Abstract
Therapeutic protein drugs are an important class of medicines serving patients most in need of novel therapies. Recently approved recombinant protein therapeutics have been developed to treat a wide variety of clinical indications, including cancers, autoimmunity/inflammation, exposure to infectious agents, and genetic disorders. The latest advances in protein-engineering technologies have allowed drug developers and manufacturers to fine-tune and exploit desirable functional characteristics of proteins of interest while maintaining (and in some cases enhancing) product safety or efficacy or both. In this review, we highlight the emerging trends and approaches in protein drug development by using examples of therapeutic proteins approved by the U.S. Food and Drug Administration over the previous five years (2011–2016, namely January 1, 2011, through August 31, 2016).
Keywords: therapeutic protein drugs, protein therapeutics, cancer therapeutics, biosimilar, recombinant DNA-derived therapeutic proteins
Protein engineering
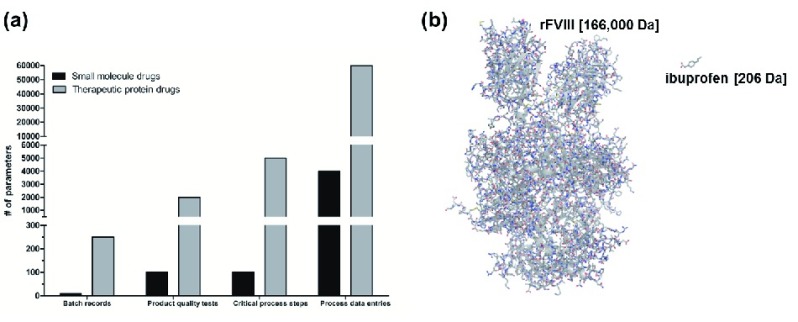
The manufacturing and production of therapeutic proteins are highly complex processes 1– 3. For example, a typical protein drug may include in excess of 5,000 critical process steps, many times greater than the number required for manufacturing a small-molecule drug 4 ( Figure 1a).
Figure 1. Complexity of therapeutic protein drugs.
( a) Graphical representation of the complexity of the manufacture of a therapeutic protein drug compared with a small-molecule drug. The number of batch records, product quality tests, critical process steps, and process data entries associated with small-molecule drugs (black) and therapeutic protein drugs (grey) as bars 4. ( b) Illustration depicting the differences in size and complexity of a protein therapeutic (recombinant (r) analogue of human coagulation factor VIII (FVIII); Novoeight, Novo Nordisk; molecular weight = 166,000 Da) and a small-molecule drug (ibuprofen; molecular weight = 206 Da) by molecular model.
Similarly, protein therapeutics, which include monoclonal antibodies as well as large or fusion proteins, can be orders-of-magnitude larger in size than small-molecule drugs, having molecular weights exceeding 100 kDa ( Figure 1b). In addition, protein therapeutics exhibit complex secondary and tertiary structures that must be maintained. Protein therapeutics cannot be completely synthesized by chemical processes and have to be manufactured in living cells or organisms; consequently, the choices of the cell line, species origin, and culture conditions all affect the final product characteristics 5– 7. Moreover, most biologically active proteins require post-translational modifications that can be compromised when heterologous expression systems are used. Additionally, as the products are synthesized by cells or organisms, complex purification processes are involved. Furthermore, viral clearance processes such as removal of virus particles by using filters or resins, as well as inactivation steps by using low pH or detergents, are implemented to prevent the serious safety issue of viral contamination of protein drug substances 8. Given the complexity of therapeutic proteins with respect to their large molecular size, post-translational modifications, and the variety of biological materials involved in their manufacturing process, the ability to enhance particular functional attributes while maintaining product safety and efficacy achieved through protein-engineering strategies is highly desirable.
While the integration of novel strategies and approaches to modify protein drug products is not a trivial matter 9, the potential therapeutic advantages have driven the increased use of such strategies during drug development. A number of protein-engineering platform technologies are currently in use to increase the circulating half-life, targeting, and functionality of novel therapeutic protein drugs as well as to increase production yield and product purity ( Table 1) 5– 7, 10– 12. For example, protein conjugation and derivatization approaches, including Fc-fusion 13, 14, albumin-fusion 15, and PEGylation 16, are currently being used to extend a drug’s circulating half-life 17. Longer in vivo half-lives are of particular importance to patients undergoing factor/enzyme/hormone replacement therapy, in which frequent dosing regimens can result in substantial negative impacts on patient well-being in terms of ease of administration and compliance, especially in young children 18. Protein-engineering approaches have also been employed to target drugs through the addition of signaling peptides or the generation of antibody-drug conjugates 19, thereby limiting toxicity and increasing drug efficacy. Additionally, exploiting particular functional characteristics of a protein drug can be accomplished through protein engineering. For example, influencing a protein’s glycosylation pattern through engineering strategies can impact the protein’s receptor-binding properties and overall effector function 20, 21. In Table 1, we have highlighted a few examples of the many technological innovations and protein-engineering platform technologies incorporated by recently approved therapeutic proteins.
Table 1. Protein-engineering platform technologies.
| Platform technology | Example of U.S. Food and Drug Administration-approved therapeutic protein |
|---|---|
| Protein production technologies | |
| Production of proteins in transgenic
animals 46 |
C1 esterase inhibitor (Ruconest) produced in transgenic rabbit milk 47 |
| Production of proteins in transgenic
plants 48 |
Human glucocerebrosidase (Elelyso) produced in carrot root cells 49, 50 |
| Rational protein structure/function technologies | |
| Glyco-engineering 20, 21 | Humanized anti-CD20 monoclonal antibody (Gazyva) 51 |
| Fc fusion 13, 14 | VEGFR Fc-fusion (Eylea) |
| CTLA-4 Fc-fusion (Nulojix) | |
| Glucagon-like peptide-
1 receptor agonist Fc-fusion (Trulicity) | |
| VEGFR Fc-fusion (Zaltrap) | |
| Recombinant factor IX Fc fusion (Alprolix) 52 | |
| Recombinant factor VIII Fc-fusion (Eloctate) 53, 54 | |
| Albumin fusion 15 | GLP-1 receptor agonist-albumin fusion (Tanzeum) |
| Recombinant factor IX albumin fusion (Idelvion) | |
| PEGylation 55 | PEGylated IFNβ-1a (Plegridy) |
| Recombinant factor VIII PEGylated (Adynovate) | |
| Antibody-drug conjugates 19 | Humanized anti-HER2/neu conjugated to emtansine (Kadcyla) |
| Mouse/human chimeric anti-CD30 (Adcetris) | |
| mAb humanization/chimerism | Humanized mAbs |
| Anti-human epidermal growth factor receptor 2 (HER2) (Perjeta) | |
| Anti-HER2/neu conjugated to emtansine (Kadcyla) | |
| Anti-IL-6 receptor (Actemra) | |
| Anti-CD20 (obinutuzumab; Gazyva) | |
| Anti-integrin a4b7 (LPAM-1) (Entyvio) | |
| Anti-PD-1 (Keytruda) | |
| Anti-dabigatran (Praxbind) | |
| Anti-IL-5 (Nucala) | |
| Anti-CD319 (SLAMF7) (Empliciti) | |
| Anti-IL-17a (Taltz) | |
| Anti-IL-5 (Cinqair) | |
| Anti-PD-L1 (Tecentriq) | |
| Anti-CD25 (Zinbryta) | |
| Mouse/human chimeric mAbs | |
| Anti-CD30 (Adcetris) | |
| Anti-IL-6 (Sylvant) | |
| Anti-GD2 (Unituxin) | |
| Anti- Bacillus anthracis (Anthim) | |
| Anti-TNFα (Inflectra) | |
Listing of commonly used protein-engineering platform technologies and examples of U.S. Food and Drug Administration-approved therapeutic proteins (2011–2016, namely January 1, 2011, through August 31, 2016) that employ each strategy. CD, cluster of differentiation; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; Fc, fragment crystallizable; GD2, disialoganglioside; GLP-1, glucagon-like peptide-1; HER2, human epidermal growth factor receptor 2; IFNb, interferon beta; IL, interleukin; LPAM-1, lymphocyte Peyer’s Patch adhesion molecule; mAb, monoclonal antibody; PD-1, programmed death receptor-1; PD-L1, programmed death-ligand 1; PEG, polyethylene glycol; SLAMF7, SLAM family member 7; TNFα, tumor necrosis factor alpha; VEGFR, vascular endothelial growth factor receptor.
Overview of recently approved protein therapeutics (2011–2016*)
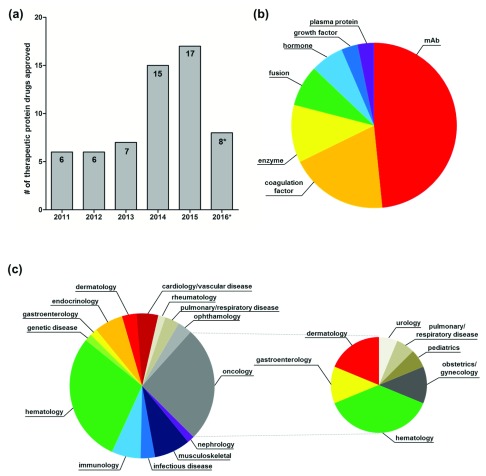
Since 2011, the U.S. Food and Drug Administration Center for Drug Evaluation and Review (CDER) and the Center for Biologics Evaluation and Review (CBER) combined have approved 62 recombinant therapeutic proteins (*January 1, 2011, through August 31, 2016; “Purple Book” list of licensed biological products, including biosimilar and interchangeable biological products 22) ( Figure 2a). Of these 62 therapeutic proteins, almost half (48%) were monoclonal antibodies (for this analysis, we included antibody-drug conjugates and antibody fragment antigen binding in this group). Coagulation factors were the next largest class (19%) of approved protein drugs over this time period. Replacement enzymes comprised 11% of all approvals. Remaining approvals (22%) were divided among fusion proteins, hormones, growth factors, and plasma proteins ( Figure 2b). These U.S. Food and Drug Administration (FDA)-approved therapeutic proteins are indicated for a wide variety of therapeutic areas. Over half of the approved therapeutic proteins were indicated for oncology (26%) and hematology (29%), whereas the remaining 45% had primary indications in cardiology/vascular disease (5%), dermatology (3%), endocrinology (6%), gastroenterology (2%), genetic disease (2%), immunology (6%), infectious diseases (3%), musculoskeletal (8%), nephrology (2%), ophthalmology (3%), pulmonary/respiratory disease (3%), and rheumatology (2%) ( Figure 2c, left). Of the 16 oncology drugs, six were approved to treat hematologic malignancies, whereas the remaining therapeutics were indicated for dermatology (3), gastroenterology (2), obstetrics/gynecology (2), pediatrics (1), pulmonary/respiratory disease (1), and urology (1) ( Figure 2c, right). For a complete listing of the approved products, see Table 2. It is evident that recently approved therapeutic proteins serve a wide spectrum of patient populations and are of great benefit to public health.
Figure 2. U.S. Food and Drug Administration (FDA)-approved therapeutic proteins (2011–2016*).
( a) Bar graph showing the number of therapeutic protein FDA approvals by year (2011–2016*). ( b) Pie chart showing the distribution of FDA-approved therapeutic proteins (2011–2016*) by drug class. ( c) (Left) Pie chart showing the distribution of FDA-approved therapeutic proteins (2011–2016*) by therapeutic area. (Right) Pie chart showing the distribution of secondary therapeutic area for oncology drugs. *January 1, 2011, through August 31, 2016.
Table 2. U.S. Food and Drug Administration-approved protein therapeutics (2011–2016).
| CDER approved protein therapeutics [2011–2016*] | |||
|---|---|---|---|
| #
Approval Date |
Drug
[Market Name; Sponsor] |
Class
[Description] |
Therapeutic Area
[General Indication] |
|
1
3/9/2011 |
belimumab
[ Benlysta; Human Genome Sciences] |
mAb
[human anti-B-cell activating factor (BAFF)] |
immunology
[autoimmunity (lupus)] |
|
2
3/25/2011 |
ipilimumab
[ Yervoy; Bristol Myers Squibb] |
mAb
[human anti-CTLA-4] |
dermatology/oncology
[cancer (melanoma)] |
|
3
6/15/2011 |
belatacept
[ Nulojix; Bristol Myers Squibb] |
Fc fusion
[CTLA-4 Fc-fusion] |
immunology/nephrology
[transplant rejection (kidney)] |
|
4
8/19/2011 |
brentuximab vedotin
[ Adcetris; Seattle Genetics] |
antibody-drug conjugate
[mouse/human chimeric anti- CD30] |
hematology/oncology
[cancer (lymphoma)] |
|
5
11/18/2011 |
afilbercept
[ Eylea; Regeneron Pharmaceuticals] |
Fc fusion
[VEGFR Fc-fusion] |
ophthalmology
[macular degeneration] |
|
6
11/18/2011 |
asparaginase erwinia
chrysanthemi [ Erwinaze; Jazz Pharmaceuticals] |
enzyme
[asparaginase erwinia chrysanthemi] |
hematology/oncology
[cancer (leukemia)] |
|
7
1/17/2012 |
glucarpidase
[ Voraxaze; BTG International] |
enzyme
[glucarpidase] |
nephrology
[kidney failure] |
|
8
5/1/2012 |
taliglucerase alfa
[ Elelyso; Pfizer] |
enzyme
[β-glucocerebrosidase] |
endocrinology/gastroenterology
[genetic disorder (Gaucher)] |
|
9
6/8/2012 |
pertuzumab
[ Perjeta; Genentech] |
mAb
[humanized anti-human epidermal growth factor receptor 2 (HER2)] |
obstetrics, gynecology/oncology
[cancer (breast)] |
|
10
8/3/2012 |
ziv-afilbercept
[ Zaltrap; Sanofi-Aventis U.S.] |
Fc fusion
[VEGFR Fc fusion] |
gastroenterology/oncology
[cancer (colorectal)] |
|
11
8/29/2012 |
tbo-filgrastim
[ Granix; Cephalon] |
growth factor
[G-CSF] |
hematology/oncology
[neutropenia] |
|
12
10/17/2012 |
ocriplasmin
[ Jetrea; ThromboGenics] |
enzyme
[ocriplasmin] |
ophthalmology
[eye condition (symptomatic vitreomacular adhesion)] |
|
13
12/14/2012 |
raxibacumab
[ raxibacumab; Human Genome Sciences] |
mAb
[human anti-anthrax protective antigen (PA)] |
infections and infectious disease
[infectious disease (inhalational anthrax)] |
|
14
2/22/2013 |
ado-trastuzumab
emtansine [ Kadcyla; Genentech] |
antibody-drug conjugate
[humanized anti-HER2/neu conjugated to emtansine] |
obstetrics, gynecology/oncology
[cancer (breast)] |
|
15
7/18/2013 |
golimumab injection, for
IV use [ Simponi Aria; Janssen Biotech] |
mAb
[human anti-TNFα] |
musculoskeletal/rheumatology
[autoimmunity (rheumatoid arthritis)] |
|
16
10/21/2013 |
tocilizumab
[ Actemra; Genentech] |
mAb
[humanized anti-IL-6 receptor] |
musculoskeletal/rheumatology
[autoimmunity (rheumatoid arthritis; juvenile idiopathic arthritis)] |
|
17
11/1/2013 |
obinutuzumab
[ Gazyva; Genentech] |
mAb
[humanized anti-CD20] |
hematology/oncology
[cancer (leukemia)] |
|
18
2/14/2014 |
elosulfase alfa
[ Vimizim; BioMarin Pharmaceutical] |
enzyme
[elosulfase alfa] |
musculoskeletal/genetic disease
[genetic disorder (Morquio A)] |
|
19
2/24/2014 |
metreleptin
[ Myalept; Amylin Pharmaceuticals] |
hormone
[metreleptin] |
immunology
[lipodystrophy] |
|
20
4/15/2014 |
albiglutide
[ Tanzeum; GlaxoSmithKline] |
albumin fusion/hormone
[glucagon-like peptide-1 dimer albumin fusion] |
endocrinology
[diabetes (type 2)] |
|
21
4/21/2014 |
ramucirumab
[ Cyramza; Eli Lilly and Company] |
mAb
[human anti-VEGFR2 (KDR)] |
gastroenterology/oncology
[cancer (stomach; gastroesophageal junction)] |
|
22
4/23/2014 |
siltuximab
[ Sylvant; Janssen Biotech] |
mAb
[mouse/human chimeric anti- IL-6] |
hematology/immunology
[multicentric Castleman's disease] |
|
23
5/20/2014 |
vedolizumab
[ Entyvio; Takeda Pharmaceuticals America] |
mAb
[humanized anti-integrin a4b7 (lymphocyte Peyer's Patch adhesion molecule; LPAM-1)] |
gastroenterology/immunology
[inflammatory (ulcerative colitis/Crohn's disease)] |
|
24
8/15/2014 |
peginterferon beta-1a
[ Plegridy; Biogen Idec] |
cytokine
[PEGylated IFNb-1b] |
immunology/musculoskeletal
[multiple sclerosis] |
|
25
9/4/2014 |
pembrolizumab
[ Keytruda; Merck Sharp & Dohme] |
mAb
[humanized anti-PD-1] |
dermatology/oncology
[cancer (melanoma)] |
|
26
9/18/2014 |
dulaglutide
[ Trulicity; Eli Lilly and Company] |
Fc fusion
[glucagon-like peptide-1 receptor agonist] |
endocrinology
[diabetes (type 2)] |
|
27
12/3/2014 |
blintumomab
[ Blincyto; Amgen] |
mAb
[mouse bispecific anti-CD19/ anti-CD3] |
hematology/oncology
[cancer (leukemia)] |
|
28
12/22/2014 |
nivolumab
[ Opdivo; Bristol Myers Squibb] |
mAb
[human anti-PD-1] |
dermatology/oncology
[cancer (melanoma)] |
|
29
1/21/2015 |
secukinumab
[ Cosentyx; Novartis Pharmaceuticals] |
mAb
[human anti-IL-17A] |
dermatology/immunology
[autoimmunity (plaque psoriasis)] |
|
30
1/23/2015 |
parathyroid hormone
[ Natpara; NPS Pharmaceuticals] |
hormone
[parathyroid hormone] |
endocrinology/hematology
[hypoparathyroidism] |
|
31
3/6/2015 |
filgrastim-sndz
[ Zarxio; Sandoz] |
growth factor
[G-CSF] |
hematology/oncology
[neutropenia] |
|
32
3/10/2015 |
dinutuximab
[ Unituxin; United Therapeutics] |
mAb
[mouse/human chimeric anti- GD2] |
oncology/pediatrics/neonatalogy
[cancer (neuroblastoma)] |
|
33
7/24/2015 |
alirocumab
[ Praluent; Sanofi-Aventis U.S.] |
mAb
[human anti-proprotein convertase substilisin/kexin type 9 (PCSK9)] |
cardiology/vascular diseases
[high cholesterol] |
|
34
8/27/2015 |
evolocumab
[ Repatha; Amgen] |
mAb
[human anti-proprotein convertase substilisin/kexin type 9 (PCSK9)] |
cardiology/vascular diseases
[high cholesterol] |
|
35
10/16/2015 |
idarucizumab
[ Praxbind; Boehringer Ingelheim Pharmaceuticals] |
Fab
[humanized anti-dabigatran] |
hematology
[anticoagulant reversal] |
|
36
10/23/2015 |
asfotase-alfa
[ Strensiq; Alexion Pharmaceuticals] |
Fc fusion/enzyme
[tissue non-specific alkaline phosphatase/Fc fusion/deca- asparatate (D10) peptide] |
genetic disease/pediatrics/neonatalogy
[genetic disorder (hypophosphatasia)] |
|
37
11/4/2015 |
mepolizumab
[ Nucala; GlaxoSmithKline] |
mAb
[humanized anti-IL-5] |
pulmonary/respiratory disease
[asthma] |
|
38
11/16/2015 |
daratumumab
[ Darzalex; Janssen Biotech] |
mAb
[human anti-CD38] |
hematology/oncology
[cancer (multiple myeloma)] |
|
39
11/24/2015 |
necitumumab
[ Portrazza; Eli Lilly and Company] |
mAb
[human anti-epidermal growth factor receptor (EGFR)] |
pulmonary/respiratory disease/oncology
[cancer (lung)] |
|
40
11/30/2015 |
elotuzumab
[ Empliciti; Bristol Myers Squibb] |
mAb
[humanized anti- CD319(SLAMF7)] |
oncology
[cancer (multiple myeloma)] |
|
41
12/8/2015 |
sebelipase alfa
[ Kanuma; Alexion Pharmaceuticals] |
enzyme
[lysosomal acid lipase] |
cardiology/vascular diseases/genetic
disease [lysosomal acid lipase deficiency] |
|
42
3/18/2016 |
obiltoxaximab
[ Anthim; Elusys Therapeutics] |
mAb
[mouse/human chimeric anti- Bacillus anthracis] |
infections and infectious disease
[infectious disease (inhalational anthrax)] |
|
43
3/22/2016 |
ixekizumab
[ Taltz; Eli Lilly and Company] |
mAb
[humanized anti-IL-17a] |
dermatology/immunology
[autoimmunity (plaque psoriasis)] |
|
44
3/23/2016 |
reslizumab
[ Cinqair; Teva Respiratory] |
mAb
[humanized anti-IL-5] |
pulmonary/respiratory disease
[asthma] |
|
45
4/5/2016 |
infliximab-dyyb
[ Inflectra; Celltrion] |
mAb
[mouse/human chimeric anti- TNFα] |
musculoskeletal/rheumatology
[inflammatory (Crohn's disease/ulcerative colitis/rheumatoid arthritis/ankylosing spondylitis/psoriatic arthritis/plaque psoriasis)] |
|
46
5/18/2016 |
atezolizumab
[ Tecentriq; Genentech] |
mAb
[humanized anti-PD-L1] |
urology/oncology
[cancer (bladder)] |
|
47
5/27/2016 |
daclizumab
[ Zinbryta; Biogen] |
mAb
[humanized anti-CD25] |
musculoskeletal/neurology
[multiple sclerosis] |
|
48
8/30/2016 |
etanercept-szzs
[ Erelzi; Sandoz] |
Fc fusion
[TNFR Fc-fusion] |
rheumatology
[inflammatory (rheumatoid arthritis/juvenile idiopathic arthritis/psoriatic arthritis/ankylosing spondylitis/plaque psoriasis)] |
| CBER approved protein therapeutics [2011–2016*] | |||
| #
Approval Date |
Drug Name
[Market Name; Sponsor] |
Class
Description |
Therapeutic Area |
|
1
6/26/2013 |
coagulation factor IX
recombinant human [ Rixubis; Baxter Healthcare] |
coagulation factor
[recombinant factor IX] |
hematology
[hemophilia B] |
|
2
10/15/2013 |
antihemophilic factor
(recombinant) [ Novoeight; Novo Nordisk] |
coagulation factor
[recombinant factor VIII] |
hematology
[hemophilia A] |
|
3
12/23/2013 |
coagulation factor XIII A-
subunit (recombinant) [ Tretten; Novo Nordisk] |
coagulation factor
[recombinant factor XIII A subunit] |
hematology
[congenital factor XIII deficiency] |
|
4
3/28/2014 |
coagulation factor IX
(recombinant), Fc fusion protein [ Alprolix; Biogen] |
Fc fusion/coagulation factor
[recombinant factor IX Fc- fusion] |
hematology
[hemophilia B] |
|
5
6/6/2014 |
antihemophilic factor
(recombinant), Fc fusion protein [ Eloctate; Biogen] |
Fc fusion/coagulation factor
[recombinant factor VIII Fc- fusion] |
hematology
[hemophilia A] |
|
6
7/16/2014 |
C1 esterase inhibitor
recombinant [ Ruconest; Salix Pharmaceuticals] |
plasma protein
[recombinant C1 esterase inhibitor] |
hematology
[hereditary angioedema] |
|
7
10/23/2014 |
antihemophilic factor
porcine, B-domain truncated recombinant [ Obizur; Baxter Healthcare] |
coagulation factor
[recombinant factor VIII (porcine)] |
hematology
[hemophilia A] |
|
8
4/29/2015 |
coagulation factor IX
(recombinant) [ Ixinity; Cangene BioPharma] |
coagulation factor
[recombinant factor IX] |
hematology
[hemophilia B] |
|
9
9/4/2015 |
antihemophilic factor
(recombinant) [ Nuwiq; Octapharma USA] |
coagulation factor
[recombinant factor VIII] |
hematology
[hemophilia A] |
|
10
11/13/2015 |
antihemophilic factor
(recombinant) PEGylated [ Adynovate; Baxalta US] |
coagulation factor
[recombinant factor VIII PEGylated] |
hematology
[hemophilia A] |
|
11
12/8/2015 |
von Willebrand factor
(recombinant) [ Vonvendi; Baxalta US] |
plasma protein
[recombinant VWF] |
hematology
[von Willebrand disease] |
|
12
3/4/2016 |
coagulation factor IX
recombinant human [ Idelvion; CSL Behring Recombinant] |
coagulation factor
[recombinant factor IX albumin fusion] |
hematology
[hemophilia B] |
|
13
3/16/2016 |
antihemophilic factor
(recombinant) [ Kovaltry; Bayer HealthCare] |
coagulation factor
[recombinant factor VIII full- length] |
hematology
[hemophilia A] |
|
14
5/25/2016 |
antihemophilic factor
(recombinant) [ Afstyla; CSL Behring] |
coagulation factor
[recombinant factor VIII] |
hematology
[hemophilia A] |
Comprehensive listing of all FDA-approved therapeutic proteins granted orphan designation upon original submission from January 1, 2011, through August 31, 2016, listed in chronological order of FDA approval. In addition, the class of protein, a brief description, and orphan designation are included. CD, cluster of differentiation; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; Fab, fragment antigen binding; Fc, fragment crystallizable; GD2, disialoganglioside; IL, interleukin; mAb, monoclonal antibody; PD-1, programmed death receptor-1; VEGFR, vascular endothelial growth factor receptor.
Pathways for the development of novel therapeutics
The rapid advances in biomedical science and technology to address unmet medical needs also require that regulatory agencies ensure that such products are safe and effective. Several new pathways have emerged or have been finalized since 2011 and are summarized below ( Table 3).
Table 3. Pathways for the development of novel therapeutics.
| Pathway | Description and relevant U.S. Food and Drug Administration (FDA) guidances |
|---|---|
| Breakthrough
therapy designation |
“process designed to expedite the development and review of drugs that are intended to treat
a serious condition and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over available therapy on a clinically significant endpoint(s)” 56, 57. Guidance for industry: Expedited Programs for Serious Conditions – Drugs and Biologics 58 |
| Orphan
designation |
Rare disease or condition that affects 200,000 people or fewer per year in the U.S.
59
Guidance for industry: ( Draft) Rare Diseases: Common Issues in Drug Development 60 |
| Biosimilar | ‘an abbreviated licensure pathway for biological products that are demonstrated to be
“biosimilar” to or “interchangeable” with an FDA-licensed biological product… a biological product may be demonstrated to be “biosimilar” if data show that, among other things, the product is “highly similar” to an already-approved biological product’ 61. Guidance for industry: Quality Considerations in Demonstrating Biosimilarity of a Therapeutic Protein Product to a Reference Product 28 Scientific Considerations in Demonstrating Biosimilarity to a Reference Product 27 Biosimilars: Questions and Answers Regarding Implementation of the Biologics Price Competition and Innovation Act of 2009 29 Formal Meetings Between the FDA and Biosimilar Biological Product Sponsors or Applicants 30 |
Summary of three pathways for the development of novel therapeutics that have emerged or have been finalized since 2011.
Breakthrough therapy designation
The Food and Drug Administration Safety and Innovation Act (FDASIA) was signed on July 9, 2012. FDASIA Section 902 provided the FDA with the ability to establish breakthrough therapy designation (BTD) as a new program within the Expedited Programs for Serious Conditions 23. BTD was designed to be available for drugs intended to treat a serious condition and that have been shown to exhibit initial clinical evidence of considerable improvement over pre-existing therapies. The BTD program joined other expedited development and review programs, including fast track designation (1997), accelerated approval (1992), and priority review designation (1992), which have promoted innovation by facilitating the expedited development and review of novel medicines. Since FDASIA was signed in July 2012, CDER has approved 30 original BTDs, one third (10) of which were protein drugs. Over this same period, 26% of CDER-approved biologics (10 out of 39) have been granted BTD designation ( Table 4). Since July 2012, CBER has also approved two drugs under the BTD designation, but neither of these was a recombinant protein.
Table 4. Therapeutic proteins granted breakthrough therapy designation upon original submission.
| # | Approval
date |
Drug name
(Market name) |
Class | Description | Use |
|---|---|---|---|---|---|
| 1 | 11/1/2013 | Obinutuzumab
(Gazyva) |
mAb | Humanized anti-CD20 | Treatment of patients with previously
untreated chronic lymphocytic leukemia in combination with chlorambucil |
| 2 | 9/4/2014 | Pembrolizumab
(Keytruda) |
mAb | Humanized anti-PD-1 | Treatment of patients with unresectable
or metastatic melanoma and disease progression following ipilimumab and, if BRAF V600 mutation positive, a BRAF inhibitor |
| 3 | 12/3/2014 | Blinatumomab
(Blincyto) |
mAb | Mouse bispecific anti-
CD19/anti-CD3 |
Treatment of Philadelphia chromosome-
negative relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL) |
| 4 | 12/22/2014 | Nivolumab
(Opdivo) |
mAb | Human anti-PD-1 | Treatment of unresectable or metastatic
melanoma and disease progression following ipilimumab and, if BRAF V600 mutation positive, a BRAF inhibitor |
| 5 | 10/16/2015 | Idarucizumab
(Praxbind) |
Fab | Humanized anti-
dabigatran |
Treatment of patients treated with
Pradaxa when reversal of the anticoagulant effects of dabigatran is needed for emergency surgery/urgent procedures and in life-threatening or uncontrolled bleeding |
| 6 | 10/23/2015 | Asfotase-alfa
(Strensiq) |
Enzyme/fusion
protein |
Tissue non-specific
alkaline phosphatase/Fc fusion/deca-asparatate (D10) peptide |
Treatment of patients with perinatal/
infantile- and juvenile-onset hypophosphatasia |
| 7 | 11/16/2015 | Daratumumab
(Darzalex) |
mAb | Human anti-CD38 | Treatment of patients with multiple
myeloma who have received at least three prior lines of therapy, including a proteasome inhibitor and an immunomodulatory agent, or are double- refractory to a proteasome inhibitor and an immunomodulatory agent |
| 8 | 11/30/2015 | Elotuzumab
(Empliciti) |
mAb | Humanized anti-
CD319(SLAMF7) |
Treatment of patients with multiple
myeloma who have received one to three prior therapies |
| 9 | 12/08/2015 | Sebelipase alfa
(Kanuma) |
Enzyme | Lysosomal acid lipase | Treatment of patients with a diagnosis of
lysosomal acid lipase deficiency |
| 10 | 5/18/2016 | Atezolizumab
(Tecentriq) |
mAb | Humanized anti-PD-L1 | Treatment of locally advanced or
metastatic urothelial carcinoma who have disease progression during or following platinum-containing chemotherapy or have disease progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy |
Comprehensive listing of all FDA-approved therapeutic proteins granted breakthrough therapy designation upon original submission from July 9, 2012, through August 31, 2016, listed in chronological order of FDA approval. In addition, the class of protein, a brief description, and use are included. BRAF, B-Raf proto-oncogene, serine/threonine kinase; CD, cluster of differentiation; Fab, fragment antigen binding; mAb, monoclonal antibody; PD-1, programmed death receptor-1; PD-L1, programmed death-ligand 1; SLAMF7, SLAM family member 7.
Orphan designation
Rare diseases substantially impact public health, as an estimated 7,000 different disorders collectively affect approximately 10% of the U.S. population, young children particularly, and many lack effective treatments 24. To promote the development of medicines that specifically address unmet medical needs, an orphan designation is given for drugs indicated for the treatment of fewer than 200,000 patients in the U.S. On June 12, 2013, the final regulations amending the 1992 Orphan Drug Regulations were issued 25. These amendments clarified and instituted minor changes to regulatory language, such as defining the term “orphan subsets”, the eligibility of designation for previously approved drugs, and the scope of orphan exclusive approval ( https://www.gpo.gov/fdsys/pkg/FR-2013-06-12/pdf/2013-13930.pdf). Under the Orphan Drug Act (ODA) Final Rule, orphan designation grants incentives such as orphan exclusivity for a specific indication (7-year protection from competition), thereby promoting innovation in drug development to help treat patients in greatest need of novel medicines. This program appears to have fulfilled a real need, as 50% of the recently approved therapeutic proteins (31 out of 62) were licensed under the orphan designation ( Table 5).
Table 5. Therapeutic proteins granted orphan designation upon original submission (2011–2016).
| # | Approval
date |
Drug name
(Market name) |
Class | Description | Orphan designation |
|---|---|---|---|---|---|
| 1 | 3/25/2011 | Ipilimumab
(Yervoy) |
mAb | Human anti-CTLA-4 | Treatment of high-risk stage II, stage III,
and stage IV melanoma |
| 2 | 6/15/2011 | Belatacept
(Nulojix) |
Fusion (Fc) | CTLA-4 Fc-fusion | Prophylaxis of organ rejection in renal
allograft recipients |
| 3 | 11/18/2011 | Asparaginase erwinia
chrysanthemi (Erwinaze) |
Enzyme | Asparaginase erwinia
chrysanthemi |
Treatment of acute lymphocytic
leukemia |
| 4 | 1/17/2012 | Glucarpidase
(Voraxaze) |
Enzyme | Glucarpidase | Treatment of patients at risk of
methotrexate toxicity |
| 5 | 5/1/2012 | Taliglucerase alfa
(Elelyso) |
Enzyme | Taliglucerase | Treatment of Gaucher’s disease |
| 6 | 12/14/2012 | Raxibacumab
(raxibacumab) |
mAb | Human anti-anthrax
protective antigen (PA) |
Treatment of anthrax |
| 7 | 6/26/2013 | Coagulation factor IX
recombinant human (Rixubis) |
Coagulation factor | Recombinant factor IX | Prophylactic use to prevent or reduce
the frequency of bleeding episodes in patients with hemophilia B (routine prophylaxis in patients where there is no evidence or suspicion of bleeding) |
| 8 | 11/1/2013 | Obinutuzumab
(Gazyva) |
mAb | Humanized anti-CD20 | Treatment of chronic lymphocytic
leukemia |
| 9 | 12/23/2013 | Coagulation factor
XIII A-subunit (recombinant) (Tretten) |
Coagulation factor | Recombinant factor XIII
A subunit |
Prophylaxis of bleeding associated with
congential factor XIII deficiency |
| 10 | 2/14/2014 | Elosulfase alfa
(Vimizim) |
Enzyme | Elosulfase alfa | Treatment of mucopolysaccharidosis
type IV A (Morquio A syndrome) |
| 11 | 2/24/2014 | Metreleptin
(Myalept) |
Hormone | Metreleptin | Treatment of metabolic disorders
secondary to lipodystrophy |
| 12 | 3/28/2014 | Coagulation factor IX
(recombinant), Fc fusion protein (Alprolix) |
Coagulation factor | Recombinant factor IX
Fc fusion |
Control and prevention of hemorrhagic
episodes in patients with hemophilia B (congenital factor IX deficiency or Christmas disease) |
| 13 | 4/21/2014 | Ramucirumab
(Cyramza) |
mAb | Human anti-VEGFR2
(KDR) |
Treatment of gastric cancer |
| 14 | 4/23/2014 | Siltuximab (Sylvant) | mAb | Mouse/human chimeric
anti-IL-6 |
Treatment of Castleman’s disease |
| 15 | 4/23/2014 | Pembrolizumab
(Keytruda) |
mAb | Humanized anti-PD-1 | Treatment of stage IIB through IV
malignant melanoma |
| 16 | 6/6/2014 | Antihemophilic
factor (recombinant), Fc fusion protein (Eloctate) |
Coagulation factor | Recombinant factor VIII
Fc-fusion |
Treatment of hemophilia A |
| 17 | 7/16/2014 | C1 esterase inhibitor
recombinant (Ruconest) |
Plasma protein | Recombinant C1
esterase inhibitor |
Treatment of (acute attacks of)
angioedema caused by hereditary or acquired C1-esterase inhibitor deficiency |
| 18 | 10/23/2014 | Antihemophilic factor
porcine, B-domain truncated recombinant (Obizur) |
Coagulation factor | Recombinant factor VIII
(porcine) |
Treatment and prevention of episodic
bleeding in patients with inhibitor antibodies to human coagulation factor VIII |
| 19 | 12/3/2014 | Blinatumomab
(Blincyto) |
mAb | Mouse bispecific anti-
CD19/anti-CD3 |
Treatment of acute lymphocytic
leukemia |
| 20 | 12/22/2014 | Nivolumab
(Opdivo) |
mAb | Human anti-PD-1 | Treatment of stage IIb to IV melanoma |
| 21 | 1/23/2015 | Parathyroid hormone
(Natpara) |
Hormone | Parathyroid hormone | Treatment of hypoparathyroidism |
| 22 | 3/10/2015 | Dinutuximab
(Unituxin) |
mAb | Mouse/human chimeric
anti-GD2 |
Treatment of neuroblastoma |
| 23 | 8/27/2015 | Evolocumab
(Repatha) |
mAb | Human anti-proprotein
convertase substilisin/ kexin type 9 (PCSK9) |
Treatment of homozygous familial
hypercholesterolemia |
| 24 | 10/16/2015 | Idarucizumab
(Praxbind) |
Fab | Humanized anti-
dabigatran |
To reverse the anticoagulant effect of
dabigatran due to uncontrolled life- threatening bleeding requiring urgent intervention or a need to undergo an emergency surgery/urgent invasive procedure |
| 25 | 10/23/2015 | Asfotase-alfa
(Strensiq) |
Enzyme/fusion
protein |
Tissue non-specific
alkaline phosphatase/ Fc fusion/deca- asparatate (D10) peptide |
Treatment of hypophosphatasia |
| 26 | 11/16/2015 | Daratumumab
(Darzalex) |
mAb | Human anti-CD38 | Treatment of multiple myeloma |
| 27 | 11/24/2015 | Necitumumab
(Portrazza) |
mAb | Human anti-epidermal
growth factor receptor |
Treatment of squamous non-small cell
lung cancer |
| 28 | 12/8/2015 | Sebelipase alfa
(Kanuma) |
Enzyme | Lysosomal acid lipase | Treatment of lysosomal acid lipase
deficiency |
| 29 | 12/8/2015 | von Willebrand Factor
(Recombinant) (Vonvendi) |
Plasma protein | Recombinant von
Willebrand Factor |
Treatment of von Willebrand disease |
| 30 | 3/4/2016 | Coagulation factor IX
recombinant human (Idelvion) |
Coagulation factor | Recombinant factor IX
albumin fusion |
Treatment of patients with congenital
factor IX deficiency (hemophilia B) |
| 31 | 3/18/2016 | Obiltoxaximab
(Anthim) |
mAb | Mouse/human chimeric
anti- Bacillus anthracis |
Treatment of exposure to
B. anthracis
spores |
Comprehensive listing of all FDA-approved therapeutic proteins granted orphan designation upon original submission from January 1, 2011, through August 31, 2016, listed in chronological order of FDA approval. In addition, the class of protein, a brief description, and orphan designation are included. CD, cluster of differentiation; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; Fab, fragment antigen binding; Fc, fragment crystallizable; GD2, disialoganglioside; IL, interleukin; mAb, monoclonal antibody; PD-1, programmed death receptor-1; VEGFR, vascular endothelial growth factor receptor.
Biosimilars
Therapeutic protein drugs are now a critical component of the overall health-care industry and have revolutionized therapy options in many disease areas. These medications, however, are also some of the most expensive in the marketplace. It had become imperative to extend the generic concept for the licensure of therapeutic protein drugs because, while there existed only a few biopharmaceuticals when the Drug Price Competition and Patent Term Restoration Act of 1984 (Waxman-Hatch Act) was passed, these now account for almost a third of pharmaceutical sales, represent a core component of modern pharmacotherapy, and include the most expensive drugs on the market 26. As many biopharmaceuticals are poised to go off-patent, it has been recognized in both the U.S. and Europe that replicating the highly successful model of generic drugs to contain the costs of these therapeutics is a desirable goal, because biosimilar biological products (biosimilars) could potentially reduce the costs of therapeutic protein drugs.
In 2010, as part of the Patient Protection and Affordable Care Act (Affordable Care Act), biologics deemed to be “biosimilar” to an existing FDA-approved reference product were granted an abbreviated drug review and licensure pathway. Over the last few years, the FDA has issued several guidances to help sponsors navigate this novel regulatory pathway 27– 30. As of August 31, 2016, three biosimilars have been approved by the FDA (Purple Book 22). The first FDA-approved biosimilar, filgrastim-sndz (Zarxio; Sandoz), a biosimilar of filgrastim (Neupogen; Amgen), was approved on March 6, 2015, as a leukocyte growth factor for use in several neutropenia-related indications 31. This landmark approval was followed by the approval of infliximab-dyyb (Inflectra; Celltrion), a biosimilar of infliximab (Remicade; Janssen), on April 5, 2016 32, and the approval of etanercept-szzs (Erelzi; Sandoz), a biosimilar of etanercept (Enbrel; Amgen), on August 30, 2016 33; both are tumor necrosis factor (TNF) blockers indicated for use in inflammatory conditions. With the upcoming patent cliff approaching for several “blockbuster” therapeutic protein drugs, there is the expectation that the number of approved biosimilars will increase over the coming years. This trend has already been observed in Europe, where 21 biosimilar medicines have been authorized by the European Medicines Agency since 2006.
Although a drug product is off-patent, competitors do not get direct access to the originator company’s proprietary data or to resources such as the DNA sequence or cell lines used during the manufacture. Typically, the developer of a biosimilar has to retrieve the reference protein as a finished drug product, purify the drug substance, and reverse-engineer the process. Consequently, the FDA biosimilar framework does not require an identical manufacturing process for the innovator product and the biosimilar. Therefore, it is not expected that, in the demonstration of biosimilarity, quality attributes such as protein structure and post-translational modifications measured in comparative physiochemical and functional studies will be identical between the biosimilar and reference product, but highly similar. It has, on the contrary, been argued that deviations from the technology of the innovator may actually be desirable 9, 34. This is because since the introduction of the first recombinant DNA-derived therapeutic proteins, the technology to produce and purify these products has greatly improved. An increased focus upon innovation and streamlining of upstream and downstream processing has been reported by the widely respected “Biopharmaceutical benchmarks” series 3, 35. Thus, it is not necessary for manufacturers of biosimilars to be locked into the obsolete technologies of the manufacturers of the original products for whom changing methods have major financial and regulatory consequences. With this in mind, it seems much more desirable for biosimilars to be produced and analyzed by the best technology on offer.
Emerging trends, challenges, and opportunities
As protein-engineering technologies and regulatory frameworks evolve over time, so do protein therapeutics. Optimized versions of existing therapies can be achieved through better drug targeting as well as enhancing potency and functionality. By understanding the mechanism of action as well as the structure-function relationship of a protein, rational design and engineering strategies allow the modification of its activity or the introduction of new activities, leading to customization of existing proteins or the generation of novel therapeutics for specific clinical applications. Here, we will highlight just two examples of rational modifications to existing protein drugs achieved through protein engineering that have led to the approval of novel second-generation therapeutics (see Box 1 and Box 2).
Box 1. Engineering a second-generation cytotoxic T lymphocyte-associated protein 4 (CTLA4)-Fc fusion.
Abatacept, a CTLA4-Fc fusion protein therapeutic, was developed by Bristol-Myers Squibb and approved by the U.S. Food and Drug Administration (FDA) in December 2005 as the first selective modulator of co-stimulation for the treatment of rheumatoid arthritis 62. CTLA4-Fc competitively inhibits CD28 on the surface of T cells for binding to the B7 family co-stimulatory receptors CD80 (B7-1) and CD86 (B7-2) expressed on the surface of antigen-presenting cells. Although this Fc-fusion has been shown to be efficacious in the treatment of rheumatoid arthritis, abatacept was not as effective when tested pre-clinically in non-human primate transplant models 63, 64. Experimental data suggested that abatacept did not completely block B7 co-stimulatory receptor-mediated T-cell activation; therefore, a similar molecule with enhanced affinity for CD80/CD86 could be of therapeutic benefit as an immunosuppressant to prevent organ transplant rejection. Belatacept, formerly known as LEA29Y, was therefore developed by Bristol-Myers Squibb as a second-generation CTLA4-Fc. Two amino acid substitutions in the CTLA-4 ligand-binding region (L104E and A29Y) resulted in enhanced in vitro binding to CD80 (about two fold more avidly) and CD86 (about four fold more avidly) in addition to greater immunosuppression of T-cell activation in vitro (about 10-fold) as compared with the parent molecule (abatacept) 65. Belatacept’s enhanced activity was also observed in vivo with prolonged renal allograft survival in non-human primates (rhesus monkeys) compared with abatacept 63, 65. In clinical studies of kidney allograft recipients, belatacept was shown to be associated with similar levels of patient and graft survival but superior renal function and reduced renal and non-renal toxicities compared with cyclosporine at 12 months after transplant 66. The rationally designed analog with enhanced CD80 and CD86 binding, belatacept, a second-generation selective co-stimulation blocker, was approved by the FDA on June 15, 2011, for the prophylaxis of kidney transplant rejection 67.
Box 2. Engineering a second-generation anti-CD20 monoclonal antibody (mAb).
Rituximab, a chimeric mouse/human type I anti-CD20 mAb, was developed by Genentech and approved by the U.S. Food and Drug Administration (FDA) in November 1997 for the treatment of B-cell non-Hodgkin’s lymphoma. Rituximab binds to CD20 expressed on the surface of many B cells (but not plasma cells), resulting in B-cell depletion via antibody-dependent cellular cytotoxicity (ADCC), complement-mediated cytotoxicity (CMA), and the induction of direct cell death (apoptosis) 68, 69. Both ADCC and CMA are dependent upon the Fc region of the mAb interacting with Fc gamma receptor IIIA (FcγRIIIA) or complement component 1q (C1q), respectively. In the case of ADCC, antibody-bound CD20 + B cells are targeted for cellular depletion by FcγRIIIA-expressing monocytes, macrophages, and natural killer cells. Given the importance of FcγRIIIA engagement and signaling in the mechanism of B-cell depletion, specifically engineering a next-generation mAb 70– 72 with enhanced functional activity would be of clinical benefit. Gazyva, formerly known as GA101, a type II anti-CD20 mAb, was engineered and developed by Genentech. This second-generation medicine contains a CD20-binding variable region introduced through protein engineering to take advantage of the potent induction of direct cell death and limited C1q binding typical of type II anti-CD20 antibodies 51. The Fc region of this mAb has also been glyco-engineered by producing the protein drug in an expression cell line that overexpresses the glycosylation enzymes β1,4- N-acetylglucosaminyltransferase III (GnTIII) and Golgi α-mannosidase II (ManII) 73, thereby enriching for afucosylated oligosaccharides. Changes to the Fc glycosylation at Asn297 can lead to changes in FcγR binding, phagocytosis, and cytotoxicity 74. In fact, afucosylated antibodies have higher-affinity FcγRIIIA binding and enhanced ADCC activity compared with parent fucosylated counterparts 20, 75. The effects of these protein-engineering strategies can be observed in pre-clinical studies as Gazyva demonstrated higher affinity to FcγRIIIA by SPR and induced more potent ADCC when compared with rituximab 51. These significant improvements were also observed in phase 3 clinical studies as patients with CD20 + chronic lymphocytic leukemia treated with Gazyva with chlorambucil had prolonged median progression-free survival time (26.7 months) when compared with patients treated with rituximab with chlorambucil (15.2 months) 76. On November 1, 2013, Gazyva became the first glyco-engineered mAb drug to be approved by the FDA. This second-generation anti-CD20 mAb for treatment of chronic lymphocytic leukemia was also the first therapeutic protein to receive breakthrough therapy designation. In addition, Gazyva was granted orphan designation upon approval and contains pharmacogenetics information included on the drug label.
Over this examination of the recently approved therapeutic protein drug landscape, several emerging trends have become apparent. We anticipate that the inclusion of pharmacogenetics information in drug labeling and the importance of “companion diagnostics” will become the focus of increased attention. The FDA has been encouraging drug developers to collect and submit pharmacogenomics data through a guidance, “Pharmacogenomic Data Submissions”, issued in March 2005 36. Pharmacogenetics can play an important role in identifying responders and non-responders to medications, avoiding adverse events, and optimizing drug dose. Drug labeling may contain information on genomic biomarkers and can describe drug exposure and clinical response variability, risk for adverse events, genotype-specific dosing, mechanisms of drug action, and polymorphic drug target and disposition genes. Therefore, pharmacogenetic profiling is of particular importance when potential drug candidates exhibit highly variable safety, efficacy, or pharmacokinetics profiles. In January 2013, the FDA issued a guidance, titled “Clinical Pharmacogenomics: Premarket Evaluation in Early-Phase Clinical Studies and Recommendations for Labeling”, to assist drug developers with conducting exploratory pharmacogenomic investigations, enrichment strategies for clinical trials, adaptive trial designs, or companion diagnostics 37. Pharmacogenetic information and changes in drug labeling can lead to drugs targeted for different populations, personalized dosing regimens, and companion diagnostics. There have been 11 therapeutic proteins approved since 2011 that have included pharmacogenetic biomarkers in their drug labels ( Table 6). For a complete listing of drugs with available pharmacogenetics information, see the FDA’s Table of Pharmacogenomic Biomarkers in Drug Labeling 38. The FDA’s review process will continue to adapt as the incorporation of pharmacogenetic information becomes more commonplace. This will also require a coordinated cross-center review to incorporate the companion diagnostic/sequencing in the drug development/licensure process.
Table 6. Therapeutic proteins with pharmacogenetic biomarkers in drug labeling.
| # | Approval
date |
Drug name
(Market name) |
Class | Description | Pharmacogenetic
biomarker |
Therapeutic area |
|---|---|---|---|---|---|---|
| 1 | 6/8/2012 | Pertuzumab
(Perjeta) |
mAb | Humanized anti-
human epidermal growth factor receptor 2 (HER2) |
HER2 protein
overexpression positive |
Oncology |
| 2 | 2/22/2013 | Ado-trastuzumab
emtansine (Kadcyla) |
Antibody-
drug conjugate |
Humanized
anti-HER2/neu conjugated to emtansine |
HER2 protein
overexpression or gene amplification positive |
Oncology |
| 3 | 11/1/2013 | Obinutuzumab
(Gazyva) |
mAb | Humanized
anti-CD20 |
CD20 antigen positive | Oncology |
| 4 | 2/14/2014 | Elosulfase alfa
(Vimizim) |
Enzyme | Elosulfase alfa | N-acetylgalactosamine-6-
sulfatase deficient |
Inborn errors of
metabolism |
| 5 | 9/4/2014 | Pembrolizumab
(Keytruda) |
mAb | Humanized anti-PD-1 | (1) BRAF V600 mutation
positive, (2) PD-L1 protein expression positive |
Oncology |
| 6 | 12/3/2014 | Blinatumomab
(Blincyto) |
mAb | Mouse bispecific
anti-CD19/anti-CD3 |
Philadelphia chromosome
negative |
Oncology |
| 7 | 12/22/2014 | Nivolumab
(Opdivo) |
mAb | Human anti-PD-1 | (1) BRAF V600 mutation
positive, (2) PD-L1 protein expression positive |
Oncology |
| 8 | 1/23/2015 | Parathyroid
hormone (Natpara) |
Hormone | Parathyroid hormone | Calcium sensing receptor
mutation positive |
Inborn errors of
metabolism |
| 9 | 3/10/2015 | Dinutuximab
(Unituxin) |
mAb | Mouse/human
chimeric anti-GD2 |
MYCN amplification positive | Oncology |
| 10 | 7/24/2015 | Alirocumab
(Praluent) |
mAb | Human anti-
proprotein convertase substilisin/kexin type 9 (PCSK9) |
LDL receptor mutation
heterozygotes |
Endocrinology |
| 11 | 8/27/2015 | Evolocumab
(Repatha) |
mAb | Human anti-
proprotein convertase substilisin/kexin type 9 (PCSK9) |
LDL receptor mutation
heterozygotes and homozygotes |
Endocrinology |
Comprehensive listing of all FDA-approved therapeutic proteins with pharmacogenetics biomarkers in drug labeling from January 1, 2011, through August 31, 2016, listed in chronological order of FDA approval. In addition, the class of protein, a brief description, pharmacogenetics biomarker, and therapeutic area are included. BRAF, B-Raf proto-oncogene, serine/threonine kinase; CD, cluster of differentiation; GD2, disialoganglioside; LDL, low-density lipoprotein; mAb, monoclonal antibody; MYCN, v-myc avian myelocytomatosis viral oncogene neuroblastoma derived homolog; PD-1, programmed death receptor-1; PD-L1, programmed death-ligand 1.
It is reasonable to anticipate that proteins will be more extensively engineered in the future. This means that the new generation of therapeutic proteins will carry neo-sequences not found in nature. Thus, the potential risks of immunogenicity (undesirable immune responses to therapeutic proteins) 39 will also increase and, in turn, demand new technologies for immunogenicity risk assessment and mitigation 39, 40. Protein engineering is no longer restricted to altering the primary sequence of proteins. On the other hand, the rapidly growing trend of codon optimization involves the substitution of synonymous codons to improve protein synthesis and increase protein production 41, 42. A growing scientific literature suggests that although synonymous codons do not alter protein sequence they can have profound effects on protein folding and function 43– 45. Consequently, these therapeutic proteins designed by using such strategies will have to be carefully evaluated.
Finally, a confluence of computational and high-throughput experimental methods for protein-engineering and “off the shelf” platform technologies has ushered in unprecedented opportunities to develop safe, effective, and more convenient protein therapeutics. These opportunities do come with risks but rapid advances in new technologies as well as the underlying science suggest that these risks can be managed.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Yusuf Tutar, Division of Biochemistry, Department of Basic Pharmaceutical Sciences, Faculty of Pharmacy, Cumhuriyet University, Sivas, 58140, Turkey
Janice Reichert, The Antibody Society, Framingham, MA, USA
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; referees: 2 approved]
References
- 1. Kimchi-Sarfaty C, Schiller T, Hamasaki-Katagiri N, et al. : Building better drugs: developing and regulating engineered therapeutic proteins. Trends Pharmacol Sci. 2013;34(10):534–48. 10.1016/j.tips.2013.08.005 [DOI] [PubMed] [Google Scholar]
- 2. Carter PJ: Introduction to current and future protein therapeutics: a protein engineering perspective. Exp Cell Res. 2011;317(9):1261–9. 10.1016/j.yexcr.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 3. Walsh G: Biopharmaceutical benchmarks 2014. Nat Biotechnol. 2014;32(10):992–1000. 10.1038/nbt.3040 [DOI] [PubMed] [Google Scholar]
- 4. Schellekens H: Biosimilar therapeutics-what do we need to consider? NDT Plus. 2009;2(Suppl_1):i27–i36. 10.1093/ndtplus/sfn177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bandaranayake AD, Almo SC: Recent advances in mammalian protein production. FEBS Lett. 2014;588(2):253–60. 10.1016/j.febslet.2013.11.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Butler M, Meneses-Acosta A: Recent advances in technology supporting biopharmaceutical production from mammalian cells. Appl Microbiol Biotechnol. 2012;96(4):885–94. 10.1007/s00253-012-4451-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhu J: Mammalian cell protein expression for biopharmaceutical production. Biotechnol Adv. 2012;30(5):1158–70. 10.1016/j.biotechadv.2011.08.022 [DOI] [PubMed] [Google Scholar]
- 8. Cipriano D, Burnham M, Hughes JV: Effectiveness of various processing steps for viral clearance of therapeutic proteins: database analyses of commonly used steps. Methods Mol Biol. 2012;899:277–92. 10.1007/978-1-61779-921-1_18 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 9. Chirino AJ, Mire-Sluis A: Characterizing biological products and assessing comparability following manufacturing changes. Nat Biotechnol. 2004;22(11):1383–91. 10.1038/nbt1030 [DOI] [PubMed] [Google Scholar]
- 10. Tobin PH, Richards DH, Callender RA, et al. : Protein engineering: a new frontier for biological therapeutics. Curr Drug Metab. 2014;15(7):743–56. 10.2174/1389200216666141208151524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wurm FM: Production of recombinant protein therapeutics in cultivated mammalian cells. Nat Biotechnol. 2004;22(11):1393–8. 10.1038/nbt1026 [DOI] [PubMed] [Google Scholar]
- 12. Lutz S: Beyond directed evolution--semi-rational protein engineering and design. Curr Opin Biotechnol. 2010;21(6):734–43. 10.1016/j.copbio.2010.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Levin D, Golding B, Strome SE, et al. : Fc fusion as a platform technology: potential for modulating immunogenicity. Trends Biotechnol. 2015;33(1):27–34. 10.1016/j.tibtech.2014.11.001 [DOI] [PubMed] [Google Scholar]
- 14. Rath T, Baker K, Dumont JA, et al. : Fc-fusion proteins and FcRn: structural insights for longer-lasting and more effective therapeutics. Crit Rev Biotechnol. 2015;35(2):235–54. 10.3109/07388551.2013.834293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Andersen JT, Pehrson R, Tolmachev V, et al. : Extending half-life by indirect targeting of the neonatal Fc receptor (FcRn) using a minimal albumin binding domain. J Biol Chem. 2011;286(7):5234–41. 10.1074/jbc.M110.164848 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 16. Turecek PL, Bossard MJ, Schoetens F, et al. : PEGylation of Biopharmaceuticals: A Review of Chemistry and Nonclinical Safety Information of Approved Drugs. J Pharm Sci. 2016;105(2):460–75. 10.1016/j.xphs.2015.11.015 [DOI] [PubMed] [Google Scholar]
- 17. Strohl WR: Fusion Proteins for Half-Life Extension of Biologics as a Strategy to Make Biobetters. BioDrugs. 2015;29(4):215–39. 10.1007/s40259-015-0133-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Coppola A, Di Capua M, De Simone C: Primary prophylaxis in children with haemophilia. Blood Transfus. 2008;6(Suppl 2):s4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Casi G, Neri D: Antibody-drug conjugates: basic concepts, examples and future perspectives. J Control Release. 2012;161(2):422–8. 10.1016/j.jconrel.2012.01.026 [DOI] [PubMed] [Google Scholar]
- 20. Jefferis R: Glycosylation as a strategy to improve antibody-based therapeutics. Nat Rev Drug Discov. 2009;8(8):226–34. 10.1038/nrd2804 [DOI] [PubMed] [Google Scholar]
- 21. Costa AR, Rodrigues ME, Henriques M, et al. : Glycosylation: impact, control and improvement during therapeutic protein production. Crit Rev Biotechnol. 2014;34(4):281–99. 10.3109/07388551.2013.793649 [DOI] [PubMed] [Google Scholar]
- 22. Food and Drug Administration: Purple Book: Lists of Licensed Biological Products with Reference Product Exclusivity and Biosimilarity or Interchangeability Evaluations.2016; 2016 Reference Source [Google Scholar]
- 23. Food and Drug Administration: Fact Sheet: Breakthrough Therapies.2016; 2016 Reference Source [Google Scholar]
- 24. Schieppati A, Henter JI, Daina E, et al. : Why rare diseases are an important medical and social issue. Lancet. 2008;371(9629):2039–41. 10.1016/S0140-6736(08)60872-7 [DOI] [PubMed] [Google Scholar]
- 25. Food and Drug Administration: Orphan Drug Regulations: Regulatory History.2015; 2016 Reference Source [Google Scholar]
- 26. Roger SD: Biosimilars: current status and future directions. Expert Opin Biol Ther. 2010;10(7):1011–8. 10.1517/14712591003796553 [DOI] [PubMed] [Google Scholar]
- 27. Food and Drug Administration: Scientific Considerations in Demonstrating Biosimilarity to a Reference Product (Guidance for Industry).2015; 2016 Reference Source [Google Scholar]
- 28. Food and Drug Administration: Quality Considerations in Demonstrating Biosimilarity of a Therapeutic Protein Product to a Reference Product (Guidance for Industry).2015; 2016 Reference Source [Google Scholar]
- 29. Food and Drug Administration: Biosimilars: Questions and Answers Regarding Implementation of the Biologics Price Competition and Innovation Act of 2009 (Guidance for Industry).2015; 2016 Reference Source [Google Scholar]
- 30. Food and Drug Administration: Formal Meetings Between the FDA and Biosimilar Biological Product Sponsors or Applicants (Guidance for Industry).2015; 2016 Reference Source [Google Scholar]
- 31. Food and Drug Administration: FDA approves first biosimilar product Zarxio.2015; 2016 Reference Source [Google Scholar]
- 32. Food and Drug Administration: FDA approves Inflectra, a biosimilar to Remicade.2016; 2016 Reference Source [Google Scholar]
- 33. Food and Drug Administration: FDA approves Erelzi, a biosimilar to Enbrel.2016; 2016 Reference Source [Google Scholar]
- 34. Schellekens H, Moors E: Clinical comparability and European biosimilar regulations. Nat Biotechnol. 2010;28(1):28–31. 10.1038/nbt0110-28 [DOI] [PubMed] [Google Scholar]
- 35. Walsh G: Biopharmaceutical benchmarks 2010. Nat Biotechnol. 2010;28(9):917–24. 10.1038/nbt0910-917 [DOI] [PubMed] [Google Scholar]
- 36. Food and Drug Administration: Guidance for Industry: Pharmacogenomic Data Submissions.2005; 2016 Reference Source [Google Scholar]
- 37. Food and Drug Administration: Guidance for Industry: Clinical Pharmacogenomics: Premarket Evaluation in Early-Phase Clinical Studies and Recommendations for Labeling.2013; 2016 Reference Source [Google Scholar]
- 38. Food and Drug Administration: Table of Pharmacogenomic Biomarkers in Drug Labeling.2016; 2016 Reference Source [Google Scholar]
- 39. Shankar G, Pendley C, Stein KE: A risk-based bioanalytical strategy for the assessment of antibody immune responses against biological drugs. Nat Biotechnol. 2007;25(5):555–61. 10.1038/nbt1303 [DOI] [PubMed] [Google Scholar]
- 40. Yin L, Chen X, Vicini P, et al. : Therapeutic outcomes, assessments, risk factors and mitigation efforts of immunogenicity of therapeutic protein products. Cell Immunol. 2015;295(2):118–26. 10.1016/j.cellimm.2015.03.002 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 41. Maertens B, Spriestersbach A, von Groll U, et al. : Gene optimization mechanisms: a multi-gene study reveals a high success rate of full-length human proteins expressed in Escherichia coli. Protein Sci. 2010;19(7):1312–26. 10.1002/pro.408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mauro VP, Chappell SA: A critical analysis of codon optimization in human therapeutics. Trends Mol Med. 2014;20(11):604–13. 10.1016/j.molmed.2014.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 43. Hunt R, Sauna ZE, Ambudkar SV, et al. : Silent (synonymous) SNPs: should we care about them? Methods Mol Biol. 2009;578:23–39. 10.1007/978-1-60327-411-1_2 [DOI] [PubMed] [Google Scholar]
- 44. Tsai C, Sauna ZE, Kimchi-Sarfaty C, et al. : Synonymous mutations and ribosome stalling can lead to altered folding pathways and distinct minima. J Mol Biol. 2008;383(2):281–91. 10.1016/j.jmb.2008.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sauna ZE, Kimchi-Sarfaty C: Understanding the contribution of synonymous mutations to human disease. Nat Rev Genet. 2011;12(10):683–91. 10.1038/nrg3051 [DOI] [PubMed] [Google Scholar]
- 46. Houdebine LM: Production of pharmaceutical proteins by transgenic animals. Comp Immunol Microbiol Infect Dis. 2009;32(2):107–21. 10.1016/j.cimid.2007.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. van Veen HA, Koiter J, Vogelezang CJ, et al. : Characterization of recombinant human C1 inhibitor secreted in milk of transgenic rabbits. J Biotechnol. 2012;162(2–3):319–26. 10.1016/j.jbiotec.2012.09.005 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 48. Hellwig S, Drossard J, Twyman RM, et al. : Plant cell cultures for the production of recombinant proteins. Nat Biotechnol. 2004;22(11):1415–22. 10.1038/nbt1027 [DOI] [PubMed] [Google Scholar]
- 49. Grabowski GA, Golembo M, Shaaltiel Y: Taliglucerase alfa: an enzyme replacement therapy using plant cell expression technology. Mol Genet Metab. 2014;112(1):1–8. 10.1016/j.ymgme.2014.02.011 [DOI] [PubMed] [Google Scholar]
- 50. Fox JL: First plant-made biologic approved. Nat Biotech. 2012;30(6):472 10.1038/nbt0612-472 [DOI] [Google Scholar]
- 51. Mössner E, Brünker P, Moser S, et al. : Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood. 2010;115(22):4393–402. 10.1182/blood-2009-06-225979 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 52. Peters RT, Low SC, Kamphaus GD, et al. : Prolonged activity of factor IX as a monomeric Fc fusion protein. Blood. 2010;115(10):2057–64. 10.1182/blood-2009-08-239665 [DOI] [PubMed] [Google Scholar]
- 53. Dumont JA, Liu T, Low SC, et al. : Prolonged activity of a recombinant factor VIII-Fc fusion protein in hemophilia A mice and dogs. Blood. 2012;119(13):3024–30. 10.1182/blood-2011-08-367813 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 54. Powell JS, Josephson NC, Quon D, et al. : Safety and prolonged activity of recombinant factor VIII Fc fusion protein in hemophilia A patients. Blood. 2012;119(13):3031–7. 10.1182/blood-2011-09-382846 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 55. Pelegri-O'Day EM, Lin EW, Maynard HD: Therapeutic protein-polymer conjugates: advancing beyond PEGylation. J Am Chem Soc. 2014;136(41):14323–32. 10.1021/ja504390x [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 56. Food and Drug Administration: Breakthrough Therapy.2014; 2016 Reference Source [Google Scholar]
- 57. Food and Drug Administration: Fact Sheet: Breakthrough Therapies.2014; 2016 Reference Source [Google Scholar]
- 58. Food and Drug Administration: Guidance for Industry Expedited Programs for Serious Conditions – Drugs and Biologics.2014; 2016 Reference Source [Google Scholar]
- 59. Food and Drug Administration: Designating an Orphan Product: Drugs and Biological Products.2016; 2016 Reference Source [Google Scholar]
- 60. Food and Drug Administration: Rare Diseases: Common Issues in Drug Development (Guidance for Industry- Draft).2015; 2016 Reference Source [Google Scholar]
- 61. Food and Drug Administration: Information on Biosimilars.2016; 2016 Reference Source [Google Scholar]
- 62. Moreland L, Bate G, Kirkpatrick P: Abatacept. Nat Rev Drug Discov. 2006;5(3):185–6. 10.1038/nrd1989 [DOI] [PubMed] [Google Scholar]
- 63. Kirk AD, Harlan DM, Armstrong NN, et al. : CTLA4-Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proc Natl Acad Sci U S A. 1997;94(16):8789–94. 10.1073/pnas.94.16.8789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Levisetti MG, Padrid PA, Szot GL, et al. : Immunosuppressive effects of human CTLA4Ig in a non-human primate model of allogeneic pancreatic islet transplantation. J Immunol. 1997;159(11):5187–91. [PubMed] [Google Scholar]
- 65. Larsen CP, Pearson TC, Adams AB, et al. : Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. Am J Transplant. 2005;5(3):443–53. 10.1111/j.1600-6143.2005.00749.x [DOI] [PubMed] [Google Scholar]
- 66. Vincenti F, Charpentier B, Vanrenterghem Y, et al. : A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). Am J Transplant. 2010;10(3):535–46. 10.1111/j.1600-6143.2009.03005.x [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 67. Vincenti F, Dritselis A, Kirkpatrick P: Belatacept. Nat Rev Drug Discov. 2011;10:655–6. 10.1038/nrd3536 [DOI] [PubMed] [Google Scholar]
- 68. Pescovitz MD: Rituximab, an anti-cd20 monoclonal antibody: history and mechanism of action. Am J Transplant. 2006;6(5 Pt 1):859–66. 10.1111/j.1600-6143.2006.01288.x [DOI] [PubMed] [Google Scholar]
- 69. Reff ME, Carner K, Chambers KS, et al. : Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. 1994;83(2):435–45. [PubMed] [Google Scholar]
- 70. Jiang XR, Song A, Bergelson S, et al. : Advances in the assessment and control of the effector functions of therapeutic antibodies. Nat Rev Drug Discov. 2011;10(2):101–11. 10.1038/nrd3365 [DOI] [PubMed] [Google Scholar]
- 71. Nelson AL, Dhimolea E, Reichert JM: Development trends for human monoclonal antibody therapeutics. Nat Rev Drug Discov. 2010;9(10):767–74. 10.1038/nrd3229 [DOI] [PubMed] [Google Scholar]
- 72. Beck A: Biosimilar, biobetter and next generation therapeutic antibodies. MAbs. 2011;3(2):107–10. 10.4161/mabs.3.2.14785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Niwa R, Satoh M: The current status and prospects of antibody engineering for therapeutic use: focus on glycoengineering technology. J Pharm Sci. 2015;104(3):930–41. 10.1002/jps.24316 [DOI] [PubMed] [Google Scholar]
- 74. Herter S, Birk MC, Klein C, et al. : Glycoengineering of therapeutic antibodies enhances monocyte/macrophage-mediated phagocytosis and cytotoxicity. J Immunol. 2014;192(5):2252–60. 10.4049/jimmunol.1301249 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 75. Ferrara C, Grau S, Jäger C, et al. : Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcgammaRIII and antibodies lacking core fucose. Proc Natl Acad Sci U S A. 2011;108(31):12669–74. 10.1073/pnas.1108455108 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 76. Goede V, Fischer K, Busch R, et al. : Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370(12):1101–10. 10.1056/NEJMoa1313984 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation