Abstract
Background:
Platelet-rich plasma (PRP) has been extensively used as a treatment in tissue healing in tendinopathy, muscle injury, and osteoarthritis. However, there is variation in methods of extraction, and this produces different types of PRP.
Purpose:
To determine the composition of PRP obtained from 4 commercial separation kits, which would allow assessment of current classification systems used in cross-study comparisons.
Study Design:
Controlled laboratory study.
Methods:
Three normal adults each donated 181 mL of whole blood, some of which served as a control and the remainder of which was processed through 4 PRP separation kits: GPS III (Biomet Biologics), Smart-Prep2 (Harvest Terumo), Magellan (Arteriocyte Medical Systems), and ACP (Device Technologies). The resultant PRP was tested for platelet count, red blood cell count, and white blood cell count, including differential in a commercial pathology laboratory. Glucose and pH measurements were obtained from a blood gas autoanalyzer machine.
Results:
Three kits taking samples from the “buffy coat layer” were found to have greater concentrations of platelets (3-6 times baseline), while 1 kit taking samples from plasma was found to have platelet concentrations of only 1.5 times baseline. The same 3 kits produced an increased concentration of white blood cells (3-6 times baseline); these consisted of neutrophils, leukocytes, and monocytes. This represents high concentrations of platelets and white blood cells. A small drop in pH was thought to relate to the citrate used in the sample preparation. Interestingly, an unexpected increase in glucose concentrations, with 3 to 6 times greater than baseline levels, was found in all samples.
Conclusion:
This study reveals the variation of blood components, including platelets, red blood cells, leukocytes, pH, and glucose in PRP extractions. The high concentrations of cells are important, as the white blood cell count in PRP samples has frequently been ignored, being considered insignificant. The lack of standardization of PRP preparation for clinical use has contributed at least in part to the varying clinical efficacy in PRP use.
Clinical Relevance:
The variation of platelet and other blood component concentrations between commercial PRP kits may affect clinical treatment outcomes. There is a need for standardization of PRP for clinical use.
Keywords: platelet-rich plasma, PRP, leukocyte, osteoarthritis, tendinopathy
Platelet-rich plasma (PRP) is defined as a platelet-rich concentrate with higher-than-baseline levels of platelets when compared with whole blood. PRP is increasingly used in prospective clinical studies to improve tissue healing, particularly with regard to tendinitis.5,7,13,21,22,30,33,36 A small number of randomized controlled trials have shown the positive benefit of PRP in tendinopathy.13,22,25,33 It has been hypothesized that this is due to platelet-derived growth factor (PDGF), transforming growth factor beta (TGF-β), vascular endothelial growth factor (VEGF), insulin-like growth factor 1 (IGF-1), and hepatocyte growth factor (HGF), which are released from the alpha granules during in vivo activation of platelets3,5,6,9,10,12,26,41 or subsequently produced by the cellular matrix of the tendon.
DeLong et al14 considered that PRP preparations can be divided into 2 forms: 1 plasma based, the other based on buffy coat preparations. Plasma-based preparations aim to capture platelets from the plasma after centrifugation and exclude red and white blood cells. Generally these kits produce smaller increases in platelets than the kits that take platelets from both the plasma and the more cellular “buffy coat.”8,9,18
There has been some discussion about whether the efficacy of the PRP is affected by the inclusion of the white blood cells.2,27 Moojen et al31 considered that there may be positive effects from the white blood cells acting as antimicrobial agents. Other authors have suggested that the platelets themselves may already have this property.38 There may also be negative effects from these white blood cells in causing further inflammation, leading to fibrosis, or from the release of catabolic cytokines.2,32 This effect may be more prevalent with neutrophils than other white blood cells.2 Recent meta-analyses of PRP in tendinopathy identified that leukocyte-rich PRP had a strongly positive outcome in the treatment of tendinopathies.19,34
There has also been discussion about whether the pH of the resultant PRP will affect platelet function,39 and thus whether the PRP produced should be “buffered.”
Because it is likely to be important in the management of different conditions to have certain types of PRP used,15 all commercial kits should be validated for cell and PRP type, but this has not always been the case. The purpose of this study was to validate all kits available in Australia for their composition of platelet, red and white blood cell counts, pH, and glucose levels using a single-donor model. A recommendation could then be made as to which PRP kits/types are associated with the best results in the treatment of different musculoskeletal conditions such as tendinopathy and osteoarthritis.
Methods
Three healthy adult human subjects were recruited and consented for this trial (2 women, 1 man; age range, 25-35 years).
Description of Common Commercial Kits
A review of all kits was undertaken as shown in Table 1 based on the International Olympic Committee (IOC) consensus paper on the use of PRP in sports medicine.16 It was decided that only kits producing PRP, autologous conditioned plasma, or pure platelets would be assessed. Only kits producing PRP from whole blood for use in musculoskeletal conditions such as tendinitis, muscle injuries, or osteoarthritis were selected. Kits were excluded if they produced platelet-rich fibrin or bone marrow samples. Thus, 8 potential kits were available for testing.
TABLE 1.
Commercially Available Kits for the Production of Platelet Productsa
| Device Name | Company | Name of Product | Comments |
|---|---|---|---|
| GPS III | Biomet | Platelet-rich plasma | Tested |
| SmartPrep2 | Harvest | Platelet-rich plasma | Tested |
| Magellan | Arteriocyte Medical | Platelet-rich plasma | Tested |
| Angel | Sorin | Platelet-rich plasma | Not available for testing |
| CS | Genesis | Platelet-rich plasma | Not available for testing |
| ACP | Arthrex | Autologous conditioned plasma | Tested |
| PRFM Fibrinet System | Cascade | Platelet-rich fibrin | Not tested, fibrin membrane |
| PRF and Vivostat | Choukroun’s | Platelet-rich fibrin | Not tested, fibrin membrane |
| BMAC | DePuy | Platelet-rich plasma and stem cells | Not tested, bone marrow |
| Cell saver–based systems Electa, Haemonetics, CATS, BRAT | Several | Pure platelets | Not tested, volume required >200 mL |
| Caption | Not yet marketed | Pure platelets | Not tested, not available |
| Total | 12 companies | 4 tested |
aTable derived from Engebretson et al.16
Cell saver–based pure platelet systems requiring a minimum sample of 200 mL of whole blood for processing24 were not deemed appropriate to study, as this large sample was regarded as impractical for office use. The Caption pure platelet kit was not commercially available at the time of testing, and therefore, 6 potential kits were available for study. Of these, only 4 were commercially available in Australia at the time of testing: GPS III (Biomet Biologics), SmartPrep2 (Terumo Harvest), Magellan (Arteriocyte Medical Systems), and ACP (Device Technologies, Arthrex). All companies agreed for their kits to be used in the trial and provided the kits.
Sample Collection and Processing
All samples were collected from the subjects by the senior author (J.F.) and were processed immediately. A total of 181 mL of blood was drawn from each subject: 5 mL was used for the control sample, 52 mL for each of the PRP-based kits (GPS III, SmartPrep2, and Magellan), and 15 mL for the ACP kit. The samples were processed according to the manufacturers’ instructions to produce 6 to 7 mL of finished product, as shown in Table 2.
TABLE 2.
Preparation of PRP Samplesa
| System | Blood Volume, mL | Anticoagulant Volume, mL | Centrifugal Force, g-force | Centrifuge Time, min | Volume Produced, mL |
|---|---|---|---|---|---|
| GPS III | 52 | ACD-A 8 | 1100 | 15 | 6-7 |
| SmartPrep2 | 52 | ACD-A 8 | 1250/1050 | 14 | 6-7 |
| Magellan | 52 | ACD-A 8 | 1200 | 17 | 6-7 |
| ACP | 15 | ACD-A 2 | 1500 | 5 | 6-7 |
aACD-A, anticoagulant citrate dextrose solution A; PRP, platelet-rich plasma.
The samples were then processed: 1.5 mL from the PRP samples and the control blood were put into a tube for analysis on a blood gas testing machine (ABL800 Flex; Radiometer), generating results for pH, K+, Na+, Cl–, glucose, and lactate. The remaining control blood and PRP samples were placed into a collection tube for analysis on a Coulter LH 250 automated analyzer (Beckman Coulter Inc) within 30 minutes of collection to measure full blood count and white blood cell count with differential.
Classification of the PRP Produced
The results from the analysis were assessed based on the PAW (platelet, activation, white blood cells)15 and the Mishra sports medicine PRP classification29 systems. The PAW system classifies PRP based on platelet numbers, the manner in which activation occurs, and the presence or absence of white blood cells. The Mishra sports medicine PRP classification system is based on platelet concentration, the presence or absence of white blood cells, and whether the PRP has been activated with exogenous thrombin or calcium chloride.
Statistical Analysis
All statistical analyses were performed using STATA version 13 (Stata Corp). All variables had a calculated mean and standard deviation. Each subject was used as their own control, and thus, change from mean was relative to their own control result.
Results
Comparison of Cellular Components
We first compared the cellular components of platelets, leukocytes, and red blood cells between these 4 kits using standard methods on 3 human subjects. A summary of data is presented in Table 3. The values for total platelet count as well as red and white blood cell counts are presented compared with controls.
TABLE 3.
Cellular Dataa
| Kit | Cell Type | Mean, ×109/L | SD, ×109/L | Median, ×109/L | Min, ×109/L | Max, ×109/L |
|---|---|---|---|---|---|---|
| Control | Platelets | 269 | 106 | 290 | 154 | 362 |
| WBC | 8.73 | 3.75 | 8.9 | 4.9 | 12.4 | |
| RBC | 4.7 | 0.436 | 4.5 | 4.4 | 5.2 | |
| ACP | Platelets | 412 | 140 | 424 | 266 | 546 |
| WBC | 1.3 | 0.781 | 7.7 | 0.4 | 1.8 | |
| RBC | 0.0333 | 0.0577 | 0 | 0 | 0.1 | |
| GPS | Platelets | 964 | 551 | 760 | 544 | 1588 |
| WBC | 35.8 | 10.8 | 41.8 | 23.3 | 42.3 | |
| RBC | 1.03 | 0.289 | 1.2 | 0.7 | 1.2 | |
| SmartPrep | Platelets | 1224 | 560 | 1262 | 646 | 1764 |
| WBC | 24.7 | 8.69 | 26.1 | 15.4 | 32.6 | |
| RBC | 1.43 | 0.306 | 1.5 | 1.1 | 1.7 | |
| Magellan | Platelets | 1266 | 831 | 1153 | 497 | 2148 |
| WBC | 31.4 | 9.4 | 35.2 | 20.7 | 38.3 | |
| RBC | 1.03 | 0.153 | 1.0 | 0.9 | 1.2 |
aRBC, red blood cell count; WBC, total white blood cell count.
Platelets
An increase in platelet production was demonstrated compared with baseline in all kits (Figure 1). The ACP kit produced a 1 to 1.7 times baseline level of platelets (412 × 109/L), which is consistent with the literature for this kit and open-tube single- or double-spin systems.4,8,37,41 The Magellan (1266 × 109/L), GPS (964 × 109/L), and SmartPrep (1224 × 109/L) kits produce 3 and 6 times baseline platelet concentrations, consistent with previous data.8,9,17,18,23,24
Figure 1.
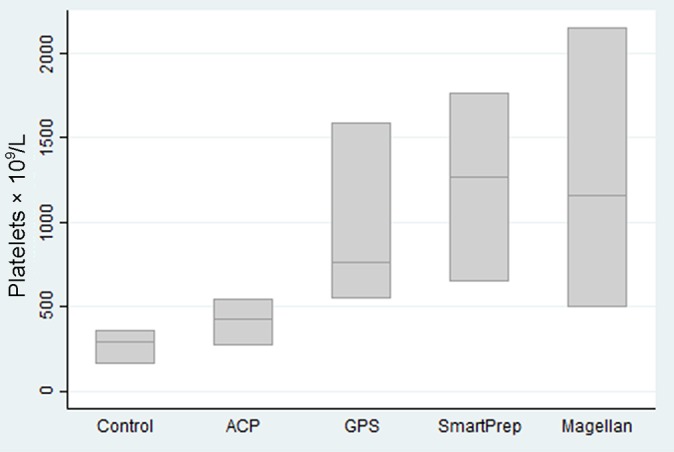
Platelet counts by kit type.
Red Blood Cells
All kits significantly reduced red blood cell counts compared with controls, as seen in Figure 2. The ACP kit virtually eliminated red blood cells. The GPS, SmartPrep, and Magellan kits reduced the red blood cells by 3 to 6 times baseline levels.
Figure 2.
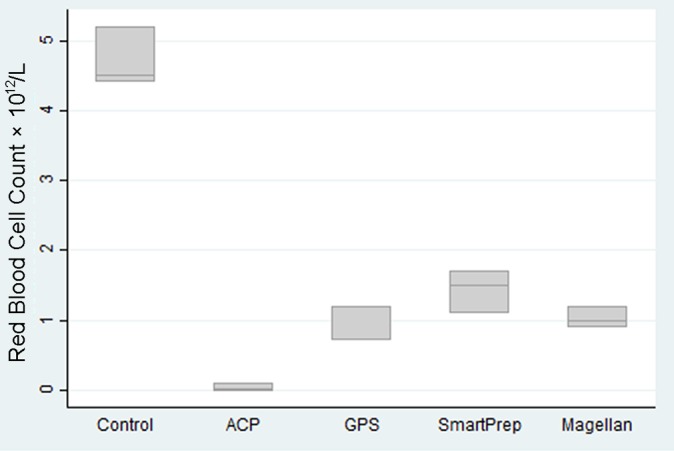
Red blood cell counts by kit type.
White Blood Cells
White blood cell counts are of great importance. Compared with controls (white blood cell count, 8.73 × 109/L), the only kit to reduce the white blood cell count was the plasma system (ACP) (1.3 × 109/L), which reduced the white blood cell count by 5 to 22 times, almost eliminating the white blood cells. The GPS III (35.8 × 109/L), SmartPrep2 (24.7 × 109/L), and Magellan (31.4 × 109/L) kits actively concentrated white blood cells 3 to 5 times baseline levels (Figure 3). This is consistent with the results found by Carmona et al.7 Similar increases across all 3 kits were demonstrated. Our results showed much higher levels of white blood cell concentration than have been indicated by others.7,8
Figure 3.
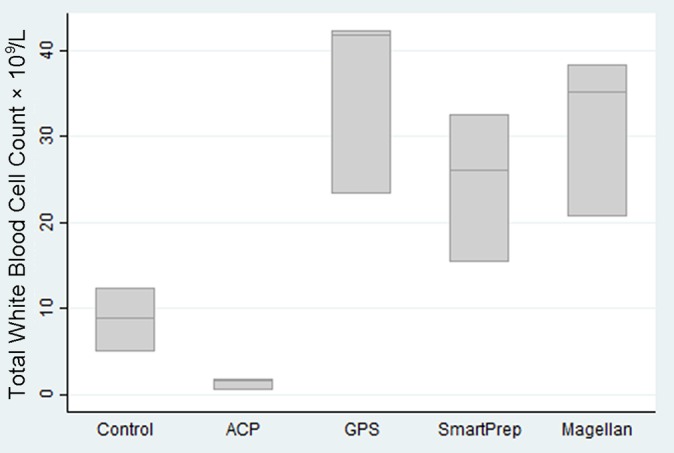
Total white blood cell counts by kit type.
When the white blood cell count is broken into a differential white blood cell count, the majority of cells are neutrophils and lymphocytes (Table 4 and Figure 4). Compared with controls (5.5 × 109/L), the GPS and Magellan kits contained greater mean neutrophil counts (15.4 and 15.1 × 109/L, respectively). The SmartPrep kit had a lower mean neutrophil count (6.47 × 109/L), and the ACP kit had a negligible mean neutrophil count (0.4 × 109/L). Compared with controls (2.37 × 109/L), the mean lymphocyte counts of the GPS (15.9 × 109/L), SmartPrep (14.0 × 109/L), and Magellan (12.5 × 109/L) were higher but similar across kits. The ACP kit had negligible lymphocytes (0.7 × 109/L). The increase in total white blood cell count was similar across the 3 buffy coat layer kits (GPS, SmartPrep, and Magellan). However, the relative increase in neutrophils was much greater for the GPS and the Magellan kits.
TABLE 4.
White Blood Cell Differential Countsa
| Kit | Mean ± SD, ×109/L | Median (Range), ×109/L |
|---|---|---|
| Control | ||
| WBC | 8.73 ± 3.75 | 8.9 (4.9-12.4) |
| Neutrophils | 5.5 ± 2.91 | 5.3 (2.7-8.5) |
| Lymphocytes | 2.37 ± 0.85 | 2.4 (1.5-3.2) |
| Monocytes | 0.6 ± 0.173 | 0.5 (0.5-0.8) |
| ACP | ||
| WBC | 1.3 ± 0.781 | 1.7 (0.4-1.8) |
| Neutrophils | 0.4 ± 0.265 | 0.5 (0.1-0.6) |
| Lymphocytes | 0.7 ± 0.436 | 0.9 (0.2-1.0) |
| Monocytes | 0.167 ± 0.115 | 0.1 (0.1-0.3) |
| GPS | ||
| WBC | 35.8 ± 10.8 | 41.8 (23.3-42.3) |
| Neutrophils | 15.4 ± 5.05 | 14 (11.2-21) |
| Lymphocytes | 15.9 ± 7.73 | 14.6 (8.9-24.2) |
| Monocytes | 3.8 ± 1.1 | 3.8 (2.7-4.9) |
| Smart Prep | ||
| WBC | 24.7 ± 8.69 | 26.1 (15.4-32.6) |
| Neutrophils | 6.47 ± 1.86 | 7 (4.4-8) |
| Lymphocytes | 14 ± 6.36 | 13 (8.2-20.8) |
| Monocytes | 3.57 ± 1 | 3.5 (2.6-4.6) |
| Magellan | ||
| WBC | 31.4 ± 9.4 | 35.2 (20.7-38.3) |
| Neutrophils | 15.1 ± 3.93 | 16.6 (10.6-18) |
| Lymphocytes | 12.5 ± 5.72 | 12 (7.1-18.5) |
| Monocytes | 3.27 ± 1.03 | 3 (2.4-4.4) |
aWBC, total white blood cell count.
Figure 4.
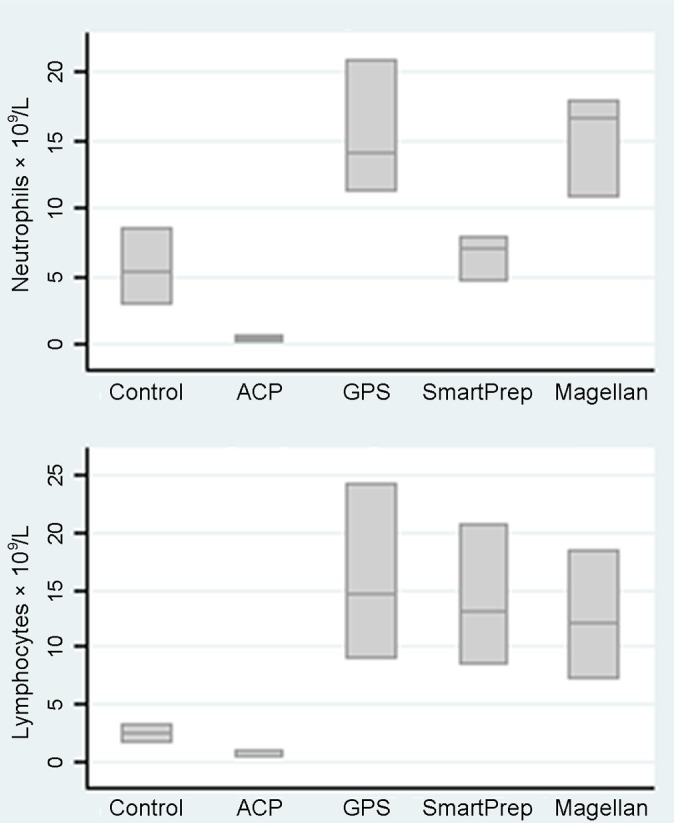
Neutrophil and lymphocyte counts by kit type.
Comparison of Chemical Composition
PRP from the kits was assessed for glucose and pH using the Radiometer ABL800 Flex. The data for pH and glucose are shown in Table 5 and Figures 5 and 6.
TABLE 5.
pH and Glucose Data
| Kit | Mean ± SD, mmol/L | Median (Range), mmol/L |
|---|---|---|
| Control | ||
| pH | 7.1 ± 0.28 | 7.12 (7.07-7.12) |
| Glucose | 4.2 ± 0.529 | 4.4 (3.6-4.6) |
| ACP | ||
| pH | 6.87 ± 0.256 | 6.99 (6.57-7.04) |
| Glucose | 18.5 ± 2.73 | 17.7 (16.2-21.5) |
| GPS | ||
| pH | 7.05 ± 0.02 | 7.05 (7.03-7.07) |
| Glucose | 15.8 ± 1.07 | 15.2 (15.1-17.0) |
| Smart Prep | ||
| pH | 6.59 ± 0.329 | 6.61 (6.55-6.61) |
| Glucose | 23.6 ± 1.36 | 23.8 (22.2-24.9) |
| Magellan | ||
| pH | 6.66 ± 0.102 | 6.69 (6.54-6.74) |
| Glucose | 22.3 ± 0.907 | 22.7 (21.3-23.0) |
Figure 5.
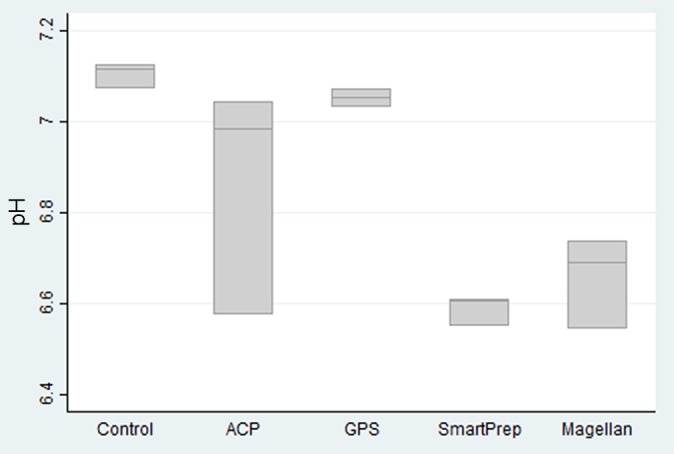
pH by kit type.
Figure 6.
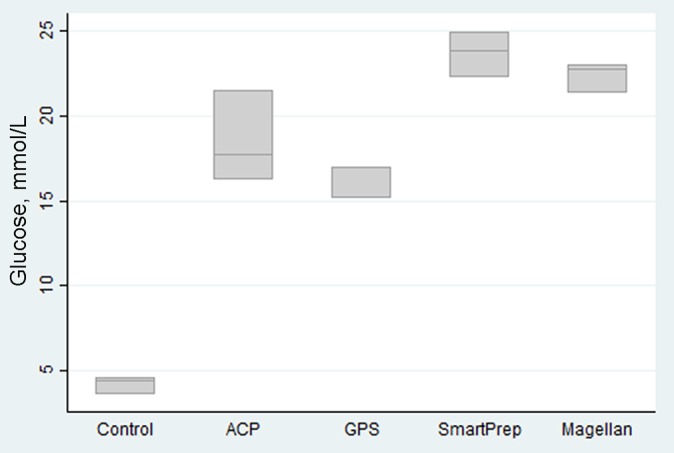
Glucose by kit type.
Compared with the glucose control of 4.2 mmol/L, all PRP produced contained a high level of glucose ranging from 15.8 to 23.6 mmol/L. This reflects an increase in glucose of 4 to 6 times baseline.
The mean pH of the controls was 7.1. The mean pH of the PRP produced ranged from 6.59 (SmartPrep) to 7.05 (GPS). The lower pH in the kit samples is related to the use of the anticoagulant citrate dextrose solution–formula A (ACD-A) anticoagulant. The amount of ACD-A used was the same ratio for all kits by volume.
Discussion
There are various kits available for the separation of PRP in clinical practice. As a specific “dose” of platelets may be required to achieve a clinical effect, it is important to identify the kits that produce different doses.15 Using a single-donor protocol, we have analyzed the blood components of 4 of the most common commercial kits available to medical practitioners in Australia. We have shown an increase in platelets from baseline in all the kits with large variations. This was found to be 1 to 1.5 for plasma-type kits (ACP) and between 3 and 6 times for buffy coat layer kits (GPS III, Magellan, SmartPrep2). This confirms the work of Castillo et al,9 who similarly compared 3 kits (Magellan, Cascade, and GPS). In addition, there is variation in the numbers of neutrophils, leukocytes, and monocytes between the kits. The plasma system (ACP) reduced the white blood cell count by 5 to 22 times, almost eliminating the white blood cells. The buffy coat kits (GPS III, SmartPrep2, and Magellan) actively concentrated white blood cells 3 to 5 times baseline. The increase in total white blood cell count was similar across the 3 buffy coat layer kits (GPS, SmartPrep, and Magellan). However, the relative increase in neutrophils was much greater for the GPS and Magellan kits.
A small reduction in pH was thought to relate to the citrate used in the sample preparation. This reduction is not thought to be of clinical significance. Based on the small drop in pH, it does not seem necessary to buffer the PRP unless this change in pH can be shown to negatively impact the production of growth factors.
No studies have reported the level of glucose in PRP produced previously. One of the surprising findings in this study was the significant increases in glucose concentration of 4 to 6 times baseline in all kits. This has not been previously reported as a significant variable, and the clinical significance of this factor is unknown. It is of interest that glucose solutions at concentrations between 12% and 20% have been used in prolotherapy injections with varying results.1,11,20 It is likely this is derived from the use of the ACD-A and is thus related to the preparation technique consistent to all kits. If one takes 5 mL of a 10% glucose solution (2.8-mmol solution) for injection, this would contain 0.5 g of glucose. If we take 5 mL of PRP produced in any of the studied kits at 20 mmol/L (70% glucose solution), this would give us 3 g of glucose. In simple terms, our PRP samples are producing a 6-times glucose concentration compared with glucose solutions used in prolotherapy. This may be important as part of the factors producing a clinical response.
Essentially there are 2 main types of PRP preparation methods. After centrifugation, there are 3 key layers, as shown in Figure 7. Plasma-based systems take product from the yellow relatively acellular plasma layer. These systems aim to exclude red and white blood cells from the preparation and to collect as many platelets from the remaining “plasma” layer as possible. As many of the platelets are in the buffy coat layer, the resultant product is low in red and white blood cells and has only a 1.5 to 1.7 times baseline level of platelets. This is well demonstrated by the results from the ACP kit in our study. The second type of PRP product is made from the buffy coat floating above the red blood cell layer. Levels of platelets at 3 to 6 times baseline levels are expected as the product is coming from a more platelet-dense environment.6,9 Again, this is confirmed by our testing, with the GPS III, Magellan, and SmartPrep2 kits having much higher platelet concentrations.
Figure 7.
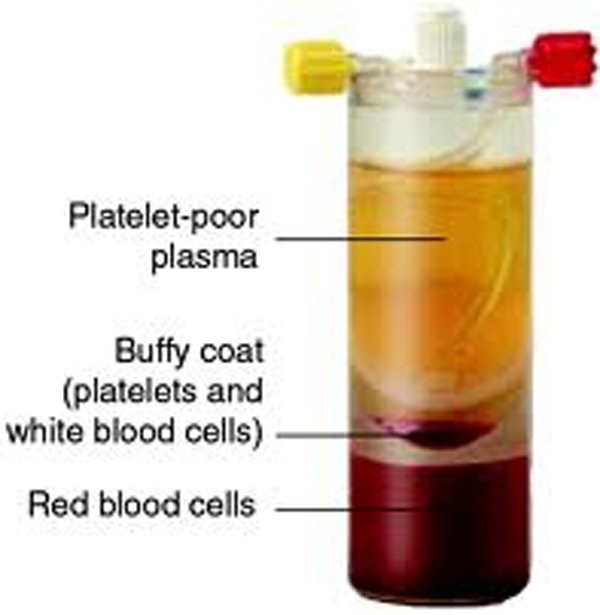
Platelet-rich plasma in GPS kit after centrifugation.
In producing PRP, all kits aim to reduce the red blood cell count6,9,12 and increase the collection of platelets. Some white blood cells are captured at the same time.6,9 Due to the addition of citrate to the blood being collected, there is likely to be a drop in pH of the sample produced. This is the first paper to identify how other variables like lactate or glucose are changed by this process. Generally, in most literature review papers, the white blood cell count is ignored or regarded as negligible. We feel that these aspects of PRP systems should be more highly noted in future literature reports as the concentration of white blood cells is as great as that of the platelets and there is glucose present in the end product.
Studies by others have also shown variation of platelet levels, growth factor and cytokine levels,32 and total white blood cell counts across PRP preparation methods.8,9,17,18,24,27,37,40,41 However, the most important finding in our study is that the white blood cell counts are significantly more concentrated than previously thought. The ACP kit was the only one in our series that reduced the white blood cell count by a factor of about 9. This may be an important point of difference if the white blood cells are not beneficial. The other 3 kits (GPS III, Magellan, SmartPrep2) concentrated the white blood cell count by 3 to 5 times, a similar increase to platelet concentration. Thus, these white blood cells are not contaminants as their levels are as high as the primary ingredient: platelets. Their levels may be regarded as potentially clinically significant. Furthermore, we assessed the white blood cell differential count and found that the cellular concentration of white blood cells was up to 40% neutrophils and lymphocytes each and a further 10% made up of monocytes. The remainder of the cells were basophils and eosinophils in small quantities. An increase in the growth factor VEGF would be expected as the number of lymphocytes increases. These lymphocytes may play an important role in further enhancing the tissue repair processes, but they may also lead to increased local inflammation.
White blood cells may contribute to the modulation of inflammatory and platelet activation, thereby acting to potentiate the tissue repair mechanism. It is possible that the white blood cells may confer an advantage to the patient in reducing the chance of infection or modulating the inflammatory response.31,35 This may be an important consideration in those clinical settings where the patient is at greater risk, such as with intra-articular procedures or at the time of surgery. Furthermore, Zimmermann et al41 found that the increased white blood cell count was responsible for between one-third and one-half of the variation on growth factors found in their samples. They found a positive correlation between the white blood cell count and VEGF (known to come from the white blood cells) and PDGF.
On the other hand, others have shown that white blood cells appear to have a deleterious effect on the tissue,27 resulting in increased inflammation and further scarring. These negative effects are largely due to neutrophils and include the release of oxygen-free radicals, catabolic cytokines, matrix metalloproteinases (MMPs), and interleukin B, which degrade tissue.14
A recent meta-analysis of the effectiveness of PRP in tendinopathy has shown that leukocyte-rich PRP (LR-PRP) is the most effective in the treatment of tendinopathy.19 This study allows us to recommend PRP produced by the GPS III, SmartPrep2, and Magellan kits in the treatment of tendinopathy. By contrast, 2 recent reviews of the effectiveness of PRP in osteoarthritis have shown that leukocyte-poor PRP (LP-PRP) may be more effective.28,34
One of the surprising new findings in this study was the significant increases in glucose concentration of 4 to 6 times baseline in all kits. This has not been previously reported as a significant variable, and the clinical significance of this factor is unknown. It is of interest that glucose solutions at concentrations of between 12% and 20% have been used in prolotherapy injections with varying results.1,11,20 The clinical significance of this remains uncertain. This was thought to be important as prolotherapy with glucose has been used in the treatment of musculoskeletal injuries.
Kit validation studies help to classify kits into those deemed similar enough to allow results from papers using kits to be compared. Some kit classification systems have been proposed in the past.15,29 Table 6 shows the PRP kits classified according to both the PAW and Mishra classification systems. The classification proposed by Mishra et al29 allows for platelet concentrations >5 or <5 times baseline. We found 3 to 6 times baseline values in the buffy coat kits and 1.5 in the plasma kits. This classification system with a cutoff of ±5 times concentration does not fit for either buffy coat or plasma system results from our study. The white blood cells are recorded only as present or absent, and there is no accounting for the level of white blood cells. The PAW classification system proposed by DeLong et al15 is appropriate for the classification of platelets, classifying our tested kits into 3 different groups, but it simply classifies the white blood cells as absent or present. Given the high concentration of white blood cells found in our laboratory analysis, this classification system does not adequately account for the high numbers of white blood cells, including both neutrophils and lymphocytes found in the PRP preparations studied. If these cells are found to be significant for efficacy, a further breakdown of types of PRP to adequately include white blood cells will be needed. Kits classified in the same class would then be able to have their results compared as a group when meta-analysis or comparison studies are being undertaken.
TABLE 6.
PRP kits by PAW and Mishra (Sports Medicine) Classificationsa
| PAW Classification | Mishra Classification | |||||||
|---|---|---|---|---|---|---|---|---|
| Kit | Platelets | WBC | Neutrophils | Resultb | Activation (PAW/Mishra) | WBC | Platelets | Resultc |
| ACP | P2 | B | b | P2Bb | N/A | Minimal | <5 | 3B |
| Magellan | P4 | A | a | P4Aa | N/A | Increased | <5 | 1B |
| GPS III | P3 | A | a | P3Aa | N/A | Increased | <5 | 1B |
| SmartPrep2 | P3 | A | a | P3Aa | N/A | Increased | <5 | 1B |
aN/A, not applicable; WBC, total white blood cell count.
bPAW classification: platelet counts: P2 = baseline to 750,000; P3 = 750,000-1,250,000; P4 = >1,250,000. Total WCC: A = above baseline, B = below or equal to baseline. Neutrophil count: a = above baseline, b = below baseline.
cMishra classification: type 1B = increased WCC, no activation, platelet count <5 times baseline; type 3B = minimal WCC, no activation, platelet count <5 times baseline.
Conclusion
This study identifies the large variations in composition and concentration of platelets, white blood cell counts, and the differential count of neutrophils and lymphocytes as well as the presence of high levels of glucose between 4 commercial PRP kits. The clinical significance of this is that these variations must be taken into account when assessing the results of clinical trials and in the choice of preparation by practitioners. This study highlights the need for standardization of platelet-rich plasma extraction for clinical use.
Acknowledgment
The authors extend thanks to Dr Ellen Maxwell, Melbourne Pathology, for advice and the pathology services. The authors acknowledge the Epworth Hospital, Melbourne, Australia, for the provision of the Radiometer ABL800 Flex for the pH and glucose readings, in addition to Suzie Moreton, Epworth librarian, for her ongoing support.
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: All 4 of the commercial kits used in this study were supplied by the manufacturers as follows: GPS III (Biomet Biologics), Smart-Prep2 (Harvest Terumo), Magellan (Arteriocyte Medical Systems), and ACP (Device Technologies).
Ethical approval for this study was waived by the University of Western Australia Human Research Ethics Committee (RA/4/1/6209).
References
- 1. Alderman D. The new age of prolotherapy. Pract Pain Manag. 2010;2010:54–72. [Google Scholar]
- 2. Andia I, Abate M. Platelet-rich plasma injections for tendinopathy and osteoarthritis. Int J Clin Rheumatol. 2012;7:397–412. [Google Scholar]
- 3. Anitua E, Andia I, Sanchez M, et al. Autologous preparations rich in growth factors promote proliferation and induce VEGF and HGF production by human tendon cells in culture. J Orthop Res. 2005;23:281–286. [DOI] [PubMed] [Google Scholar]
- 4. Arthrex Inc. Arthrex ACP kit series I – ABS-10011 https://www.arthrex.com/products/ABS-10011. Accessed February 12, 2015. [Google Scholar]
- 5. Baksh N, Hannon CP, Murawski CD, Smyth NA, Kennedy JG. Platelet-rich plasma in tendon models: a systematic review of basic science literature. Arthroscopy. 2013;29:596–607. [DOI] [PubMed] [Google Scholar]
- 6. Boswell SG, Cole BJ, Sundman EA, Karas V, Fortier LA. Platelet-rich plasma: a milieu of bioactive factors. Arthroscopy. 2012;28:429–439. [DOI] [PubMed] [Google Scholar]
- 7. Brooke K, Coombes LB, Vincenzo B. Efficacy and safety of corticosteroid injections and other injections for management of tendinopathy: a systematic review of randomised controlled trials. Lancet. 2010;376:1751–1767. [DOI] [PubMed] [Google Scholar]
- 8. Carmona UJ, Lopez C, Sandoval JA. Review of the currently available systems to obtain platelet related products to treat equine musculoskeletal injuries. Rec Patents Regen Med. 2013;3:148–159. [Google Scholar]
- 9. Castillo TN, Pouliot MA, Kim HJ, Dragoo JL. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am J Sports Med. 2011;39:266–271. [DOI] [PubMed] [Google Scholar]
- 10. Creaney L, Wallace A, Curtis M, Connell D. Growth factor-based therapies provide additional benefit beyond physical therapy in resistant elbow tendinopathy: a prospective, single-blind, randomised trial of autologous blood injections versus platelet-rich plasma injections. Br J Sports Med. 2011;45:966–971. [DOI] [PubMed] [Google Scholar]
- 11. Dagenais S, Yelland MJ, Del Mar C, Schoene ML. Prolotherapy injections for chronic low-back pain. Cochrane Database Syst Rev. 2007;2:CD004059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Vos RJ, van Veldhoven PL, Moen MH, Weir A, Tol JL, Maffulli N. Autologous growth factor injections in chronic tendinopathy: a systematic review. Br Med Bull. 2010;95:63–77. [DOI] [PubMed] [Google Scholar]
- 13. de Vos RJ, Weir A, van Schie HT, et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA. 2010;303:144–149. [DOI] [PubMed] [Google Scholar]
- 14. DeLong JM, Beitzel K, Mazzocca AD, Shepard D, Roller BL, Hanypsiak BT. Update on platelet-rich plasma. Curr Orthop Pract. 2011;22:514–523. [Google Scholar]
- 15. DeLong JM, Russell RP, Mazzocca AD. Platelet-rich plasma: the PAW classification system. Arthroscopy. 2012;28:998–1009. [DOI] [PubMed] [Google Scholar]
- 16. Engebretsen L, Steffen K, Alsousou J, et al. IOC consensus paper on the use of platelet-rich plasma in sports medicine. Br J Sports Med. 2010;44:1072–1081. [DOI] [PubMed] [Google Scholar]
- 17. Eppley BL, Woodell JE, Higgins J. Platelet quantification and growth factor analysis from platelet-rich plasma: implications for wound healing. Plast Reconstr Surg. 2004;114:1502–1508. [DOI] [PubMed] [Google Scholar]
- 18. Everts PA, Brown Mahoney C, Hoffmann JJ, et al. Platelet-rich plasma preparation using three devices: implications for platelet activation and platelet growth factor release. Growth Factors. 2006;24:165–171. [DOI] [PubMed] [Google Scholar]
- 19. Fitzpatrick J, Bulsara M, Zheng MH. The effectiveness of platelet-rich plasma in the treatment of tendinopathy: a meta-analysis of randomized controlled clinical trials [published online June 6, 2016]. Am J Sports Med. doi:10.1177/0363546516643716. [DOI] [PubMed] [Google Scholar]
- 20. Fullerton BD, Reeves KD. Ultrasonography in regenerative injection (prolotherapy) using dextrose, platelet-rich plasma, and other injectants. Phys Med Rehabil Clin N Am. 2010;21:585–605. [DOI] [PubMed] [Google Scholar]
- 21. Gosens T, Den Oudsten BL, Fievez E, van ‘t Spijker P, Fievez A. Pain and activity levels before and after platelet-rich plasma injection treatment of patellar tendinopathy: a prospective cohort study and the influence of previous treatments. Int Orthop. 2012;36:1941–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gosens T, Peerbooms JC, van Laar W, den Oudsten BL. Ongoing positive effect of platelet-rich plasma versus corticosteroid injection in lateral epicondylitis: a double-blind randomized controlled trial with 2-year follow-up. Am J Sports Med. 2011;39:1200–1208. [DOI] [PubMed] [Google Scholar]
- 23. Harvest. SmartPReP 2 centrifuge system for platelet and bone marrow cell concentration: operator’s and service manual. https://www.harvesttech.com/clinician/clinician-home/prp/products. Accessed October 13, 2015.
- 24. Kevy SV, Jacobson MS. Comparison of methods for point of care preparation of autologous platelet gel. J Extra Corpor Technol. 2004;36:28–35. [PubMed] [Google Scholar]
- 25. Krogh TP, Fredberg U, Stengaard-Pedersen K, Christensen R, Jensen P, Ellingsen T. Treatment of lateral epicondylitis with platelet-rich plasma, glucocorticoid, or saline: a randomized, double-blind, placebo-controlled trial. Am J Sports Med. 2013;41:625–635. [DOI] [PubMed] [Google Scholar]
- 26. McCarrel T, Fortier L. Temporal growth factor release from platelet-rich plasma, trehalose lyophilized platelets, and bone marrow aspirate and their effect on tendon and ligament gene expression. J Orthop Res. 2009;27:1033–1042. [DOI] [PubMed] [Google Scholar]
- 27. McCarrel TM, Minas T, Fortier LA. Optimization of leukocyte concentration in platelet-rich plasma for the treatment of tendinopathy. J Bone Joint Surg Am. 2012;94:e143. [DOI] [PubMed] [Google Scholar]
- 28. Meheux CJ, McCulloch PC, Lintner DM, Varner KE, Harris JD. Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: a systematic review. Arthroscopy. 2016;32:495–505. [DOI] [PubMed] [Google Scholar]
- 29. Mishra A, Harmon K, Woodall J, Vieira A. Sports medicine applications of platelet rich plasma. Curr Pharm Biotechnol. 2012;13:1185–1195. [DOI] [PubMed] [Google Scholar]
- 30. Mishra A, Pavelko T. Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am J Sports Med. 2006;34:1774–1778. [DOI] [PubMed] [Google Scholar]
- 31. Moojen DJ, Everts PA, Schure RM, et al. Antimicrobial activity of platelet-leukocyte gel against Staphylococcus aureus. J Orthop Res. 2008;26:404–410. [DOI] [PubMed] [Google Scholar]
- 32. Oh JH, Kim W, Park KU, Roh YH. Comparison of the cellular composition and cytokine-release kinetics of various platelet-rich plasma preparations. Am J Sports Med. 2015;43:3062–3070. [DOI] [PubMed] [Google Scholar]
- 33. Peerbooms JC, Sluimer J, Bruijn DJ, Gosens T. Positive effect of an autologous platelet concentrate in lateral epicondylitis in a double-blind randomized controlled trial: platelet-rich plasma versus corticosteroid injection with a 1-year follow-up. Am J Sports Med. 2010;38:255–262. [DOI] [PubMed] [Google Scholar]
- 34. Riboh JC, Saltzman BM, Yanke AB, Fortier L, Cole BJ. Effect of leukocyte concentration on the efficacy of platelet-rich plasma in the treatment of knee osteoarthritis. Am J Sports Med. 2016;44:792–800. [DOI] [PubMed] [Google Scholar]
- 35. Saad Setta H, Elshahat A, Elsherbiny K, Massoud K, Safe I. Platelet-rich plasma versus platelet-poor plasma in the management of chronic diabetic foot ulcers: a comparative study. Int Wound J. 2011;8:307–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Seijas R, Ares O, Alvarez P, Cusco X, Garcia-Balletbo M, Cugat R. Platelet-rich plasma for calcific tendinitis of the shoulder: a case report. J Orthop Surg. 2012;20:126–130. [DOI] [PubMed] [Google Scholar]
- 37. Tamimi FM, Montalvo S, Tresguerres I, Blanco Jerez L. A comparative study of 2 methods for obtaining platelet-rich plasma. J Oral Maxillofac Surg. 2007;65:1084–1093. [DOI] [PubMed] [Google Scholar]
- 38. Tohidnezhad M, Varoga D, Wruck CJ, et al. Platelets display potent antimicrobial activity and release human beta-defensin 2. Platelets. 2012;23:217–223. [DOI] [PubMed] [Google Scholar]
- 39. Wahlstrom O, Linder C, Kalen A, Magnusson P. Variation of pH in lysed platelet concentrates influence proliferation and alkaline phosphatase activity in human osteoblast-like cells. Platelets. 2007;18:113–118. [DOI] [PubMed] [Google Scholar]
- 40. Weibrich G, Kleis WK, Curasan PRP. kit vs. PCCS PRP system collection efficiency and platelet counts of two different methods for the preparation of platelet-rich plasma. Clin Oral Implants Res. 2002;13:437–443. [DOI] [PubMed] [Google Scholar]
- 41. Zimmermann R, Jakubietz R, Jakubietz M, et al. Different preparation methods to obtain platelet components as a source of growth factors for local application. Transfusion. 2001;41:1217–1224. [DOI] [PubMed] [Google Scholar]


