Abstract
The role of serotonin in major depressive disorder (MDD) is the focus of accumulating clinical and preclinical research. The results of these studies reflect the complexity of serotonin signaling through many receptors, in a large number of brain regions, and throughout the lifespan. The role of the serotonin transporter in MDD has been highlighted in gene by environment association studies as well as its role as a critical player in the mechanism of the most effective antidepressant treatments – selective serotonin reuptake inhibitors. While the majority of the 15 known receptors for serotonin have been implicated in depression or depressive-like behavior, the serotonin 1A (5-HT 1A) and 1B (5-HT 1B) receptors are among the most studied. Human brain imaging and genetic studies point to the involvement of 5-HT 1A and 5-HT 1B receptors in MDD and the response to antidepressant treatment. In rodents, the availability of tissue-specific and inducible knockout mouse lines has made possible the identification of the involvement of 5-HT 1A and 5-HT 1B receptors throughout development and in a cell-type specific manner. This, and other preclinical pharmacology work, shows that autoreceptor and heteroreceptor populations of these receptors have divergent roles in modulating depression-related behavior as well as responses to antidepressants and also have different functions during early postnatal development compared to during adulthood.
Keywords: serotonin, MDD, major depressive disorder, serotonin receptor, 5-HT1A, 5-HT1B, 5-HTT, selective serotonin reuptake inhibitors, antidepressant
Introduction
The serotonin hypothesis of depression has dominated the field of depression for over four decades 1. This theory is centered on the idea that reduced serotonin signaling is a risk factor in the etiology and/or pathophysiology of major depressive disorder (MDD) 2. However, the most robust body of evidence for the role of serotonin in depression is the efficacy of increasing extracellular serotonin for the treatment of depression. The discovery that the efficacy of tricyclic antidepressants (TCAs) and monoamine oxidase inhibitor (MAOI) antidepressants was largely due to their serotonergic actions, which prompted the use of serotonin selective reuptake inhibitors (SSRIs), the first among them fluoxetine, to treat depression 3– 6. These drugs act at the serotonin transporter (5-HTT, also known as SERT) and cause increases in extracellular serotonin, which is the purported mechanism of action 6– 8. Many subsequent drugs inhibiting serotonin reuptake have shown behavioral efficacy as antidepressant drugs, suggesting that increasing synaptic serotonin levels may lead to the treatment of depression 6, 9.
Despite the relative success in treating depression by increasing extracellular serotonin, there is a lack of strong evidence supporting a direct correlation between low serotonin signaling and depression. While some studies report an association between levels of platelet serotonin and depression, this has not been a consistent finding in large sample sets, and it is also unclear how platelet levels are related to brain levels of serotonin 10, 11. Additionally, few studies report direct correlations between cerebrospinal fluid 5-hydroxyindoleacetic acid (5-HIAA), a serotonin metabolite, and depression 12, 13. Low levels of tryptophan have been consistently linked to depression; however, these effects could be independent of serotonin 14, 15. The lack of consistent clear-cut abnormalities in global measures of serotonin signaling isn’t surprising if one considers the complexity of the receptors at which serotonin binds, the intricate neuroanatomical circuitry of the serotonin system, and the developmental role serotonin plays as a neurotrophic factor 16– 18. Many recent studies have focused on understanding the mechanisms through which serotonin affects depression by studying the impact of 5-HTT and the 15 known receptors through gene-association studies, human brain imaging, and pharmacological and genetic mouse models 19.
The success in treating depression by targeting the transporter with SSRIs prompted investigations into whether variability in 5-HTT expression levels could be involved in the etiology of depression. A highly cited study showed that there is an association between a polymorphism in the serotonin transporter (5-HTTLPR) and susceptibility to developing depression 20. This and other studies have shown that the short “s” allele, which results in lower levels of 5-HTT expression (at least in vitro) and therefore increased extracellular 5-HT, is associated with a higher risk of depression when combined with stressful life events 21, 22. This discovery would be unexpected if developmental considerations were not considered. Although inhibiting the function of the transporter during adulthood decreases depressive symptoms as in the case of SSRIs, reduced expression of 5-HTT during development may increase depressive behavior in adulthood. A human functional magnetic resonance imaging (fMRI) study supports this, showing that short allele carriers show morphological and functional alterations in limbic circuits 23. Additionally, mice lacking 5-HTT throughout life display increased depressive-like behaviors, and pharmacological blockade of 5-HTT in mice exclusively during early postnatal development resulted in increased adult depressive behavior 24. These results highlight the differences in developmental versus adult effects of altered serotonin neurotransmission on depression.
In addition to the serotonin transporter, the majority of the 15 serotonin receptors have been implicated in the modulation of depression, depressive-like behaviors, or the response to anti-depressant treatment 19. There are numerous pre-clinical studies which have investigated the role of serotonin receptors using pharmacological manipulations and genetic knockout (KO) models in rodents ( Table 1). Given the breadth of this literature, this review will focus on two receptors that are among the most extensively studied for their role in modulating depression, the 5-HT 1A and 5-HT 1B receptor subtypes. In addition, attention will be paid to population-dependent and development-dependent effects of serotonin signaling at these receptors and will draw from both rodent and human studies.
Table 1. Preclinical evidence supporting the role for serotonin receptors in depression.
| Receptor | PubMed
Hits * |
Pharmacological studies on
depression |
Genetic effects on
depression |
Other behavioral
phenotypes |
|---|---|---|---|---|
| 5-HT 2A | 588 | Antagonists have
antidepressant-like effects and potentiate the effects of SSRIs 133, 134 |
No known effect of 5-HT
2A
KO on depressive-like behavior 135 |
Agonists are hallucinogenic;
antagonists are antipsychotic and anxiolytic; KO mouse has reduced anxiety-like behavior 135– 137 |
| 5-HT 2B | 52 | Agonists have
antidepressant-like effects 138 |
Required for behavioral
effects of SSRIs 138, 139 |
KO mouse shows increased
impulsivity 140 |
| 5-HT 2C | 282 | Antagonists have
antidepressant-like effects; agonists have pro-depressive effects 141, 142 |
No known effect of 5-HT
2C
KO on depressive-like behavior |
Antagonists have anxiolytic
effects; agonists decrease impulsivity and motivation for drug and food consumption; KO mouse has reduced anxiety-like behavior 143– 145 |
| 5-HT 3A | 252 | Antagonist has
antidepressant-like effects 146 |
5-HT
3 required for
exercise-induced antidepressant effects; KO has antidepressant-like phenotype 147, 148 |
Antagonists are anxiolytic 149 |
| 5-HT 4 | 81 | Agonists have rapid
antidepressant-like effects 150, 151 |
KO has attenuated
responses to stress 152 |
Agonists are anxiolytic;
agonists improve cognitive performance and reduce feeding 151, 153 |
| 5-HT 5A | 5 | Unknown | Unknown | KO mice display increased
exploratory behavior 154 |
| 5-HT 6 | 62 | Agonists produce
antidepressant-like effects and antagonists block the effects of SSRIs 155, 156 |
Unknown | Antagonists enhance
cognitive performance; blockade of signaling is anxiogenic 157, 158 |
| 5-HT 7 | 137 | Antagonists have
antidepressant-like effects 159 |
KOs have an
antidepressant-like phenotype 159 |
Antagonists have
pro-cognitive effects 160 |
*Number of PubMed hits based on the search terms including “depression” and the receptor as of August 25, 2016.
N.B. 5-HT1D, 1E, 1F, 3B, and 5B are not included in the chart owing to a lack of published research concerning the role of these receptors in behavior.
5-HT, serotonin; KO, knockout; SSRI, selective serotonin reuptake inhibitor.
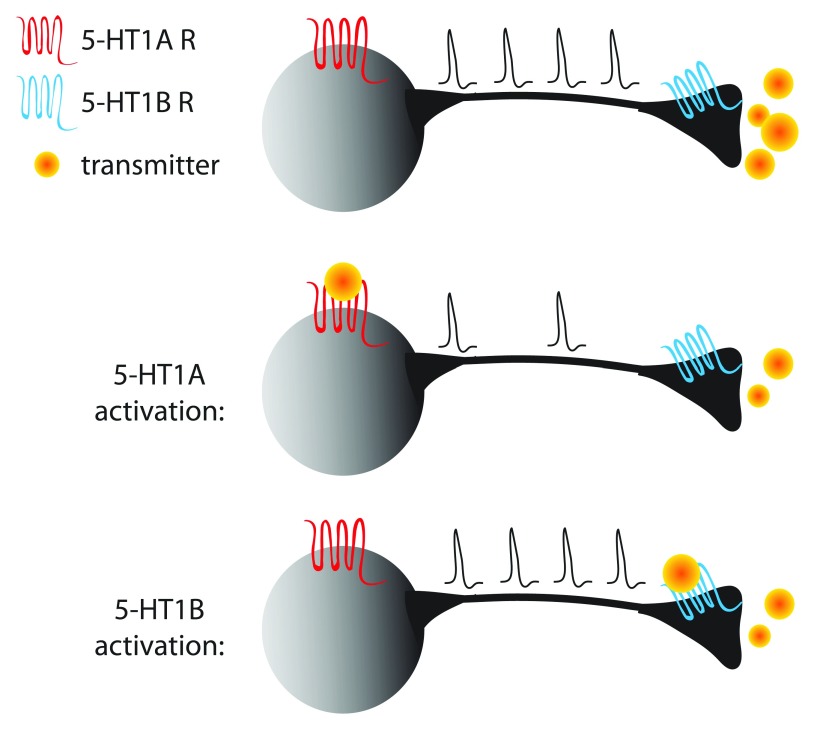
The 5-HT 1A and 5-HT 1B receptors are both inhibitory Gi/o-coupled seven transmembrane receptors that are located throughout the brain 25– 27. A major difference between these two receptors is their subcellular distribution 28. 5-HT 1A receptors are somatodendritic, while 5-HT 1B receptors are located on axon terminals 27, 29. This difference is also reflected in their mechanisms of inhibitory action ( Figure 1). Activation of either receptor causes decreased neurotransmitter release; however, 5-HT 1A receptor activation causes hyperpolarization, leading to decreased firing, while 5-HT 1B receptors inhibit voltage-gated calcium channels in the presynaptic terminal 30– 32. Another mechanism for 5-HT 1B receptor-mediated inhibition is via effects on 5-HTT, and activation of the 5-HT 1B receptor increases serotonin reuptake 33, 34.
Figure 1. Schematic illustrating the inhibitory effects of serotonin (5-hydroxytryptamine, 5-HT) 1A (5-HT 1A) (red) and 5-HT 1B (blue) receptors on the normal firing and neurotransmitter release of a neuron (top).
Activation of 5-HT 1A receptors results in decreased firing (middle), while activation of 5-HT 1B receptors causes decreased neurotransmitter release through actions in the presynaptic terminal (bottom).
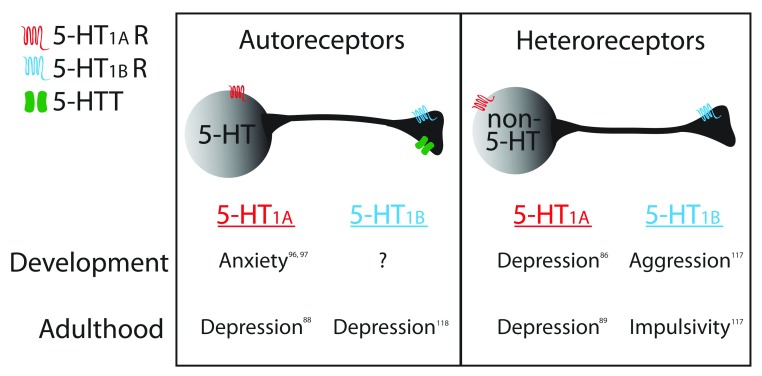
Both 5-HT 1A and 5-HT 1B receptors act as autoreceptors located on serotonin neurons and also have heteroreceptor populations located on non-serotonin receptors ( Figure 2). Although the mRNA in the raphe (corresponding to autoreceptors) is comparable between the two receptors, their heteroreceptors have distinct patterns of expression 35. 5-HT 1A receptors are enriched in the hippocampus and cortex, while 5-HT 1B receptors are highly expressed in the basal ganglia 36, 37. These differences in mechanism of action and localization may play a role in the different functional effects of these receptors.
Figure 2. Diagram summarizing the roles of autoreceptor and heteroreceptor populations of serotonin (5-hydroxytryptamine, 5-HT) 1A (5-HT 1A) and 5-HT 1B receptors on behavior during development and adulthood.
5-HTT, serotonin transporter.
While this review focuses on the contribution of 5-HT 1A and 5-HT 1B receptors in depression and depressive-like behaviors, these receptors also modulate other psychiatric-relevant phenotypes. For example, alterations in 5-HT 1A receptor expression influence anxiety behavior, and 5-HT 1B receptor signaling affects reward- and impulsivity-related phenotypes. These receptor-based differences in serotonergic regulation of emotional behavior, which segment into endophenotypes, could contribute to the heterogeneity of symptoms found in MDD 38. Understanding the neural circuits that subserve these receptor-based and endophenotype-based differences can help clarify the often confusing and sometimes contradictory findings from various preclinical approaches. From a behavioral perspective, these phenotypes can be segmented through formal unsupervised factor analyses to better divide depressive behaviors into meaningful endophenotypes. Then predictors of the different endophenotypes could be tested by including genetic or pharmacological manipulations.
5-HT 1A and depression
Of the 15 known serotonin receptors, the 5-HT 1A receptor is the most studied for its role in depression 39. Quantification of 5-HT 1A receptor levels in humans from post mortem and positron emission tomography (PET) imaging studies reveals an increased level of 5-HT 1A receptors in patients diagnosed with MDD 40– 42. Gene association studies have linked a polymorphism in the 5-HT 1A regulatory region (rs6295; G-1019C) with receptor levels in the brain and also to increased risk for depression 43– 47. The GG genotype at this single nucleotide polymorphism (SNP) is associated with altered levels of 5-HT 1A receptor expression and reduced responsiveness to antidepressant treatment 43, 48. Additionally, clinical studies have revealed antidepressant effects of buspirone and other 5-HT 1A receptor agonists 49, 50.
Rodent models have also shown that 5-HT 1A receptor agonists, such as 8-hydroxy-2-(di-n-propylamino) tetralin (8-OH-DPAT), can have acute antidepressant-like effects 51– 53. These effects are blocked by 5-HT 1A receptor antagonists, suggesting that the antidepressant-like response is specific to 5-HT 1A receptor signaling 54. 5-HT 1A heteroreceptors, expressed throughout the limbic system, are the likely site of action for these acute 5-HT 1A receptor-mediated effects 50, 55. On the other hand, 5-HT 1A autoreceptors work in opposition to the heteroreceptors, leading to pro-depressive effects. Specifically, activation results in hyperpolarization and reduced firing of raphe neurons, leading to diminished serotonin release in projection regions 56. Therefore, stimulation of 5-HT 1A autoreceptors from increased extracellular serotonin following SSRI treatment is thought to oppose SSRI actions by downregulating serotonin neuron activity 57. Over the first few weeks of treatment, these receptors desensitize, which may underlie the delayed behavioral efficacy of SSRIs 58. Therefore, blocking 5-HT 1A autoreceptor activation has been introduced as an adjunctive therapy to SSRIs. 5-HT 1A receptor partial agonists such as pindolol, and more recently vilazodone, have been shown to be an effective adjunctive therapy to SSRIs in clinical studies 59– 62. The development of new agonists that preferentially target subpopulations of 5-HT 1A receptors, for example autoreceptors versus heteroreceptors, potentially through biased agonism, may be useful tools for the treatment of MDD 63.
Differences in receptor levels have also been modeled in mice by using genetic loss-of-function models and have allowed causal links between receptor expression levels and depressive-like behavior. 5-HT 1A receptor KO mice have an anti-depressive phenotype 64, 65. Tissue-specific KOs have been especially valuable for the dissection of this phenotype and have allowed investigations into the distinct roles of different populations of receptors 66. The absence of heteroreceptors results in increased depressive-like behavior- as measured in the forced swim test. This mouse model also allowed for temporal control of receptor expression, which revealed a developmental sensitive period for the effect of heteroreceptors on depressive-like behavior. Specifically, knockdown of 5-HT 1A heteroreceptors in adulthood was not sufficient to produce the depressive-like behavior. On the other hand, reduction of autoreceptors in adulthood increased mobility in the forced swim test, suggesting an “anti-depressed” phenotype.
Preclinical studies have also confirmed a causal role for alterations in 5-HT 1A receptor expression in antidepressant efficacy. 5-HT 1A receptor KO mice do not show a behavioral response to fluoxetine 67. As expected from the pharmacology work, this effect is not mediated by autoreceptors, since reduced expression of 5-HT 1A autoreceptors actually increases the speed and efficacy of SSRI response, requiring only 8 days of fluoxetine treatment to show a behavioral antidepressant-like response 68. Recent data show that 5-HT 1A heteroreceptors are critical for an effective behavioral response to an SSRI in mice 69. Genetic or viral deletion of 5-HT 1A receptors specifically in the dentate gyrus of the hippocampus reduced the behavioral response to fluoxetine. Furthermore, expression of 5-HT 1A receptors only in the dentate gyrus was sufficient for normal antidepressant-like responses. These results importantly demonstrate a mechanism for 5-HT 1A-mediated antidepressant effects localized in the mature granule cells of the dentate gyrus.
5-HT 1A and other psychiatric-relevant phenotypes
Anxiety behavior is also modulated strongly by the 5-HT 1A receptor, and, among depressed patients, almost half have a comorbid anxiety disorder 70. In preclinical studies, 5-HT 1A receptor agonists have anxiolytic effects, and 5-HT 1A receptor KO mice display increased anxiety-like behavior 64, 65, 71, 72. The effect has a developmental sensitive period, since early developmental but not adult rescue of the receptor was sufficient to restore the normal phenotype in the KO 73. Consistent with this, early postnatal blockade of 5-HT 1A receptors, through genetic or pharmacological methods, also leads to increased anxiety 74, 75. Recent work has shown that the sensitive period is peri-pubertal, and tissue-specific KO mice point to a role for autoreceptors during this period of development 66, 76, 77.
Other psychiatric disorders have also been linked to the 5-HT 1A receptor, including bipolar disorder and post-traumatic stress disorder 78, 79. Additionally, the SNP rs6295 found in the premotor region that is associated with risk for depression is also linked with psychiatric hospitalization, a history of substance abuse, and prior suicide attempts 43. Consistent with the studies in depression, the G allele is associated with reduced expression of the 5-HT 1A receptor in the prefrontal cortex and an increased risk for psychiatric outcomes. Interestingly, the effects on receptor expression were also seen in the brain during early human embryonic development, suggesting its potential importance in mediating developmental contributions to adult depression. Finally, there were associations with childhood maltreatment with trends towards significant genotype by environment interactions 40.
5-HT 1B and depression
While the 5-HT 1B receptor is best known for its role in regulating aggressive and impulsive behavior, it also plays an important role in modulating depression. Activation of the 5-HT 1B receptor decreases serotonin levels in the brain through effects on release, synthesis, and reuptake 33, 80, 81. In humans, reduced 5-HT 1B receptor function is associated with MDD 82. Additionally, patients with MDD are less responsive to 5-HT 1B receptor agonists, suggesting reduced expression or desensitization 83, 84. This is consistent with clinical studies showing that 5-HT 1B receptor agonists produce antidepressant effects in humans 85– 87. This has also been shown in mice, with specific agonists resulting in antidepressant-like behavior 88, 89. However, genetic KO of the receptor also results in antidepressant-like behavior, suggesting that this is possibly caused by compensatory effects 90– 94.
Both autoreceptor and heteroreceptor populations of 5-HT 1B receptors have been implicated in depressive-like behaviors using rodent models. However, since 5-HT 1B receptors are located on presynaptic terminals, heteroreceptors and autoreceptors have overlapping localization 95. This rules out brain imaging and pharmacological manipulations in preclinical models as tools to differentiate the role of the two populations of receptors. Therefore, it has been only the recent availability of a tissue-specific genetic mouse model that has allowed the dissection of the role of 5-HT 1B receptors in the regulation of behavior 96.
Our recent studies show that selective ablation of 5-HT 1B autoreceptors results in decreased depressive-like behaviors in mice 97. These mice also show increased elevations in serotonin levels compared to controls following SSRI administration, suggesting a potential mechanism of action for the behavioral effects. Specifically, removing the terminal auto-inhibition may result in increased serotonin in projection regions that are relevant to depressive behavior. Furthermore, we also showed that the impact of 5-HT 1B autoreceptors on behavior was not due to developmental expression, since the phenotype was not recapitulated in a mouse with developmental knockdown. These data are consistent with other evidence suggesting a pro-depressive role for the activation of 5-HT 1B autoreceptors 98, 99. For example, 5-HT 1B mRNA is elevated in the raphe of rats following stress and in models of depression such as learned helplessness, and viral overexpression of 5-HT 1B receptors in the raphe results in depressive-like behavior following stress 100. In rats, reductions in 5-HT 1B receptor mRNA in the raphe are seen following SSRI treatment in post mortem brains 101, 102. This effect isn’t seen in other brain regions such as the cortex, hippocampus, or striatum, suggesting that this effect is specific to autoreceptors. Additionally, another study showed that 5-HT 1B autoreceptors may desensitize following SSRI treatment, similar to 5-HT 1A autoreceptors 103. Finally, a recent PET study in humans reported that following effective cognitive behavioral therapy for depression, 5-HT 1B receptor binding was reduced in the brainstem 104.
There is evidence which suggests an opposing role for 5-HT 1B heteroreceptors in depressive behaviors. Activation of 5-HT 1B heteroreceptors in a rodent serotonin depletion model (to remove the contribution of autoreceptors) results in an antidepressant-like effect 105. Additionally, reduced expression of 5-HT 1B heteroreceptors in the ventral striatum is associated with depression in humans 82. Finally, 5-HT 1B receptors located in the ventral striatum have been suggested to interact with p11 (a 5-HT 1B receptor-binding protein) to affect depression-related behaviors 106, 107.
5-HT 1B and other psychiatric-related phenotypes
Reward dysfunction is a major symptom of MDD which is mediated, in part, by altered signaling in the mesolimbic reward system 108– 112. 5-HT 1B receptors have been implicated in the neural basis of dysregulated reward sensitivity in a number of human studies and preclinical models 113– 116, and both 5-HT 1B receptor protein and mRNA are located within the mesolimbic pathway in the nucleus accumbens (NAc) and ventral tegmental area (VTA) 95. Additionally, activation of 5-HT 1B receptors in the VTA increases dopamine levels in the NAc, potentially via effects on GABAergic signaling in the VTA 117.
Many studies linking the receptor to functional deficits in reward processing have focused on addiction. Polymorphisms in the 5-HT 1B receptor gene have also been associated with drug and alcohol abuse 118– 120. Additionally, a PET imaging study revealed increased 5-HT 1B receptor binding in pathological gamblers, who have known deficits in reward sensitivity, and gambling disorder is highly comorbid with depression and alcohol and substance use disorders 116, 121. Another PET imaging study shows that there is reduced 5-HT 1B receptor binding in cocaine-dependent participants compared to in healthy controls 122. In preclinical models, 5-HT 1B receptor KO mice are more motivated to self-administer cocaine 123. Consistent with this, 5-HT 1B receptor agonists attenuate the motivation for cocaine but paradoxically increase the rewarding effects of cocaine 124. These effects are mediated by 5-HT 1B receptor expression on medium spiny neurons in the NAc, likely through their projections to the VTA 125, 126. Additionally, 5-HT 1B receptors are required for the rewarding properties of social interaction, supporting an impact on general reward systems 114.
5-HT 1B receptors are also implicated in impulsive aggression. In humans, polymorphisms in the gene encoding 5-HT 1B receptors have been associated with aggression, suicide, and disorders that include impulsivity as a core phenotype, including attention deficit hyperactivity disorder and substance use disorder 115, 120, 127. In mice, 5-HT 1B receptor KOs are highly aggressive in tests of male and female aggression and also display increased impulsivity 128– 130. Additionally, 5-HT 1B receptor agonists are known as “serenics” because they decrease aggression 131. While the aggressive and impulsive phenotype was originally thought to be modulated by the same underlying circuits, our recent work shows that distinct populations of 5-HT 1B receptors modulate aggression and impulsivity 96. Furthermore, developmental expression of the 5-HT 1B receptor influences aggression, while adult expression modulates impulsive behavior.
Conclusion
There is a considerable body of research that implicates serotonin in the modulation of depression and depression-related behaviors. The preclinical work delineating the effects of signaling through the 5-HT 1A and 5-HT 1B receptors has been made possible because of careful pharmacological studies as well as the development of transgenic mouse models that have allowed for tissue-specific and inducible knockdown. These studies have highlighted the complexity of serotonin receptors, showing that their role varies through the lifespan and by cell-type population. Additionally, the availability of specific radioligands for PET imaging of these receptors has allowed for the translation of findings from preclinical work to humans. The large number of studies concerning the role of these receptors is partially due to the fact that the 5-HT 1 receptor subtypes were some of the first discovered, and it may be only a matter of time before the roles of more newly discovered receptors are clarified 17.
Despite the amassing of evidence of serotonin receptor-specific involvement in depression, the primary pharmaceutical treatment strategy for depression remains the inhibition of serotonin reuptake. The lack of new treatment options is surprising given the need for them, since current SSRI treatments are ineffective in one-third of patients 132. Additionally, the majority of patients, as seen in the STAR*D study, don’t respond to administration of the first SSRI treatment, requiring multi-step treatment plans that take months 132. Furthermore, the considerable differences in treatment outcome also emphasize the heterogeneity of the depressed patient population. A better understanding of receptor signaling and neural circuit mechanisms by which serotonin affects depression may inform the development of novel, more targeted drugs that influence specific receptors, signaling cascades, or time periods. Also, personalized treatment plans could be developed based on symptoms, biomarkers, or pathophysiological presentation.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Irwin Lucki, Department of Psychiatry, Department of Pharmacology, University of Pennsylvania, Philadelphia, PA, USA
Randy Blakely, Department of Biomedical Sciences, Charles E. Schmidt College of Medicine, Florida Atlantic University, Jupiter, FL, 33458, USA
John Neumaier, Department of pharmacology, University of Washington, Seattle, WA, USA
Funding Statement
Support for René Hen was provided by Hope for Depression Research Foundation (RGA 13-003), NIH R37MH068542, and R01MH083862. Funding for Katherine Nautiyal was provided by NIH K99 MH106731 and a NARSAD Young Investigator Award.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 3 approved]
References
- 1. Coppen A: The biochemistry of affective disorders. Br J Psychiatry. 1967;113(504):1237–64. 10.1192/bjp.113.504.1237 [DOI] [PubMed] [Google Scholar]
- 2. Albert PR, Benkelfat C: The neurobiology of depression--revisiting the serotonin hypothesis. II. Genetic, epigenetic and clinical studies. Philos Trans R Soc Lond B Biol Sci. 2013;368(1615):20120535. 10.1098/rstb.2012.0535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. De Montigny C: Enhancement of the 5-HT neurotransmission by antidepressant treatments. J Physiol (Paris). 1981;77(2–3):455–61. [PubMed] [Google Scholar]
- 4. AZIMA H, VISPO RH: Imipramine; a potent new anti-depressant compound. Am J Psychiatry. 1958;115(3):245–6. 10.1176/ajp.115.3.245 [DOI] [PubMed] [Google Scholar]
- 5. Messing RB, Phebus L, Fisher LA, et al. : Analgesic effect of fluoxetine hydrochloride (Lilly 110140), a specific inhibitor of serotonin uptake. Psychopharmacol Commun. 1975;1(5):511–21. [PubMed] [Google Scholar]
- 6. de Montigny C, Blier P: Effects of antidepressant treatments on 5-HT neurotransmission: electrophysiological and clinical studies. Adv Biochem Psychopharmacol. 1984;39:223–39. [PubMed] [Google Scholar]
- 7. Lemberger L, Rowe H, Carmichael R, et al. : Pharmacologic effects in man of a specific serotonin-reuptake inhibitor. Science. 1978;199(4327):436–7. 10.1126/science.619465 [DOI] [PubMed] [Google Scholar]
- 8. Nackenoff AG, Moussa-Tooks AB, McMeekin AM, et al. : Essential Contributions of Serotonin Transporter Inhibition to the Acute and Chronic Actions of Fluoxetine and Citalopram in the SERT Met172 Mouse. Neuropsychopharmacology. 2016;41(7):1733–41. 10.1038/npp.2015.335 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 9. Asberg M, Mårtensson B: Serotonin selective antidepressant drugs: past, present, future. Clin Neuropharmacol. 1993;16 Suppl 3:S32–44. [PubMed] [Google Scholar]
- 10. Mann JJ, McBride PA, Anderson GM, et al. : Platelet and whole blood serotonin content in depressed inpatients: correlations with acute and life-time psychopathology. Biol Psychiatry. 1992;32(3):243–57. 10.1016/0006-3223(92)90106-A [DOI] [PubMed] [Google Scholar]
- 11. Coppen A, Turner P, Rowsell AR, et al. : 5-Hydroxytryptamine (5-HT) in the whole-blood of patients with depressive illness. Postgrad Med J. 1976;52(605):156–8. 10.1136/pgmj.52.605.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van Praag HM, de Haan S: Central serotonin metabolism and frequency of depression. Psychiatry Res. 1979;1(3):219–24. 10.1016/0165-1781(79)90002-7 [DOI] [PubMed] [Google Scholar]
- 13. Asberg M, Bertilsson L, Mårtensson B, et al. : CSF monoamine metabolites in melancholia. Acta Psychiatr Scand. 1984;69(3):201–19. 10.1111/j.1600-0447.1984.tb02488.x [DOI] [PubMed] [Google Scholar]
- 14. Karege F, Widmer J, Bovier P, et al. : Platelet serotonin and plasma tryptophan in depressed patients: effect of drug treatment and clinical outcome. Neuropsychopharmacology. 1994;10(3):207–14. 10.1038/npp.1994.23 [DOI] [PubMed] [Google Scholar]
- 15. Ogawa S, Fujii T, Koga N, et al. : Plasma L-tryptophan concentration in major depressive disorder: new data and meta-analysis. J Clin Psychiatry. 2014;75(9):e906–15. 10.4088/JCP.13r08908 [DOI] [PubMed] [Google Scholar]
- 16. Gaspar P, Cases O, Maroteaux L: The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci. 2003;4(12):1002–12. 10.1038/nrn1256 [DOI] [PubMed] [Google Scholar]
- 17. Barnes NM, Sharp T: A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38(8):1083–152. 10.1016/S0028-3908(99)00010-6 [DOI] [PubMed] [Google Scholar]
- 18. Bang SJ, Jensen P, Dymecki SM, et al. : Projections and interconnections of genetically defined serotonin neurons in mice. Eur J Neurosci. 2012;35(1):85–96. 10.1111/j.1460-9568.2011.07936.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Carr GV, Lucki I: The role of serotonin receptor subtypes in treating depression: a review of animal studies. Psychopharmacology (Berl). 2011;213(2–3):265–87. 10.1007/s00213-010-2097-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Caspi A, Sugden K, Moffitt TE, et al. : Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–9. 10.1126/science.1083968 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 21. Karg K, Burmeister M, Shedden K, et al. : The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Arch Gen Psychiatry. 2011;68(5):444–54. 10.1001/archgenpsychiatry.2010.189 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 22. Risch N, Herrell R, Lehner T, et al. : Interaction between the serotonin transporter gene ( 5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. JAMA. 2009;301(23):2462–71. 10.1001/jama.2009.878 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 23. Pezawas L, Meyer-Lindenberg A, Drabant EM, et al. : 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8(6):828–34. 10.1038/nn1463 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 24. Ansorge MS, Zhou M, Lira A, et al. : Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science. 2004;306(5697):879–81. 10.1126/science.1101678 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 25. Albert PR, Robillard L: G protein specificity: traffic direction required. Cell Signal. 2002;14(5):407–18. 10.1016/S0898-6568(01)00259-5 [DOI] [PubMed] [Google Scholar]
- 26. Laporte AM, Lima L, Gozlan H, et al. : Selective in vivo labelling of brain 5-HT 1A receptors by [ 3H]WAY 100635 in the mouse. Eur J Pharmacol. 1994;271(2–3):505–14. 10.1016/0014-2999(94)90812-5 [DOI] [PubMed] [Google Scholar]
- 27. Boschert U, Amara DA, Segu L, et al. : The mouse 5-hydroxytryptamine 1B receptor is localized predominantly on axon terminals. Neuroscience. 1994;58(1):167–82. 10.1016/0306-4522(94)90164-3 [DOI] [PubMed] [Google Scholar]
- 28. Ghavami A, Stark KL, Jareb M, et al. : Differential addressing of 5-HT1A and 5-HT1B receptors in epithelial cells and neurons. J Cell Sci. 1999;112(Pt 6):967–76. [DOI] [PubMed] [Google Scholar]
- 29. Riad M, Garcia S, Watkins KC, et al. : Somatodendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B serotonin receptors in adult rat brain. J Comp Neurol. 2000;417(2):181–94. [DOI] [PubMed] [Google Scholar]
- 30. Beck SG, Choi KC, List TJ: Comparison of 5-hydroxytryptamine1A-mediated hyperpolarization in CA1 and CA3 hippocampal pyramidal cells. J Pharmacol Exp Ther. 1992;263(1):350–9. [PubMed] [Google Scholar]
- 31. Knobelman DA, Kung HF, Lucki I: Regulation of extracellular concentrations of 5-hydroxytryptamine (5-HT) in mouse striatum by 5-HT 1A and 5-HT 1B receptors. J Pharmacol Exp Ther. 2000;292(3):1111–7. [PubMed] [Google Scholar]
- 32. Mizutani H, Hori T, Takahashi T: 5-HT 1B receptor-mediated presynaptic inhibition at the calyx of Held of immature rats. Eur J Neurosci. 2006;24(7):1946–54. 10.1111/j.1460-9568.2006.05063.x [DOI] [PubMed] [Google Scholar]
- 33. Hagan CE, McDevitt RA, Liu Y, et al. : 5-HT 1B autoreceptor regulation of serotonin transporter activity in synaptosomes. Synapse. 2012;66(12):1024–34. 10.1002/syn.21608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Montanez S, Munn JL, Owens WA, et al. : 5-HT 1B receptor modulation of the serotonin transporter in vivo: studies using KO mice. Neurochem Int. 2014;73:127–31. 10.1016/j.neuint.2013.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 35. Clark MS, McDevitt RA, Neumaier JF: Quantitative mapping of tryptophan hydroxylase-2, 5-HT 1A, 5-HT 1B, and serotonin transporter expression across the anteroposterior axis of the rat dorsal and median raphe nuclei. J Comp Neurol. 2006;498(5):611–23. 10.1002/cne.21073 [DOI] [PubMed] [Google Scholar]
- 36. Maroteaux L, Saudou F, Amlaiky N, et al. : Mouse 5HT1B serotonin receptor: cloning, functional expression, and localization in motor control centers. Proc Natl Acad Sci U S A. 1992;89(7):3020–4. 10.1073/pnas.89.7.3020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pompeiano M, Palacios JM, Mengod G: Distribution and cellular localization of mRNA coding for 5-HT1A receptor in the rat brain: correlation with receptor binding. J Neurosci. 1992;12(2):440–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Donaldson ZR, Hen R: From psychiatric disorders to animal models: a bidirectional and dimensional approach. Biol Psychiatry. 2015;77(1):15–21. 10.1016/j.biopsych.2014.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Savitz J, Lucki I, Drevets WC: 5-HT 1A receptor function in major depressive disorder. Prog Neurobiol. 2009;88(1):17–31. 10.1016/j.pneurobio.2009.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Parsey RV, Ogden RT, Miller JM, et al. : Higher serotonin 1A binding in a second major depression cohort: modeling and reference region considerations. Biol Psychiatry. 2010;68(2):170–8. 10.1016/j.biopsych.2010.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 41. Kaufman J, Sullivan GM, Yang J, et al. : Quantification of the Serotonin 1A Receptor Using PET: Identification of a Potential Biomarker of Major Depression in Males. Neuropsychopharmacology. 2015;40(7):1692–9. 10.1038/npp.2015.15 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 42. Stockmeier CA, Shapiro LA, Dilley GE, et al. : Increase in serotonin-1A autoreceptors in the midbrain of suicide victims with major depression-postmortem evidence for decreased serotonin activity. J Neurosci. 1998;18(18):7394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Donaldson ZR, Le Francois B, Santos TL, et al. : The functional serotonin 1a receptor promoter polymorphism, rs6295, is associated with psychiatric illness and differences in transcription. Transl Psychiatry. 2016;6:e746. 10.1038/tp.2015.226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Albert PR, Lemonde S: 5-HT1A receptors, gene repression, and depression: guilt by association. Neuroscientist. 2004;10(6):575–93. 10.1177/1073858404267382 [DOI] [PubMed] [Google Scholar]
- 45. Albert PR: Transcriptional regulation of the 5-HT 1A receptor: implications for mental illness. Philos Trans R Soc Lond B Biol Sci. 2012;367(1601):2402–15. 10.1098/rstb.2011.0376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Strobel A, Gutknecht L, Rothe C, et al. : Allelic variation in 5-HT 1A receptor expression is associated with anxiety- and depression-related personality traits. J Neural Transm (Vienna). 2003;110(12):1445–53. 10.1007/s00702-003-0072-0 [DOI] [PubMed] [Google Scholar]
- 47. Lemonde S, Turecki G, Bakish D, et al. : Impaired repression at a 5-hydroxytryptamine 1A receptor gene polymorphism associated with major depression and suicide. J Neurosci. 2003;23(25):8788–99. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 48. Parsey RV, Olvet DM, Oquendo MA, et al. : Higher 5-HT 1A receptor binding potential during a major depressive episode predicts poor treatment response: preliminary data from a naturalistic study. Neuropsychopharmacology. 2006;31(8):1745–9. 10.1038/sj.npp.1300992 [DOI] [PubMed] [Google Scholar]
- 49. Robinson DS, Rickels K, Feighner J, et al. : Clinical effects of the 5-HT1A partial agonists in depression: a composite analysis of buspirone in the treatment of depression. J Clin Psychopharmacol. 1990;10(3 Suppl):67S–76S. [DOI] [PubMed] [Google Scholar]
- 50. Lucki I: Behavioral studies of serotonin receptor agonists as antidepressant drugs. J Clin Psychiatry. 1991;52 Suppl:24–31. [PubMed] [Google Scholar]
- 51. Kennett GA, Dourish CT, Curzon G: Antidepressant-like action of 5-HT 1A agonists and conventional antidepressants in an animal model of depression. Eur J Pharmacol. 1987;134(3):265–74. 10.1016/0014-2999(87)90357-8 [DOI] [PubMed] [Google Scholar]
- 52. Detke MJ, Rickels M, Lucki I: Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl). 1995;121(1):66–72. 10.1007/BF02245592 [DOI] [PubMed] [Google Scholar]
- 53. López-Rubalcava C, Lucki I: Strain differences in the behavioral effects of antidepressant drugs in the rat forced swimming test. Neuropsychopharmacology. 2000;22(2):191–9. 10.1016/S0893-133X(99)00100-1 [DOI] [PubMed] [Google Scholar]
- 54. Detke MJ, Wieland S, Lucki I: Blockade of the antidepressant-like effects of 8-OH-DPAT, buspirone and desipramine in the rat forced swim test by 5HT 1A receptor antagonists. Psychopharmacology (Berl). 1995;119(1):47–54. 10.1007/BF02246053 [DOI] [PubMed] [Google Scholar]
- 55. Blier P, de Montigny C: Current advances and trends in the treatment of depression. Trends Pharmacol Sci. 1994;15(7):220–6. 10.1016/0165-6147(94)90315-8 [DOI] [PubMed] [Google Scholar]
- 56. Albert PR, Lembo P, Storring JM, et al. : The 5-HT1A receptor: signaling, desensitization, and gene transcription. Neuropsychopharmacology. 1996;14(1):19–25. 10.1016/S0893-133X(96)80055-8 [DOI] [PubMed] [Google Scholar]
- 57. Blier P, Ward NM: Is there a role for 5-HT 1A agonists in the treatment of depression? Biol Psychiatry. 2003;53(3):193–203. 10.1016/S0006-3223(02)01643-8 [DOI] [PubMed] [Google Scholar]
- 58. Blier P, Piñeyro G, el Mansari M, et al. : Role of somatodendritic 5-HT autoreceptors in modulating 5-HT neurotransmission. Ann N Y Acad Sci. 1998;861:204–16. 10.1111/j.1749-6632.1998.tb10192.x [DOI] [PubMed] [Google Scholar]
- 59. Tome MB, Isaac MT, Harte R, et al. : Paroxetine and pindolol: a randomized trial of serotonergic autoreceptor blockade in the reduction of antidepressant latency. Int Clin Psychopharmacol. 1997;12(2):81–9. [PubMed] [Google Scholar]
- 60. Artigas F, Celada P, Laruelle M, et al. : How does pindolol improve antidepressant action? Trends Pharmacol Sci. 2001;22(5):224–8. 10.1016/S0165-6147(00)01682-5 [DOI] [PubMed] [Google Scholar]
- 61. Sahli ZT, Banerjee P, Tarazi FI: The Preclinical and Clinical Effects of Vilazodone for the Treatment of Major Depressive Disorder. Expert Opin Drug Discov. 2016;11(5):515–23. 10.1517/17460441.2016.1160051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Artigas F, Romero L, de Montigny C, et al. : Acceleration of the effect of selected antidepressant drugs in major depression by 5-HT 1A antagonists. Trends Neurosci. 1996;19(9):378–83. 10.1016/S0166-2236(96)10037-0 [DOI] [PubMed] [Google Scholar]
- 63. Newman-Tancredi A, Martel JC, Assié MB, et al. : Signal transduction and functional selectivity of F15599, a preferential post-synaptic 5-HT 1A receptor agonist. Br J Pharmacol. 2009;156(2):338–53. 10.1111/j.1476-5381.2008.00001.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ramboz S, Oosting R, Amara DA, et al. : Serotonin receptor 1A knockout: an animal model of anxiety-related disorder. Proc Natl Acad Sci U S A. 1998;95(24):14476–81. 10.1073/pnas.95.24.14476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Heisler LK, Chu HM, Brennan TJ, et al. : Elevated anxiety and antidepressant-like responses in serotonin 5-HT 1A receptor mutant mice. Proc Natl Acad Sci U S A. 1998;95(25):15049–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Richardson-Jones JW, Craige CP, Nguyen TH, et al. : Serotonin-1A autoreceptors are necessary and sufficient for the normal formation of circuits underlying innate anxiety. J Neurosci. 2011;31(16):6008–18. 10.1523/JNEUROSCI.5836-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Santarelli L, Saxe M, Gross C, et al. : Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301(5634):805–9. 10.1126/science.1083328 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 68. Richardson-Jones JW, Craige CP, Guiard BP, et al. : 5-HT 1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron. 2010;65(1):40–52. 10.1016/j.neuron.2009.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Samuels BA, Anacker C, Hu A, et al. : 5-HT1A receptors on mature dentate gyrus granule cells are critical for the antidepressant response. Nat Neurosci. 2015;18(11):1606–16. 10.1038/nn.4116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Fava M, Rankin MA, Wright EC, et al. : Anxiety disorders in major depression. Compr Psychiatry. 2000;41(2):97–102. 10.1016/S0010-440X(00)90140-8 [DOI] [PubMed] [Google Scholar]
- 71. de Vry J: 5-HT 1A receptor agonists: recent developments and controversial issues. Psychopharmacology (Berl). 1995;121(1):1–26. 10.1007/BF02245588 [DOI] [PubMed] [Google Scholar]
- 72. Parks CL, Robinson PS, Sibille E, et al. : Increased anxiety of mice lacking the serotonin 1A receptor. Proc Natl Acad Sci U S A. 1998;95(18):10734–9. 10.1073/pnas.95.18.10734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gross C, Zhuang X, Stark K, et al. : Serotonin 1A receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature. 2002;416(6879):396–400. 10.1038/416396a [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 74. Lo Iacono L, Gross C: α-Ca 2+/calmodulin-dependent protein kinase II contributes to the developmental programming of anxiety in serotonin receptor 1A knock-out mice. J Neurosci. 2008;28(24):6250–7. 10.1523/JNEUROSCI.5219-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Vinkers CH, Oosting RS, van Bogaert, et al. : Early-life blockade of 5-HT 1A receptors alters adult anxiety behavior and benzodiazepine sensitivity. Biol Psychiatry. 2010;67(4):309–16. 10.1016/j.biopsych.2009.08.013 [DOI] [PubMed] [Google Scholar]
- 76. Garcia-Garcia AL, Meng Q, Richardson-Jones J, et al. : Disruption of 5-HT 1A function in adolescence but not early adulthood leads to sustained increases of anxiety. Neuroscience. 2016;321:210–21. 10.1016/j.neuroscience.2015.05.076 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 77. Donaldson ZR, Piel DA, Santos TL, et al. : Developmental effects of serotonin 1A autoreceptors on anxiety and social behavior. Neuropsychopharmacology. 2014;39(2):291–302. 10.1038/npp.2013.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sullivan GM, Ogden RT, Huang Y, et al. : Higher in vivo serotonin-1a binding in posttraumatic stress disorder: a PET study with [ 11C]WAY-100635. Depress Anxiety. 2013;30(3):197–206. 10.1002/da.22019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sullivan GM, Ogden RT, Oquendo MA, et al. : Positron emission tomography quantification of serotonin-1A receptor binding in medication-free bipolar depression. Biol Psychiatry. 2009;66(3):223–30. 10.1016/j.biopsych.2009.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hjorth S, Suchowski CS, Galloway MP: Evidence for 5-HT autoreceptor-mediated, nerve impulse-independent, control of 5-HT synthesis in the rat brain. Synapse. 1995;19(3):170–6. 10.1002/syn.890190304 [DOI] [PubMed] [Google Scholar]
- 81. Trillat AC, Malagié I, Scearce K, et al. : Regulation of serotonin release in the frontal cortex and ventral hippocampus of homozygous mice lacking 5-HT 1B receptors: in vivo microdialysis studies. J Neurochem. 1997;69(5):2019–25. 10.1046/j.1471-4159.1997.69052019.x [DOI] [PubMed] [Google Scholar]
- 82. Murrough JW, Henry S, Hu J, et al. : Reduced ventral striatal/ventral pallidal serotonin 1B receptor binding potential in major depressive disorder. Psychopharmacology (Berl). 2011;213(2–3):547–53. 10.1007/s00213-010-1881-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Whale R, Clifford EM, Bhagwagar Z, et al. : Decreased sensitivity of 5-HT 1D receptors in melancholic depression. Br J Psychiatry. 2001;178(5):454–7. 10.1192/bjp.178.5.454 [DOI] [PubMed] [Google Scholar]
- 84. Cleare AJ, Murray RM, Sherwood RA, et al. : Abnormal 5-HT 1D receptor function in major depression: a neuropharmacological challenge study using sumatriptan. Psychol Med. 1998;28(2):295–300. [DOI] [PubMed] [Google Scholar]
- 85. Stern L, Zohar J, Cohen R, et al. : Treatment of severe, drug resistant obsessive compulsive disorder with the 5HT 1D agonist sumatriptan. Eur Neuropsychopharmacol. 1998;8(4):325–8. 10.1016/S0924-977X(97)00092-8 [DOI] [PubMed] [Google Scholar]
- 86. Tatarczynska E, Antkiewicz-Michaluk L, Klodzinska A, et al. : Antidepressant-like effect of the selective 5-HT 1B receptor agonist CP 94253: a possible mechanism of action. Eur J Pharmacol. 2005;516(1):46–50. 10.1016/j.ejphar.2005.04.025 [DOI] [PubMed] [Google Scholar]
- 87. Miranda H, Ortiz G, Figueroa S, et al. : Depression scores following migraine treatment in patients attending a specialized center for headache and neurology. Headache. 2001;41(7):680–4. 10.1046/j.1526-4610.2001.041007680.x [DOI] [PubMed] [Google Scholar]
- 88. Redrobe JP, Bourin M: Evidence of the activity of lithium on 5-HT1B receptors in the mouse forced swimming test: comparison with carbamazepine and sodium valproate. Psychopharmacology (Berl ). 1999;141(4):370–7. 10.1007/s002130050846 [DOI] [PubMed] [Google Scholar]
- 89. O'Neill MF, Conway MW: Role of 5-HT 1A and 5-HT 1B receptors in the mediation of behavior in the forced swim test in mice. Neuropsychopharmacology. 2001;24(4):391–8. 10.1016/S0893-133X(00)00196-2 [DOI] [PubMed] [Google Scholar]
- 90. Knobelman DA, Hen R, Lucki I: Genetic regulation of extracellular serotonin by 5-hydroxytryptamine(1A) and 5-hydroxytryptamine(1B) autoreceptors in different brain regions of the mouse. J Pharmacol Exp Ther. 2001;298(3):1083–91. [PubMed] [Google Scholar]
- 91. Malagié I, Trillat AC, Bourin M, et al. : 5-HT 1B Autoreceptors limit the effects of selective serotonin re-uptake inhibitors in mouse hippocampus and frontal cortex. J Neurochem. 2001;76(3):865–71. 10.1046/j.1471-4159.2001.00083.x [DOI] [PubMed] [Google Scholar]
- 92. Jones MD, Lucki I: Sex differences in the regulation of serotonergic transmission and behavior in 5-HT receptor knockout mice. Neuropsychopharmacology. 2005;30(6):1039–47. 10.1038/sj.npp.1300664 [DOI] [PubMed] [Google Scholar]
- 93. Bechtholt AJ, Smith K, Gaughan S, et al. : Sucrose intake and fasting glucose levels in 5-HT 1A and 5-HT 1B receptor mutant mice. Physiol Behav. 2008;93(4–5):659–65. 10.1016/j.physbeh.2007.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Mayorga AJ, Dalvi A, Page ME, et al. : Antidepressant-like behavioral effects in 5-hydroxytryptamine(1A) and 5-hydroxytryptamine(1B) receptor mutant mice. J Pharmacol Exp Ther. 2001;298(3):1101–7. [PubMed] [Google Scholar]
- 95. Sari Y: Serotonin 1B receptors: from protein to physiological function and behavior. Neurosci Biobehav Rev. 2004;28(6):565–82. 10.1016/j.neubiorev.2004.08.008 [DOI] [PubMed] [Google Scholar]
- 96. Nautiyal KM, Tanaka KF, Barr MM, et al. : Distinct Circuits Underlie the Effects of 5-HT1B Receptors on Aggression and Impulsivity. Neuron. 2015;86(3):813–26. 10.1016/j.neuron.2015.03.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Nautiyal KM, Tritschler L, Ahmari SE, et al. : A Lack of Serotonin 1B Autoreceptors Results in Decreased Anxiety and Depression-Related Behaviors. Neuropsychopharmacology. 2016;41(12):2941–50. 10.1038/npp.2016.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Neumaier JF, Petty F, Kramer GL, et al. : Learned helplessness increases 5-hydroxytryptamine1B receptor mRNA levels in the rat dorsal raphe nucleus. Biol Psychiatry. 1997;41(6):668–74. 10.1016/S0006-3223(96)00114-X [DOI] [PubMed] [Google Scholar]
- 99. Neumaier JF, Edwards E, Plotsky PM: 5-HT 1B mrna regulation in two animal models of altered stress reactivity. Biol Psychiatry. 2002;51(11):902–8. 10.1016/S0006-3223(01)01371-3 [DOI] [PubMed] [Google Scholar]
- 100. Clark MS, Sexton TJ, McClain M, et al. : Overexpression of 5-HT 1B receptor in dorsal raphe nucleus using Herpes Simplex Virus gene transfer increases anxiety behavior after inescapable stress. J Neurosci. 2002;22(11):4550–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Neumaier JF, Root DC, Hamblin MW: Chronic fluoxetine reduces serotonin transporter mRNA and 5-HT 1B mRNA in a sequential manner in the rat dorsal raphe nucleus. Neuropsychopharmacology. 1996;15(5):515–22. 10.1016/S0893-133X(96)00095-4 [DOI] [PubMed] [Google Scholar]
- 102. Anthony JP, Sexton TJ, Neumaier JF: Antidepressant-induced regulation of 5-HT 1b mRNA in rat dorsal raphe nucleus reverses rapidly after drug discontinuation. J Neurosci Res. 2000;61(1):82–7. [DOI] [PubMed] [Google Scholar]
- 103. Davidson C, Stamford JA: Effect of chronic paroxetine treatment on 5-HT 1B and 5-HT 1D autoreceptors in rat dorsal raphe nucleus. Neurochem Int. 2000;36(2):91–6. 10.1016/S0197-0186(99)00115-1 [DOI] [PubMed] [Google Scholar]
- 104. Tiger M, Rück C, Forsberg A, et al. : Reduced 5-HT 1B receptor binding in the dorsal brain stem after cognitive behavioural therapy of major depressive disorder. Psychiatry Res. 2014;223(2):164–70. 10.1016/j.pscychresns.2014.05.011 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 105. Chenu F, David DJ, Leroux-Nicollet I, et al. : Serotonin 1B heteroreceptor activation induces an antidepressant-like effect in mice with an alteration of the serotonergic system. J Psychiatry Neurosci. 2008;33(6):541–50. [PMC free article] [PubMed] [Google Scholar]
- 106. Svenningsson P, Chergui K, Rachleff I, et al. : Alterations in 5-HT 1B receptor function by p11 in depression-like states. Science. 2006;311(5757):77–80. 10.1126/science.1117571 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 107. Alexander B, Warner-Schmidt J, Eriksson T, et al. : Reversal of depressed behaviors in mice by p11 gene therapy in the nucleus accumbens. Sci Transl Med. 2010;2(54):54ra76. 10.1126/scitranslmed.3001079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Lim BK, Huang KW, Grueter BA, et al. : Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature. 2012;487(7406):183–9. 10.1038/nature11160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Brown AS, Gershon S: Dopamine and depression. J Neural Transm Gen Sect. 1993;91(2–3):75–109. 10.1007/BF01245227 [DOI] [PubMed] [Google Scholar]
- 110. D'Aquila PS, Collu M, Gessa GL, et al. : The role of dopamine in the mechanism of action of antidepressant drugs. Eur J Pharmacol. 2000;405(1–3):365–73. 10.1016/S0014-2999(00)00566-5 [DOI] [PubMed] [Google Scholar]
- 111. Nestler EJ, Carlezon WA, Jr: The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59(12):1151–9. 10.1016/j.biopsych.2005.09.018 [DOI] [PubMed] [Google Scholar]
- 112. Heshmati M, Russo SJ: Anhedonia and the brain reward circuitry in depression. Curr Behav Neurosci Rep. 2015;2(3):146–53. 10.1007/s40473-015-0044-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Barot SK, Ferguson SM, Neumaier JF: 5-HT 1B receptors in nucleus accumbens efferents enhance both rewarding and aversive effects of cocaine. Eur J Neurosci. 2007;25(10):3125–31. 10.1111/j.1460-9568.2007.05568.x [DOI] [PubMed] [Google Scholar]
- 114. Dolen G, Darvishzadeh A, Huang KW, et al. : Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature. 2013;501(7466):179–84. 10.1038/nature12518 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 115. Zouk H, McGirr A, Lebel V, et al. : The effect of genetic variation of the serotonin 1B receptor gene on impulsive aggressive behavior and suicide. Am J Med Genet B Neuropsychiatr Genet. 2007;144B(8):996–1002. 10.1002/ajmg.b.30521 [DOI] [PubMed] [Google Scholar]
- 116. Potenza MN, Walderhaug E, Henry S, et al. : Serotonin 1B receptor imaging in pathological gambling. World J Biol Psychiatry. 2013;14(2):139–45. 10.3109/15622975.2011.598559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. O'Dell LE, Parsons LH: Serotonin 1B receptors in the ventral tegmental area modulate cocaine-induced increases in nucleus accumbens dopamine levels. J Pharmacol Exp Ther. 2004;311(2):711–9. 10.1124/jpet.104.069278 [DOI] [PubMed] [Google Scholar]
- 118. Contini V, Bertuzzi GP, Polina ER, et al. : A haplotype analysis is consistent with the role of functional HTR1B variants in alcohol dependence. Drug Alcohol Depend. 2012;122(1–2):100–4. 10.1016/j.drugalcdep.2011.09.020 [DOI] [PubMed] [Google Scholar]
- 119. Proudnikov D, LaForge KS, Hofflich H, et al. : Association analysis of polymorphisms in serotonin 1B receptor (HTR1B) gene with heroin addiction: a comparison of molecular and statistically estimated haplotypes. Pharmacogenet Genomics. 2006;16(1):25–36. 10.1097/01.fpc.0000182782.87932.d6 [DOI] [PubMed] [Google Scholar]
- 120. Cao J, LaRocque E, Li D: Associations of the 5-hydroxytryptamine (serotonin) receptor 1B gene ( HTR1B) with alcohol, cocaine, and heroin abuse. Am J Med Genet B Neuropsychiatr Genet. 2013;162B(2):169–76. 10.1002/ajmg.b.32128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Petry NM, Stinson FS, Grant BF: Comorbidity of DSM-IV pathological gambling and other psychiatric disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2005;66(5):564–74. 10.4088/JCP.v66n0504 [DOI] [PubMed] [Google Scholar]
- 122. Matuskey D, Bhagwagar Z, Planeta B, et al. : Reductions in brain 5-HT 1B receptor availability in primarily cocaine-dependent humans. Biol Psychiatry. 2014;76(10):816–22. 10.1016/j.biopsych.2013.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 123. Rocha BA, Scearce-Levie K, Lucas JJ, et al. : Increased vulnerability to cocaine in mice lacking the serotonin-1B receptor. Nature. 1998;393(6681):175–8. 10.1038/30259 [DOI] [PubMed] [Google Scholar]
- 124. Pentkowski NS, Acosta JI, Browning JR, et al. : Stimulation of 5-HT 1B receptors enhances cocaine reinforcement yet reduces cocaine-seeking behavior. Addict Biol. 2009;14(4):419–30. 10.1111/j.1369-1600.2009.00162.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Neumaier JF, Vincow ES, Arvanitogiannis A, et al. : Elevated expression of 5-HT1B receptors in nucleus accumbens efferents sensitizes animals to cocaine. J Neurosci. 2002;22(24):10856–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Pentkowski NS, Cheung TH, Toy WA, et al. : Protracted withdrawal from cocaine self-administration flips the switch on 5-HT 1B receptor modulation of cocaine abuse-related behaviors. Biol Psychiatry. 2012;72(5):396–404. 10.1016/j.biopsych.2012.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Conner TS, Jensen KP, Tennen H, et al. : Functional polymorphisms in the serotonin 1B receptor gene ( HTR1B) predict self-reported anger and hostility among young men. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(1):67–78. 10.1002/ajmg.b.30955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Pattij T, Broersen LM, van der Linde J, et al. : Operant learning and differential-reinforcement-of-low-rate 36-s responding in 5-HT 1A and 5-HT 1B receptor knockout mice. Behav Brain Res. 2003;141(2):137–45. 10.1016/S0166-4328(02)00345-5 [DOI] [PubMed] [Google Scholar]
- 129. Saudou F, Amara DA, Dierich A, et al. : Enhanced aggressive behavior in mice lacking 5-HT1B receptor. Science. 1994;265(5180):1875–8. 10.1126/science.8091214 [DOI] [PubMed] [Google Scholar]
- 130. Olivier B, Mos J, van Oorschot R, et al. : Serotonin receptors and animal models of aggressive behavior. Pharmacopsychiatry. 1995;28(Suppl 2):80–90. 10.1055/s-2007-979624 [DOI] [PubMed] [Google Scholar]
- 131. de Boer SF, Koolhaas JM: 5-HT 1A and 5-HT 1B receptor agonists and aggression: a pharmacological challenge of the serotonin deficiency hypothesis. Eur J Pharmacol. 2005;526(1–3):125–39. 10.1016/j.ejphar.2005.09.065 [DOI] [PubMed] [Google Scholar]
- 132. Rush AJ, Trivedi MH, Wisniewski SR, et al. : Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905–17. 10.1176/ajp.2006.163.11.1905 [DOI] [PubMed] [Google Scholar]
- 133. Quesseveur G, Reperant C, David DJ, et al. : 5-HT 2A receptor inactivation potentiates the acute antidepressant-like activity of escitalopram: involvement of the noradrenergic system. Exp Brain Res. 2013;226(2):285–95. 10.1007/s00221-013-3434-3 [DOI] [PubMed] [Google Scholar]
- 134. Patel JG, Bartoszyk GD, Edwards E, et al. : The highly selective 5-hydroxytryptamine (5-HT) 2A receptor antagonist, EMD 281014, significantly increases swimming and decreases immobility in male congenital learned helpless rats in the forced swim test. Synapse. 2004;52(1):73–5. 10.1002/syn.10308 [DOI] [PubMed] [Google Scholar]
- 135. Weisstaub NV, Zhou M, Lira A, et al. : Cortical 5-HT 2A receptor signaling modulates anxiety-like behaviors in mice. Science. 2006;313(5786):536–40. 10.1126/science.1123432 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 136. Meltzer HY: The role of serotonin in antipsychotic drug action. Neuropsychopharmacology. 1999;21(2 Suppl):106S–115S. 10.1016/S0893-133X(99)00046-9 [DOI] [PubMed] [Google Scholar]
- 137. Halberstadt AL, Geyer MA: Multiple receptors contribute to the behavioral effects of indoleamine hallucinogens. Neuropharmacology. 2011;61(3):364–81. 10.1016/j.neuropharm.2011.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Diaz SL, Doly S, Narboux-Neme N, et al. : 5-HT 2B receptors are required for serotonin-selective antidepressant actions. Mol Psychiatry. 2012;17(2):154–63. 10.1038/mp.2011.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Diaz SL, Narboux-Neme N, Boutourlinsky K, et al. : Mice lacking the serotonin 5-HT 2B receptor as an animal model of resistance to selective serotonin reuptake inhibitors antidepressants. Eur Neuropsychopharmacol. 2016;26(2):265–79. 10.1016/j.euroneuro.2015.12.012 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 140. Bevilacqua L, Doly S, Kaprio J, et al. : A population-specific HTR2B stop codon predisposes to severe impulsivity. Nature. 2010;468(7327):1061–6. 10.1038/nature09629 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 141. Cryan JF, Lucki I: Antidepressant-like behavioral effects mediated by 5-hydroxytryptamine 2C receptors. J Pharmacol Exp Ther. 2000;295(3):1120–6. [PubMed] [Google Scholar]
- 142. Opal MD, Klenotich SC, Morais M, et al. : Serotonin 2C receptor antagonists induce fast-onset antidepressant effects. Mol Psychiatry. 2014;19(10):1106–14. 10.1038/mp.2013.144 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 143. Heisler LK, Zhou L, Bajwa P, et al. : Serotonin 5-HT 2C receptors regulate anxiety-like behavior. Genes Brain Behav. 2007;6(5):491–6. 10.1111/j.1601-183X.2007.00316.x [DOI] [PubMed] [Google Scholar]
- 144. Higgins GA, Silenieks LB, Altherr EB, et al. : Lorcaserin and CP-809101 reduce motor impulsivity and reinstatement of food seeking behavior in male rats: Implications for understanding the anti-obesity property of 5-HT 2C receptor agonists. Psychopharmacology (Berl). 2016;233(14):2841–56. 10.1007/s00213-016-4329-3 [DOI] [PubMed] [Google Scholar]
- 145. Griebel G, Perrault G, Sanger DJ: A comparative study of the effects of selective and non-selective 5-HT 2 receptor subtype antagonists in rat and mouse models of anxiety. Neuropharmacology. 1997;36(6):793–802. 10.1016/S0028-3908(97)00034-8 [DOI] [PubMed] [Google Scholar]
- 146. Ramamoorthy R, Radhakrishnan M, Borah M: Antidepressant-like effects of serotonin type-3 antagonist, ondansetron: an investigation in behaviour-based rodent models. Behav Pharmacol. 2008;19(1):29–40. 10.1097/FBP.0b013e3282f3cfd4 [DOI] [PubMed] [Google Scholar]
- 147. Kondo M, Nakamura Y, Ishida Y, et al. : The 5-HT3 receptor is essential for exercise-induced hippocampal neurogenesis and antidepressant effects. Mol Psychiatry. 2015;20(11):1428–37. 10.1038/mp.2014.153 [DOI] [PubMed] [Google Scholar]
- 148. Bhatnagar S, Nowak N, Babich L, et al. : Deletion of the 5-HT 3 receptor differentially affects behavior of males and females in the Porsolt forced swim and defensive withdrawal tests. Behav Brain Res. 2004;153(2):527–35. 10.1016/j.bbr.2004.01.018 [DOI] [PubMed] [Google Scholar]
- 149. Costall B, Naylor RJ: Anxiolytic potential of 5-HT 3 receptor antagonists. Pharmacol Toxicol. 1992;70(3):157–62. 10.1111/j.1600-0773.1992.tb00448.x [DOI] [PubMed] [Google Scholar]
- 150. Lucas G, Rymar VV, Du J, et al. : Serotonin 4 (5-HT 4) receptor agonists are putative antidepressants with a rapid onset of action. Neuron. 2007;55(5):712–25. 10.1016/j.neuron.2007.07.041 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 151. Mendez-David I, David DJ, Darcet F, et al. : Rapid anxiolytic effects of a 5-HT 4 receptor agonist are mediated by a neurogenesis-independent mechanism. Neuropsychopharmacology. 2014;39(6):1366–78. 10.1038/npp.2013.332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Compan V, Zhou M, Grailhe R, et al. : Attenuated response to stress and novelty and hypersensitivity to seizures in 5-HT 4 receptor knock-out mice. J Neurosci. 2004;24(2):412–9. 10.1523/JNEUROSCI.2806-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Jean A, Conductier G, Manrique C, et al. : Anorexia induced by activation of serotonin 5-HT 4 receptors is mediated by increases in CART in the nucleus accumbens. Proc Natl Acad Sci U S A. 2007;104(41):16335–40. 10.1073/pnas.0701471104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Waeber C, Grailhe R, Yu XJ, et al. : Putative 5-Ht 5 receptors: localization in the mouse CNS and lack of effect in the inhibition of dural protein extravasation. Ann N Y Acad Sci. 1998;861:85–90. 10.1111/j.1749-6632.1998.tb10177.x [DOI] [PubMed] [Google Scholar]
- 155. Svenningsson P, Tzavara ET, Qi H, et al. : Biochemical and behavioral evidence for antidepressant-like effects of 5-HT 6 receptor stimulation. J Neurosci. 2007;27(15):4201–9. 10.1523/JNEUROSCI.3110-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Carr GV, Schechter LE, Lucki I: Antidepressant and anxiolytic effects of selective 5-HT 6 receptor agonists in rats. Psychopharmacology (Berl). 2011;213(2–3):499–507. 10.1007/s00213-010-1798-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Mitchell ES, Neumaier JF: 5-HT 6 receptors: a novel target for cognitive enhancement. Pharmacol Ther. 2005;108(3):320–33. 10.1016/j.pharmthera.2005.05.001 [DOI] [PubMed] [Google Scholar]
- 158. Hamon M, Doucet E, Lefèvre K, et al. : Antibodies and antisense oligonucleotide for probing the distribution and putative functions of central 5-HT 6 receptors. Neuropsychopharmacology. 1999;21(2 Suppl):68S–76S. 10.1016/S0893-133X(99)00044-5 [DOI] [PubMed] [Google Scholar]
- 159. Guscott M, Bristow LJ, Hadingham K, et al. : Genetic knockout and pharmacological blockade studies of the 5-HT 7 receptor suggest therapeutic potential in depression. Neuropharmacology. 2005;48(4):492–502. 10.1016/j.neuropharm.2004.11.015 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 160. Ballaz SJ, Akil H, Watson SJ: The 5-HT 7 receptor: role in novel object discrimination and relation to novelty-seeking behavior. Neuroscience. 2007;149(1):192–202. 10.1016/j.neuroscience.2007.07.043 [DOI] [PubMed] [Google Scholar]