxiety. Journal of Experimental Psychology. 1961;61(6):489–493. doi: 10.1037/h0049220. [DOI] [PubMed] [Google Scholar]
- Deater-Deckard K. Parenting and child behavioral adjustment in early childhood: A quantitative genetic approach to studying family processes and child development. Child Development. 2000;71:468–484. doi: 10.1111/1467-8624.00158. [DOI] [PubMed] [Google Scholar]
- Deater-Deckard K, Sewell MD, Petrill SA, Thompson LA. Maternal working memory and reactive negativity in parenting. Psychological Science. 2010;21:75–79. doi: 10.1177/0956797609354073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deater-Deckard K, Wang Z, Chen N, Bell MA. Maternal executive function, harsh parenting, and child conduct problems. Journal of Child Psychology and Psychiatry. 2012;53(10):1084–1091. doi: 10.1111/j.1469-7610.2012.02582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix T. The affective organization of parenting: Adaptive and maladaptative processes. Psychological bulletin. 1991;110(1):3–25. doi: 10.1037/0033-2909.110.1.3. [DOI] [PubMed] [Google Scholar]
- Dmitrieva NO, Almeida DM, Dmitrieva J, Loken E, Pieper CF. A day-centered approach to modeling cortisol: Diurnal cortisol profiles and their associations among US adults. Psychoneuroendocrinology. 2013;38(10):2354–2365. doi: 10.1016/j.psyneuen.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Büchel C, Gross JJ. The neural bases of emotion regulation. Nature Reviews Neuroscience. 2015;16(11):693–700. doi: 10.1038/nrn4044. [DOI] [PubMed] [Google Scholar]
- Fairclough SH, Venables L, Tattersall A. The influence of task demand and learning on the psychophysiological response. International Journal of Psychophysiology. 2005;56:171–184. doi: 10.1016/j.ijpsycho.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Finkenauer C, Buyukcan-Tetik A, Baumeister RF, Schoemaker K, Bartels M, Vohs KD. Out of control: Identifying the role of self-control strength in family violence. Current Directions in Psychological Science. 2015;24(4):261–266. [Google Scholar]
- Friedman NP, Miyake A. Unity and diversity of executive functions: Individual differences as a window on cognitive structure. Cortex. doi: 10.1016/j.cortex.2016.04.023. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano RJ, Skowron EA, Berkman ET. Growth models of dyadic synchrony and mother–child vagal tone in the context of parenting at-risk. Biological Psychology. 2015;105:29–36. doi: 10.1016/j.biopsycho.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagger MS, Wood C, Stiff C, Chatzisarantis NL. Ego depletion and the strength model of self-control: a meta-analysis. Psychological bulletin. 2010;136(4):495–525. doi: 10.1037/a0019486. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Staff PAR. WCST: CV4 Wisconsin Card Sorting Test: Computer Version 4 research edition user’s manual. Lutz, FL: Psychological Assessment Resources; 2003. [Google Scholar]
- Hughes C. Changes and challenges in 20 years of research into the development of executive functions. Infant and Child Development. 2011;20(3):251–271. [Google Scholar]
- Joosen KJ, Mesman J, Bakermans-Kranenburg MJ, Pieper S, Zeskind PS, van IJzendoorn MH. Physiological reactivity to infant crying and observed maternal sensitivity. Infancy. 2013a;18(3):414–431. [Google Scholar]
- Joosen KJ, Mesman J, Bakermans-Kranenburg MJ, van IJzendoorn MH. Maternal overreactive sympathetic nervous system responses to repeated infant crying predicts risk for impulsive harsh discipline of infants. Child Maltreatment. 2013b;18(4):252–263. doi: 10.1177/1077559513494762. [DOI] [PubMed] [Google Scholar]
- Kennedy DO, Scholey AB. Glucose administration, heart rate and cognitive performance: Effects of increasing mental effort. Psychopharmacology. 2000;149:63–71. doi: 10.1007/s002139900335. [DOI] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Research Reviews. 1999;29:169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Lansford JE, Malone PS, Dodge KA, Chang L, Chaudhary N, Tapanya S, … Deater-Deckard K. Children’s perceptions of maternal hostility as a mediator of the link between discipline and children’s adjustment in four countries. International Journal of Behavioral Development. 2010;34(5):452–461. doi: 10.1177/0165025409354933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leerkes EM, Supple AJ, O’Brien M, Calkins SD, Haltigan JD, Wong MS, Fortuna K. Antecedents of maternal sensitivity during distressing tasks: integrating attachment, social information processing, and psychobiological perspectives. Child Development. 2015;86(1):94–111. doi: 10.1111/cdev.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren KA, Larson CL, Schaefer SM, Abercrombie HC, Ward RT, Oakes TR, … Davidson RJ. Thalamic metabolic rate predicts EEG alpha power in healthy control subjects but not in depressed patients. Biological Psychiatry. 1999;45(8):943–952. doi: 10.1016/s0006-3223(98)00350-3. [DOI] [PubMed] [Google Scholar]
- Lorber MF, O’Leary SG. Mediated paths to overreactive discipline: Mothers’ experienced emotion, appraisals, and physiological responses. Journal of Consulting and Clinical Psychology. 2005;73(5):972–981. doi: 10.1037/0022-006X.73.5.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martorell GA, Bugental DB. Maternal variations in stress reactivity: implications for harsh parenting practices with very young children. Journal of Family Psychology. 2006;20(4):641–647. doi: 10.1037/0893-3200.20.4.641. [DOI] [PubMed] [Google Scholar]
- McClelland GH, Judd CM. Statistical difficulties of detecting interactions and moderator effects. Psychological Bulletin. 1993;114(2):376–390. doi: 10.1037/0033-2909.114.2.376. [DOI] [PubMed] [Google Scholar]
- Melby-Lervåg M, Hulme C. Is working memory training effective? A meta-analytic review. Developmental psychology. 2013;49(2):270–291. doi: 10.1037/a0028228. [DOI] [PubMed] [Google Scholar]
- Mills-Koonce WR, Propper C, Gariepy JL, Barnett M, Moore GA, Calkins S, Cox MJ. Psychophysiological correlates of parenting behavior in mothers of young children. Developmental Psychobiology. 2009;51(8):650–661. doi: 10.1002/dev.20400. [DOI] [PubMed] [Google Scholar]
- Mitchell RL, Phillips LH. The psychological, neurochemical and functional neuroanatomical mediators of the effects of positive and negative mood on executive functions. Neuropsychologia. 2007;45(4):617–629. doi: 10.1016/j.neuropsychologia.2006.06.030. [DOI] [PubMed] [Google Scholar]
- Nix RL, Pinderhughes EE, Dodge KA, Bates JE, Pettit GS, McFadyen-Ketchum SA. The relation between mothers’ hostile attribution tendencies and children’s externalizing behavior problems: The mediating role of mothers’ harsh discipline practices. Child development. 1999;70(4):896–909. doi: 10.1111/1467-8624.00065. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA. Electroencephalography and high-density electrophysiological source localization. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of psychophysiology. 3. Cambridge, UK: Cambridge University Press; 2007. pp. 56–84. [Google Scholar]
- Rodriguez CM, Tucker MC. Predicting maternal physical child abuse risk beyond distress and social support: Additive role of cognitive processes. Journal of Child and Family Studies. 2015;24(6):1780–1790. [Google Scholar]
- Sauseng P, Klimesch W, Stadler W, Schabus M, Doppelmayr M, Hanslmayr S, … Birbaumer N. A shift of visual spatial attention is selectively associated with human EEG alpha activity. European Journal of Neuroscience. 2005;22(11):2917–2926. doi: 10.1111/j.1460-9568.2005.04482.x. [DOI] [PubMed] [Google Scholar]
- Schmeichel BJ, Tang D. Individual differences in executive functioning and their relationship to emotional processes and responses. Current Directions in Psychological Science. 2015;24(2):93–98. [Google Scholar]
- Schreckenberger M, Lange-Asschenfeldt C, Lockmann M, Mann K, Siessmeier T, Buchholz HG, Bartenstein P, Grunder G. The thalamus as the generator and modulator of EEG alpha rhythm: A combined PET/EEG study with lorazepam challenge in humans. Neuroimage. 2004;22:637–644. doi: 10.1016/j.neuroimage.2004.01.047. [DOI] [PubMed] [Google Scholar]
- Skowron EA, Loken E, Gatzke-Kopp LM, Cipriano-Essel EA, Woehrle PL, Van Epps JJ, … Ammerman RT. Mapping cardiac physiology and parenting processes in maltreating mother–child dyads. Journal of Family Psychology. 2011;25(5):663–674. doi: 10.1037/a0024528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler DP, Bell MA, Deater-Deckard K. Emotion suppression moderates the quadratic association between RSA and executive function. Psychophysiology. 2015;52(9):1175–1185. doi: 10.1111/psyp.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern RM, Ray WJ, Quigley KS. Psychophysiological recording. Oxford University Press; 2001. [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18(6):643. [Google Scholar]
- Sturge-Apple ML, Suor JH, Skibo MA. Maternal child-centered attributions and harsh discipline: The moderating role of maternal working memory across socioeconomic contexts. Journal of Family Psychology. 2014;28(5):645–654. doi: 10.1037/fam0000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahler RG, Dumas JE. Attentional problems in dysfunctional mother-child interactions: An interbehavioral model. Psychological Bulletin. 1989;105(1):116–130. doi: 10.1037/0033-2909.105.1.116. [DOI] [PubMed] [Google Scholar]
- Wilson GF, Swain CR, Ullsperger P. EEG power changes during a multiple level memory retention task. International Journal of Psychophysiology. 1999;32:107–118. doi: 10.1016/s0167-8760(99)00005-7. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Hansen AL, Saus-Rose E, Johnsen BH. Heart rate variability, prefrontal neural function, and cognitive performance: the neurovisceral integration perspective on self-regulation, adaptation, and health. Annals of Behavioral Medicine. 2009;37(2):141–153. doi: 10.1007/s12160-009-9101-z. [DOI] [PubMed] [Google Scholar]
- Zanstra YJ, Johnston DW. Cardiovascular reactivity in real life settings: Measurement, mechanisms and meaning. Biological Psychology. 2011;86(2):98–105. doi: 10.1016/j.biopsycho.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]