Abstract
Background:
Platelet-rich plasma (PRP) has emerged as a popular biologic treatment for musculoskeletal injuries and conditions. Despite numerous investigations on the efficacy of PRP therapy, current utilization of this treatment within the United States is not widely known.
Purpose:
To investigate the national utilization of PRP, including the incidence and conditions for which it is used in the clinical setting, and to determine the current charges associated with this treatment.
Study Design:
Descriptive epidemiology study.
Methods:
Using a national database (PearlDiver) of private insurance billing records, we conducted a comprehensive search using Current Procedural Terminology (CPT) codes to identify patients who received PRP injections over a 2-year period (2010-2011). Associated International Classification of Diseases, 9th Revision (ICD-9) codes were identified to determine the specific conditions the injection was used to treat. The aggregate patient data were analyzed by yearly quarter, practice setting, geographic region, and demographics. PRP therapy charges were calculated and reported as per-patient average charges (PPACs).
Results:
A total of 2571 patients who received PRP injections were identified; 51% were male and 75% were older than 35 years. The overall incidence ranged from 5.9 to 7.9 per 1000 patients over the study period. PRP was most commonly administered in hospitals (39%) and ambulatory surgical centers (37%) compared with in private offices (26%). The most common conditions treated were knee meniscus/plica disorders, followed by unspecified shoulder conditions, rotator cuff injuries, epicondylitis, and plantar fasciitis. Further evaluation revealed that 25% of all patients received injections for cartilage-related conditions, 25% meniscus, 25% unspecified, 12% tendon, 8% glenoid labrum, and 5% ligament. The PPAC for PRP treatment was US$1755 per injection.
Conclusion:
Despite a lack of consensus regarding PRP indications and efficacy, we observed widespread application of this treatment for a myriad of musculoskeletal injuries. Most treated patients were older than 35 years, and the most commonly treated conditions included cartilage and meniscus disorders. Given the current controversy surrounding this treatment, further studies are necessary to guide clinicians on the value of this therapy for each clinical diagnosis.
Keywords: platelet-rich plasma, PRP, tendinitis, cartilage, musculoskeletal injury
Platelet-rich plasma (PRP) represents a popular biologic treatment for various musculoskeletal injuries involving tendon, ligament, cartilage, and bone. PRP is derived from autologous whole blood and contains numerous growth factors and cytokines that have been shown to initiate and promote healing by stimulating cell migration, cell proliferation, angiogenesis, and matrix synthesis.45 The increased concentration of platelets and accompanying growth factors can facilitate the natural healing process, which has prompted a myriad of studies to investigate the therapeutic applications of PRP in various musculoskeletal conditions.22,38 Numerous studies have evaluated the use of PRP in the setting of joint osteotomy, anterior cruciate ligament (ACL) reconstruction, arthroplasty, plantar fasciitis, Achilles tendinopathy, degenerative spine disease, rotator cuff repair, elbow tendinitis, and knee osteoarthritis with varying results.¶ While the in vitro effects of PRP therapy on various tissues types has been promising, only a few level 1 evidence studies have demonstrated favorable clinical translation for some of the aforementioned conditions.6,14,24,29,35,41,43 Ultimately, contrasting clinical results may be due to the heterogeneity of PRP formulations being utilized in these studies, further highlighting the need for a more sophisticated understanding of preparation procedures and PRP composition.
Despite a paucity of large-scale clinical evidence to support the use of PRP therapy, there has been widespread application among the orthopaedic community due to enthusiasm about its potential. However, given the current concerns about escalating health care costs, it is important to be mindful of available resources and the efficacy of clinical treatments. Samuelson et al34 recently performed a cost-utility analysis of PRP used in the setting of rotator cuff repair and found PRP augmentation was not a cost-effective treatment to improve retear rates. Given these considerations, the purpose of this study was to determine the current utilization of PRP therapy in the United States. We sought to investigate specific trends related to PRP use, including patient demographics, practice setting, musculoskeletal conditions, and tissue types treated, as well as charges associated with the procedure.
Methods
We performed a retrospective review of a national private-payer medical record database (PearlDiver; PearlDiver Technologies Inc) over a 2-year period (2010-2011). This database is a public Health Insurance Portability and Accountability Act (HIPAA)–compliant collection of more than 6 million patient records from a single insurance payer (UnitedHealthcare). Patient records can be retrieved and analyzed by Current Procedural Terminology (CPT) and International Classification of Diseases, 9th Revision (ICD-9) codes related to specific musculoskeletal conditions.
The CPT code associated with PRP injections (CPT-0232T) was used to identify patients who received PRP therapy over the study period. Patient counts and charges for the PRP injections were compared by sex, age, geographic region, clinical setting in which the procedure was performed, and yearly quarters. Because the CPT code for PRP injections was not introduced until mid-2010, we investigated the number of PRP injections performed from 2010 to 2011 on a quarterly basis (designated Q1, Q2, Q3, and Q4) to determine a trend in utilization.
After identification of patients who received PRP injection, we conducted a comprehensive search for associated ICD-9 and CPT codes that were coded on the same day as the PRP injection to determine the primary condition being treated. The ICD-9 codes were categorized as follows: rotator cuff, biceps, glenoid labrum, shoulder cartilage, epicondylitis, knee cartilage, knee ligament, knee meniscus/plica, knee tendonitis, knee synovium, hip, plantar fasciitis, foot/ankle tendon, foot/ankle ligament, foot deformities, unspecified shoulder, unspecified knee, unspecified foot/ankle, and generally unspecified. The patient counts and charges associated with these codes were recorded. The codes were also categorized and analyzed by the primary type of tissue affected: cartilage, meniscus, tendon, ligament, glenoid labrum, and unspecified. Charges were presented as per-patient average charges (PPACs), which were calculated by dividing the total charges of a procedure by the number of patients receiving that procedure.
The overall utilization of PRP injections was compared between years using overall incidence rates (eg, number of patients receiving PRP injection compared with the total number of orthopaedic patients in the database). Descriptive statistics were used to report data as appropriate. Comparative analyses over time were performed using repeated-measures analysis of variance (ANOVA) with Tukey-adjusted pairwise comparisons. Analyses were 2-tailed, and statistical significance was defined as P < .05.
Results
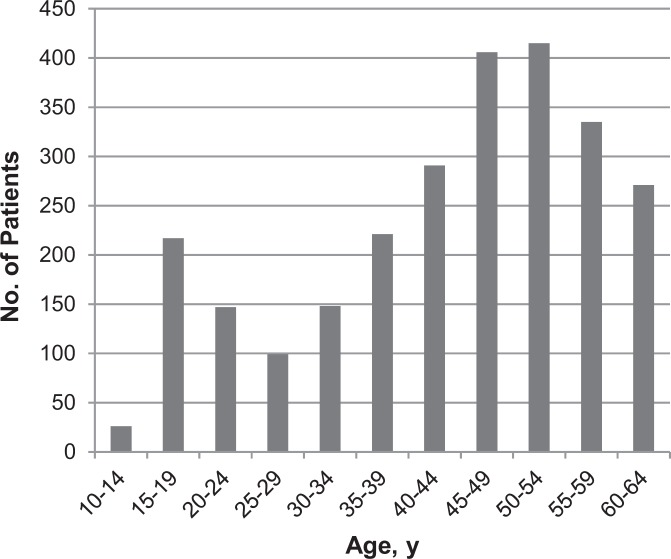
A total of 2571 patients received PRP injections from 2010 through 2011, with a total PPAC of US$1755. We noted an equal distribution among patients with regard to sex, as 49% were female and 51% were male. The age distribution of patients who underwent PRP therapy was quite wide, with patient age ranging from 10 to 64 years. Older patients received PRP therapy with greater frequency, as we noted 75% of patients who received injections over the study period were older than 35 years (Figure 1).
Figure 1.
Age distribution of patients receiving platelet-rich plasma injections.
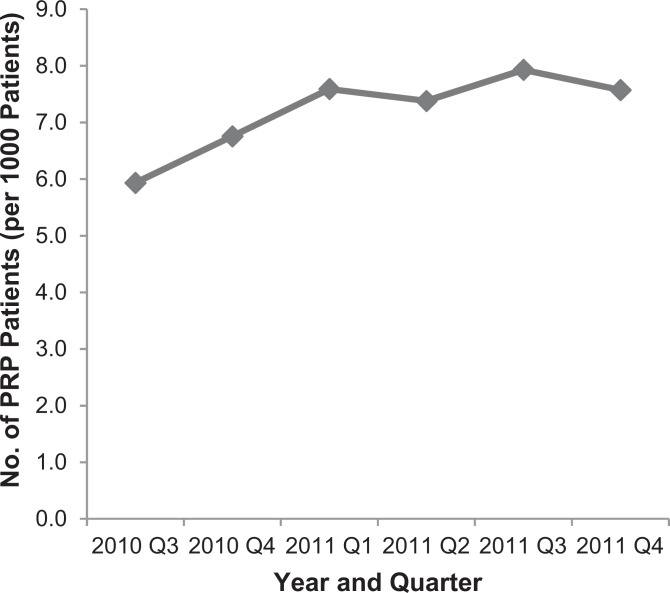
The number of patients who received PRP injections was analyzed across yearly quarters from mid-2010 through 2011. The incidence of PRP injections ranged from 5.9 to 7.9 per 1000 patients over the study period (Figure 2). While this appeared to trend up over the study period, there was no statistically significant change over time in the rate of utilization (P > .05, repeated-measures ANOVA).
Figure 2.
Number of patients treated with platelet-rich plasma (PRP) by year and quarter. The number of patients receiving PRP injections is provided for each quarter of the year from mid-2010 through 2011. The increasing trend in total patients treated was not statistically significant.
In terms of clinical setting, 39% of patients received PRP injections in hospitals, while 37% received injections in ambulatory surgical centers. PRP injections were performed in the office for 26% of patients, and the remaining 1% of patients lacked data on clinical setting. Geographic distribution showed that only 14% of patients were located in the Northeast, while the majority of patients (47%) were in the South. Patients in the Western region represented 21% of the total number of patients receiving PRP injections, while patients in the Midwest represented 18%. Table 1 provides an overview of patient distribution according to the aforementioned factors.
TABLE 1.
Characteristics of Patient Population
| No. of Patients (% of Total) | |
|---|---|
| Sex | |
| Male | 1317 (51) |
| Female | 1254 (49) |
| Clinical setting | |
| Hospital | 981 (39) |
| Ambulatory surgical center | 952 (37) |
| Office | 680 (26) |
| Unspecified | 23 (1) |
| Geographic region | |
| South | 1198 (47) |
| West | 552 (21) |
| Midwest | 452 (18) |
| Northeast | 372 (14) |
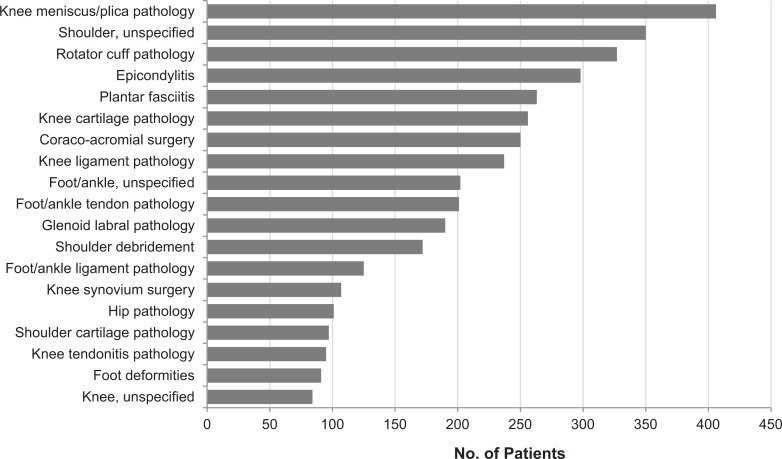
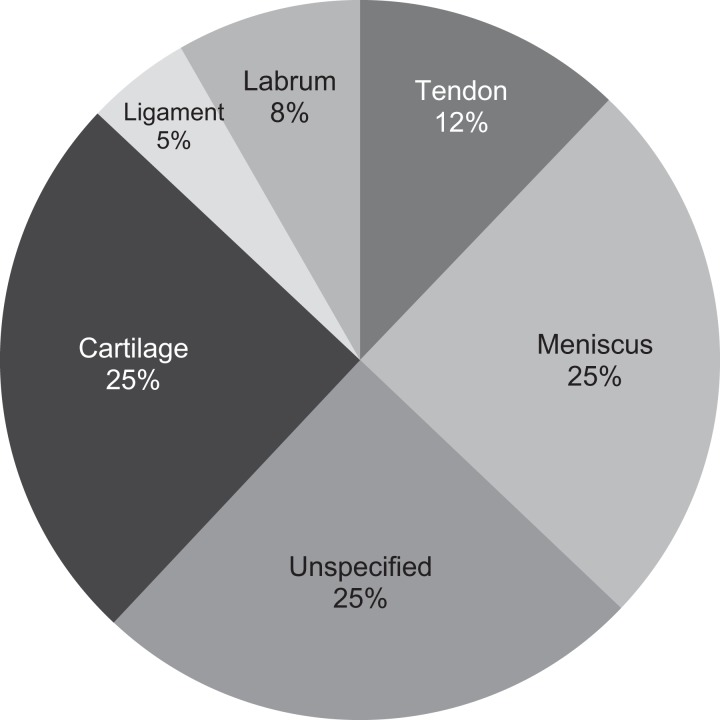
PRP therapy was used for a wide variety of orthopaedic diagnoses. Injections were most commonly performed for patients with conditions involving the knee meniscus or plica, as these patients represented 16% of all PRP injections performed over the 2-year study period. The additional conditions for which PRP injections were most commonly performed were: “unspecified” shoulder pathology (representing 14% of all same-day ICD-9 codes in patients receiving PRP injections), rotator cuff pathology (13%), and epicondylitis (12%). Figure 3 displays the full range of conditions that were evaluated and the number of patients who received PRP injections for each condition. With respect to affected tissue type (eg, cartilage, meniscus, ligament, tendon, glenoid labrum, and unspecified), 25% received injections for cartilage-related conditions, 25% for meniscus-related conditions, and 25% did not specify the type of tissue affected. The remaining 25% consisted of tendon, labrum, or ligament disorders. Figure 4 demonstrates a detailed analysis of PRP utilization according to tissue type.
Figure 3.
Distribution of patients receiving platelet-rich plasma injection by diagnosis.
Figure 4.
Breakdown of tissue types targeted for platelet-rich plasma therapy.
Discussion
There are numerous basic science and animal studies that support the notion that PRP has a favorable effect on the healing process of various musculoskeletal tissue types.8,15,21,23,36,47 Given these positive results, it is easy to understand the widespread enthusiasm for PRP among the orthopaedic community and the attempts to achieve successful clinical translation. Yet despite its exciting potential, there is currently no consensus regarding therapeutic guidelines for the utilization of PRP. This study sought to examine specific trends in utilization as well as the direct charges associated with this treatment.
To summarize the findings of this study, the PPAC for PRP injections was US$1755. We were surprised to find that the majority of PRP injections were most commonly performed in either hospitals or ambulatory surgical centers relative to the office setting. The majority of patients (75%) who received PRP injections were older than 35 years; however, we observed a wide range of ages (10-64 years) that even included pediatric patients. The 4 conditions for which PRP injections were most frequently performed were: knee meniscus/plica conditions, unspecified shoulder disorders, rotator cuff pathology, and epicondylitis. Notably, we were surprised to find that despite level 1 and 2 evidence supporting the use of PRP injections for epicondylitis, this condition was only the fourth most common condition for which PRP injections were used.14,26,27,31,41 Finally, patients more commonly received PRP injections for conditions involving the meniscus or cartilage compared with disorders of the tendon, labrum, or ligament. These observations parallel a growing body of research that has investigated the utility of PRP injections for knee osteoarthritis. In several level 1 evidence studies, PRP injections for knee osteoarthritis led to fewer symptoms and improved validated outcome scores when compared with hyaluronic acid or placebo.6,29,35,43 Over the past few years, multiple other studies have been published demonstrating clinical improvement for up to 1 year in patients who receive PRP injection for knee osteoarthritis.10–13,17,18,32,33,39 However, the long-term sustained benefits remain unclear, and significant variations in PRP formulations and frequency of administration continue to obscure conclusions in even high-quality studies.19,20,24
While there have been several studies evaluating the efficacy of PRP injections for a variety of orthopaedic conditions, to our knowledge there have not been any studies investigating the nationwide distribution of PRP utilization and the direct costs associated with these injections for musculoskeletal conditions. In 2015, Vavken et al44 assessed the cost-effectiveness of PRP injections for rotator cuff tears and concluded that PRP injections were not cost-effective for small- and medium-sized tears; however, the study relied on a sensitivity analysis that ranged from US$450 to $2500 per PRP injection due to lack of available cost data. More recently in 2016, Samuelson et al34 conducted a cost-effectiveness analysis with Markov modeling on PRP injections for rotator cuff tears. This study also concluded that augmenting rotator cuff repairs with PRP was not cost-effective. The proposed model assumed that a single PRP injection cost US$750, based on reported ranges of $500 to $1500 per PRP injection in 2014. In contrast with Vavken et al,44 this study included perioperative costs and facility fees based on local data collected from private orthopaedic practices and ambulatory surgical centers in 2014 within their analysis. While not extensively discussed in published literature, the total cost of a procedure has been shown to increase dramatically depending on the clinical setting due to the addition of significant facility fees.4,42 As a result, injections performed in a hospital outpatient department or operating room may result in higher costs due to these additional fees when compared with the private office setting. This observation may account for the significant charges associated with PRP in our study. Overall, the current study provides valuable patient-level cost data based on a national database, which will allow for greater precision in future cost-effectiveness and cost-utility research.
Application of the PearlDiver database in this study, as with any epidemiologic data, comes with some inherent limitations. First, the quality of available data for our analysis depends on the quality of the input information. The majority of patients in the database are located in the South, which may bias the results. In addition, patients older than 64 years are not included in the database due to Medicare coverage, so the results of the current study are only applicable to the non-Medicare population. Further study in similar Medicare databases might clarify any differences between these 2 populations; however, the trend noted in Figure 1 would indicate that patients older than 65 years would not be the largest consumers of PRP therapy. Additionally, the database only includes patients from a single insurance payer and does not capture a large proportion of facilities that provide this treatment using a cash-pay model. However, to determine trends in PRP utilization, the current study investigated change in use over time and proportional utilization trends within the database. We therefore were able to show these trends with more accuracy by normalizing all values to the database population. Furthermore, because the PearlDiver database sample does not account for PRP utilization in cash-paying patients, who may represent a large proportion of those using PRP, the results in the current study may underestimate the true national utilization of PRP. Therefore, the widespread use of PRP, despite controversial findings in basic science and clinical studies, is possibly even greater than we have reported in this study. Next, miscoding or absence of coding for PRP injections with CPT code 0232T would not have been captured by our search. Nonetheless, there is no reason to suspect nondifferential misclassification of these data, which are checked for accuracy before inclusion in the database. The presence of only 1 CPT code for PRP injections is in fact advantageous, as it is more likely to accurately capture patients for inclusion than if multiple codes are utilized. Despite this fact, the high proportion of “unspecified” codes limits our ability to fully understand the currently utilized indications for PRP; however, this is a limitation of all large database research. Additionally, to determine the orthopaedic condition being treated for each patient, we recorded diagnosis codes that were entered the same day as the procedural code. It is possible that some patients may have more than 1 orthopaedic condition coded with the procedure, which may lead to an overestimation of some of the conditions that were treated.
Further limitations include the fact that data and charges are provided in aggregate rather than on a per-patient basis; thus, granular statistical analysis is limited with such summary data. The database also lacks clinical outcomes data, and as a result, we cannot draw any conclusions regarding the efficacy of PRP therapy or cost-effectiveness. Future prospective clinical outcomes research is required to determine cost-effectiveness; however, the results of the current study may be used to accurately populate cost data for future calculations.
In addition to the aforementioned limitations, it is important to note that the database reports charges billed to insurance providers rather than the actual cost of care delivery. Nevertheless, because cost is typically a proportion of the submitted charge, the data reported here should closely represent the proportional cost distribution of PRP use. A few studies have used cost-to-charge ratio, or CCR, to estimate actual reimbursement amounts from hospital billing charges.1–3,37,46 However, the CCRs vary by hospital and are frequently based on a specific selection of charges and costs in the hospitals, which may not yield accurate cost estimates when applied to specific codes such as that for PRP injections or based on Medicare reimbursements as opposed to payment data from private insurers.1–3 This limitation does not affect the utilization results of the current study, which are vital in understanding the practice patterns associated with PRP use in patients younger than 65 years.
Conclusion
The PPAC for PRP injections was US$1755, with PRP injections most frequently performed in hospitals and ambulatory surgical centers. The patient population receiving PRP injections is most frequently in the fifth or sixth decade of life, although late adolescents and young adults are also being treated with PRP for various musculoskeletal disorders. Although large comparative studies have identified lateral epicondylitis as the only condition with convincing clinical improvement after PRP injection when compared with controls or comparison treatments, we found most patients receive PRP therapy for conditions involving the knee meniscus and shoulder (including rotator cuff injuries) despite a lack of high-quality evidence to support its use.
Acknowledgment
The authors thank Jeremiah R. Cohen from the UCLA David Geffen School of Medicine for assisting with the data collection for this study.
Footnotes
References
- 1. Afana M, Brinjikji W, Cloft H, Salka S. Hospitalization costs for acute myocardial infarction patients treated with percutaneous coronary intervention in the United States are substantially higher than Medicare payments. Clin Cardiol. 2015;38:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alvin MD, Miller JA, Lubelski D, et al. Variations in cost calculations in spine surgery cost-effectiveness research. Neurosurg Focus. 2014;36(6):E1. [DOI] [PubMed] [Google Scholar]
- 3. Bai G, Anderson GF. Extreme markup: the fifty US hospitals with the highest charge-to-cost ratios. Health Aff (Millwood). 2015;34:922–928. [DOI] [PubMed] [Google Scholar]
- 4. Bronstein JM, Johnson VA, Fargason CA. Impact of care setting on cost and quality under Medicaid. J Health Care Poor Underserved. 1997;8:202–213. [DOI] [PubMed] [Google Scholar]
- 5. Castricini R, Longo UG, De Benedetto M, et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med. 2011;39:258–265. [DOI] [PubMed] [Google Scholar]
- 6. Cerza F, Carni S, Carcangiu A, et al. Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am J Sports Med. 2012;40:2822–2827. [DOI] [PubMed] [Google Scholar]
- 7. Dallari D, Savarino L, Stagni C, et al. Enhanced tibial osteotomy healing with use of bone grafts supplemented with platelet gel or platelet gel and bone marrow stromal cells. J Bone Joint Surg Am. 2007;89:2413–2420. [DOI] [PubMed] [Google Scholar]
- 8. de Mos M, van der Windt AE, Jahr H, et al. Can platelet-rich plasma enhance tendon repair? A cell culture study. Am J Sports Med. 2008;36:1171–1178. [DOI] [PubMed] [Google Scholar]
- 9. de Vos RJ, Weir A, van Schie HT, et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA. 2010;303:144–149. [DOI] [PubMed] [Google Scholar]
- 10. Filardo G, Kon E, Buda R, et al. Platelet-rich plasma intra-articular knee injections for the treatment of degenerative cartilage lesions and osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2011;19:528–535. [DOI] [PubMed] [Google Scholar]
- 11. Filardo G, Kon E, Di Martino A, et al. Platelet-rich plasma vs hyaluronic acid to treat knee degenerative pathology: study design and preliminary results of a randomized controlled trial. BMC Musculoskelet Disord. 2012;13:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gobbi A, Karnatzikos G, Mahajan V, Malchira S. Platelet-rich plasma treatment in symptomatic patients with knee osteoarthritis: preliminary results in a group of active patients. Sports Health. 2012;4:162–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gobbi A, Lad D, Karnatzikos G. The effects of repeated intra-articular PRP injections on clinical outcomes of early osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc. 2015;23:2170–2177. [DOI] [PubMed] [Google Scholar]
- 14. Gosens T, Peerbooms JC, van Laar W, den Oudsten BL. Ongoing positive effect of platelet-rich plasma versus corticosteroid injection in lateral epicondylitis: a double-blind randomized controlled trial with 2-year follow-up. Am J Sports Med. 2011;39:1200–1208. [DOI] [PubMed] [Google Scholar]
- 15. Kajikawa Y, Morihara T, Sakamoto H, et al. Platelet-rich plasma enhances the initial mobilization of circulation-derived cells for tendon healing. J Cell Physiol. 2008;215:837–845. [DOI] [PubMed] [Google Scholar]
- 16. Kalaci A, Cakici H, Hapa O, Yanat AN, Dogramaci Y, Sevinc TT. Treatment of plantar fasciitis using four different local injection modalities: a randomized prospective clinical trial. J Am Podiatr Med Assoc. 2009;99:108–113. [DOI] [PubMed] [Google Scholar]
- 17. Kon E, Buda R, Filardo G, et al. Platelet-rich plasma: intra-articular knee injections produced favorable results on degenerative cartilage lesions. Knee Surg Sports Traumatol Arthrosc. 2010;18:472–479. [DOI] [PubMed] [Google Scholar]
- 18. Kon E, Mandelbaum B, Buda R, et al. Platelet-rich plasma intra-articular injection versus hyaluronic acid viscosupplementation as treatments for cartilage pathology: from early degeneration to osteoarthritis. Arthroscopy. 2011;27:1490–1501. [DOI] [PubMed] [Google Scholar]
- 19. Lai LP, Stitik TP, Foye PM, Georgy JS, Patibanda V, Chen B. Use of platelet rich plasma in intra-articular knee injections for osteoarthritis: a systematic review. PM R. 2015;7:637–648. [DOI] [PubMed] [Google Scholar]
- 20. Laudy AB, Bakker EW, Rekers M, Moen MH. Efficacy of platelet-rich plasma injections in osteoarthritis of the knee: a systematic review and meta-analysis. Br J Sports Med. 2015;49:657–672. [DOI] [PubMed] [Google Scholar]
- 21. Majewski M, Ochsner PE, Liu F, Fluckiger R, Evans CH. Accelerated healing of the rat Achilles tendon in response to autologous conditioned serum. Am J Sports Med. 2009;37:2117–2125. [DOI] [PubMed] [Google Scholar]
- 22. Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10:225–228. [DOI] [PubMed] [Google Scholar]
- 23. McCarrel T, Fortier L. Temporal growth factor release from platelet-rich plasma, trehalose lyophilized platelets, and bone marrow aspirate and their effect on tendon and ligament gene expression. J Orthop Res. 2009;27:1033–1042. [DOI] [PubMed] [Google Scholar]
- 24. Meheux CJ, McCulloch PC, Lintner DM, Varner KE, Harris JD. Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: a systematic review. Arthroscopy. 2016;32:495–505. [DOI] [PubMed] [Google Scholar]
- 25. Mei-Dan O, Carmont MR, Laver L, Mann G, Maffulli N, Nyska M. Platelet-rich plasma or hyaluronate in the management of osteochondral lesions of the talus. Am J Sports Med. 2012;40:534–541. [DOI] [PubMed] [Google Scholar]
- 26. Mishra A, Pavelko T. Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am J Sports Med. 2006;34:1774–1778. [DOI] [PubMed] [Google Scholar]
- 27. Mishra AK, Skrepnik NV, Edwards SG, et al. Efficacy of platelet-rich plasma for chronic tennis elbow: a double-blind, prospective, multicenter, randomized controlled trial of 230 patients. Am J Sports Med. 2014;42:463–471. [DOI] [PubMed] [Google Scholar]
- 28. Nin JR, Gasque GM, Azcarate AV, Beola JD, Gonzalez MH. Has platelet-rich plasma any role in anterior cruciate ligament allograft healing? Arthroscopy. 2009;25:1206–1213. [DOI] [PubMed] [Google Scholar]
- 29. Patel S, Dhillon MS, Aggarwal S, Marwaha N, Jain A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41:356–364. [DOI] [PubMed] [Google Scholar]
- 30. Peerbooms JC, de Wolf GS, Colaris JW, Bruijn DJ, Verhaar JA. No positive effect of autologous platelet gel after total knee arthroplasty. Acta Orthop. 2009;80:557–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Peerbooms JC, Sluimer J, Bruijn DJ, Gosens T. Positive effect of an autologous platelet concentrate in lateral epicondylitis in a double-blind randomized controlled trial: platelet-rich plasma versus corticosteroid injection with a 1-year follow-up. Am J Sports Med. 2010;38:255–262. [DOI] [PubMed] [Google Scholar]
- 32. Raeissadat SA, Rayegani SM, Hassanabadi H, et al. Knee osteoarthritis injection choices: platelet- rich plasma (PRP) versus hyaluronic acid (a one-year randomized clinical trial). Clin Med Insights Arthritis Musculoskelet Disord. 2015;8:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sampson S, Reed M, Silvers H, Meng M, Mandelbaum B. Injection of platelet-rich plasma in patients with primary and secondary knee osteoarthritis: a pilot study. Am J Phys Med Rehabil. 2010;89:961–969. [DOI] [PubMed] [Google Scholar]
- 34. Samuelson EM, Odum SM, Fleischli JE. The cost-effectiveness of using platelet-rich plasma during rotator cuff repair: a Markov model analysis. Arthroscopy. 2016;32:1237–1244. [DOI] [PubMed] [Google Scholar]
- 35. Sanchez M, Fiz N, Azofra J, et al. A randomized clinical trial evaluating plasma rich in growth factors (PRGF-Endoret) versus hyaluronic acid in the short-term treatment of symptomatic knee osteoarthritis. Arthroscopy. 2012;28:1070–1078. [DOI] [PubMed] [Google Scholar]
- 36. Schnabel LV, Mohammed HO, Miller BJ, et al. Platelet rich plasma (PRP) enhances anabolic gene expression patterns in flexor digitorum superficialis tendons. J Orthop Res. 2007;25:230–240. [DOI] [PubMed] [Google Scholar]
- 37. Skolasky RL, Riley LH., 3rd Medicare charges and payments for cervical spine surgery: association with hospital characteristics. Spine (Phila Pa 1976). 2015;40:E936–E942. [DOI] [PubMed] [Google Scholar]
- 38. Smyth NA, Murawski CD, Fortier LA, Cole BJ, Kennedy JG. Platelet-rich plasma in the pathologic processes of cartilage: review of basic science evidence. Arthroscopy. 2013;29:1399–1409. [DOI] [PubMed] [Google Scholar]
- 39. Spakova T, Rosocha J, Lacko M, Harvanova D, Gharaibeh A. Treatment of knee joint osteoarthritis with autologous platelet-rich plasma in comparison with hyaluronic acid. Am J Phys Med Rehabil. 2012;91:411–417. [DOI] [PubMed] [Google Scholar]
- 40. Sys J, Weyler J, Van Der Zijden T, Parizel P, Michielsen J. Platelet-rich plasma in mono-segmental posterior lumbar interbody fusion. Eur Spine J. 2011;20:1650–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Thanasas C, Papadimitriou G, Charalambidis C, Paraskevopoulos I, Papanikolaou A. Platelet-rich plasma versus autologous whole blood for the treatment of chronic lateral elbow epicondylitis: a randomized controlled clinical trial. Am J Sports Med. 2011;39:2130–2134. [DOI] [PubMed] [Google Scholar]
- 42. Vallier HA, Patterson BM, Meehan CJ, Lombardo T. Orthopaedic traumatology: the hospital side of the ledger, defining the financial relationship between physicians and hospitals. J Orthop Trauma. 2008;22:221–226. [DOI] [PubMed] [Google Scholar]
- 43. Vaquerizo V, Plasencia MA, Arribas I, et al. Comparison of intra-articular injections of plasma rich in growth factors (PRGF-Endoret) versus Durolane hyaluronic acid in the treatment of patients with symptomatic osteoarthritis: a randomized controlled trial. Arthroscopy. 2013;29:1635–1643. [DOI] [PubMed] [Google Scholar]
- 44. Vavken P, Sadoghi P, Palmer M, et al. Platelet-rich plasma reduces retear rates after arthroscopic repair of small- and medium-sized rotator cuff tears but is not cost-effective. Am J Sports Med. 2015;43:3071–3076. [DOI] [PubMed] [Google Scholar]
- 45. Wasterlain AS, Braun HJ, Harris AH, Kim HJ, Dragoo JL. The systemic effects of platelet-rich plasma injection. Am J Sports Med. 2013;41:186–193. [DOI] [PubMed] [Google Scholar]
- 46. Yeh JL, Wu S, Wu BU. Regional cost variation for acute pancreatitis in the U.S. JOP. 2014;15:448–454. [DOI] [PubMed] [Google Scholar]
- 47. Zhang J, Wang JH. Platelet-rich plasma releasate promotes differentiation of tendon stem cells into active tenocytes. Am J Sports Med. 2010;38:2477–2486. [DOI] [PubMed] [Google Scholar]