Abstract
Background
Type-2 diabetes and obesity independently increases the risk of heart failure via incompletely understood mechanisms. We propose that hyperinsulinemia might promote adverse consequences in hearts of subjects with type-2 diabetes and obesity.
Methods
High fat diet feeding was used to induce obesity and diabetes in wild type mice or mice lacking β2-adrenergic receptor (β2AR) or β-arrestin2. Wild type mice fed with high fat diet were treated with β-blocker carvedilol or G-protein receptor kinase 2 (GRK2) inhibitor. We examined the signaling and cardiac contractile function.
Results
High fat diet feeding selectively increases the expression of phosphodiesterase 4D (PDE4D) in mouse hearts, in concert with reduced PKA phosphorylation of phospholamban, which contributes to systolic and diastolic dysfunction. The expression of PDE4D is also elevated in human hearts with diabetes. The induction of PDE4D expression is mediated by an insulin receptor, insulin receptor substrate, and (GRK2) and β-arrestin2-dependent transactivation of a β2AR-ERK signaling cascade. Thus pharmacological inhibition of β2AR or GRK2, or genetic deletion of β2AR or β-arrestin2, all significantly attenuate insulin-induced phosphorylation of ERK and PDE4D induction, to prevent diabetes-related contractile dysfunction.
Conclusions
These studies elucidate a novel mechanism by which hyperinsulinemia contributes to heart failure by increasing PDE4D expression and identify β2AR or GRK2 as plausible therapeutic targets for preventing or treating heart failure in subjects with type-2 diabetes.
Keywords: beta-adrenergic receptors, insulin, diabetic cardiomyopathy, heart failure, phosphodiesterase
Cardiovascular disease remains the greatest unmet challenge in reducing mortality in subjects with type 2 diabetes and the metabolic syndrome 1. Heart failure (HF) risk remains substantially elevated in diabetes even after adjusting for traditional risk factors such as ischemic heart disease and hypertension 2. Indeed, the term diabetic cardiomyopathy has been used to describe intrinsic pathophysiological mechanisms in cardiomyocytes, which are exacerbated by diabetes that directly leads to cardiac dysfunction or may exacerbate cardiac dysfunction in the face of stressors such as cardiac ischemia or hypertrophy 2, 3. Multiple mechanisms have been suggested including altered substrate metabolism, mitochondrial dysfunction and altered calcium signaling 4. Altered myocardial insulin signaling has emerged as a potential pathophysiological mechanism 5, 6. The specific mechanisms linking insulin resistance and heart failure have been challenging to unravel given complex interactions between insulin signaling pathways such as Akt signaling, chronic activation of which may accelerate ventricular remodeling 7-9, versus impaired activation of FOXO proteins a hall-mark of cellular insulin resistance, which may also exacerbate heart failure in insulin resistant states 10-12. Moreover, in type 2 diabetes, hyperinsulinemia has been associated with depressed adrenergic signaling, that may lead to decreased contractility despite elevated catecholamine levels, presumably due to either dysfunctional calcium handling within cardiomyocytes or catecholamine-induced β adrenergic receptor (βAR) desensitization13-16.
Human studies have suggested that insulin receptor (IR)-mediated signaling is enhanced in heart failure9. We also recently demonstrated that reducing IR signaling in cardiomyocytes attenuated LV dysfunction and adverse LV remodeling following transverse aortic constriction 7. A novel mechanism by which hyperinsulinemia might impair myocardial contractility is via inhibition of adrenergic signaling. We recently provided direct molecular evidence for an inhibitory effect of insulin signaling on beta-adrenergic responsiveness in the heart that was mediated by β2AR signaling pathways17. Interestingly some clinical trials have raised the possibility that insulin treatment or drugs that may raise circulating insulin concentrations might increase mortality or hospitalization rates in HF patients18-21. Although, β blockade with either beta-1 selective agents such as metoprolol22 or β1AR and β2AR nonselective agents such as carvedilol 23 reduces mortality in diabetic patients with heart failure, the mortality reduction benefit is attenuated relative to non-diabetics and some trials suggest that agents with β2AR blocking properties could be superior 24. Therefore, additional mechanistic studies are required to elucidate the complex interactions between insulin and beta-adrenergic signaling that may increase heart failure risk in diabetes.
Biochemically, IRs and βARs induce heterologous signal transduction pathways leading to different cellular processes. IRs, a member of the large receptor tyrosine kinase (RTK) family, phosphorylates insulin receptor substrate (IRS-1) leading to Akt and ERK activation 25-27. Ligand binding to βARs, which are prototypical members of the G protein-coupled receptor superfamily, induces cAMP-dependent protein kinase A (PKA) activation 28-31. Studies in adipocytes and liver cells have suggested that insulin and adrenergic stimulation act reciprocally to blunt each other's signaling. Recently, we have shown that IRs and β2ARs form a membrane receptor complex to coordinate signal transduction induced by insulin and catecholamines in the heart17, 32. The mechanism and consequence of cross-talk between IRs and βARs appears to be cell and tissue-specific, and remains incompletely understood in cardiac tissue. In particular, little is known about how IR signaling may affect βAR signaling in the myocardium, to modulate cardiac function in clinically relevant states such as diabetes and heart failure.
We hypothesized that diabetes-associated heart failure is due in part to decreased myocardial contractility that is mediated by insulin-induced depression of βAR signaling in cardiomyocytes, which decreases cAMP/PKA activities and substrate phosphorylation that are required to sustain cardiac contractility. In the present study, we reveal that hyperinsulinemia selectively induces cardiac expression of PDE4D, an enzyme that is critically involved in regulating cAMP-PKA activity in cardiac adrenergic signaling and function33, 34. We show that hyperinsulinemia-induced PDE4D expression impairs adrenergic regulation and promotes cardiac dysfunction in a diabetic model induced by high fat diet (HFD) feeding and identify the molecular pathways that mediate this. We also demonstrate that pharmacological or genetic inhibition of these pathways can effectively prevent or treat obesity or diabetes-related heart failure.
Methods
Experimental animals and in vivo treatment
The animal care and experimental protocols followed US National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committees (IACUC) of the University of California at Davis, the University of Utah and the Carver College of Medicine of the University of Iowa. C57BL/6 mice were purchased from Charles River. Six-week-old male WT, β2AR global knockout (β2KO) and β-arrestin2 global knockout (β-arr2 KO) mice were randomly assigned to two groups fed ad libitum with either a low-fat diet or a matched high-fat diet (Research Diets Inc.) for six months (n=26). The low fat diet was D12450J (3.85 kcal/g; 10% of calories from fat, 20% of calories from protein and 70% of calories from carbohydrate) and the high fat diet (HFD) was D12492 (5.24 kcal/g; 60% of calories from fat, 20% of calories from protein and 20% of calories from carbohydrate). Blood glucose levels were measured after a fast of 6 hours. Cardiac function was assessed before and after 24 wk. HFD or chow diet by echocardiography under isoflurane anesthesia. Mice were subjected to intraperitoneal glucose tolerance testing (IPGTT) following each echocardiography study. Echocardiography was performed using a Vevo 2100 imaging system from VisualSonics (Toronto, ON, Canada) with a 22-55 MHz MS550D transducer.
Primary adult cardiomyocyte isolation and culture
The isolation of adult cardiomyocytes was carried out as described previously 17. Freshly isolated adult cardiomyocytes were loaded with Fluo-4 AM (5 μM; Molecular Probes, Grand Island, NY,) for 30 min before measuring calcium transients and contractility as described 32.
Statistical analysis
All data are expressed as mean ± SEM. All statistical analysis was performed in SPSS statistical software, version 22.0. The sample size for each group is shown in the figure legends or online supplementary tables. The in vitro studies were done with at least three sets of independent experiments. All data were normally distributed. The differences between two groups were then evaluated by 2 -tailed Student's t-test; and the comparisons of multiple groups were performed using either one-way or two-way ANOVA followed by post hoc Tukey's test. For the time course animal studies, data were analyzed with repeated measures ANOVA. P < 0.05 was defined as statistically significant.
Extended methods can be found in the online supplementary materials.
Results
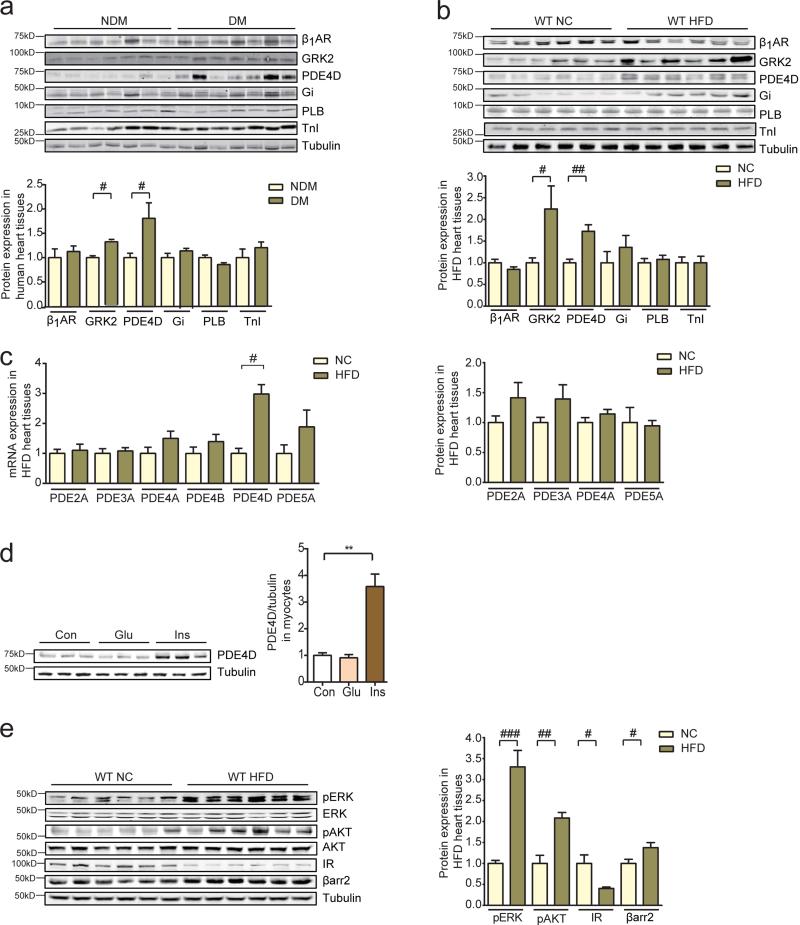
We examined if diabetes mellitus (DM) is associated with modification of adrenergic signaling in human hearts. In right atrial appendage tissues from patients with type 2 DM or non-diabetics obtained at the time of coronary artery bypass surgery, phosphodiesterase 4 (PDE4) and GRK2 were significantly increased relative to patients without DM (Figure 1a). We then examined a murine model of HFD feeding that develops obesity, hyperglycemia and hyperinsulinemia (Supplementary Figure 1a and 1b). After 6 months of high-fat feeding, these animals developed cardiac hypertrophy and fibrosis when compared to normal chow (NC) controls; they also displayed a small but significant increase in apoptosis in myocardium (Supplementary Figure 2a and 2b). In concordance with data from human tissues, both mRNA and protein levels of a PDE4 family gene PDE4D were specifically induced in HFD hearts relative to those fed with NC (Figure 1b and 1c). In contrast, the other PDE isoforms were not altered (Figure 1c). In isolated adult ventricular myocytes (AVMs), insulin, but not glucose induced PDE4D protein levels (Figure 1d), suggesting that insulin-induced signaling may modulate PDE4D expression in HFD hearts. We also examined the insulin signaling pathways in HFD hearts. The expression of IR and IRS2 were reduced in HFD hearts relative to those fed with NC (Figure 1e and Supplementary Figure 1c). Accordingly, in comparison to isolated myocytes from NC hearts, myocytes from HFD hearts displayed a blunted response to insulin stimulation (Supplementary Figure 1d). Importantly, basal ERK and Akt signaling, as well as downstream S6K1 and GSK3 phosphorylation, were significantly elevated in HFD hearts relative to those fed with NC (Figure 1e). These data suggest that despite insulin resistance, ambient hyperinsulinemia may activate the remaining IRs to promote downstream signaling that might regulate gene expression.
Figure 1.
Myocardial phosphodiesterase 4D expression is increased in insulin resistant states. a) Right atrial appendage tissues from patients with diabetes mellitus display increased PDE4D protein levels relative to patients without diabetes mellitus (n=6). b and c) 6 months of HFD increases protein and mRNA levels of PDE4D in mouse hearts (n=12). d) Overnight treatment with insulin (100 nM), but not glucose (400 mg/dL) increases PDE4D expression levels in mouse adult ventricle myocytes (AVMs) (n=6). **p< 0.01by one-way ANOVA followed by post hoc Tukey's test. e) HFD induces activation of ERK (T202/Y204) and Akt (S473) despite a decrease in IR expression (n=12). #p< 0.05, ##p < 0.01, and ###p < 0.001 by student t-test between paired groups.
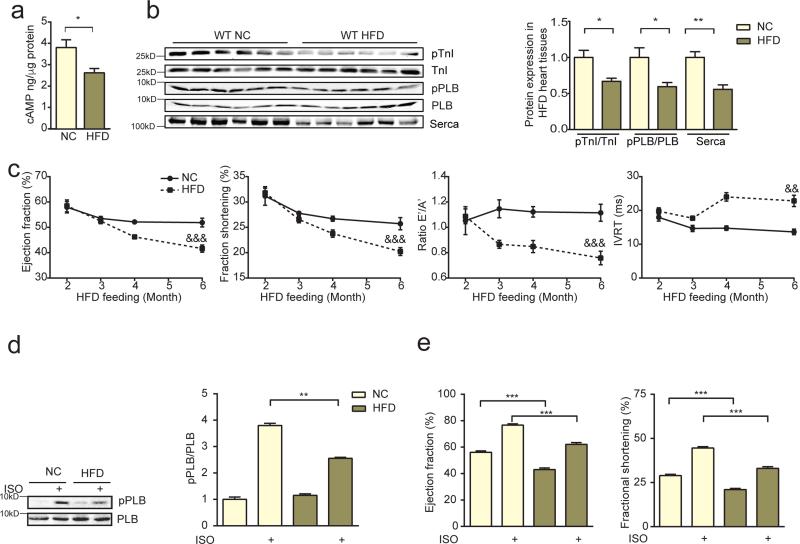
PDE4D plays an essential role in controlling cAMP levels in hearts via hydrolysis of cyclic nucleotides. In HFD hearts with elevated PDE4D expression, cAMP levels and cAMP-dependent PKA phosphorylation of substrates such as phospholamban (PLB) and troponin I (TnI) that are involved in cardiac contractility were reduced (Figure 2a and 2b). Consequently, HFD mice developed time-dependent diastolic and systolic cardiac dysfunction (Figure 2c and online supplementary Table 1). In addition, HFD mice displayed reduced PKA phosphorylation of PLB and cardiac reserve in response to beta-adrenergic stimulation (Figure 2d and 2e). Taken together, this data link hyperinsulinemia with PDE4D induction, impaired inotropic signaling to adrenergic stimulation, and diastolic and systolic dysfunction in murine hearts.
Figure 2.
HFD impairs adrenergic reserve, and induces diastolic and systolic dysfunction in murine hearts. a and b) HFD leads to reduced cAMP and PKA phosphorylation at serine 16 of PLB and at serine 23/24 of troponin I (TnI) in mouse hearts. HFD also leads to reduced serca expression (n=12). *p < 0.05 and **p < 0.01by Student's t-test c) HFD promotes time-dependent systolic and diastolic dysfunction in mouse hearts (n=12). &&p < 0.01, &&&p < 0.001 by repeated measures ANOVA. d and e) After 6 month of HFD, mouse hearts display reduced PKA phosphorylation of PLB (S16, n=6) and contractile response to β adrenergic stimulation (ISO, 0.2 mg/kg in vivo, n=12). ** p < 0.01, and *** p < 0.001 by one-way ANOVA followed by post hoc Tukey's test.
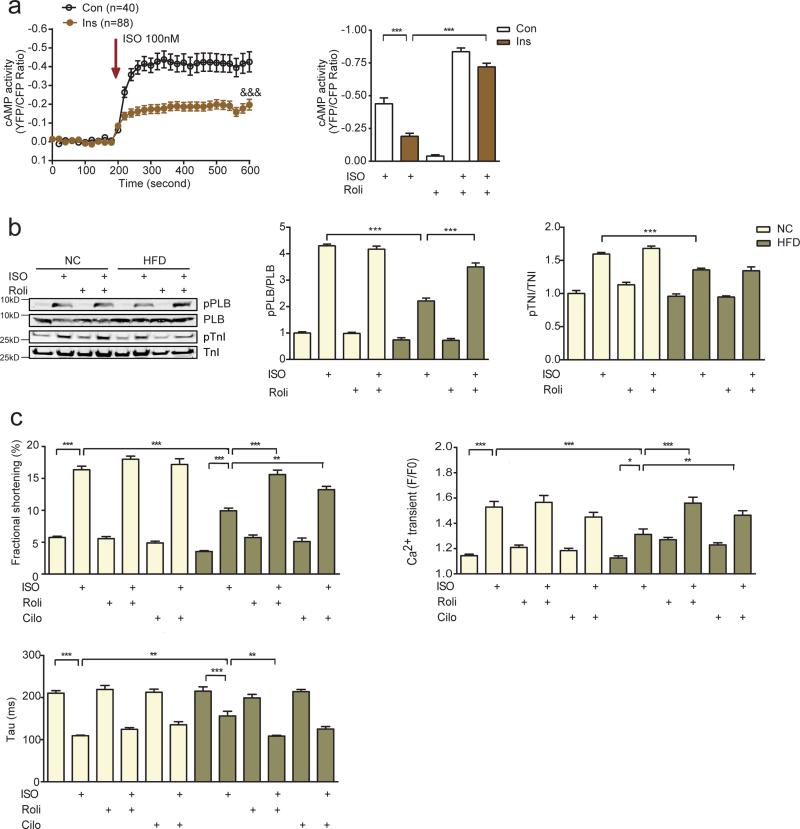
We therefore hypothesized that increased cAMP degradation resulting from increased PDE4D expression in HFD hearts will impair cardiac contractile function in response to adrenergic stimulation. In isolated naïve AVMs, 12-hour incubation with insulin significantly reduced adrenergic (isoproterenol)-induced cAMP levels, which was rescued by a specific PDE4 inhibitor rolipram (Figure 3a). In AVMs isolated from HFD hearts, PKA phosphorylation of both PLB and TnI in response to adrenergic stimulation was significantly impaired relative to those from NC controls (Figure 3b). In parallel, calcium transients and contractile shortening in response to adrenergic stimulation were also impaired in AVMs from HFD hearts (Figure 3c). The PDE4 inhibitor Rolipram, rescued PKA phosphorylation of PLB, calcium transients and contractile shortening in HFD myocytes (Figure 3b and 3c). These data suggest that hyperinsulinemia-induced PDE4D expression contributes to impaired inotropic signaling in cardiomyocytes.
Figure 3.
Impaired adrenergic stimulation of cardiac function in hyperinsulinemic states is mediated in part by increased PDE4D expression. a) Insulin treatment (100 nM, 12hrs) impairs adrenergic stimulation of cAMP in mouse AVMs, which is rescued by pretreating cells with the PDE4 specific inhibitor rolipram (100 nM, n=6). &&& p <0.001 by two-way ANOVA, * p< 0.05, ** p < 0.01, and *** p < 0.001 by one-way ANOVA followed by post hoc Tukey's test. b and c) HFD leads to reduced PKA phosphorylation of PLB (S16) and TnI (S23/24), calcium signaling, and contractility in mouse AVMs in response to β adrenergic stimulation (ISO, 100 nM for AVM stimulation, n=6), which are rescued by the PDE4 inhibitor rolipram (100 nM). ** p < 0.01, and *** p < 0.001 by one-way ANOVA followed by post hoc Tukey's test
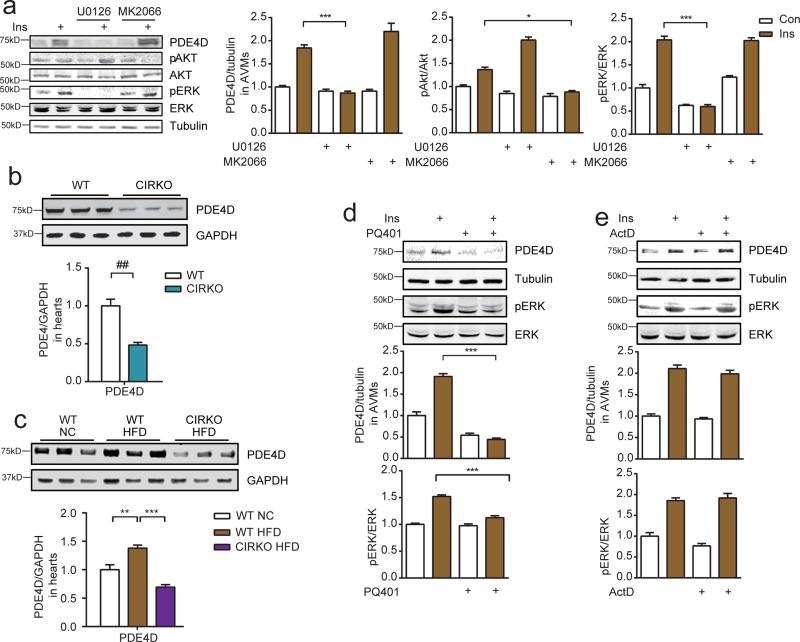
IR activation promotes two major signaling cascades that modulate cellular responses namely ERK and Akt. Both ERK and Akt phosphorylation were elevated in HFD hearts. We examined which signaling branch is involved in the induction of PDE4D expression. In isolated AVMs, inhibition of ERK, but not Akt completely blocked insulin-induced induction of PDE4D (Figure 4a). We then sought to map the signaling nodes required for ERK activation and PDE4D induction in HFD hearts using strategies in vivo and in cultured cardiomyocytes. We first examined PDE4D expression in the hearts of mice with cardiomyocyte-restricted deletion of IR (CIRKO) 25. As shown in Figure 4b, PDE4D expression was reduced by 50% in CIRKO mice fed a NC diet and remained repressed following HFD (Figure 4b and 4c). Inhibition of IRS, but not GRB2 significantly blocked insulin-induced ERK activation and PDE4D induction in isolated AVMs (Figure 4d and 4e). These data suggest that in this experimental context, ERK activity induced by insulin might be independent of the classical IRS-GRB2-SOS cascade.
Figure 4.
Insulin induces PDE4D expression in an IR-and IRS-dependent manner. a) Insulin (100 nM) induces PDE4D expression in mouse AVMs that is dependent on ERK (T202/Y204), but not Akt (S473) signaling (n=3). b and c) Decreased PDE4D content in the hearts of mice with cardiomyocyte-restricted deletion of IR (CIRKO) on normal chow or after 36-weeks of a 45% HFD (n=6). d and e) Insulin-induced ERK activation and PDE4D expression in mouse AVMs is IRS-dependent, but GRB2-independent (n=3). PQ401 is an IRS inhibitor and actinomycin D (ActD) is an inhibitor of GRB2.* p< 0.05, ** p < 0.01, and *** p < 0.001 by one-way ANOVA followed by post hoc Tukey's test.
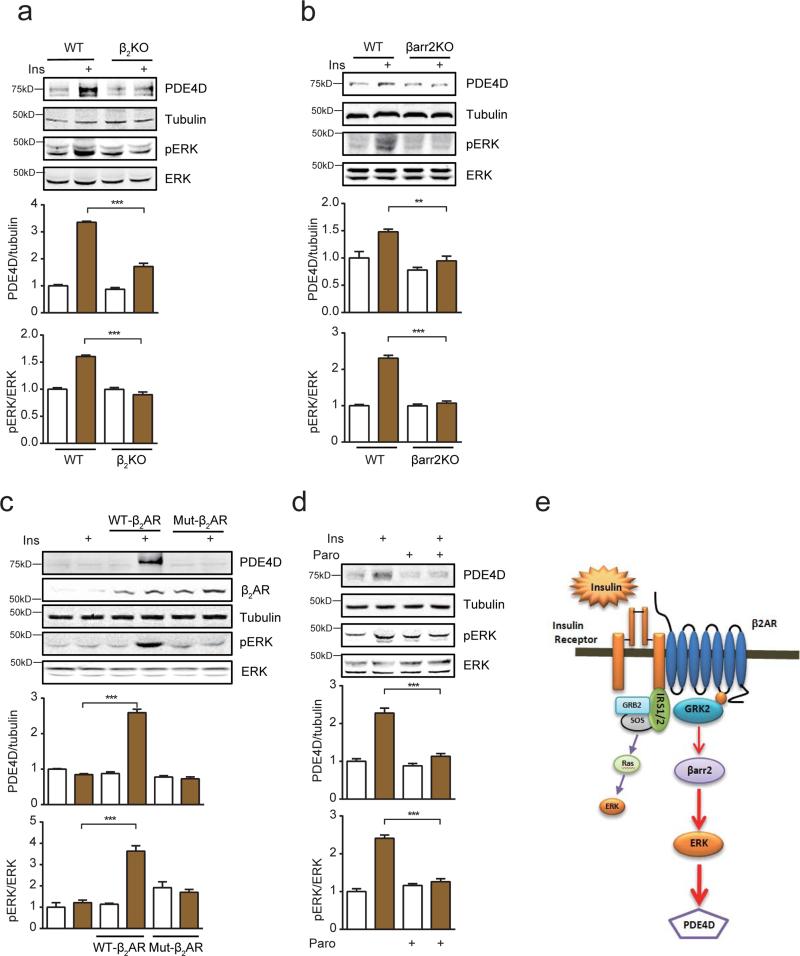
To further explore the underlying mechanisms by which insulin induces PDE4D, we examined the recently characterized membrane IR-β2AR complex by which stimulation of the IR leads to an IRS-dependent and GRK2-mediated phosphorylation of the β2AR17, 32. We hypothesized that the insulin-induced and GRK2-phosphorylated β2AR might promote arrestin-dependent ERK activation 35. Indeed, β2AR formed a complex with β-arrestin2 and ERK in cardiac myocytes after insulin stimulation; the receptor-bound ERK displayed increased activity (Supplementary Figure 3a). Deletion of either β2AR or β-arrestin2 almost completely blocked insulin-induced ERK activation and PDE4D induction (Figure 5a and 5b). In contrast, deletion of β1AR did not affect insulin-induced ERK activation and PDE4D induction (Supplementary Figure 3b). Meanwhile, a clinical non-selective β-blocker carvedilol with arrestin-biased properties and a neutral non-selective β-blocker timolol both attenuated insulin-induced ERK activation and PDE4D induction (Supplementary Figure 3c). Moreover, in β2AR-KO AVMs, reintroduction of wild-type, but not mutant β2AR lacking the GRK phosphorylation sites restored insulin-induced ERK activation and PDE4D induction (Figure 5c). Furthermore, a selective serotonin reuptake inhibitor paroxetine that also acts as a specific GRK2 inhibitor,36 significantly blocked insulin-induced ERK activation and PDE4D induction (Figure 5d), whereas another selective serotonin reuptake inhibitor fluoxetine did not affect insulin-induced ERK activation and PDE4D induction (Supplementary Figure 3d). These data support the existence of a novel IR/IRS-induced and GRK-mediated transactivation of a β2AR-β-arrestin2-ERK signaling cascade that is the primary mediator of insulin-induced ERK activation in cardiomyocytes, which is also necessary for insulin-induction of PDE4D expression (Figure 5e).
Figure 5.
Insulin promotes PDE4D expression via transactivation of a β2AR- β arrestin2-ERK signaling cascade. a and b) Insulin-induced ERK activation and PDE4D expression in mouse AVMs is dependent on β2AR and β-arrestin2 (n=3). c) In β2AR knockout AVMs, reintroduction of wild type, but not β2AR-GRKmut lacking serine 355/356 rescues insulin-induced ERK activation and PDE4D expression (n=3). d) Insulin-induced ERK activation (T202/Y204) and PDE4D expression in mouse AVMs is blocked by a GRK2 inhibitor paroxetine (Paro, 100μM) (n=3). e) Cartoon depicts the signaling intermediates involved in insulin-induced ERK activation and PDE4D expression. ** p< 0.01 and *** p < 0.001 by one-way ANOVA followed by post hoc Tukey's test.
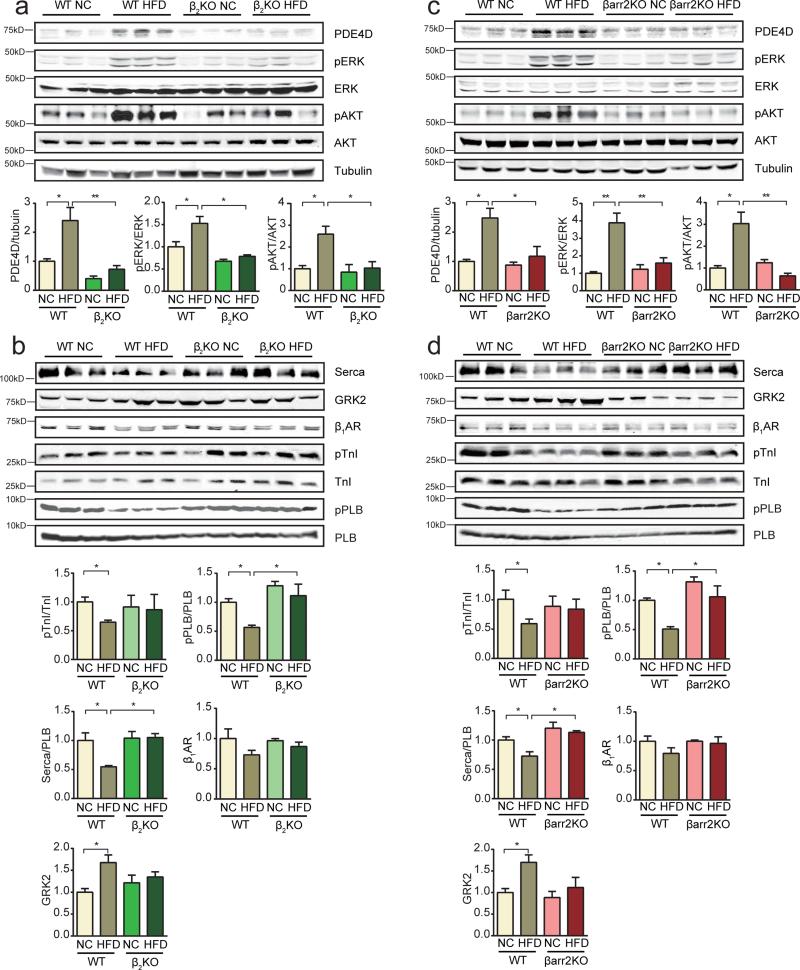
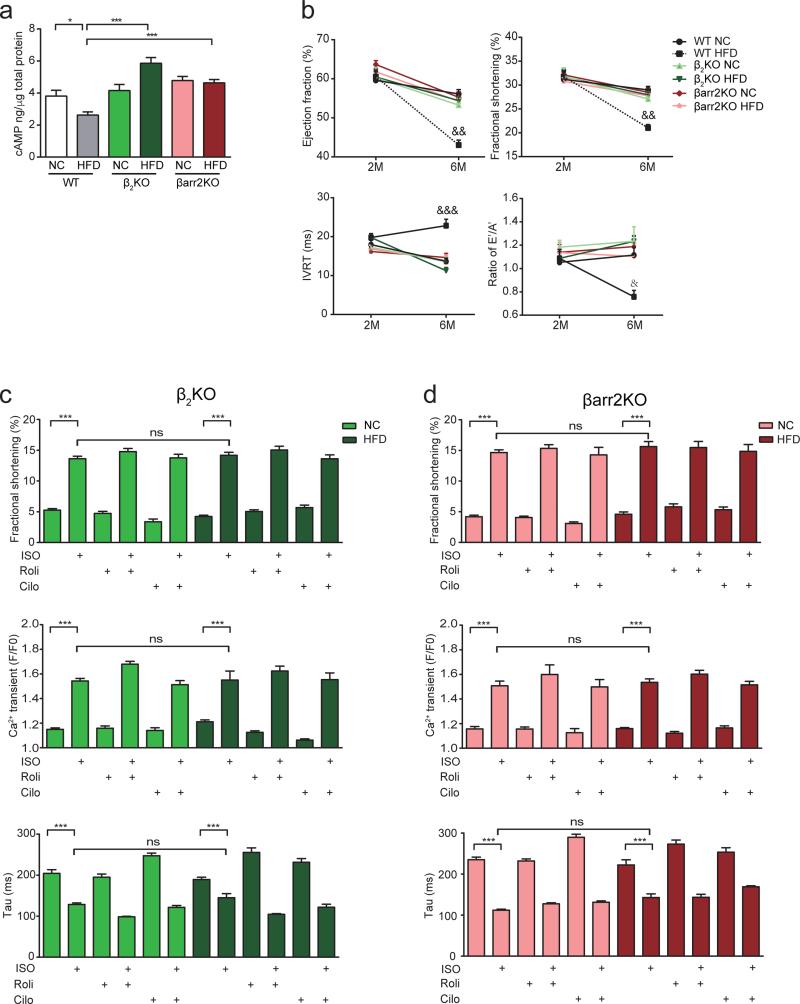
To determine if this novel IR signaling cascade impairs the activation of signaling mediators of EC coupling and contributes to cardiac dysfunction associated with diabetes, mice with germline deletion of either β2AR or β-arrestin2 gene were fed with HFD or NC. On the HFD, these mutant mice still became obese and diabetic (supplementary Figure 4a-4d), and developed cardiac hypertrophy, but myocardial fibrosis and apoptosis were prevented (supplementary Figure 5a-5d, supplementary Table 2). Deletion of these genes blocked activation of ERK and Akt phosphorylation, and PDE4D induction in HFD hearts (Figure 6a and 6c, supplementary Figure 6a and 6b). Deletion of either gene normalized PKA phosphorylation of PLB and TnI (Figure 6b and 6d). Deletion of either gene also normalized the ratio of serca2 to PLB, which was reduced in wild-type HFD hearts (Figure 6b and 6d). Accordingly, deletion of either gene blocked the decrease in cAMP levels in HFD hearts (Figure 7a). Moreover, deletion of either β2AR or β-arrestin2 genes significantly ameliorated cardiac dysfunction induced by HFD feeding (Figure 7b). In AVMs from HFD hearts lacking the β2AR or β-arrestin2 gene, calcium transients and contractile shortening in response to adrenergic stimulation were normal relative to those from NC hearts (Figure 7c and 7d). The PDE4 inhibitor rolipram did not further enhance calcium transients and contractile shortening in HFD myocytes (Figure 7c and 7d). These data suggest that deletion of either β2AR or β-arrestin2 significantly restored contractility in cardiomyocytes and ameliorated cardiac dysfunction in hearts.
Figure 6.
Deletion of β2AR and β-arrestin2 prevents PDE4D induction in hearts of HFD mice. a) Deletion of β2AR prevented HFD-induced activation of ERK (T202/Y204) and Akt (S473), and PDE4D induction in mouse hearts (n=12). b) Deletion of β2AR normalizes PKA phosphorylation of PLB (S16) and TnI (S23/24), the ratio of serca2/PLB in mouse hearts and attenuates the induction of GRK2 (n=12). c) Deletion of β-arrestin2 prevents HFD-induced activation of ERK and Akt, and PDE4D induction in mouse hearts (n=12). d) Deletion of β-arrestin2 normalizes PKA phosphorylation of PLB (S16) and TnI (S23/24), the ratio of serca2/PLB in mouse hearts and prevents the induction of GRK2 (n=12). * p< 0.05 and ** p < 0.01 by one-way ANOVA followed by post hoc Tukey's test. &&&p< 0.001 by two-way ANOVA followed by post hoc Tukey's test.
Figure 7.
Deletion of β2AR and β-arrestin2 ameliorates cardiac dysfunction in HFD mice. a) Deletion of β2AR or β-arrestin2 prevents downregulation of cAMP activity (n=12). b) Deletion of β2AR or β-arrestin2 ameliorates both systolic and diastolic dysfunction induced by HFD (n=12). &p< 0.05, &&p< 0.01 and &&&p< 0.001 by repeated measures ANOVA. c) Deletion of β2AR normalizes calcium signaling and contractile shortening in myocytes after HFD feeding (n=4). d) Deletion of β-arrestin2 normalizes calcium signaling and contractile shortening in myocytes after HFD feeding (n=4). * p< 0.05 and *** p < 0.001 by one-way ANOVA followed by post hoc Tukey's test
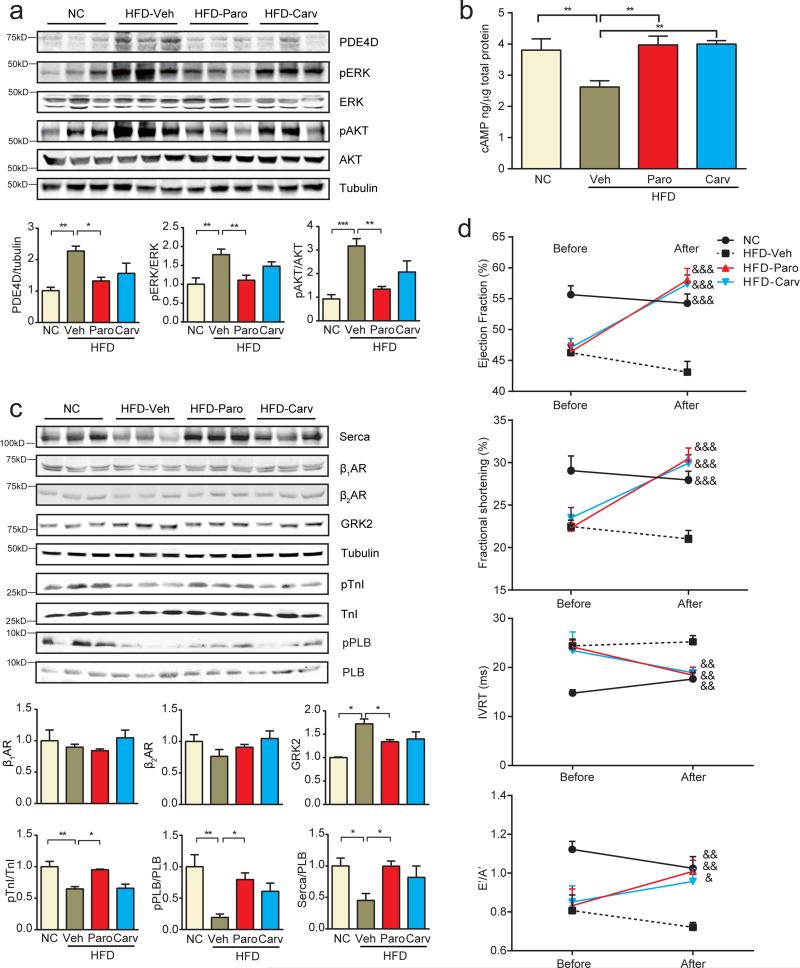
This novel IR-induced transactivation of the β2AR-β-arrestin2-ERK signaling module presents new therapeutic opportunities to treat/prevent cardiac dysfunction associated with DM. We therefore tested whether blocking GRK2-mediated transactivation of the β2AR would be effective in attenuating cardiac dysfunction induced by HFD. Animals fed with HFD for 4 months displayed cardiac dysfunction (Figure 2c); mice were then subjected to one-month therapy with vehicle, the GRK2 specific inhibitor paroxetine that was recently shown to be effective in treating myocardial infarction-induced heart failure 37, or carvedilol, the clinically effective nonselective β-blocker that blocks both β1AR and β2AR. Both paroxetine and carvedilol treatments attenuated ERK and Akt activities and PDE4D induction in hearts relative to animals treated with vehicle alone, with paroxetine treatment being more effective (Figure 8a). Both treatments normalized cAMP levels and PKA phosphorylation of PLB and TnI in HFD hearts relative to vehicle-treated mice (Figure 8b and 8c), and normalized the ratio of serca2 and PLB (Figure 8c). Paroxetine and carvedilol did not alter fasting glucose concentrations but partially improved glucose tolerance although hyperinsulinemia persisted (supplementary Figure 7a and 7b). Despite modest effects on systemic metabolic homeostasis cardiac fibrosis and apoptosis were largely reversed in HFD mice, although cardiac hypertrophy persisted (Supplementary Figure 8a-8c). Importantly, paroxetine and carvedilol significantly improved cardiac dysfunction in comparison to control HFD mice treated with vehicle (Figure 8d and supplementary Table 3).
Figure 8.
The GRK2 inhibitor paroxetine and the nonselective β-blocker carvedilol prevent HFD-induced PDE4D induction and cardiac dysfunction. a) The GRK2 inhibitor paroxetine (2.5 mg/kg, 1 month) and the β-blocker carvedilol (2.5 mg/kg, 1 month) blocks HFD-induced ERK and Akt activation and PDE4D induction in mouse hearts (n=12). b and c) Paroxetine and carvedilol block HFD-induced reduction of cAMP, PKA phosphorylation of PLB (S16) and TnI (S23/24), the ratio of serca2/PLB and prevents the induction of GRK2 in mouse hearts (n=12). d) Paroxetine and carvedilol treatment for 1 month ameliorates cardiac dysfunction in HFD models (n=12). * p< 0.05, ** p < 0.01, and *** p < 0.001 by one-way ANOVA followed by post hoc Tukey's test. &p< 0.05, &&p< 0.01, &&&p< 0.001 by repeated measures ANOVA.
Discussion
Hyperinsulinemia is an independent risk factor for ischemic cardiac disease and predicts coronary atherosclerosis38, 39. Moreover, hyperinsulinemia has been correlated with depressed adrenergic signaling, which may contribute to systolic and diastolic dysfunction in diabetes, despite elevated catecholamine levels, presumably due to either dysfunctional calcium handling within cardiomyocytes or catecholamine-induced βAR down-regulation 13-16.The present study demonstrates that hyperinsulinemia directly impairs cardiac function by depressing cardiomyocyte adrenergic signaling. We describe a novel mechanism, whereby hyperinsulinemia remodels adrenergic signal transduction pathways by selectively increasing the expression of PDE4D, but not of βARs. Hyperinsulinemia drives a novel IR signaling cascade via IRS- and GRK2-dependent transactivation of a β2AR-arrestin-ERK pathway to promote expression of the PDE4D gene. The elevated PDE4D enzyme reduces cAMP activity and PKA phosphorylation of its substrates that may contribute to reduced myocyte calcium cycling and contractile shortening, and systolic and diastolic cardiac dysfunction. More interesting, pharmacological inhibition of GRK2 or the β2AR can effectively reverse cardiac dysfunction induced by HFD.
Mechanistically, IR and β2AR form a membrane complex in cardiomyocytes17, 32. Activation of IR promotes IRS and GRK2-mediated phosphorylation of the β2AR17, 32, which leads to arrestin-dependent ERK activation (Figure 4). Therefore, IR induces arrestin-biased transactivation of the β2AR in a GRK2 phosphorylation-dependent manner. Surprisingly, this new signaling branch is the major mediator of ERK activity induced by insulin, whereas the classic IRS-GRB2-SOS axis appears to play a minor role of in promoting insulin-induced ERK activation in the heart (Figure 5). Moreover, our data suggest that only the β2AR-dependent activation of ERK is necessary for gene expression of PDE4 in cardiomyocytes and in hearts, suggesting that insulin signaling may activate distinct pools of ERK, leading to diverse cellular responses. Insulin-induced transactivation of the β2AR may also play a role in Akt activation, which could promote cardiac hypertrophy independently of the ERK-PDE4D axis. Hyperinsulinemia and hyperglycemia persist in both WT and β2AR-KO hearts after HFD feeding. Despite these metabolic defects, β2AR-KO myocytes and mice after HFD feeding display normalized contractile shortening and cardiac function, respectively, arguing that the improved cardiac function is largely independent of changes in systemic metabolism, but may be due in part to the currently characterized cardiac insulin-β2AR-arrestin-ERK pathway that induces PDE4D expression. Interestingly, a recent study shows that genetic ablation of GRK2 is effective in ameliorating adiposity, and improving plasma levels of insulin, insulin signaling, and glucose intolerance in a HFD-induced diabetes animal model 40, 41. We also observed attenuation of glucose intolerance in HFD mice after therapy with the GRK2 inhibitor paroxetine (Supplementary Figure 3), although not to the same extent. Thus, global improvement in insulin signaling could also contribute in part to the beneficial effects of GRK2 inhibition on cardiac function. Genetic and pharmacological inhibition of the cardiac insulin-β2AR-arrestin pathway also attenuates the expression of cardiac GRK2 levels, which may also contribute to the improved cardiac function. Together, these data suggest that adrenergic signaling and metabolic regulation may represent two independent therapeutic modalities to prevent or treat heart failure that is associated with obesity or type 2 diabetes.
Phosphodiesterases play essential roles in modulating ion channel activity such as L-type calcium channels, ryanodine receptors and the serca2 pump, which controls E-C coupling in cardiomyocytes42-47. PDE3 and PDE4 are the major PDEs in both rodent and human hearts48. Recent studies indicated that the expression of PDEs is altered in diverse disease models49, potentially as an adaptation to early stage disease. In contrast, deletion of the PDE4D gene leads to spontaneous heart failure 42. These observations suggest that phosphodiesterases play an essential role in maintaining a fine balance of tonic cAMP-PKA activity for tissue integrity and function in hearts. Here we show that in a model of insulin resistance and type 2 diabetes, activation of insulin signaling pathways mediates the induction of PDE4D. While myocytes isolated from mice after HFD feeding display impaired calcium signaling and contractile shortening, inhibition of PDE4, but not PDE3 completely rescued calcium signaling and contractile function after adrenergic stimulation. Taken together with prior observations indicating hyperactivation of insulin signaling in failing human hearts 9 and the attenuation of heart failure when insulin signaling is reduced in a model of transverse aortic constriction7; these observations suggest that insulin-induced induction of PDE4 may be a central mechanism that contributes to heart failure. Further studies are needed to directly assess a role for PDE4 in obesity and diabetes associated cardiac dysfunction in vivo.
A detailed mechanistic understanding of the interactions between hyperinsulinemia and heart failure remains to be achieved. For example, the divergence between fibrosis, which was attenuated by inhibiting β2AR and GRK2 signaling, despite persistence of cardiac hypertrophy, underlies the complex interactions of these signaling pathways on distinct elements of cardiac remodeling. Thus mechanisms linking cardiomyocyte ERK activation to the development of cardiac fibrosis in the context of metabolic disorders will be the focus of future investigations. Moreover, we have also previously shown that acute insulin treatment inhibits cardiac contractility by inducing Gi-biased β2AR signaling in hearts 17, 32. Whether this Gi pathway contributes to insulin-induced transactivation of β2AR-arrestin-ERK leading to cardiac dysfunction remains to be examined. Furthermore, despite reduced IR levels in HFD hearts, the remaining IRs are still sufficient to activate ERK and Akt signaling in the face of persistent hyperinsulinemia. These signaling changes may lead to defects in cardiac metabolism and/or structural remodeling including cardiac fibrosis. Indeed, although the focus of this study is on the relationship between ERK activation and PDE4D induction, which clearly might contribute to decreased cardiac contractility, it is possible that the cardiac fibrosis observed, while dependent on ERK signaling could be independent of the induction of PDE4D.
These studies have important translational implications given the large number of individuals with the metabolic syndrome and the significant enrichment of heart failure among subjects with diabetes 50. Hyperinsulinemia is a cardinal characteristic of patients with heart failure and also of those with the metabolic syndrome and type 2 diabetes, many of who require high doses of insulin to achieve metabolic control. Thus, hyperinsulinemia by increasing the expression of PDE4, may exacerbate heart failure by limiting ventricular contractile reserve on the basis of reduced myocardial cAMP/PKA activity. Epidemiological and clinical observations have linked insulin treatment with increased mortality in HF patients with diabetes21. Conversely, blocking the IR-induced β2AR-ERK signaling pathway either genetically or pharmacologically, significantly alleviates the cardiac dysfunction (Figure 7 and 8). In agreement, β blockade with either metoprolol22 or carvedilol23 reduces mortality in diabetic subjects with heart failure with the possibility that agents that possess β2 selective inhibition could be somewhat more effective24. The present study suggests a specific role for the β2AR in hyperinsulinemia-induced cardiac dysfunction. Despite its property as an arrestin-biased ligand, carvedilol can attenuate insulin induced ERK activation and PDE4 induction in vitro and in vivo. It is also possible that the β1AR could be activated as a consequence of the compensatory elevation of sympathetic drive, thereby indirectly contributing to cardiac dysfunction in hyperinsulinemic states. It remains to be determined whether the β1AR selective agent metoprolol is effective in ameliorating cardiac systolic and diastolic dysfunction. Paroxetine is a FDA-approved selective serotonin reuptake inhibitor that also inhibits GRK236. Paroxetine was recently shown to be effective in treating myocardial infarction-induced heart failure whereas another selective serotonin reuptake inhibitor fluoxetine did not 37. Our data shows that inhibition of GRK2 with paroxetine can also be effective in ameliorating cardiac dysfunction in HFD mice. Thus in addition to providing new insights into the pathophysiology of heart failure in insulin resistant states our study provides a strong rationale for targeting β2AR and GRK2-mediated signaling as an attractive therapeutic modality to prevent or treat heart failure that is associated with type 2 diabetes.
Supplementary Material
Clinical Impact.
- What is new?
- Hyperinsulinemia, which characterizes insulin resistant states, synergistically interacts with β2AR signaling pathways via induction of the phosphodiesterase PDE4D that degrades cAMP. This pathway is activated in human hearts in diabetes
- Hyperinsulinemia-induced β2AR signaling pathway inhibits cardiac function and promotes structural injury
- In a mouse model of obesity and type 2 diabetes myocardial injury and dysfunction can be reversed by pharmacologically inhibiting GRK2 signaling with the drug Paroxetine (currently used clinically as an antidepressant) or by inhibiting β2AR signaling with Carvedilol, independently of changes in systemic metabolic parameters (i.e. hyperglycemia and hyperinsulinemia).
- What is clinical implication?
- This is the first demonstration that targeting this novel insulin-b2AR pathway therapeutically may materially prevent diabetes-associated heart failure, which remains a clinically intractable problem, that may be exacerbated following treatment with most existing diabetes therapeutics, particularly those that cause hyperinsulinemia.
- This study has significant implications for the use of beta-blockers in the management of diabetes –associated heart failure and opens up a new therapeutic role for an existing drug that could be repurposed to treat heart failure in diabetes.
Acknowledgment
None
Funding sources:
Studies were supported by NIH grants HL127764 and HL112413 (EDA and YKX), S10 OD10389 (YKX), and DK092065 (EDA), VA Merit grant 01BX002900 to (YKX) and by China NSFC grants 81330081(WW) and 81202541 (QTW), 81473212 (QF), and 81428022 (YKX). EDA and YKX are established investigators of the American Heart Association, and YKX is a Shanghai Eastern Scholar. YZ is supported by a post-doctoral fellowship from the American Heart Association.
Footnotes
Disclosure:
None
Reference
- 1.Gregg EW, Li Y, Wang J, Burrows NR, Ali MK, Rolka D, Williams DE, Geiss L. Changes in diabetes-related complications in the United States, 1990-2010. The New England journal of medicine. 2014;370:1514–23. doi: 10.1056/NEJMoa1310799. [DOI] [PubMed] [Google Scholar]
- 2.Bugger H, Abel ED. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia. 2014;57:660–71. doi: 10.1007/s00125-014-3171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayat SA, Patel B, Khattar RS, Malik RA. Diabetic cardiomyopathy: mechanisms, diagnosis and treatment. Clin Sci (Lond) 2004;107:539–57. doi: 10.1042/CS20040057. [DOI] [PubMed] [Google Scholar]
- 4.Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–23. doi: 10.1161/CIRCULATIONAHA.106.679597. [DOI] [PubMed] [Google Scholar]
- 5.Abel ED, O'Shea KM, Ramasamy R. Insulin resistance: metabolic mechanisms and consequences in the heart. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:2068–76. doi: 10.1161/ATVBAHA.111.241984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taegtmeyer H, Beauloye C, Harmancey R, Hue L. Insulin resistance protects the heart from fuel overload in dysregulated metabolic states. Am J Physiol Heart Circ Physiol. 2013;305:H1693–7. doi: 10.1152/ajpheart.00854.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimizu I, Minamino T, Toko H, Okada S, Ikeda H, Yasuda N, Tateno K, Moriya J, Yokoyama M, Nojima A, Koh GY, Akazawa H, Shiojima I, Kahn CR, Abel ED, Komuro I. Excessive cardiac insulin signaling exacerbates systolic dysfunction induced by pressure overload in rodents. J Clin Invest. 2010;120:1506–14. doi: 10.1172/JCI40096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wende AR, O'Neill BT, Bugger H, Riehle C, Tuinei J, Buchanan J, Tsushima K, Wang L, Caro P, Guo A, Sloan C, Kim BJ, Wang X, Pereira RO, McCrory MA, Nye BG, Benavides GA, Darley-Usmar VM, Shioi T, Weimer BC, Abel ED. Enhanced cardiac Akt/protein kinase B signaling contributes to pathological cardiac hypertrophy in part by impairing mitochondrial function via transcriptional repression of mitochondrion-targeted nuclear genes. Molecular and cellular biology. 2015;35:831–46. doi: 10.1128/MCB.01109-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cook SA, Varela-Carver A, Mongillo M, Kleinert C, Khan MT, Leccisotti L, Strickland N, Matsui T, Das S, Rosenzweig A, Punjabi P, Camici PG. Abnormal myocardial insulin signalling in type 2 diabetes and left-ventricular dysfunction. Eur Heart J. 2010;31:100–11. doi: 10.1093/eurheartj/ehp396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Battiprolu PK, Hojayev B, Jiang N, Wang ZV, Luo X, Iglewski M, Shelton JM, Gerard RD, Rothermel BA, Gillette TG, Lavandero S, Hill JA. Metabolic stress-induced activation of FoxO1 triggers diabetic cardiomyopathy in mice. J Clin Invest. 2012;122:1109–18. doi: 10.1172/JCI60329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qi Y, Zhu Q, Zhang K, Thomas C, Wu Y, Kumar R, Baker KM, Xu Z, Chen S, Guo S. Activation of Foxo1 by insulin resistance promotes cardiac dysfunction and beta-myosin heavy chain gene expression. Circ Heart Fail. 2015;8:198–208. doi: 10.1161/CIRCHEARTFAILURE.114.001457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qi Y, Xu Z, Zhu Q, Thomas C, Kumar R, Feng H, Dostal DE, White MF, Baker KM, Guo S. Myocardial loss of IRS1 and IRS2 causes heart failure and is controlled by p38alpha MAPK during insulin resistance. Diabetes. 2013;62:3887–900. doi: 10.2337/db13-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bell DS. Heart failure: the frequent, forgotten, and often fatal complication of diabetes. Diabetes Care. 2003;26:2433–41. doi: 10.2337/diacare.26.8.2433. [DOI] [PubMed] [Google Scholar]
- 14.Ganguly PK, Pierce GN, Dhalla KS, Dhalla NS. Defective sarcoplasmic reticular calcium transport in diabetic cardiomyopathy. Am J Physiol. 1983;244:E528–35. doi: 10.1152/ajpendo.1983.244.6.E528. [DOI] [PubMed] [Google Scholar]
- 15.Giacomelli F, Wiener J. Primary myocardial disease in the diabetic mouse. An ultrastructural study. Lab Invest. 1979;40:460–73. [PubMed] [Google Scholar]
- 16.Bell DS. Diabetic cardiomyopathy. A unique entity or a complication of coronary artery disease? Diabetes Care. 1995;18:708–14. doi: 10.2337/diacare.18.5.708. [DOI] [PubMed] [Google Scholar]
- 17.Fu Q, Xu B, Liu Y, Parikh D, Li J, Li Y, Zhang Y, Riehle C, Zhu Y, Rawlings T, Shi Q, Clark RB, Chen X, Abel ED, Xiang YK. Insulin inhibits cardiac contractility by inducing a Gi-biased beta2-adrenergic signaling in hearts. Diabetes. 2014;63:2676–89. doi: 10.2337/db13-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nichols GA, Hillier TA, Erbey JR, Brown JB. Congestive heart failure in type 2 diabetes: prevalence, incidence, and risk factors. Diabetes Care. 2001;24:1614–9. doi: 10.2337/diacare.24.9.1614. [DOI] [PubMed] [Google Scholar]
- 19.Castagno D, Baird-Gunning J, Jhund PS, Biondi-Zoccai G, MacDonald MR, Petrie MC, Gaita F, McMurray JJ. Intensive glycemic control has no impact on the risk of heart failure in type 2 diabetic patients: evidence from a 37,229 patient meta-analysis. Am Heart J. 2011;162:938–948 e2. doi: 10.1016/j.ahj.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 20.Scirica BM, Braunwald E, Raz I, Cavender MA, Morrow DA, Jarolim P, Udell JA, Mosenzon O, Im K, Umez-Eronini AA, Pollack PS, Hirshberg B, Frederich R, Lewis BS, McGuire DK, Davidson J, Steg PG, Bhatt DL, Committee S-TS and Investigators* Heart failure, saxagliptin, and diabetes mellitus: observations from the SAVOR-TIMI 53 randomized trial. Circulation. 2014;130:1579–88. doi: 10.1161/CIRCULATIONAHA.114.010389. [DOI] [PubMed] [Google Scholar]
- 21.Smooke S, Horwich TB, Fonarow GC. Insulin-treated diabetes is associated with a marked increase in mortality in patients with advanced heart failure. Am Heart J. 2005;149:168–74. doi: 10.1016/j.ahj.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Deedwania PC, Giles TD, Klibaner M, Ghali JK, Herlitz J, Hildebrandt P, Kjekshus J, Spinar J, Vitovec J, Stanbrook H, Wikstrand J. Efficacy, safety and tolerability of metoprolol CR/XL in patients with diabetes and chronic heart failure: experiences from MERIT-HF. Am Heart J. 2005;149:159–67. doi: 10.1016/j.ahj.2004.05.056. [DOI] [PubMed] [Google Scholar]
- 23.Bell DS, Lukas MA, Holdbrook FK, Fowler MB. The effect of carvedilol on mortality risk in heart failure patients with diabetes: results of a meta-analysis. Current medical research and opinion. 2006;22:287–96. doi: 10.1185/030079906X80459. [DOI] [PubMed] [Google Scholar]
- 24.Torp-Pedersen C, Metra M, Charlesworth A, Spark P, Lukas MA, Poole-Wilson PA, Swedberg K, Cleland JG, Di Lenarda A, Remme WJ, Scherhag A, investigators C Effects of metoprolol and carvedilol on pre-existing and new onset diabetes in patients with chronic heart failure: data from the Carvedilol Or Metoprolol European Trial (COMET). Heart. 2007;93:968–73. doi: 10.1136/hrt.2006.092379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Belke DD, Betuing S, Tuttle MJ, Graveleau C, Young ME, Pham M, Zhang D, Cooksey RC, McClain DA, Litwin SE, Taegtmeyer H, Severson D, Kahn CR, Abel ED. Insulin signaling coordinately regulates cardiac size, metabolism, and contractile protein isoform expression. J Clin Invest. 2002;109:629–39. doi: 10.1172/JCI13946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiojima I, Yefremashvili M, Luo Z, Kureishi Y, Takahashi A, Tao J, Rosenzweig A, Kahn CR, Abel ED, Walsh K. Akt signaling mediates postnatal heart growth in response to insulin and nutritional status. J Biol Chem. 2002;277:37670–7. doi: 10.1074/jbc.M204572200. [DOI] [PubMed] [Google Scholar]
- 27.Laustsen PG, Russell SJ, Cui L, Entingh-Pearsall A, Holzenberger M, Liao R, Kahn CR. Essential role of insulin and insulin-like growth factor 1 receptor signaling in cardiac development and function. Mol Cell Biol. 2007;27:1649–64. doi: 10.1128/MCB.01110-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiang Y, Kobilka BK. Myocyte adrenoceptor signaling pathways. Science. 2003;300:1530–2. doi: 10.1126/science.1079206. [DOI] [PubMed] [Google Scholar]
- 29.Xiao RP, Zhu W, Zheng M, Cao C, Zhang Y, Lakatta EG, Han Q. Subtype-specific alpha1- and beta-adrenoceptor signaling in the heart. Trends Pharmacol Sci. 2006;27:330–7. doi: 10.1016/j.tips.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 30.Perrino C, Rockman HA. Reversal of cardiac remodeling by modulation of adrenergic receptors: a new frontier in heart failure. Curr Opin Cardiol. 2007;22:443–9. doi: 10.1097/HCO.0b013e3282294d72. [DOI] [PubMed] [Google Scholar]
- 31.Brittsan AG, Kranias EG. Phospholamban and cardiac contractile function. J Mol Cell Cardiol. 2000;32:2131–9. doi: 10.1006/jmcc.2000.1270. [DOI] [PubMed] [Google Scholar]
- 32.Fu Q, Xu B, Parikh D, Cervantes D, Xiang YK. Insulin induces IRS2-dependent and GRK2-mediated beta2AR internalization to attenuate betaAR signaling in cardiomyocytes. Cell Signal. 2015;27:707–15. doi: 10.1016/j.cellsig.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiang Y, Naro F, Zoudilova M, Jin SL, Conti M, Kobilka B. Phosphodiesterase 4D is required for beta2 adrenoceptor subtype-specific signaling in cardiac myocytes. Proc Natl Acad Sci U S A. 2005;102:909–14. doi: 10.1073/pnas.0405263102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richter W, Day P, Agrawal R, Bruss MD, Granier S, Wang YL, Rasmussen SG, Horner K, Wang P, Lei T, Patterson AJ, Kobilka B, Conti M. Signaling from beta1- and beta2-adrenergic receptors is defined by differential interactions with PDE4. Embo J. 2008;27:384–93. doi: 10.1038/sj.emboj.7601968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin FT, Miller WE, Luttrell LM, Lefkowitz RJ. Feedback regulation of beta-arrestin1 function by extracellular signal-regulated kinases. J Biol Chem. 1999;274:15971–4. doi: 10.1074/jbc.274.23.15971. [DOI] [PubMed] [Google Scholar]
- 36.Thal DM, Homan KT, Chen J, Wu EK, Hinkle PM, Huang ZM, Chuprun JK, Song J, Gao E, Cheung JY, Sklar LA, Koch WJ, Tesmer JJ. Paroxetine is a direct inhibitor o f g protein-coupled receptor kinase 2 and increases myocardial contractility. ACS Chem Biol. 2012;7:1830–9. doi: 10.1021/cb3003013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schumacher SM, Gao E, Zhu W, Chen X, Chuprun JK, Feldman AM, JJ GT, Koch WJ. Paroxetine-mediated GRK2 inhibition reverses cardiac dysfunction and remodeling after myocardial infarction. Science translational medicine. 2015;7:277ra31. doi: 10.1126/scitranslmed.aaa0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Despres JP, Lamarche B, Mauriege P, Cantin B, Lupien PJ, Dagenais GR. Risk factors for ischaemic heart disease: is it time to measure insulin? European heart journal. 1996;17:1453–4. doi: 10.1093/oxfordjournals.eurheartj.a014700. [DOI] [PubMed] [Google Scholar]
- 39.Pyorala M, Miettinen H, Laakso M, Pyorala K. Hyperinsulinemia predicts coronary heart disease risk in healthy middle-aged men: the 22-year follow-up results of the Helsinki Policemen Study. Circulation. 1998;98:398–404. doi: 10.1161/01.cir.98.5.398. [DOI] [PubMed] [Google Scholar]
- 40.Garcia-Guerra L, Nieto-Vazquez I, Vila-Bedmar R, Jurado-Pueyo M, Zalba G, Diez J, Murga C, Fernandez-Veledo S, Mayor F, Jr., Lorenzo M. G protein-coupled receptor kinase 2 plays a relevant role in insulin resistance and obesity. Diabetes. 2010;59:2407–17. doi: 10.2337/db10-0771. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Vila-Bedmar R, Cruces-Sande M, Lucas E, Willemen HL, Heijnen CJ, Kavelaars A, Mayor F, Jr., Murga C. Reversal of diet-induced obesity and insulin resistance by inducible genetic ablation of GRK2. Sci Signal. 2015;8:ra73. doi: 10.1126/scisignal.aaa4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lehnart SE, Wehrens XH, Reiken S, Warrier S, Belevych AE, Harvey RD, Richter W, Jin SL, Conti M, Marks AR. Phosphodiesterase 4D deficiency in the ryanodine-receptor complex promotes heart failure and arrhythmias. Cell. 2005;123:25–35. doi: 10.1016/j.cell.2005.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beca S, Helli PB, Simpson JA, Zhao D, Farman GP, Jones PP, Tian X, Wilson LS, Ahmad F, Chen SR, Movsesian MA, Manganiello V, Maurice DH, Conti M, Backx PH. Phosphodiesterase 4D regulates baseline sarcoplasmic reticulum Ca2+ release and cardiac contractility, independently of L-type Ca2+ current. Circ Res. 2011;109:1024–30. doi: 10.1161/CIRCRESAHA.111.250464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghigo A, Perino A, Mehel H, Zahradnikova A, Jr., Morello F, Leroy J, Nikolaev VO, Damilano F, Cimino J, De Luca E, Richter W, Westenbroek R, Catterall WA, Zhang J, Yan C, Conti M, Gomez AM, Vandecasteele G, Hirsch E, Fischmeister R. Phosphoinositide 3-kinase gamma protects against catecholamine-induced ventricular arrhythmia through protein kinase A-mediated regulation of distinct phosphodiesterases. Circulation. 2012;126:2073–83. doi: 10.1161/CIRCULATIONAHA.112.114074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leroy J, Abi-Gerges A, Nikolaev VO, Richter W, Lechene P, Mazet JL, Conti M, Fischmeister R, Vandecasteele G. Spatiotemporal dynamics of beta-adrenergic cAMP signals and L-type Ca2+ channel regulation in adult rat ventricular myocytes: role of phosphodiesterases. Circ Res. 2008;102:1091–100. doi: 10.1161/CIRCRESAHA.107.167817. [DOI] [PubMed] [Google Scholar]
- 46.Leroy J, Richter W, Mika D, Castro LR, Abi-Gerges A, Xie M, Scheitrum C, Lefebvre F, Schittl J, Mateo P, Westenbroek R, Catterall WA, Charpentier F, Conti M, Fischmeister R, Vandecasteele G. Phosphodiesterase 4B in the cardiac L-type Ca(2)(+) channel complex regulates Ca(2)(+) current and protects against ventricular arrhythmias in mice. J Clin Invest. 2011;121:2651–61. doi: 10.1172/JCI44747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Timofeyev V, Myers RE, Kim HJ, Woltz RL, Sirish P, Heiserman JP, Li N, Singapuri A, Tang T, Yarov-Yarovoy V, Yamoah EN, Hammond HK, Chiamvimonvat N. Adenylyl cyclase subtype-specific compartmentalization: differential regulation of L-type Ca2+ current in ventricular myocytes. Circ Res. 2013;112:1567–76. doi: 10.1161/CIRCRESAHA.112.300370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Richter W, Xie M, Scheitrum C, Krall J, Movsesian MA, Conti M. Conserved expression and functions of PDE4 in rodent and human heart. Basic research in cardiology. 2011;106:249–62. doi: 10.1007/s00395-010-0138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abi-Gerges A, Richter W, Lefebvre F, Mateo P, Varin A, Heymes C, Samuel JL, Lugnier C, Conti M, Fischmeister R, Vandecasteele G. Decreased expression and activity of cAMP phosphodiesterases in cardiac hypertrophy and its impact on beta-adrenergic cAMP signals. Circ Res. 2009;105:784–92. doi: 10.1161/CIRCRESAHA.109.197947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haas SJ, Vos T, Gilbert RE, Krum H. Are beta-blockers as efficacious in patients with diabetes mellitus as in patients without diabetes mellitus who have chronic heart failure? A meta-analysis of large-scale clinical trials. Am Heart J. 2003;146:848–53. doi: 10.1016/S0002-8703(03)00403-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.