Highlights
-
•
Maintenance of adequate portal inflow is essential for the graft regeneration in adult LDLT.
-
•
Portal inflow steal may occur due to presence of huge spontaneous porto-systemic collaterals.
-
•
If the portal inflow to the liver graft is inadequate after adult LDLT, post-transplant impairment of the graft regeneration and eventually graft failure would occur.
-
•
A surgical procedure to increase the portal inflow is rarely necessary in adult LDLT.
-
•
We report a case of prophylactic surgical interruption of spontaneous huge porto-systemic collateral to prevent PFS during adult LDLT procedure.
Abbreviations: LDLT, living donor liver transplantation; PFS, portal inflow steal; DDLT, deceased donor liver transplantation; MELD, model for end stage liver disease; US, ultrasonography; CT, computed tomography; GRWR, graft weight to recipient weight ratio; TIPS, transjugular intrahepatic porto-systemic shunt
Keywords: Living donor liver transplantation, Portal inflow steal, Lieno-renal collaterals
Abstract
Introduction
In adult living donor liver transplantation (LDLT), maintenance of adequate portal inflow is essential for the graft regeneration. Portal inflow steal (PFS) may occur due to presence of huge spontaneous porto-systemic collaterals. A surgical procedure to increase the portal inflow is rarely necessary in adult LDLT.
Presentation
A 52 years male patient with end-stage liver disease due to chronic hepatitis C virus infection. Preoperative portography showed marked attenuated portal vein and its two main branches, patent tortuous splenic vein, multiple splenic hilar collaterals, and large lieno-renal collateral. He received a right hemi-liver graft from his nephew. Exploration revealed markedly cirrhotic liver, moderate splenomegaly with multiple collaterals and large lieno-renal collateral. Upon dissection of the hepato-duodenal ligament, a well-developed portal vein could be identified with a small mural thrombus.
The recipient portal vein stump was anastomosed, in end to end fashion, to the graft portal vein. Doppler US showed reduced portal vein flow, so ligation of the huge lieno-renal collateral that allows steal of the portal inflow. After ligation of the lieno-renal collateral, improvement of the portal vein flow was observed in Doppler US.
Discussion
There is no accepted algorithm for managing spontaneous lieno-renal shunts before, during, or after liver transplantation, and evidence for efficacy of treatments remains limited. We report a case of surgical interruption of spontaneous huge porto-systemic collateral to prevent PFS during adult LDLT.
Conclusion
Complete interruption of large collateral vessels might be needed as a part of adult LDLT procedure to avoid devastating postoperative PFS.
1. Introduction
Living donor liver transplantation (LDLT) has been a useful therapeutic option for end-stage liver disease because of the shortage of deceased donor liver grafts [1].
In adult LDLT, maintenance of adequate portal inflow is essential for the graft regeneration. Portal inflow steal (PFS) may occur due to presence of huge spontaneous porto-systemic collaterals. If the portal inflow to the liver graft is inadequate after adult LDLT, through persistent spontaneous porto-systemic collaterals, post-transplant impairment of the graft regeneration and eventually graft failure would occur [2].
A surgical procedure to increase the portal inflow is rarely necessary in adult LDLT. Unlike pediatric LDLT where a ligation of the porto-systemic collaterals is sometimes necessary to maximize the portal inflow to a relatively large graft [3], and deceased donor liver transplantation (DDLT) where a sufficient liver vascular bed is usually present [4].
We report a case of prophylactic surgical interruption of spontaneous huge porto-systemic collateral to prevent PFS during adult LDLT procedure. This work has been reported in line with the SCARE criteria [5].
2. Case presentation
52 years male patient with end-stage liver disease due to chronic hepatitis C virus infection. He His preoperative Child-Pugh score was 7 (class B), and his model for end stage liver disease (MELD) was 13. He was planned for living donor liver transplantation.
Preoperative abdominal ultrasonography (US) showed marked cirrhotic liver with no ascites, moderate enlargement of the spleen, and dilated splenic vein with multiple hilar collaterals. Doppelr US showed marked attenuated portal vein along its course (5 mm) with absent flow inside and large lieno-renal shunt siphoning the portal circulation.
Preoperative triphasic abdominal computed tomography (CT) showed shrunken cirrhotic liver, marked attenuated portal vein, moderate enlarged uniform spleen, and dilated splenic vein with multiple hilar collaterals. CT portography showed marked attenuated portal vein and its two main branches, dilated patent tortuous splenic vein, normal patent superior mesenteric vein, multiple dilated splenic hilar collaterals, and large lieno-renal collateral (Fig. 1).
Fig. 1.
Preoperative CT portography showing markedly attenuated portal vein (thin arrow) with huge lieno-renal collateral (thick arrow).
He received an ABO identical right hemi-liver graft without the middle hepatic vein from his nephew. The actual graft weight was 983 g and graft weight to recipient weight ratio (GRWR) was 1.06.
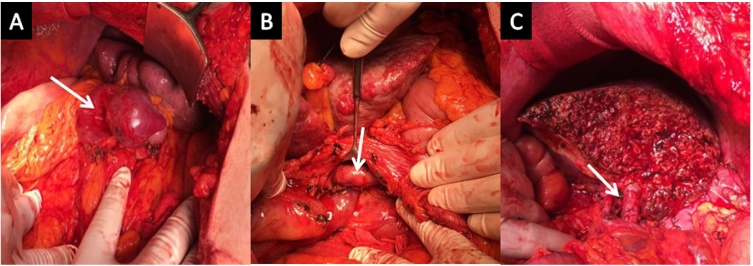
Exploration of the patient revealed markedly cirrhotic liver, moderate splenomegaly with multiple collaterals and large lieno-renal collateral. Upon dissection of the hepato-duodenal ligament, a well-developed portal vein could be identified with a small mural thrombus, unlike the preoperative CT findings (Fig. 2). Thrombectomy was done, and the forward flow from the portal vein stump was evaluated before the implantation of the graft and found to be sufficient for the reperfusion of the graft.
Fig. 2.
Operative photos showing; A- Huge lieno-renal collateral (arrow), B- After dissection of hepatoduodenal ligament and exposure of healthy portal vein (arrow), C- After completion of graft implantation (arrow at portal vein anastomosis).
The graft had a single dominant right hepatic vein, and was anastomosed to the recipient right hepatic vein with venoplasty.
The recipient portal vein stump was anastomosed, in end to end fashion, to the graft portal vein. Doppler US showed reduced portal vein flow (52 cm/s). The decision was to ligate the huge lieno-renal collateral that allowed steal of the portal inflow. After ligation of the lieno-renal collateral, improvement of the portal vein flow was observed in Doppler US (92 cm/sec).
Then arterial reconstruction was done between the recipient right hepatic artery and graft right hepatic artery. Biliary reconstruction was done between the recipient right and left hepatic ducts to the graft right anterior and posterior hepatic ducts, respectively. Completion Doppler US showed sound anastomoses and adequate portal inflow.
The postoperative course was uneventful. Regular follow-up Doppler US showed adequate portal blood flow and he was discharged 15 days after transplantation. 6 month after liver transplantation, the patient is doing well with normal liver function tests (serum albumin: 4.5 gm/dL, serum bilirubin: 0.8 mg/dL, serum alanine aminotransferase: 46 IU/ml, serum aspartate aminotransferase: 38 IU/ml, serum international normalized ratio: 1, serum alkaline phosphatase: 5 KAU), and adequate Doppler US examination. Follow-up triphasic abdominal CT showed normal liver graft, and moderate splenomegaly. CT angiography showed normal all vascular anastomoses (Fig. 3).
Fig. 3.
Follow up CT portography after 6 month showing adequate portal vein anastomosis and its intraparenchymal branching pattern.
3. Discussion
Postoperative PFS by residual porto-systemic collaterals is an important cause of graft dysfunction after liver transplantation. These collaterals siphon portal inflow and decrease portal perfusion pressure of the liver impairing the graft regeneration and may cause graft loss [6].
In adult LDLT, PFS is not a rare problem, because the partial liver grafts have much smaller volume to accommodate hyperdynamic portal blood flow, and rapid regeneration of the liver graft to meet the metabolic demands of recipient transiently increase intrahepatic resistance to the portal inflow [7].
Preoperative radiological studies should assess the site and the size of any collaterals, so a decision can be made about if and when to intervene [8]. In our case, the preoperative Doppler US and CT portography could identify the presence of large lieno-renal shunt and lack of portal vein flow. This was essential to map superficial veins of the lower limbs, during preoperative preparation, that may be used for reconstructing the portal vein.
There is no accepted algorithm for managing spontaneous lieno-renal shunts before, during, or after liver transplantation, and evidence for efficacy of treatments remains limited [8]. Most published literature in this aspect consists mainly of case reports, and the experience of most transplant teams is limited to few cases [2], [9], [10].
Treatment options include preoperative transjugular intrahepatic porto-systemic shunt (TIPS) to decompress the shunts, routine intraoperative ligation of the shunts, intraoperative assessment of portal inflow with decision to intervene if there is evidence of inadequate portal inflow to the graft, close follow-up of the shunt and the graft functions without intervention, creation of porto-renal anastomosis in the case of portal vein thrombosis, or postoperative radiological interventions such as embolization of the shunt [8], [9].
Little attention is paid to prophylactic ligation of the collateral vessels in adult LDLT, and few reports addressed this topic. Moon et al. reported 2 cases of PFS after adult LDLT. They recommended prophylactic ligation of large porto-systemic collaterals in adult LDLT, if it is equal to or more than 10 mm in diameter, or less than 10 mm in diameter associated with portal vein stenosis on the preoperative CT scan [2].
Sadamori et al. recommended the transection of the huge spleno-renal shunt by a splenectomy because ligation of the shunt alone was insufficient to reverse the PFS [10]. In our case, splenectomy was avoided because the portal inflow was restored by collateral ligation alone.
Shirouzu et al. recommended ligation of a huge porto-systemic collaterals at the time of transplantation, even when a relatively small graft is implanted. They also recommended simultaneous modulation of portal inflow, with splenic artery ligation, when the occlusion of huge porto-systemic collaterals produces an excessive portal inflow [9].
On the other hand, an excessive portal inflow due to occlusion of the porto-systemic collaterals may have a negative impact on the graft outcome in the immediate postoperative period. This is attributed to increased splanchnic blood flow to the smaller grafts in adult LDLT [9].
In conclusion, complete interruption of large collateral vessels might be needed as a part of adult LDLT procedure to avoid devastating postoperative PFS. Preoperative radiological evaluation of the portal vein condition might be misleading; however, we should be ready to different alternatives to reconstruct the portal flow.
Conflicts of interest
No conflicts of interest were declared.
Financial support
No external funding resources.
This work has been reported in line with the SCARE criteria.
Acknowledgments
None
References
- 1.Eguchi S., Takatsuki M., Hidaka M. Evolution of living donor liver transplantation over 10 years: experience of a single center. Surg. Today. 2008;38:795–800. doi: 10.1007/s00595-007-3729-8. [DOI] [PubMed] [Google Scholar]
- 2.Moon D.B., Lee S.G., Kim K.H. The significance of complete interruption of large spontaneous portosystemic collaterals in adult living donor liver transplantation as a graft salvage procedure. Transpl. Int. 2008;21(7):698–700. doi: 10.1111/j.1432-2277.2008.00639.x. [DOI] [PubMed] [Google Scholar]
- 3.Fujimoto M., Moriyasu F., Nada T. Influence of spontaneous portosystemic collateral pathways on portal hemodynamics in living-related liver transplantation in children. Doppler ultrasonographic study. Transplantation. 1995;60:41–45. doi: 10.1097/00007890-199507150-00008. [DOI] [PubMed] [Google Scholar]
- 4.Carlis L.D., Favero E.D., Rondinara G. The role of spontaneous portosystemic shunts in the course of orthotopic liver transplantation. Transpl. Int. 1992;5:9. doi: 10.1007/BF00337182. [DOI] [PubMed] [Google Scholar]
- 5.Agha R.A., Fowler A.J., Saeta A. The SCARE statement: consensus-based surgical case report guidelines. Int. J. Surg. 2016;6 doi: 10.1016/j.ijsu.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 6.Miyamoto A., Kato T., Dono K. Living-related liver transplantation with renoportal anastomosis for a patient with large spontaneous splenorenal collateral. Transplantation. 2003;75(9):1596–1598. doi: 10.1097/01.TP.0000061769.78127.5D. [DOI] [PubMed] [Google Scholar]
- 7.Kita Y., Harihara Y., Sano K. Reversible hepatofugal portal flow after liver transplantation using a small-for-size graft from a living donor. Transpl. Int. 2001;14:217. doi: 10.1007/s001470100327. [DOI] [PubMed] [Google Scholar]
- 8.Awad N., Horrow M.M., Parsikia A. Perioperative management of spontaneous splenorenal shunts in orthotopic liver transplant patients. Exp. Clin. Transplant. 2012;10(5):475–481. doi: 10.6002/ect.2011.0201. [DOI] [PubMed] [Google Scholar]
- 9.Shirouzu Y., Ohya Y., Tsukamoto Y., Yaet al. How to handle a huge portosystemic shunt in adult living donor liver transplantation with a small-for-size graft: report of a case. Surg. Today. 2009;39(7):637–640. doi: 10.1007/s00595-008-3886-4. [DOI] [PubMed] [Google Scholar]
- 10.Sadamori H., Yagi T., Matsukawa H. The outcome of living donor liver transplantation with prior spontaneous large portosystemic shunts. Transpl. Int. 2008;21:156–162. doi: 10.1111/j.1432-2277.2007.00593.x. [DOI] [PubMed] [Google Scholar]