Abstract
Herein we report acid-directed β-C(sp3)–H arylation of α-amino acids enabled by pyridine-type ligands. This reaction does not require the installation of an exogenous directing group, is scalable, and enables the preparation of Fmoc-protected unnatural amino acids in three steps. The pyridine-type ligands are crucial in the development of this new C(sp3)–H arylation.
Keywords: arylation, amino acids, C–H activation, palladium, pyridine ligands
Graphical abstract
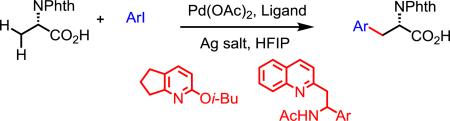
The palladium(II)-catalyzed β-C(sp3)–H arylation of α-amino acids of free carboxylic acids enabled by pyridine or quinoline-based ligands has been developed. The arylation is highly scalable and allows for the rapid synthesis of non-natural amino acids. The β–methylene C–H bonds of carbocyclic rings and other aliphatic acids is also reported.
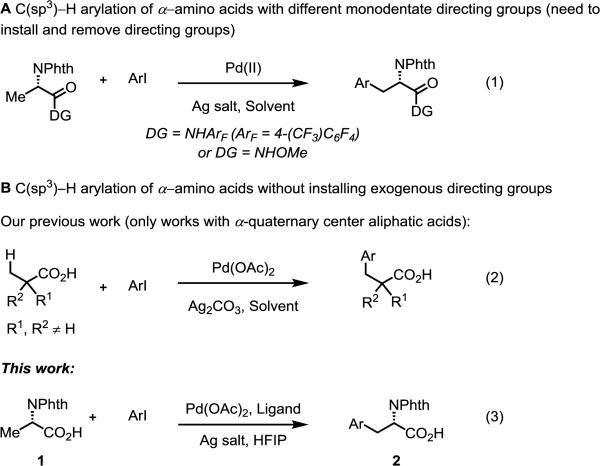
Compared to directing groups that traditionally promote cyclometallation, carboxylic acids form relatively weak interactions with palladium (Pd), thereby hindering reactivity. Practical C–H activation directed by carboxylic acids would represent a huge achievement for the field as carboxylic acids are an important class of compounds widely prevalent in natural products, pharmaceuticals, and materials. Moreover, carboxylic acids are an important functional group handle, which may be readily converted into orthogonal functionality.[1] Insofar as acid-directed C–H activation is concerned, myriad examples of functionalization of C(sp2)–H bonds has been detailed extensively and are reviewed elsewhere.[2] On the contrary, examples of acid-directed C(sp3)–H activation are sparse: Sen, Sames, and Chang have reported K2PtCl4-catalyzed carboxyl-directed lactonization of aromatic[3] and aliphatic acids, albeit with poor isolated yields. Our group first reported Pd-catalyzed C(sp3)–H arylation with simple aliphatic acids, yet the substrate scope was limited.[4] Later, Martin and colleagues reported Pd-catalyzed benzylic C(sp3)–H activation/C(sp3)–O bond formation, producing benzolactones.[5] Despite these significant advances, Pd-catalyzed β-C(sp3)–H functionalization of aliphatic acids has not been well developed, and is largely an unsolved problem.
Previously, the research in our group has focused on monodentate weak-coordinating groups for C–H activation; these directing groups, compared to bidentate directing groups[6] or strong monodentate-directing groups, offer the advantage of benefiting from ligand-accelerated catalysis.[7] Our work has been continuously influenced by our early studies on oxazoline-directed C(sp3)–H activation,[8] in which we used stereochemical information obtained in the C–H insertion step to deduce ideal geometric, steric, and electronic requirements of the reaction pretransition-state.
This information has guided our efforts in the design of novel monodentate directing groups, including (A) CONHArF (ArF = 4-(CF3)C6F4) and (B) a more simple and practical CONHOMe auxiliary (Scheme 1, [Eq. (1)]),[9,10] both of which serve as carboxylate surrogates in the Pd-catalyzed C–H activation of alanine derivatives, enabling the preparation of unnatural α-amino acids. Both of these reactions benefit from ligand acceleration by either pyridine- or quinoline-based ligands, though the installation and removal of these exogenous directing groups hampers the broad application of these methods. To address this limitation, and building upon our previous work in carboxylic acid-directed C(sp3)–H arylation,[4] we recently reported the enantioselective C(sp3)–H arylation of methylene C–H bonds with free carboxylic acids,[11] however, in both papers only aliphatic acids with α-quaternary center could be applied to this reaction [Eq. (2)].
Scheme 1.
Ary lation of α-Amino Acids
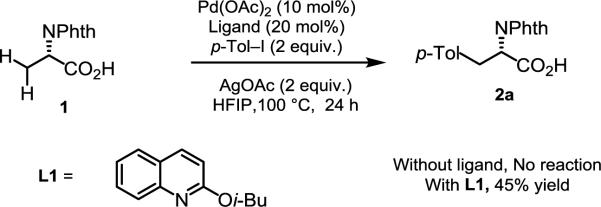
Given the weak directing nature of carboxylic acids, we elected to explore carboxylic acid-directed C(sp3)–H activation on N-phthaloyl alanine substrates by exploiting ligand acceleration from pyridine- and quinoline-based ligands [Eq. (3)]. Employing similar conditions to those reported in our CONHOMe-directed arylation,[10] we were pleased to find that L1 provided the desired arylated product in 45% yield (Scheme 2). A control experiment showed that no reaction occurred in the absence of ligand.
Scheme 2.
Preliminary Discovery for Ligand-Promoted C–H Ary lation
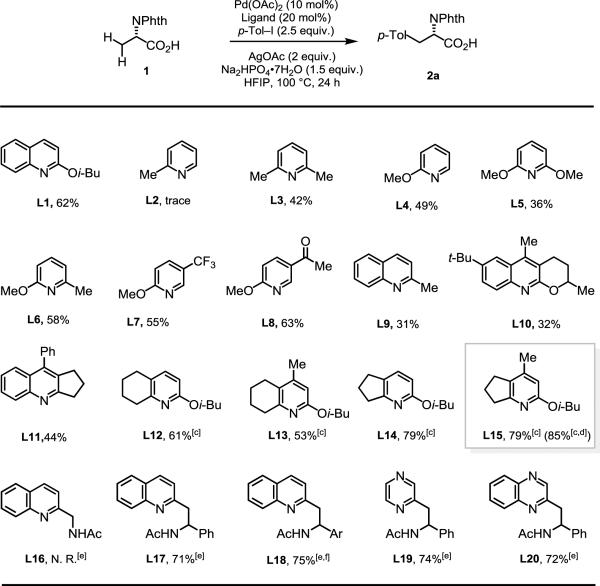
Encouraged by this initial result, we screened different reaction conditions, including solvents and bases (see supporting information for details). It is evident that 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) is crucial for the reaction. HFIP has excellent hydrogen–bonding properties and may be (A) aptly suited to interact with acidic directing groups and/or (B) could serve an ancillary ligand, coordinating and stabilizing catalytically-active electrophilic Pd-species, thereby improving the overall efficiency of the reaction.[12] Base additives were very important in this reaction: Na2HPO4•7H2O afforded the desired product in 62% yield. We suspect that this base is capable of deprotonating the acid substrates, allowing for adequate Pd-coordination, as shown previously.[4]
With these results in hand, we began to screen various ligands (Table 1). Surprisingly, 2-picoline (L2), which is highly reactive in both CONHArF and CONHOMe directed arylation of amino acid substrates, was found to be inactive in this C–H arylation. However, the bulkier 2,6-lutidine (L3) afforded the desired product in 42% yield. By changing the methyl group of L2 to a methoxy group (L4) the reaction proceeded in 49% yield. An additional methoxy group at C6 (L5) failed to improve the yield (36%); L6, possessing a methyl group at C6 improved the yield (58%); utilizing C5 acetylated ligand L8 resulted in a further increase in reactivity. Neither 2-methyl quinoline (L9) nor quinoline-based ligands (L10, L11) improved the yield. Since simple substitutions on pyridine- and quinoline-based ligands failed to increase reactivity; consequently, we prepared several fused bicyclic pyridine ligands (L12-L15). Whereas ligands with fused six-membered rings (L12, L13) afforded similar results to those of L1, ligands with fused five-membered rings afforded a dramatic increase in reactivity (L14, L15 = 79% yield). Inspired by multiligand promoted C–N bond formation from the Buchwald group,[13] we tried a variety of mixed ligands to further improve the yield (see supporting information). We discovered that a combination of L15 (10 mol%) and L8 (10 mol%) gave the desired product in 85% yield. While bidentate ligands that form 5-membered chelates with Pd were unreactive (L16), bidentate ligands that form 6-membered chelates – which were previously found to promote enantioselective methylene C–H activation[11] – were also very reactive in this reaction (L17 = 71%, L18 = 75% yield). Efforts to change the heterocyclic nucleus of these ligands (L19, L20) did not enhance reactivity, but still afforded the desired product in high yields.
Table 1.
Experiments were performed with substrate 1 (0.1 mmol), Pd(OAc)2 (10 mol%), AgOAc (0.2 mmol), Ar–I (0.25 mmol), Na2HPO4·7H2O (0.15 mmol), Ligand (20 mol%), HFIP (1.0 mL), 100 °C, 24 h.
Determined by 1H NMR analysis ofthe crude product using CH2Br2 as an internal standard.
[c] HFIP (2.0 mL).
[d] L15 (10 mol%)+L8 (10 mol%).
[e] Conditions: substrate (0.1 mmol), Pd(OAc)2 (10 mol%), Ag2CO3 (0.2 mmol), Ar–I (0.25 mmol), ligand (10 mol%), K2HPO4 (0.1 mmol), HFIP (1.0 mL), 100 °C, 24 h.
[f] Ar = 3,5-di-t-butylphenyl
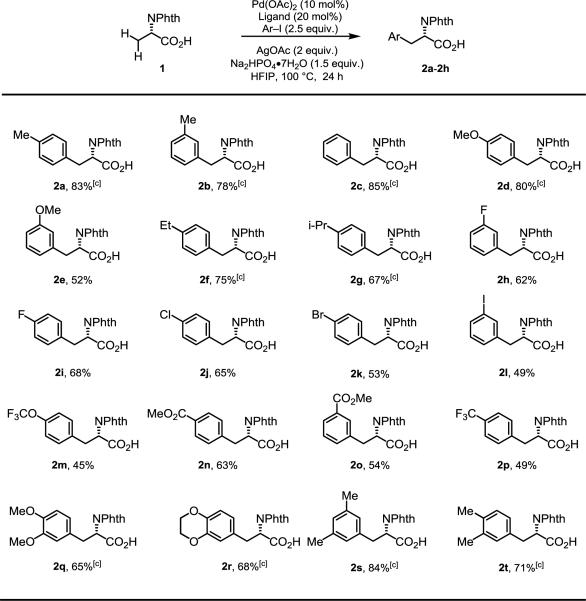
Various aryl iodides were next subjected to the optimized reaction conditions (Table 2). Arylation of para- and meta-methyl aryl iodides provided the corresponding products 2a and 2b in 83% and 78% yields, respectively. Simple iodobenzene gave 2c in 85% yield, and other electron-donating aryl iodides (methoxy, ethyl, iso-propyl) gave the desired products in good to excellent yields (2d, 2f, 2g), though meta-methoxy aryl iodide only produced 2e in a moderate yield (52%). Different halogen substitution patterns were all tolerated, and moderate-to-good yields were obtained (2h-2l); these results are attractive in that the chloro-, bromo-, and iodo-groups could be further functionalized. Aryl iodides containing electron-withdrawing groups (trifluoromethoxy, ester, trifluoromethyl) were also compatible with the optimized reaction conditions, providing desired product (2m-2p) in lower yields compared with electron-donating aryl iodides (2d, 2f, 2g). Di-substituted aryl iodides afforded the arylation products (2q-2t) in good-to-excellent yields. Unfortunately, heteroaryl iodides are presently incompatible with the arylation conditions.
Table 2.
Experiments were performed with substrate 1 (0.1 mmol), Pd(OAc)2 (10 mol %), AgOAc (0.2 mmol), Ar–I (0.25 mmol), Na2HPO4·7H2O (0.15 mmol), L15 (20 mol%), HFIP (1.0 mL), 100 °C, 24 h.
Isolated yields are shown based on corresponding methyl ester.
[c] L15 (10 mol%)+L8 (10 mol%)
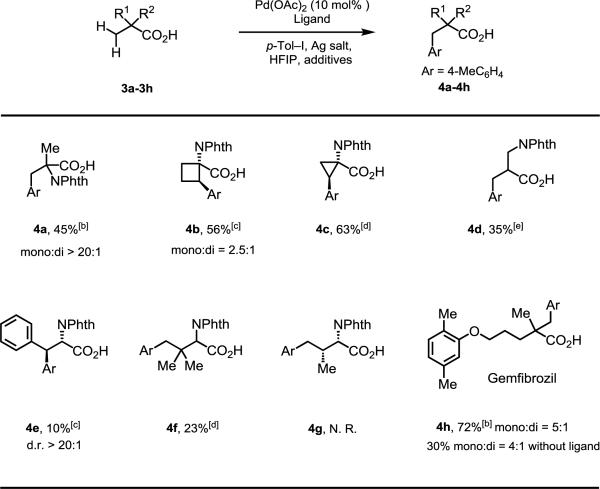
We next tested acid-directed arylation in non-alanine susbtrates (Table 3): Isovaline derivatives afforded mono-arylation product (4a) in 45% yield under the same condition for the arylation of the alanine substrate. Arylation of 1-aminocyclobutane-1-carboxylic acid and 1-aminocyclopropane-1-carboxylic acid under the same reaction conditions proceeded in moderate yields (4b = 42% [mono: di = 2.2:1]; 4c = 45%), but switching to L18 provided a boost in reactivity (4b = 56% [mono: di = 2.5:1]; 4c = 63%). The β-amino acid substrate 3d afforded the desired product 4d in poor yields (35%). 3e was selected as a substrate to further test the limitations of the acid-directed methylene C–H arylation (demonstrated in substrates 3b and 3c): Unfortunately, N-phthaloyl phenylalanine produced 4e in 10% yield when L18 was used. γ-C–H arylation was next attempted with substrates 3f and 3g: while 4f was obtained in23% yield, N-phthaloyl valine (3g) was not reactive. We suspect this is due to the Thorpe-Ingold Effect which favors cyclopalladation.[14] It is worth noting that this chemistry is applicable to α-quaternary acid substrates: Gemfibrozil (3h), an oral drug used to lower lipid levels,[15] was arylated in good yields (72%); in the absence of ligand, 4h was only obtained in 30% yield. We attempted to expand the substrate scope to other carboxylic acids, however the yields were generally low (see Table S4 in supporting information).
Table 3.
Arylation of Other Amino Acids and Carboxylic Acids.[a]
Isolated yields are shown based on corresponding methyl ester.
[b] Conditions: substrate (0.1 mmol), Pd(OAc)2 (10 mol %), AgOAc (0.2 mmol), Ar–I (0.25 mmol), Na2HPO4·7H2O (0.15 mmol), L15 (20 mol%), HFIP (1.0 mL), 100 °C, 24 h.
[c] Conditions: substrate (0.1 mmol), Pd(OAc)2 (10 mol%), AgOAc (0.2 mmol), Ar–I (0.25 mmol), Na2HPO4·7H2O (0.15 mmol), L18 (12 mol%), HFIP (1.0 mL), 100 °C, 24 h.
[d] Conditions: substrate (0.1 mmol), Pd(OAc)2 (10 mol%), Ag2CO3 (0.2 mmol), Ar–I (0.25 mmol), L18 (12 mol%), K2HPO4 (0.1 mmol), HFIP (1.0 mL), 100 °C, 24 h.
[e] Conditions: substrate (0.1 mmol), Pd(OAc)2 (10 mol%), AgOAc (0.2 mmol), Ar–I (0.25 mmol), Na2HPO4·7H2O (0.15 mmol), L8 (20 mol%), HFIP (1.0 mL), 100 °C, 24 h.
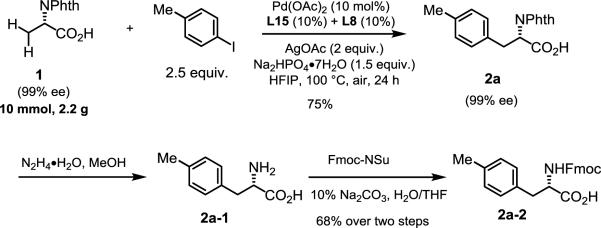
There are several advantages to directly using free amino acids as substrates for gram-scale preparation of unnatural amino acids: the installation and removal of exogenous and often times cumbersome directing groups are entirely avoided, thus shortening synthetic routes and eliminating chemical waste. To demonstrate the utility of the herein reported reaction, we performed the reaction on gram scale with 1 and para-tolyl iodides (Scheme 3) under the aforementioned reaction conditions, the desired product 2a was obtained in 75% yield. It is noteworthy that the α-center chirality was preserved (see supporting information). The phthalimide group was next removed in the presence of hydrazine to generate the free amine 2a-1, which was subsequently converted to final Fmoc-protected amino acid 2a-2 (68% over two steps) which is widely used in peptide synthesis.
Scheme 3.
Gram-Scale Synthesis of Unnatural Amino Acid.
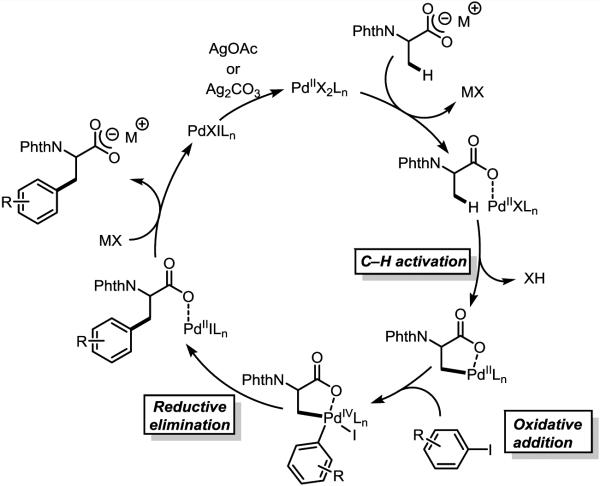
We propose that the transformation herein disclosed proceeds through the mechanism [6a, 9] outlined in Figure 1: Pd(II) coordinates the carboxylate of the substrate, followed by (1) C–H cleavage; (2) oxidative addition by aryl iodides, generating a fleeting Pd(IV) intermediate; (3) reductive elimination, (4) product dissociation; (5) regeneration of the active Pd(II) species by silver salts. At this stage, we are currently investigating the precise role of monodentate pyridine and bidentate heterocyclic ligands in enabling this mechanism.
Figure 1.
Plausible mechanism for C(sp3)–H ary lation.
In conclusion, we have developed β-C–H arylation of α-amino acids using innate carboxylic acid functionality as a directing group. This reaction was easily scaled up to prepare unnatural amino acid building blocks. Further development of more efficient ligands to expand the scope of substrates – so as to achieve methylene-C–H and γ-C–H arylation – as well as coupling partners (e.g. heteroaryl iodides) is ongoing in our laboratory.
Experimental Section
General procedure for arylation with aryl iodides
The starting material 1 (0.1 mmol, 22 mg), Pd(OAc)2 (10 mol%, 2.2 mg), and AgOAc (0.2 mmol, 33.4 mg) were weighted in air and placed in a sealed tube (10 mL) with a magnetic stir bar. To the reaction mixture, aryl iodide (0.25 mmol), Na2HPO4 •7H2O (1.5 equiv.), ligand (20 mol%), HFIP (2.0 mL) were added. The reaction mixture was first stirred at room temperature for 10 min and then heated to 100 °C for 24 hours under vigorous stirring. Upon completion, the reaction mixture was cooled to room temperature, added AcOH (0.05 mL), and filtered through celite using DCM. The solvents were removed under reduced pressure and the resulting mixture was added with DCM (3 mL), cat. DMF, and (COCl)2 (2 equiv.). After 2 hours, MeOH (0.5 mL) was added the reaction mixture and stirred for another 1 hour. The solvents were removed under reduced pressure and the resulting mixture w as purified by preparative TLC.
Supplementary Material
Acknowledgements
We gratefully acknowledge The Scripps Research Institute and Bristol-Myers Squibb for financial support. This work was supported by NIH (NIGMS, 2R01 GM084019). We thank SIOC, Zhejiang Medicine and Pharmaron (fellowships to G. Chen). T. G. Saint-Denis is supported by the NSF GRFP and the TSRI Dean's Fellowship.
Footnotes
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.201xxxxxx.
Contributor Information
Dr. Gang Chen, Department of Chemistry, The Scripps Research Institute (TSRI) 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)
Zhe Zhuang, Department of Chemistry, The Scripps Research Institute (TSRI) 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA).
Gen-Cheng Li, Department of Chemistry, The Scripps Research Institute (TSRI) 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA).
Tyler G. Saint-Denis, Department of Chemistry, The Scripps Research Institute (TSRI) 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA)
Dr. Yi Xiao, Chemical and Synthetic Development, Bristol-Myers Squibb, 1 Squibb Drive, New Brunswick, New Jersey 08903 (USA)
Dr. Candice L. Joe, Chemical and Synthetic Development, Bristol-Myers Squibb, 1 Squibb Drive, New Brunswick, New Jersey 08903 (USA)
Prof. Dr. Jin-Quan Yu, Department of Chemistry, The Scripps Research Institute (TSRI) 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA).
References
- 1. For selected reviews and discussion on utility of carboxylic acids, see. [Google Scholar]; a Gooßen LJ, Rodriguez N, Gooßen K. Angew. Chem. Int. Ed. 2008;47:3100. doi: 10.1002/anie.200704782. [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2008;120:3144. [Google Scholar]; b Qin T, Cornella J, Li C, Malins LR, Edwards JT, Kawamura S, Maxwell BD, Eastgate MD, Baran PS. Science. 2016;352:801. doi: 10.1126/science.aaf6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. For reviews of acid-directed C(sp2)–H activation, see. [Google Scholar]; a Engle KM, Mei T-S, Wasa M, Yu J-Q. Acc. Chem. Res. 2012;45:788. doi: 10.1021/ar200185g. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Chen Z, Wang B, Zhang J, Yu W, Liu Z, Zhang Y. Org. Chem. Front. 2015;2:1107. [Google Scholar]
- 3.Kao L, Sen A. J. Chem. Soc, C.C. 1991:1242. [Google Scholar]; b Dangel BD, Johnson JA, Sames D. J. Am. Chem. Soc. 2001;123:8149. doi: 10.1021/ja016280f. [DOI] [PubMed] [Google Scholar]; c Lee JM, Chang S. Tetrahedron Lett. 2006;47:1375. [Google Scholar]
- 4.Giri R, Maugel N, Li J-J, Wang D-H, Breazzano SP, Saunder LB, Yu J-Q. J. Am. Chem. Soc. 2007;129:3510. doi: 10.1021/ja0701614. [DOI] [PubMed] [Google Scholar]
- 5.Novák P, Correa A, Gallardo-Donaire J, Martin R. Angew. Chem. Int. Ed. 2011;50:12236. doi: 10.1002/anie.201105894. [DOI] [PubMed] [Google Scholar]; Angew.Chem. 2011;123:12444. [Google Scholar]
- 6.a Zaitsev VG, Shabashov D, Daugulis O. J. Am. Chem. Soc. 2005;127:13154. doi: 10.1021/ja054549f. [DOI] [PubMed] [Google Scholar]; b Daugulis O, Do H-Q, Shabashov D. Acc. Chem. Res. 2009;42:1074. doi: 10.1021/ar9000058. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Tran LD, Daugulis O. Angew. Chem., Int. Ed. 2012;51:5188. doi: 10.1002/anie.201200731. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew.Chem. 2012;124:5278. [Google Scholar]; d Reddy BVS, Reddy LR, Corey EJ. Org. Lett. 2006;8:3391. doi: 10.1021/ol061389j. [DOI] [PubMed] [Google Scholar]; e Wang B, Nack WA, He G, Zhang S, Chen G. Chem. Sci. 2014;5:3952. [Google Scholar]; f Zhang Q, Chen K, Rao W, Zhang Y, Chen F-J, Shi B-F. Angew.Chem. Int. Ed. 2013;52:13588. doi: 10.1002/anie.201306625. [DOI] [PubMed] [Google Scholar]; Angew.Chem. 2013;125:13833. [Google Scholar]
- 7.Engle KM, Yu J-Q. J. Org. Chem. 2013;78:8927. doi: 10.1021/jo400159y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giri R, Chen X, Yu J-Q. Angew. Chem. Int. Ed. 2005;44:2112. doi: 10.1002/anie.200462884. [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2005;117:2150. [Google Scholar]
- 9.He J, Li S, Deng Y, Fu H, Laforteza BN, Spangler JE, Homs A, Yu J-Q. Science. 2014;343:1216. doi: 10.1126/science.1249198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen G, Shigenari T, Jain P, Zhang Z, Jin Z, He J, Li S, Mapelli C, Miller MM, Poss MA, Scola PM, Yeung K-S, Yu J-Q. J. Am. Chem. Soc. 2015;137:3338. doi: 10.1021/ja512690x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen G, Gong W, Zhuang Z, Andra MS, Chen Y-Q, Hong X, Yang Y-F, Liu T, Houk KN, Yu J-Q. Science. 2016;353:1023. doi: 10.1126/science.aaf4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wencel-Delord J, Colobert F. Org. Chem. Front. 2016;3:394. For a review on HFIP-enabled C–H activation, see the following. [Google Scholar]
- 13.Fors BP, Buchwald SL. J. Am. Chem. Soc. 2010;132:15914. doi: 10.1021/ja108074t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.a Li S, Chen G, Feng C-G, Gong W, Yu J-Q. J. Am. Chem. Soc. 2014;136:5267. doi: 10.1021/ja501689j. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Li S, Zhu R-Y, Xiao K-J, Yu J-Q. Angew.Chem. Int. Ed. 2016;55:4317. doi: 10.1002/anie.201512020. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew.Chem. 2016;128:4389. [Google Scholar]
- 15.Jun M, Foote C, Lu J, Neal B, Patel A, Nicholls S, Grobbee DE, Cass A, Chalmers J, Perkovic V. The Lancet. 2010;375:1875. doi: 10.1016/S0140-6736(10)60656-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.