Abstract
F1‐ATPase (F1) is a multisubunit water‐soluble domain of FoF1‐ ATP synthase and is a rotary enzyme by itself. Earlier genetic studies using yeast suggested that two factors, Atp11p and Atp12p, contribute to F1 assembly. Here, we show that their mammalian counterparts, AF1 and AF2, are essential and sufficient for efficient production of recombinant bovine mitochondrial F1 in Escherichia coli cells. Intactness of the function and conformation of the E. coli‐expressed bovine F1 was verified by rotation analysis and crystallization. This expression system opens a way for the previously unattempted mutation study of mammalian mitochondrial F1.
Keywords: F1‐ATPase, FoF1‐ATP synthase, molecular chaperone
Abbreviations
- AMPPNP
adenylyl‐imidodiphosphate
- Au‐bead
colloidal gold particles
- CBB
Coomassie Brilliant Blue
- F1
F1‐ATPase
- FoF1
FoF1‐ATP synthase
- fps
frames per second
- IF1
inhibitory factor‐1
- Pi
inorganic phosphate
- rps
revolutions per second
FoF1‐ATP synthase (FoF1) is ubiquitously found in membranes of bacteria, chloroplasts, and mitochondria, and synthesizes ATP from ADP and inorganic phosphate (Pi) driven by downhill proton flow across the membranes 1, 2, 3. F1‐ATPase (F1) is a water‐soluble catalytic domain of FoF1, which has a subunit composition of α3β3γδε. F1 is a rotary motor, where net hydrolysis of one ATP molecule drives a 120° rotation of a central rotor shaft composed of γδε‐subunits relative to a surrounding stator ring of α3β3‐subunits (eukaryotic subunit composition) 4, 5. Extensive studies on rotation of bacterial F1 revealed six‐step rotation in one revolution, that is, repetition of an 80° rotation by ATP binding to one of the three catalytic β‐subunits and a 40° rotation by release of Pi from another β‐subunit 4, 6, 7, 8, 9. Understanding the rotation mechanism of F1 requires knowledge on how chemical events occurring to F1 induce its structural changes and trigger rotation. In this respect, much structural information has been accumulated for bovine F1, rather than bacterial F1, by X‐ray crystallography 10, 11. However, rotation of mitochondrial F1 was not demonstrated until recently because of the absence of in vitro expression system of mitochondrial F1 genes that enables genetic modification necessary for single‐molecule observation, such as introduction of the His‐tag.
We recently succeeded in expressing human mitochondrial F1 in Escherichia coli cells and reported its nine‐step rotation in one revolution 5. Following up this work, here, we report the expression of bovine mitochondrial F1 in E. coli. Early genetic works using Saccharomyces cerevisiae identified two mitochondrial proteins of Atp11p and Atp12p as molecular chaperones necessary for assembly of F1 12. Analyses using a yeast two‐hybrid system and immunoprecipitation further showed direct interaction of Atp11p 13 with a β‐subunit and of Atp12p with an α‐subunit 14. Mammalian homologs of these chaperones are ATPAF1 and ATPAF2 (AF1 and AF2, hereafter), and their coding genes, ATP11 and ATP12, can respectively complement genetic deficiencies of ATP11 15 and ATP12 16 of yeast. AF1 and AF2 have antiaggregation activity toward reduced insulin 17, 18 and citrate synthase in vitro, respectively 19. However, whether AF1 and AF2 are essential for the production of mammalian F1 was not tested directly. We thus expressed the five subunits of bovine mitochondrial F1 in E. coli cells with or without coexpression of AF1 and AF2. The results clearly show that AF1 and AF2 are essential and sufficient for the production of bovine F1 in E. coli. ATP‐driven rotation and crystallization confirmed intactness of E. coli‐expressed bovine F1.
Experimental procedures
Expression of bovine F1 in E. coli
The expression plasmid for bovine F1 was constructed in the same manner as performed previously for human F1 5; five genes coding subunits of bovine F1 (α, β, γ, δ, and ε) 20 and two genes, ATP11 and ATP12, were amplified by PCR from the cDNA library prepared from the total RNA of bovine heart muscle. The genes were tandemly introduced in the order of α‐γ‐β‐δ‐ε‐ATP11‐ATP12 into the expression vector pTR19 21, which are transcribed from the trc promoter. A histidine tag composed of 10 histidine residues was genetically introduced into the N terminus of the β‐subunit of F1 as performed previously 4. The resulting plasmid, pBF1, was introduced into FoF1‐deficient E. coli strain, DK8 21. The recombinant E. coli strain was cultivated in 2 × YT medium containing 100 μg·mL−1 ampicillin for 40 h at 29 °C. The culture flasks were shaken for aeration because respiration of cells is necessary for efficient expression even though growth is dependent on glycolysis. As observed in the case of expression of F1 from thermophilic Bacillus PS3 in E. coli 22, the growth rate of the E. coli was not significantly affected by the expression of bovine F1. It is assumed that submillimolar concentration of ADP in cytoplasm is enough to keep F1 in the inactive state of so‐called MgADP‐inhibition, a general feature of F1 from any sources 23. The cells were disrupted and the water‐soluble fraction was subjected to Ni‐affinity column chromatography and gel‐filtration column chromatography. Purification procedures for bovine F1 are the same as those for human F1, except the buffers for cell lysis (20 mm potassium phosphate (pH 7.5), 100 mm KCl and 0.1 mm ATP) and for gel‐filtration (40 mm Tris/HCl (pH 8.0), 200 mm NaCl, 1 mm EDTA and 0.1 mm ATP). After gel‐filtration with Superdex200 10/300GL column (GE Healthcare, Uppsala, Sweden), fractions of a peak having the ATPase activity were collected, concentrated with a centrifugal concentrator (50 kDa, Centricon50; Millipore Corp., Billerica, MA, USA), and used for further analyses. Yield of the purified recombinant bovine F1 was about 2–3 mg per 6‐L‐culture. Authentic bovine F1 was prepared from bovine heart as reported 24 with a modification; gel‐filtration was performed with a Superdex200 column in 20 mm Tris/HCl (pH8.0), 200 mm NaCl, 0.1 mm ATP, and 0.5 mm EDTA. To avoid cold dissociation of bovine F1, all procedures were carried out at a temperature higher than 20 °C. Mutated IF1 (IF1‐GFP) used in this study, I60GFPHis, was prepared as reported previously 25.
Rotation of E. coli‐expressed bovine F1
Rotation of a single molecule of bovine F1 was observed by the procedures described in ref. 5. Two cysteine residues were introduced into a globular domain of γ‐subunit (γAla99Cys and γSer191Cys). Images of a rotating submicron polystyrene bead attached to the γ‐subunit of immobilized bovine F1 were captured with a CCD camera (ICL‐B0620M; Implex, Minneapolis, MN, USA) at 500 frames per sec (fps) under illumination of a mercury lamp. Rotation of the Au‐bead (40 nm diameter) was observed at 25 000 fps with a laser‐illuminated center‐shielded dark‐field microscopic system equipped with a high‐speed camera (MEMRECAM GX‐8S; NAC Image Technology Inc., Tokyo, Japan) 5.
Crystallization of E. coli‐expressed bovine F1
Concentrated recombinant bovine F1 was supplemented with 0.5 mm AMPPNP and 20 mm MgCl2 (the final bovine F1 concentration was 10 mg·mL−1) and used for crystallization. Reservoir solution (70 μL) containing 100 mm Tris/HCl (pH 8.5), 200 mm LiSO4, and 21–23% PEG3350 (Hampton Research, Aliso Viejo, CA, USA) was put into a sitting‐drop dish, and the bovine F1 solution and the reservoir solution (each 0.25 μL) were mixed to make one sitting drop. Crystals with the size of 0.05–0.3 mm were grown in approximately 3 weeks at 20 °C. For analysis of the crystals with polyacrylamide gel electrophoresis in the presence of sodium dodecylsulfate (SDS/PAGE), 10–20 crystals were collected from crystallization drops using a cryoloop, washed four times with 100 μL of wash solution (the reservoir solution supplemented with 0.5 mm AMPPNP, 20 mm MgCl2, and 25 mm NaCl), and dissolved in the SDS/PAGE sample buffer. After electrophoresis, the gel was stained with silver.
Other methods
The ATPase activity was measured in 50 mm HEPES/KOH buffer (pH 7.5) containing 100 mm KCl, 1 mm MgCl2, 1 mm ATP, and the ATP‐regenerating system 21 supplemented with 0.2 mm NADH and 0.2 mg·mL−1 lactate dehydrogenase 26. The reaction was initiated by adding F1, and the change in absorbance at 340 nm was recorded. The ATPase activity was calculated from the slope of absorbance decrease during 400–500 s. For the assay of IF1 inhibition, IF1‐GFP was added to the reaction mixture prior to the measurement at the indicated concentration. Previous studies of authentic bovine F1 25 showed that IC50 of IF1‐GFP is 65 nm, while that of wild‐type IF1 is approximately 10 nm 5. Protein concentrations were determined by protein assay kit (Pierce Biotechnology Inc., Rockford, IL, USA), with bovine serum albumin as a standard. All SDS/PAGE and native‐PAGE in this study were performed with a gradient polyacrylamide gel (10–20%) and nongradient gel (12%). The proteins were visualized by Coomassie Brilliant Blue (CBB) or by immunoblotting with anti‐β and anti‐δ antibodies. All data used for this study were measured at least in triplicate.
Results and Discussion
Escherichia coli expression of bovine F1 depends on AF1 and AF2
The five genes for bovine F1 were introduced into the E. coli expression vector in the same order as in the E. coli FoF1 operon, α‐γ‐β‐δ‐ε, to generate a plasmid pBF1(‐AFs). A set of genes, ATP11 and ATP12, were further introduced at the end of the operon as α‐γ‐β‐δ‐ε‐ATP11‐ATP12 to generate a plasmid pBF1(+AFs). These plasmids were individually introduced into the E. coli strain that lacks the whole FoF1 operon in the chromosome, and resultant recombinant strains were cultured. The water‐soluble fraction of harvested cells was analyzed with polyacrylamide gel electrophoresis in the absence of SDS (native‐PAGE) using authentic bovine F1 purified from bovine heart as a control (Fig. 1A–D). Native‐PAGE followed by immunoblotting with anti‐β antibodies showed that pBF1(+AFs)‐harboring cells produced a significant amount of bovine F1 while pBF1(‐AFs)‐harboring cells produced very little, if any, amount of bovine F1 (Fig. 1A). The band arising from the monomeric β‐subunit was seen in all samples. The immunoblotting with anti‐δ antibodies confirms pBF1(+AFs)‐dependent production of bovine F1 (Fig. 1B). F1 isolated from bovine heart appeared as two split bands in native‐PAGE for an unknown reason and bovine F1 produced in E. coli also gives two bands. The monomeric β‐subunit of bovine F1 produced in E. coli migrates in the gel more slowly than that of authentic bovine F1 due to the attached histidine tag (Fig. 1A). Production of bovine F1 in pBF1(+AFs)‐harboring cells was confirmed by protein staining as a faint, but distinct band (Fig. 1C, D). These results show that expression of the ATP11 and ATP12 is essential for efficient production of bovine F1. We purified bovine F1 from pBF1(+AFs)‐harbored E. coli cells and confirmed that it has the same subunit composition as authentic bovine F1 by SDS/PAGE analysis (Fig. 1E). The ATPase activity of mitochondrial F1 is known to be inhibited by a specific inhibitor protein of mitochondria, IF1 25. Sensitivity of E. coli‐expressed bovine F1 to IF1 was tested by using bovine IF1 fused to GFP. As shown in Fig. 1F, the ATPase activity of E. coli‐expressed bovine F1 was inhibited by IF1‐GFP in the same manner as observed for authentic bovine F1 (Fig. 1F).
Figure 1.

Expression of bovine F1 in Escherichia coli. (A–D) Native‐PAGE analysis of water‐soluble fraction of E. coli cells expressing bovine F1 without (plasmid pBF1(‐AFs), lane 2) or with (pBF1(+AFs), lane 3) simultaneous expression of the two genes, ATP11 and ATP12. Lane 1 represents authentic bovine F1 purified from bovine heart muscle. The gels were analyzed by immunoblotting using anti‐β‐subunit (A) or anti‐δ‐subunit antibodies (B), or by protein staining with CBB (C, D). (D) The region containing a band of bovine F1 in C is enlarged. Arrows indicate the band of bovine F1. (E) SDS/PAGE analysis of authentic bovine F1 (lane 1) and purified E. coli‐expressed bovine F1 (lane 2). (F) Inhibition of ATPase activity of bovine F1 by IF1. GFP‐fused IF1 was used. The ATPase activity in the absence of IF1‐GFP is set to 100%. Solid line, E. coli‐expressed bovine F1; dotted line, authentic bovine F1.
Rotation of E. coli‐expressed bovine F1
To verify the function of E. coli‐expressed bovine F1, ATPase‐driven rotation was observed by microscopic single‐molecule analysis. For this purpose, a submicron polystyrene bead was attached to two introduced cysteine residues of the γ‐subunit as a rotation probe. At a low ATP concentration (1 μm), bovine F1 rotates at a speed 2.5 ± 0.3 rps and rotation takes three dwells per revolution, approximately at every 120° rotation (Fig. 2A). The dwells become shorter as the ATP concentration increased, indicating that bovine F1 waits for ATP binding during the dwell to drive the next cycle of 120° rotation. The rate constant of ATP binding (k on) calculated from the lifetime of the dwell (τ=56 ± 3 ms) is 1.8 ± 0.3 × 107 m −1·s−1 (Fig. 2B). Rotation at a saturating ATP concentration (4 mm) was observed with a rapid camera (a frame per 40 μs) (Fig. 2C). By using colloidal gold particles (diameter, 40 nm) as a rotation probe, viscous friction of the rotating particle did not slow down rotation under the experimental conditions and the rotation speed, 655 ± 38 rps (N = 7 molecules), directly reflects the maximum turnover rate of ATP hydrolysis by a single molecule of bovine F1, that is, ~ 2000 per second. The presence of dwells is suggested from the angle histogram of rotation that awaits extensive analysis. As expected from high sequence conservation between bovine F1 and human F1, these motor characteristics of bovine F1 are similar to those of human F1 (k on, 2.7 ± 0.3 × 107 m −1·s−1; rotation speed, 705 ± 75 rps) 5.
Figure 2.
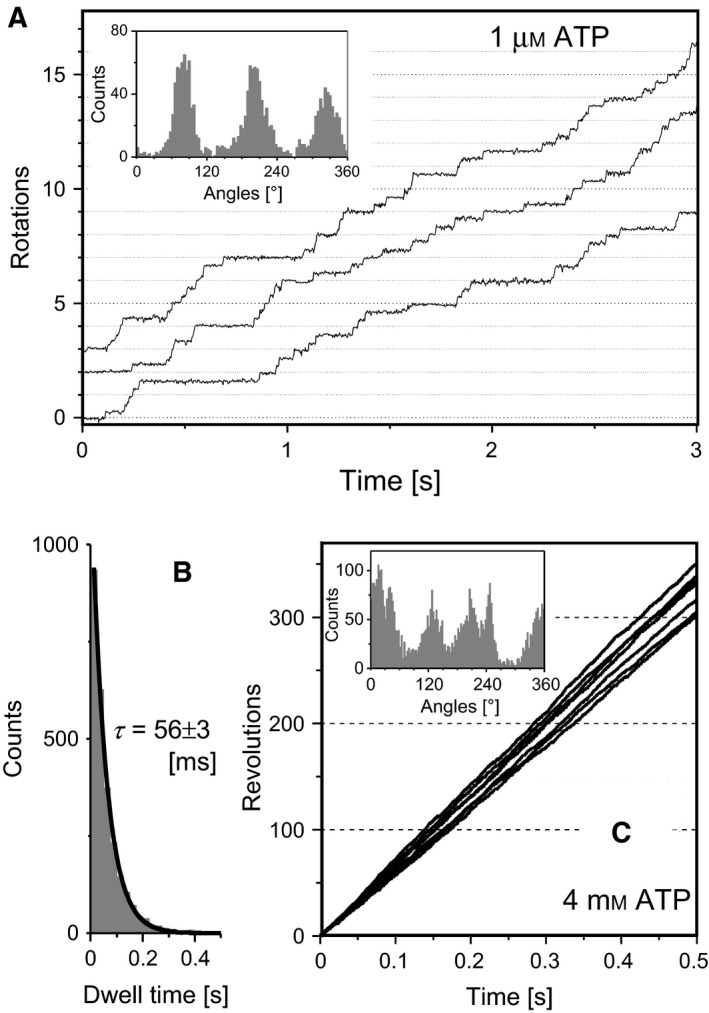
Single‐molecule analysis of rotation of Escherichia coli‐expressed bovine F1. (A, B) Rotation of bovine F1 at 1 μm ATP observed with a camera 500 fps. (A) Time‐courses of the rotation. The inset is an angle histogram of the rotation. (B) A histogram of duration of dwells (N = 3672 dwells, three molecules) observed in the rotation. The bin width was 0.25 s. The histogram was best simulated with a single‐exponential decay function with a lifetime of 56 ± 3 ms. (C) Time‐courses of rotation at a saturating ATP concentration, 4 mm. Rotation was analyzed at 25k fps using Au particles (diameter was 40 nm) as a rotation probe. The inset is an angle histogram of the rotation. The averaged rotation speed over 0.5 s was 655 ± 38 rps (N = 6 molecules).
Crystallization of E. coli‐expressed bovine F1
Bovine F1 (without a Cys mutation) purified from E. coli cells was subjected to crystallization. A crystal was not made under the reported conditions for crystallization of authentic bovine F1 27 probably because of the histidine‐tag of the β‐subunit of E. coli‐expressed bovine F1. After screening crystallization conditions, we found that crystals were reproducibly formed in the solution containing 0.5 mm AMPPNP and 20 mm MgCl2 with PEG3350 as precipitant (Fig. 3A). Crystals were collected from the drops, washed, and analyzed by SDS/PAGE (Fig. 3B). All the five F1 subunits were detected in the gel, confirming that the crystals were of bovine F1. The result shows that the purified bovine F1 has a quality high enough to grow crystals.
Figure 3.
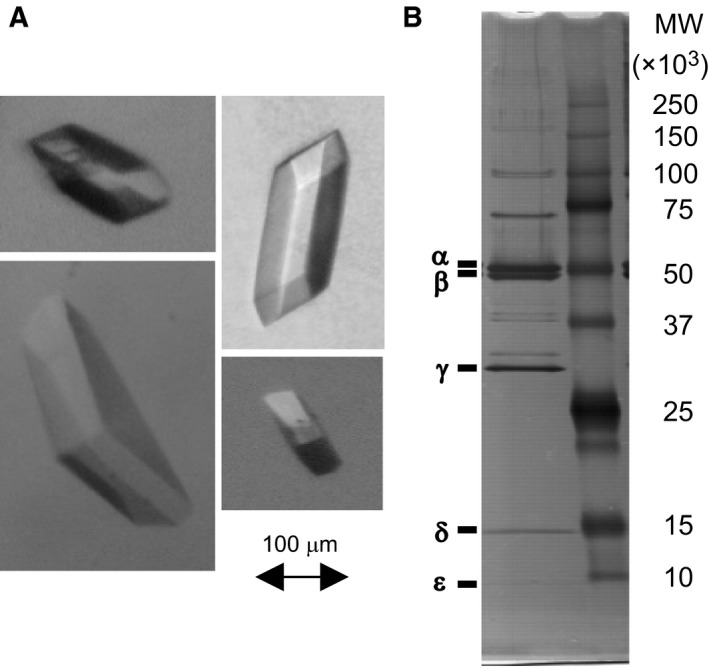
Crystals of Escherichia coli‐expressed bovine F1. (A) Crystals of bovine F1 were routinely obtained by sitting‐drop vapor diffusion method as stated in Experimental Procedures. (B) SDS/PAGE analysis of the crystals. The gel was stained with silver.
Conclusions
Bovine F1, for the first time, was successfully expressed in E. coli cells. As expected, purified bovine F1 exhibits motor characteristics similar to those of human F1. It forms good crystals rather easily. We previously spent time and efforts for crystallization of bacterial F1 28, but now realize that bovine F1 is superior to bacterial F1 in crystallization for the detailed structural study of F1. Availability of mutants adds a further advantage to E. coli‐expressed bovine F1 over the native protein.
Without AF1 and AF2, only very little, if any, bovine F1 was produced by E. coli, indicating that AF1 and AF2 are required for efficient production of bovine F1. This expression is the first demonstration of the chaperone function of these two factors for assembly of mammalian F1. As speculated in a previous yeast study 29, mammalian AF2 would bind to α‐subunit by mimicking the coiled‐coil region of the γ‐subunit and then, α‐ and β‐subunit eject their cognate chaperone factors by switching their partner on the way of the assembly. In relation to this, a metabolic disease with a decreased amount of FoF1 in mitochondria is attributed to a mutation in the ATP12 gene, suggesting a critical physiological role of these assembly factors in production of functional FoF1 30. Although we did not test human ATP11 and ATP 12 for expressing bovine F1, sequence similarities of AF1 and AF2 are 93% and 88% such that we would expect them to be interchangeable. We expect that the development and improvement of the present bovine F1 expression system would open a way to the study of detailed mechanisms of the assembly and a structure‐mechanism relationship of mitochondrial F1.
Author contributions
TS and MY conceived and designed the experiments and wrote the paper. TS developed the E. coli expression system of bovine F1 with NI and JS and the X‐ray crystallographic system with YW and TE, and single‐molecule analytical system. TH gave critical suggestions for experimental systems and interpretations throughout this study.
Acknowledgements
We thank our colleagues in Waseda University, Drs. K. Kinosita, K. Adachi, M. Bertz, R. Chiwata, T. Ogawa in Kyoto‐Sangyo University, Drs. Y. Araiso and S. Matsumoto, and those in The University of Tokyo, Drs. H. Noji, R. Watanabe and N. Soga, for valuable discussions. This work was partly supported by JSPS KAKENHI Grant Number 24570149 (TS) and 90049073 (MY) and by the Platform Project for Supporting in Drug Discovery and Life Science Research from Japan Agency for Medical Research and Development (AMED) (TS).
Contributor Information
Toshiharu Suzuki, Email: toshisuz@appchem.t.u-tokyo.ac.jp.
Masasuke Yoshida, Email: masasuke.yoshida@cc.kyoto-su.ac.jp.
References
- 1. Yoshida M, Muneyuki E and Hisabori T (2001) ATP synthase – a marvellous rotary engine of the cell. Nat Rev Mol Cell Biol 2, 669–677. [DOI] [PubMed] [Google Scholar]
- 2. Boyer PD (2002) A research journey with ATP synthase. J Biol Chem 277, 39045–39061. [DOI] [PubMed] [Google Scholar]
- 3. Senior AE, Nadanaciva S and Weber J (2002) The molecular mechanism of ATP synthesis by F1F0‐ATP synthase. Biochim Biophys Acta 1553, 188–211. [DOI] [PubMed] [Google Scholar]
- 4. Noji H, Yasuda R, Yoshida M and Kinosita K Jr (1997) Direct observation of the rotation of F1‐ATPase. Nature 386, 299–302. [DOI] [PubMed] [Google Scholar]
- 5. Suzuki T, Tanaka K, Wakabayashi C, Saita E and Yoshida M (2014) Chemomechanical coupling of human mitochondrial F1‐ATPase motor. Nat Chem Biol 10, 930–936. [DOI] [PubMed] [Google Scholar]
- 6. Yasuda R, Noji H, Kinosita K Jr and Yoshida M (1998) F1‐ATPase is a highly efficient molecular motor that rotates with discrete 120 degree steps. Cell 93, 1117–1124. [DOI] [PubMed] [Google Scholar]
- 7. Yasuda R, Noji H, Yoshida M, Kinosita K Jr and Itoh H (2001) Resolution of distinct rotational substeps by submillisecond kinetic analysis of F1‐ATPase. Nature 410, 898–904. [DOI] [PubMed] [Google Scholar]
- 8. Adachi K, Oiwa K, Nishizaka T, Furuike S, Noji H, Itoh H, Yoshida M and Kinosita K Jr (2007) Coupling of rotation and catalysis in F1‐ATPase revealed by single‐molecule imaging and manipulation. Cell 130, 309–321. [DOI] [PubMed] [Google Scholar]
- 9. Furuike S, Hossain MD, Maki Y, Adachi K, Suzuki T, Kohori A, Itoh H, Yoshida M and Kinosita K Jr (2008) Axle‐less F1‐ATPase rotates in the correct direction. Science 319, 955–958. [DOI] [PubMed] [Google Scholar]
- 10. Walker JE and Dickson VK (2006) The peripheral stalk of the mitochondrial ATP synthase. Biochim Biophys Acta 1757, 286–296. [DOI] [PubMed] [Google Scholar]
- 11. Bason JV, Montgomery MG, Leslie AG and Walker JE (2015) How release of phosphate from mammalian F1‐ATPase generates a rotary substep. Proc Natl Acad Sci USA 112, 6009–6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ackerman SH (2002) Atp11p and Atp12p are chaperones for F1‐ATPase biogenesis in mitochondria. Biochim Biophys Acta 1555, 101–105. [DOI] [PubMed] [Google Scholar]
- 13. Wang ZG and Ackerman SH (2000) The assembly factor Atp11p binds to the beta‐subunit of the mitochondrial F1‐ATPase. J Biol Chem 275, 5767–5772. [DOI] [PubMed] [Google Scholar]
- 14. Wang ZG, Sheluho D, Gatti DL and Ackerman SH (2000) The α‐subunit of the mitochondrial F1‐ATPase interacts directly with the assembly factor Atp12p. EMBO J 19, 1486–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang ZG, Schmid KJ and Ackerman SH (1999) The Drosophila gene 2A5 complements the defect in mitochondrial F1‐ATPase assembly in yeast lacking the molecular chaperone Atp11p. FEBS Lett 452, 305–308. [DOI] [PubMed] [Google Scholar]
- 16. Wang ZG, White PS and Ackerman SH (2001) Atp11p and Atp12p are assembly factors for the F1‐ATPase in human mitochondria. J Biol Chem 276, 30773–30778. [DOI] [PubMed] [Google Scholar]
- 17. Hinton A, Zuiderweg ER and Ackerman SH (2003) A purified subfragment of yeast Atp11p retains full molecular chaperone activity. J Biol Chem 278, 34110–34113. [DOI] [PubMed] [Google Scholar]
- 18. Sheluho D and Ackerman SH (2001) An accessible hydrophobic surface is a key element of the molecular chaperone action of Atp11p. J Biol Chem 276, 39945–39949. [DOI] [PubMed] [Google Scholar]
- 19. Hinton A, Gatti DL and Ackerman SH (2004) The molecular chaperone, Atp12p, from Homo sapiens. In vitro studies with purified wild type and mutant (E240K) proteins. J Biol Chem 279, 9016–9022. [DOI] [PubMed] [Google Scholar]
- 20. Knowles AF and Penefsky HS (1972) The subunit structure of beef heart mitochondrial adenosine triphosphatase. Isolation procedures. J Biol Chem 247, 6617–6623. [PubMed] [Google Scholar]
- 21. Suzuki T, Ueno H, Mitome N, Suzuki J and Yoshida M (2002) Fo of ATP synthase is a rotary proton channel. Obligatory coupling of proton translocation with rotation of c‐subunit ring. J Biol Chem 277, 13281–13285. [DOI] [PubMed] [Google Scholar]
- 22. Matsui T and Yoshida M (1995) Expression of the wild‐type and the Cys‐/Trp‐less α3β3γ complex of thermophilic F1‐ATPase in Escherichia coli . Biochim Biophys Acta 1231, 139–146. [DOI] [PubMed] [Google Scholar]
- 23. Jault JM, Dou C, Grodsky NB, Matsui T, Yoshida M and Allison WS (1996) The α3β3γ subcomplex of the F1‐ATPase from the thermophilic Bacillus PS3 with the betaT165S substitution does not entrap inhibitory MgADP in a catalytic site during turnover. J Biol Chem 271, 28818–28824. [DOI] [PubMed] [Google Scholar]
- 24. Walker JE, Fearnley IM, Gay NJ, Gibson BW, Northrop FD, Powell SJ, Runswick MJ, Saraste M and Tybulewicz VL (1985) Primary structure and subunit stoichiometry of F1‐ATPase from bovine mitochondria. J Mol Biol 184, 677–701. [DOI] [PubMed] [Google Scholar]
- 25. Bason JV, Runswick MJ, Fearnley IM and Walker JE (2011) Binding of the inhibitor protein IF1 to bovine F1‐ATPase. J Mol Biol 406, 443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Suzuki T, Suzuki J, Mitome N, Ueno H and Yoshida M (2000) Second stalk of ATP synthase. Cross‐linking of γ subunit in F1 to truncated Fo b subunit prevents ATP hydrolysis. J Biol Chem 275, 37902–37906. [DOI] [PubMed] [Google Scholar]
- 27. Lutter R, Abrahams JP, van Raaij MJ, Todd RJ, Lundqvist T, Buchanan SK, Leslie AG and Walker JE (1993) Crystallization of F1‐ATPase from bovine heart mitochondria. J Mol Biol 229, 787–790. [DOI] [PubMed] [Google Scholar]
- 28. Shirakihara Y, Shiratori A, Tanikawa H, Nakasako M, Yoshida M and Suzuki T (2015) Structure of a thermophilic F1‐ATPase inhibited by an ε‐subunit: deeper insight into the ε‐inhibition mechanism. FEBS J 282, 2895–2913. [DOI] [PubMed] [Google Scholar]
- 29. Ludlam A, Brunzelle J, Pribyl T, Xu X, Gatti DL and Ackerman SH (2009) Chaperones of F1‐ATPase. J Biol Chem 284, 17138–17146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. De Meirleir L, Seneca S, Lissens W, De Clercq I, Eyskens F, Gerlo E, Smet J and Van Coster R (2004) Respiratory chain complex V deficiency due to a mutation in the assembly gene ATP12 . J Med Genet 41, 120–124. [DOI] [PMC free article] [PubMed] [Google Scholar]


