Abstract
Objective:
To evaluate an early home-supported discharge service for stroke patients.
Design:
We carried out a prospective, randomised, open-label, blinded-endpoint trial (allocation ratio of 1:1) with patients assigned to either an early home-supported discharge service or usual care.
Setting:
The study was undertaken in Aveiro, Portugal, between April 2009 and April 2013.
Subjects:
We included stroke patients aged 25–85 years admitted to the stroke unit with an initial Functional Independence Measure of up to 100, who gave informed consent.
Interventions:
Patients in the early home-supported discharge group began their rehabilitation intervention in the stroke unit and the early home-supported discharge team worked with them at home for a maximum of one month. Patients in the control group received usual services.
Main measures:
The primary outcome measure was the Functional Independence Measure at six months after stroke.
Results:
We randomised 190 patients of whom 34 were lost to follow-up. There were no significant differences (p > 0.5) in the average scores of Functional Independence Measure between the early home-supported discharge (69 ±22; mean ±SD) and the control groups (71 ±17) measured at baseline; and between the early home-supported discharge (107 ±20) and the control groups (107 ±25) measured at six months. The number of individuals with a low Functional Independence Measure score (<60) in the early home-supported discharge group compared with the control group was higher at admission (34/95 vs. 26/95) and lower at follow-up (2/74 vs. 5/78).
Conclusions:
It was feasible to implement early home-supported discharge procedures in a Southern European setting, but we have not shown convincing differences in disability at six months.
Keywords: Stroke, early home-supported discharge, randomised controlled trial, Functional Independence Measure, clinical effectiveness, home rehabilitation, Portugal, care continuity
Introduction
Early home-supported discharge (EHSD) schemes (also called early supported discharge services) for people who have had a stroke have been shown to be effective in well-resourced European countries, but their effectiveness in countries such as Portugal has yet to be demonstrated. These services, provided by well-organised multidisciplinary teams, seem to be capable of improving long-term independence1–7 and may also deliver better health outcomes at equivalent or lower costs,8–10 particularly when combined with stroke unit care.11
In Portugal, stroke patients discharged from acute care hospitals are faced with a number of rehabilitation possibilities.12 Since the establishment of the National Network of Long-Term Integrated Care in 2006, they could be discharged to a convalescence unit where they would undergo up to one month of intensive, inpatient rehabilitation, after which a new assessment of their condition and further needs would be made. However, by the time this study was carried out, many patients were being discharged directly home, with variable access to further ambulatory rehabilitation performed either at the hospital or other public institution or at private clinics. Several commentators have recognised that there was a need to improve the interface between hospital and home and to develop this particular field.13–15
While EHSD services for stroke patients have been researched in Scandinavia and the United Kingdom, no trials have taken place in the health systems environment of Southern Europe.7 The present HOMECARE study was developed as part of a European project on integrated care. This included adapting EHSD services implemented in Denmark9,16 to the conditions of Portugal, with an implementation project in the District of Aveiro.
The purpose of this trial was to establish if we could implement an EHSD service in Portugal and evaluate its impact on the functional independence of the users.
Methods
This parallel-group, observer-blinded randomised controlled trial, which was part of an EU FP7 funded project (HOMECARE – Clinical Continuity by Integrated Care, grant agreement 2222954), was carried out in Aveiro, Portugal, between April 2009 and April 2013. The service was modelled on the Danish EHSD9,16 component of the project. However, major adaptations were required for the Portuguese service because of the specific organisational and legal situation in Portugal (see below). Our hypothesis was that patients receiving the EHSD service would have better functional outcomes six months after stroke and require less inpatient rehabilitation.
Participants
We recruited patients with a clinical definition of stroke (confirmed on brain imaging) who were admitted to the stroke unit of the Hospital Infante D. Pedro (HIP) (District of Aveiro, Centre Region, Portugal), between October 2009 and January 2012. We included patients who fulfilled the inclusion criteria and provided signed informed consent. For patients unable to write as a consequence of stroke, we collected other evidence of consent, always with a family member present as testimony. Additionally, we sought the consent of a family member living in the same dwelling or a future informal caregiver who would be present during rehabilitation at home.
Inclusion and exclusion criteria
We included stroke patients aged between 25 and 85 years admitted to the stroke unit who had some residual disability in the form of an initial Functional Independence Measure (FIM)17 of up to 100, no significant previous neurological disability and who resided within the district of Aveiro.
Exclusion criteria included major speech and language problems preventing participation in the study, major psychological illness or dementia (such as psychotic disorders and Alzheimer’s disease), other severe comorbidity, pregnancy or transfer to another acute care hospital for more than five days.
Trial design
We carried out a prospective, randomised, open-label, blinded-endpoint trial in which patients were randomised equally to either EHSD service or usual care (control group) within 72 hours of admission. Allocation of patients to each group was done by taking one folded sheet of paper from a prefilled opaque envelope containing folded sheets of paper with either the letter H or the letter C written inside. This was done by a staff member not involved in the trial.
The study was approved by the Ethics Committee of the Hospital Infante D. Pedro.
Intervention
The main goal of this study was to adapt an EHSD service model developed and implemented in Denmark to the conditions of Portugal, and then to evaluate the impact of this service. The Portuguese intervention was based on early-supported discharge services,7 but had to be adapted to cope with the structural and legal aspects, and the complex and fragmented system of postdischarge rehabilitation in Portugal.
By the time we started the HOMECARE study, usual care rehabilitation was being provided in convalescence units (rehabilitation units with protocols with the National Network of Long-Term Integrated Care) or outpatient services, which could be operated by public or private organisations.
In the context of this study the ‘early home-supported discharge (EHSD)’ service fulfilled most of the features of the consensus description of early-supported discharge services.18 In Portugal, a community-based team of therapists would meet the patient, their informal caregivers and family and the healthcare professionals involved in their rehabilitation at the stroke unit and/or the rehabilitation setting, and collaborate in the development of a home rehabilitation plan for the patient and support the transition to the patient’s home. By the time we designed the HOMECARE study, this arrangement was part of the planned allocation of responsibilities and activities for the recently launched National Network of Long-Term Integrated Care, therefore we adapted to what was expected to be the future practice in the country.
The intervention started in the stroke unit, where the team coordinator at the hospital identified potential patients for the study according to the inclusion and exclusion criteria, verified treatment diagnosis and other significant diseases. They also completed the Functional Independence Measure.16 Final consensus scoring took place by the third day. They would then inform the patient, and if possible their family, about the study, present the informed consent form and invite their participation. At the same time, they would contact the case manager informing them about a possible admission to the trial and between them they would schedule a visit to the patient, should they finally agree on participating to the study. After obtaining the informed consent, the patient would be randomised.
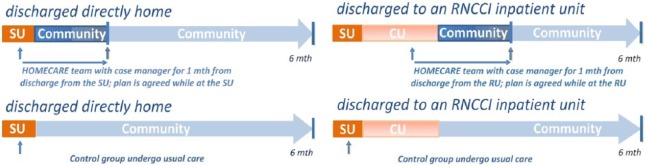
Early rehabilitation was provided to all patients as part of the standard care provided by the stroke unit. The EHSD intervention started during their stay at the stroke unit, where the patient and informal caregiver were first met by their assigned EHSD case manager. The EHSD team of therapists included two physiotherapists, two occupational therapists and a psychologist, whose input was selected according to the needs of a particular patient. All received training in the EHSD procedures and all of them filled in forms describing, in detail, the context and the content of each session with patient. The assigned case manager was one of two gerontologists who were included in the study, mainly to help negotiate the fragmented nature of the Portuguese health and social care systems. The case manager met the patient in the stroke unit and scheduled a meeting with the main carer. The case manager was also responsible for the administration of the EHSD team, providing back office help for the therapists and the patients. For patients discharged to their homes, the intervention continued directly after discharge in order to provide a seamless transfer from the hospital to home. This included an individual rehabilitation plan with provision of aids and modifications at home, where economically feasible. For patients discharged to an inpatient rehabilitation setting for further inpatient rehabilitation, contact with the EHSD team was reinitiated when discharge home was planned. Each EHSD team intervention was planned taking into consideration the particular patient’s needs and expectations. The EHSD team worked with patients to provide approximately eight home-based training sessions for a maximum of one month (Figure 1).
Figure 1.
Patients’ pathways to rehabilitation in the HOMECARE and the control groups.
RNCCI: Rede Nacional de Cuidados Continuados Integrados (National Network of Long-Term Integrated Care); SU: Stroke Unit; CU: Convalescence Unit.
In the EHSD group, patients and carers received education on healthy behaviours and information about stroke, its consequences, how to best participate in rehabilitation and how to find help within their communities. The team provided information and training tailored to the patient’s needs; the mix of physiotherapy, occupational therapy and psychology sessions was also adapted to the specific condition of each patient. Rehabilitation was focused on daily activities valued by the patient in their usual context. This was done in order to improve adherence, transfer of effort and adaptation to daily life. The content could include personally meaningful activities (e.g. ‘to paint my nails again’, ‘to ride my bike again’), personal care, outdoor walking, shopping or leisure activities. Caregivers were trained and made aware of the competencies and ability of the patient and were encouraged to follow their progress.
Patients in the usual care group were contacted in the stroke unit, introduced to the study and assigned a case manager to ensure they could be tracked after being discharged. They received information from the case manager about services available in the community, but no further specific input was provided. They began their rehabilitation as part of standard care in the stroke unit and then accessed the standard rehabilitation available in the region following discharge (Figure 1). The usual care rehabilitation frequently focused on components of training of impairments, such as ambulatory rehabilitation, with less emphasis on understanding how skills would be transferred into normal living. Access to healthcare professionals was less easy for the usual care group and it was to address questions arising during rehabilitation.
In the stroke unit, readiness for discharge was assessed by the team of specialists. Frequently a social worker was involved, especially in the case of those discharged directly home. When discharged directly home, most patients would be referred for intensive ambulatory rehabilitation, which would be performed in public units or private clinics. In those cases, predefined packages of sessions were being prescribed and paid by the NHS.
Those usual care patients who were transferred to a convalescence unit for intensive inpatient rehabilitation would be automatically prescribed a first package of 30 days in a unit of the National Network of Long-Term Integrated Care paid by the NHS. There was no incentive for these units to discharge patients earlier. In the convalescence units, readiness for discharge was also assessed by a team of specialists. Informal caregiver and/or family members of the patient would be involved and a meeting held with care professionals. The patient could be discharged either home with or without prescription of further ambulatory rehabilitation, prescribed more inpatient rehabilitation in the convalescence unit or discharged to long-term care unit, where care would be mostly of a social nature.
The aim of the study in Portugal was not so much to help discharge the patients in the study group earlier from the convalesce units, but try to avoid prolonged admission in convalescent units or packages of NHS-supported ambulatory rehabilitation while guaranteeing rehabilitation and re-adaptation to home conditions and social activities and life.
Outcome measures
The primary outcome of the study was independence in physical and cognitive activities as assessed by FIM,17 which was recorded at admission and discharge from the stroke unit and at two and six months after randomisation.
No standard recommendation is available about the level required for inpatient rehabilitation. We therefore proposed that a patient with three points in each variable (total score of less than 60) would require inpatient rehabilitation. Secondary outcome measures19 included the Frenchay Activity Index (FAI),20 the World Health Organization WHOQOL-BREF quality of life assessment (WHOQOL-BREF),21 Short Form-6D,22 Barthel Index23 and Mini-Mental State Examination.24 Outcome measures were collected at the patients homes by the case managers. We collected length of stay at the stroke unit and the convalescence units from the clinical records. We have attempted two approaches to collect rehabilitation effort per patient in terms of ambulatory sessions: (a) by giving a diary to each patient and their closest informal caregiver and asking them to register all events related to the stroke episode, including rehabilitation sessions, consultations, medication, travelling, as well as cost involved, and; (b) by contacting all entities that had provided ambulatory rehabilitation and transportation service to our patients. Both methods proved to be difficult to implement and prone to missing data and questionable data quality.
Statistical analysis
Descriptive statistics were used to compute means, standard deviations, lowest and highest values. Independent-samples t-tests were used to compare differences between groups and significance of the differences (sig.). All analyses were performed with IBM SPSS Statistics Version 21.25
A sample size of 190 was sufficient to have 90% power at the 5% level to detect a 10 point difference (with a standard deviation of 20) in the FIM with a 10% drop-out.
Results
A total of 190 patients admitted to the stroke unit of the Hospital Infante D. Pedro were included in the EHSD trial (Figure 2). From the 571 patients screened for inclusion after admission to the stroke unit, 381 were found to be ineligible for the trial (see Table 1 for detailed reasons). A description of the two groups of 95 participants is provided in Table 2. From these, a total of 34 (18 EHSD; 16 usual care) were lost to follow-up (see Figure 2 for detailed reasons).
Figure 2.
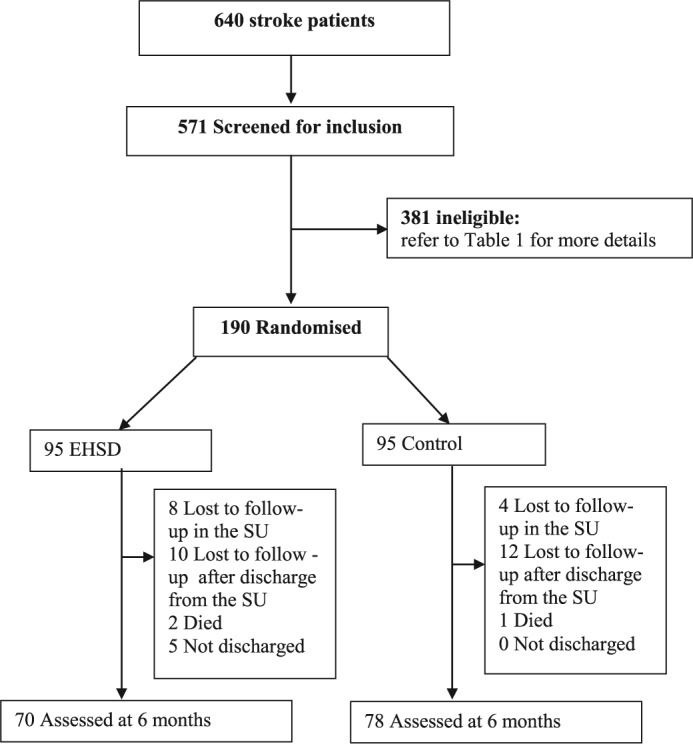
Trial profile.
SU: stroke unit; EHSD: early home-supported discharge.
Table 1.
Reasons for exclusion from the trial when screened at the stroke unit.
| Reason for exclusion | Number |
|---|---|
| FIM > 100 | 125 |
| Stroke during last year | 22 |
| Aphasia | 99 |
| Confusion | 28 |
| Age | 28 |
| Severe pathology that compromises rehabilitation after stroke | 40 |
| Transferred to other unit for more than five days | 9 |
| Coma | 15 |
| Moving abroad or outside the area after discharge | 3 |
| Refusal | 8 |
| Address outside the area | 2 |
| Security of the team members | 2 |
| 381 |
FIM: Functional Independence Measure.
Table 2.
Demographic features, length of stay, mean total FIM at baseline and discharge from the SU of patients lost to follow-up, and clinical outcomes.
| EHSD group | Control group | Sig. | |
|---|---|---|---|
| N = 190 | 95 | 95 | |
| Mean age (range) | 67.5 (40–84) | 66.5 (35–84) | – |
| Number of women (%) | 48 (51) | 41 (43) | – |
| Number of patients discharged to a rehabilitation unit | 17 | 20 | – |
| Deaths | 2 | 1 | – |
| Length of stay in the SU, mean (SD) | 9.8 (5.3) | 10.0 (5.3) | 0.795 |
| Length of stay in an RU, mean (SD) | 40.6 (11.1) | 39.0 (18.3) | 0.747 |
| Total FIM at baseline of patients lost to follow-up: mean (SD) (min–max) | 63.5 (22.1) (27–95) | 69.5 (9.9) (50–84) | 0.329 |
| Total FIM at discharge from the SU of patients lost to follow-up: mean (SD) (min–max) | 90.2 (30.1) (34–125) | 90.3 (16.5) (62–123) | 0.984 |
| FIM at baseline in the SU (N) | (N = 95) | (N = 95) | |
| Total FIM at baseline: mean (SD) (min–max) | 69.0 (21.3) (30–100) | 70.5 (18.7) (24–100) | 0.593 |
| FAI at baseline (N) | (N = 91) | (N = 93) | |
| Total FAI baseline: mean (SD) (min–max) | 43.4 (19.1) (16–138) | 42.9 (17.0) (15–141) | 0.856 |
| FIM at discharge (N) | (N = 92) | (N = 93) | |
| Total FIM discharge: mean (SD) (min–max) | 88.9 (27.6) (29–126) | 90.3 (23.3) (27–126) | 0.699 |
| FIM at the 2nd month (N) | (N = 80) | (N = 80) | |
| Total FIM 2nd month: mean (SD) (min–max) | 104.6 (21.6) (53–126) | 105.6 (24.0) (20–126) | 0.798 |
| FIM at the 6th month (N) | (N = 74) | (N = 78) | |
| Total FIM 6th month: mean (SD) (min–max) | 107.4 (19.9) (45–126) | 106.6 (25.5) (18–126) | 0.816 |
| FAI at the 6th month (N) | (N = 73) | (N = 74) | |
| Total FAI 6th month: mean (SD) (min–max) | 34.6 (17.6) (15–134) | 32.2 (11.4) (15–154) | 0.328 |
EHSD: early home-supported discharge; FAI: Frenchay Activities Index; FIM: Functional Independence Measure; RU: rehabilitation unit; SD: standard deviation; SU: stroke unit.
Table 2 reports the number of patients discharged to a convalescence unit for intensive rehabilitation after being treated in the stroke unit, the number of deaths, the length of stay in the stroke unit and the convalescence units, the mean total FIM at baseline and discharge from the SU of patients lost to follow-up, and the total FIM and FAI (score), by group. All values are very similar between the two groups. There are no significant differences in the average scores of FIM between the EHSD (M = 69.1, SD = 21.6) and the usual care groups (M = 70.5, SD = 18.7) measured at baseline (t(188) = –0.48, p = 0.633); and between the EHSD (M = 107.4, SD = 19.9) and the usual care groups (M = 106.6, SD = 25.5) measured at six months (t(150) = 0.23, p = 0.816). There were no statistically significant differences between the EHSD and the usual care groups in the average FAI scores at the baseline and six months.
The range of FIM values was greater in the control group by the second and the sixth months, with some of the individuals scoring very low in the FIM scale by the time of the follow-up assessments.
The analysis of the cases with low FIM (total FIM equal or lower than 60 points) shows that while the EHSD group had more of such patients at the admission (34/95 vs. 26/95) and discharge from the stroke unit, by the second month (2/80 vs. 7/80, respectively) and the sixth month (2/74 vs. 5/78) the number of patients with low FIM was higher in the control group.
We were not able to include useful data on ambulatory rehabilitation sessions collected from the several public providers and private clinics and patients’ annotations in the analysis owing to incomplete records and missing data. The information available suggested similar rehabilitation activity in the two groups.
Discussion and conclusions
The results from HOMECARE trial in Portugal show that it was feasible to implement the EHSD procedures in a Southern European setting, but we have not shown convincing changes in disability scores. There were no statistically significant differences between the EHSD and the usual care groups in the average FIM scores measured at the four assessment points, nor in the average FAI scores at the baseline and six months. This result is in line with some other studies.6 However, the number of individuals with a low FIM score (equal or lower than 60 points) at follow-up tended to be higher in the control group, which may support the efficacy of the EHSD intervention. We should acknowledge that the loss of patients to follow-up could have influenced this result. However, this result was also in line with the perceptions of the case managers that followed all the participants from their admission to the stroke unit.
The intervention of the EHSD team professionals could plausibly have produced improved results. First, after discharge, they sought to help patients to adapt to daily living routines at home, outside activities and local condition. This included finding help in the community, dealing with bouts of depression and lack of motivation to adhere to a rehabilitation routine, and providing information to informal carers and family members. Second, for those patients discharged home from the stroke unit for a few days while awaiting a place in an inpatient rehabilitation unit, the EHSD team could have played a role in their recovering and future functionality, providing therapy at home during those critical days and helping preserving their rehabilitation capacities.
The weaknesses of our study are that we lost patients to follow-up with some loss of power but also a disruption of the planned intention-to-treat analysis. We have tried to account for this as much as possible in the analysis. Also the quality of data collected regarding ambulatory rehabilitation was variable, what undermined its usefulness.
The strengths of our trial are that we recruited a substantial number of patients and used a secure randomisation system to allocate treatment. We have also applied the intervention in a flexible manner to reflect the realities of healthcare in Portugal.
The HOMECARE trial can be considered a complex intervention and in such cases the use of a single outcome measure might be problematic.26 The use of death as poor outcome as in other studies would also be questionable. In fact, all deaths in this trial were owing to unfortunate developments of comorbidities already present by the time of the randomisation. Using the FAI raised some challenges. The scale complements the FIM well in many aspects, but a number of problems arose with items that were sensitive to gender or culture, in particular those assessing ability to carry out home tasks. For example, it was common for males still active in the labour market by the time they suffered stroke to report they performed a number of home tasks after discharge they had not performed before stroke. We found that this was simply because they were at home alone while their partners/family were at work, not owing to a change in their condition. Therefore, we have analysed FIM distributions at the several assessment points in detail and computed the number of patients with FIM below a threshold of 60 points. However, the threshold was not defined a priori and should be considered exploratory.
More research work is needed in Southern European countries, whenever possible with a larger number of participants. Such studies will have to deal with the complex healthcare pathways in different countries and the lack of integrated information systems. This situation represents an enormous challenge to those researching conditions that cross the boundaries of several organisations, public and private, belonging to health and social care systems.
Despite the identified pitfalls and challenges faced during the four years of this study, including preparation of the trial, its implementation in the field and respective data handling and analysis, a number of positive outcomes were achieved. The HOMECARE trial represents the first controlled trial of stroke services in Portugal. The study was adapted to local health and social care services. For the first time, we report the experience of using case managers in Portugal, allowing a comprehensive understanding of all aspects related to an episode of stroke. This also helped to coordinate the work of community-based homecare therapists, to support patients in aspects not covered by traditional healthcare providers and to integrate information from providers in different sectors, such as health and social care, informal caregivers and patient transportation services.
Results from the HOMECARE study cannot be generalised easily across countries, but all above aspects represent valuable knowledge that might be used, not only in similar studies, but when designing policies, guidelines and working procedures, especially those involving multiple service providers and complex pathways, crossing over a number of sectors.
The research study also shows that it is possible and probably desirable to implement EHSD procedures in very complex realities, calling for more of such studies in settings outside the UK and Scandinavia.
Clinical messages.
Implementing an early home-supported discharge (EHSD) service was feasible in Portugal.
Mean disability scores did not differ significantly at six month follow-up.
Mean length of stay did not differ, but fewer EHSD patients received inpatient rehabilitation.
The use of case managers allowed a more complete understanding of the patient pathway.
Acknowledgments
We acknowledge the contributions of other members of the project group for their efforts in design, randomisation, training the patients at home and data collection.
HOMECARE case managers
Mariana Ribeiro, Gerontologist and Case Manager
Marta Viana, Gerontologist and Case Manager
HOMECARE team of professionals providing care at home
Joana Freitas, Occupational Therapist
Margarida Cerveira, Psychologist, Post-graduate in Neuropsychology
Francisco Martins, Physiotherapist
Sílvia Pinto, Occupational Therapist
Liliana Cardoso, Physiotherapist
The team of nurses in the stroke unit of the Hospital Infante D. Pedro, District of Aveiro, Portugal
National Network of Long-Term Integrated Care
Júlia Oliveira, Convalescence Unit of the RNCCI, Hospital Dr. Francisco Zagalo, Ovar, District of Aveiro, Portugal
LaSalette Matos, Regional Coordinator of the RNCCI by the time of the study, Family Health Unit LEME, Ílhavo, District of Aveiro, Portugal
We acknowledge the contribution of the medical trainee Guilherme de Oliveira, revising the article and all the figures in tables.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributors: SS has contributed to conception and design, acquisition of data, analysis and interpretation of data, drafting or revising the manuscript critically for important intellectual content, and final approval of the version to be published. PL and TL have contributed to conception and design, interpretation of data, revising the manuscript critically for important intellectual content, and final approval of the version to be published. JR and CN have contributed to acquisition of data, interpretation of data, drafting or revising the manuscript critically for important intellectual content, and final approval of the version to be published. BJ has contributed to conception and design, revising the manuscript, and final approval of the version to be published. PR and NS have contributed to acquisition of data, revising the manuscript and final approval of the version to be published. All authors have read and approved the final manuscript.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work has been funded by the European Commission [FP7-Homecare 222954]. NS was partially supported by FCT – the Portuguese Foundation for Science and Technology PhD [grant number SFRH/BD/69892/2010]. The funding sources had no role in design and conduct of the study, collection, management, analysis and interpretation of the data and preparation, review, or approval of the manuscript.
References
- 1. Indredavik B, Fjaertoft H, Ekeberg G, Loge AD, Morch B. Benefit of an extended stroke unit service with early supported discharge: A randomized, controlled trial. Stroke 2000; 31(12): 2989–2994. [DOI] [PubMed] [Google Scholar]
- 2. Fjaertoft H, Rohweder G, Indredavik B. Stroke unit care combined with early supported discharge improves 5-year outcome: A randomized controlled trial. Stroke 2011; 42(6): 1707–1711. [DOI] [PubMed] [Google Scholar]
- 3. Early Supported Discharge Trialists. Services for reducing duration of hospital care for acute stroke patients. Cochrane Database Syst Rev 2005: CD000443. [DOI] [PubMed] [Google Scholar]
- 4. Langhorne P, Holmqvist LW. Early supported discharge after stroke. J Rehabil Med 2007; 39(2): 103–108. [DOI] [PubMed] [Google Scholar]
- 5. Bautz-Holter E, Sveen U, Rygh J, Rodgers H, Wyller TB. Early supported discharge of patients with acute stroke: A randomized controlled trial. Disabil Rehabil 2002; 24(7): 348–355. [DOI] [PubMed] [Google Scholar]
- 6. Mayo NE, Scott S. Evaluating a complex intervention with a single outcome may not be a good idea: An example from a randomised trial of stroke case management. Age Ageing 2011; 40(6): 718–724. [DOI] [PubMed] [Google Scholar]
- 7. Fearon P, Langhorne P. Services for reducing duration of hospital care for acute stroke patients. Cochrane Database Syst Rev 2012: CD000443. [DOI] [PubMed] [Google Scholar]
- 8. Larsen T, Olsen TS, Sorensen J. Early home-supported discharge of stroke patients: A health technology assessment. Int J Technol Assess Health Care 2006; 22(3): 313–320. [DOI] [PubMed] [Google Scholar]
- 9. Larsen T, Jaarsma T, Strömberg A, et al. Integrated Homecare in Europe for frail elderly somatic patients – focusing on stroke, heart failure and COPD. A Health Technology Assessment: Deliverable D12 to HOMECARE EU FP7 funded project, http://static.sdu.dk/mediafiles/E/A/A/%7BEAA5D715–3B2A-4176–9BB7–8197D54DE48D%7DHomecare222954_D12_HTAofIHC.pdf (accessed 1 December 2012).
- 10. Opara J, Blaszczyszyn M, Barszcz J. The continuation of post-stroke rehabilitation according to the model of early home supported discharge. Med Rehabil 2009; 13(3): 25–30. [Google Scholar]
- 11. Saka O, Serra V, Samyshkin Y, McGuire A, Wolfe CC. Cost-effectiveness of stroke unit care followed by early supported discharge. Stroke 2009; 40(1): 24–29. [DOI] [PubMed] [Google Scholar]
- 12. Santana S, Szczygiel N, Redondo P. Integration of care systems in Portugal: Anatomy of recent reforms. Int J Integr Care 2014; 14: e014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lopes M, Mendes F, Escoval A, et al. Plano Nacional de Saúde 2011–2016. Cuidados Continuados Integrados, 2010. [Google Scholar]
- 14. Nogueira JMA, Henriques JL, Gomes AF, Leitão AL. Enquadramento das Unidades de Reabilitação de AVC. Unidade de Missão para os Cuidados Continuados Integrados, editor2007. [Google Scholar]
- 15. Tavares JPA, Silva AL. Barreiras e Soluções nos Cuidados Continuados a Pessoas Idosas: Percepções de Enfermeiros(as). Revista de Enfermagem Referência 2010; 3(1): 17–28. [Google Scholar]
- 16. Langhorne P, Jepsen BG, P M, Santana S, et al. Early home supported discharge (EHSD) services for stroke patients. A practical problem-based guide linking clinical evidence to clinical rehabilitation: Deliverable D9 to HOMECARE EU FP7 funded project, http://static.sdu.dk/mediafiles/0/9/6/%7B0962B82A-D7FD-4C1B-8972–6A496DF63427%7DHOMECARE%20222954_EHSDguide%28D9%29.pdf (accessed 1 December 2011).
- 17. Dodds TA, Martin DP, Stolov WC, Deyo RA. A validation of the functional independence measurement and its performance among rehabilitation inpatients. Arch Phys Med Rehabil 1993; 74(5): 531–536. [DOI] [PubMed] [Google Scholar]
- 18. Fisher RJ, Gaynor C, Kerr M, et al. A consensus on stroke: Early supported discharge. Stroke 2011; 42(5): 1392–1397. [DOI] [PubMed] [Google Scholar]
- 19. Fitzpatrick R, Bowling A, Gibbons E, Haywood K, Jenkinson C, Mackintosh AEA. A structured review of patient-reported measures in relation to selected chronic conditions, perceptions of quality of care and carer impact. National Centre for Health Outcomes Development (Oxford site) UoH-CEDoPH, editor. Oxford: University of Oxford, 2006. [Google Scholar]
- 20. Holbrook M, Skilbeck CE. An activities index for use with stroke patients. Age Ageing 1983; 12(2): 166–170. [DOI] [PubMed] [Google Scholar]
- 21. Skevington SM, Lotfy M, O’Connell KA. The World Health Organization’s WHOQOL-BREF quality of life assessment: Psychometric properties and results of the international field trial. A report from the WHOQOL group. Qual Life Res 2004; 13(2): 299–310. [DOI] [PubMed] [Google Scholar]
- 22. Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ 2002; 21(2): 271–292. [DOI] [PubMed] [Google Scholar]
- 23. Mahoney FI, Barthel DW. Functional evaluation: The Barthel Index. MD State Med J 1965; 14: 61–65. [PubMed] [Google Scholar]
- 24. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12(3): 189–198. [DOI] [PubMed] [Google Scholar]
- 25. IBM Corporation Inc. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp, http://www-01.ibm.com/software/analytics/spss/products/statistics/index.html (accessed 11 November 2015). [Google Scholar]
- 26. Bagiella E. Clinical trials in rehabilitation: single or multiple outcomes? Arch Phys Med Rehabil 2009; 90(11 Suppl): S17–21. [DOI] [PubMed] [Google Scholar]