Abstract
Plant-herbivore interactions shape community dynamics across marine, freshwater, and terrestrial habitats. From amphipods to elephants and from algae to trees, plant-herbivore relationships are the crucial link generating animal biomass (and human societies) from mere sunlight. These interactions are, thus, pivotal to understanding the ecology and evolution of virtually any ecosystem. Here, we briefly highlight recent advances in four areas of plant-herbivore interactions: (1) plant defense theory, (2) herbivore diversity and ecosystem function, (3) predation risk aversion and herbivory, and (4) how a changing climate impacts plant-herbivore interactions. Recent advances in plant defense theory, for example, highlight how plant life history and defense traits affect and are affected by multiple drivers, including enemy pressure, resource availability, and the local plant neighborhood, resulting in trait-mediated feedback loops linking trophic interactions with ecosystem nutrient dynamics. Similarly, although the positive effect of consumer diversity on ecosystem function has long been recognized, recent advances using DNA barcoding to elucidate diet, and Global Positioning System/remote sensing to determine habitat selection and impact, have shown that herbivore communities are probably even more functionally diverse than currently realized. Moreover, although most diversity-function studies continue to emphasize plant diversity, herbivore diversity may have even stronger impacts on ecosystem multifunctionality. Recent studies also highlight the role of risk in plant-herbivore interactions, and risk-driven trophic cascades have emerged as landscape-scale patterns in a variety of ecosystems. Perhaps not surprisingly, many plant-herbivore interactions are currently being altered by climate change, which affects plant growth rates and resource allocation, expression of chemical defenses, plant phenology, and herbivore metabolism and behavior. Finally, we conclude by noting that although the field is advancing rapidly, the world is changing even more rapidly, challenging our ability to manage these pivotal links in the food chain.
Keywords: plant defense theory, herbivore diversity, climate impact, plant-herbivore interactions
Introduction
Plant-herbivore interactions are important for understanding community dynamics and ecosystem function given that they are the critical link between primary production and food webs. Plant-herbivore studies are also the backbone of multiple fields within ecology and evolution, including co-evolution 1, 2, chemical ecology 3– 5, nutritional ecology 6, 7, and ecological stoichiometry 8– 10. The topic crosses ecosystem boundaries (freshwater, terrestrial, and marine) 11, huge ranges of organismal size (aphids to elephants and phytoplankton to trees) 12, 13 ( Figure 1), and vast productivity gradients (deserts to tropical rain forests) 14– 16, resulting in broadly applicable ecological theories.
Figure 1. Plant-herbivore interactions shape community dynamics across terrestrial, freshwater, and marine habitats.
These interactions encompass a huge range of taxa and organismal size for both plants and herbivores. Common herbivores across these ecosystems are ( A) spicebush swallowtail caterpillar ( Papilio troilus) feeding on spicebush ( Lindera benzoin); ( B) impala ( Aepyceros melampus) browsing shrubs in an African savanna; ( C) white rhinoceros ( Ceratotherium simum) grazing African grasses; ( D) Daphnia dentifera, an important grazer on freshwater phytoplankton; ( E) the white tuberculed crayfish ( Procambarus spiculifer) consuming a freshwater macrophyte ( Egeria densa); ( F) Canada goose ( Branta canadensis), an important herbivore on freshwater macrophytes and terrestrial grasses; ( G) the isopod Erichsonella attenuata, a mesograzer of epiphytes and seaweeds in seagrass meadows; ( H) blue parrotfish ( Scarus coeruleus) grazing filamentous algae on a coral reef; and ( I) West Indian manatee ( Trichechus manatus) curiously eating a red mangrove plant ( Rhizophora mangle). Photo credits: Eric Lind ( A), John Parker ( B, E, F, G, I), Deron Burkepile ( C), Alan Wilson ( D), Thomas Adam ( H).
Recent technological and statistical advances have resulted in a rapidly advancing field of study, including (1) the genetic and phylogenetic basis of plant-herbivore interactions and chemical defenses 17– 21, (2) DNA barcoding to elucidate herbivore diets 22, 23, (3) Global Positioning System (GPS) and remote sensing technology to understand landscape-level predator-herbivore-plant interactions 24, and (4) statistical advances that allow for comprehensive analyses of multiple co-varying drivers in both observational and experimental studies 25. Given the array of topics comprising the study of plant-herbivore interactions, our focus was not to exhaustively review this literature. Instead, we point out several exciting and growing areas of research from the past 3 years. We focus on briefly highlighting four areas of plant-herbivore interactions: (1) plant defense theory, (2) herbivore diversity and ecosystem function, (3) the context dependency of herbivory and predation risk, and (4) how a changing climate impacts plant-herbivore interactions. We strived to include a broad array of examples from across ecosystems and, in the process, are likely to have missed many worthy studies. We conclude that the study of plant-herbivore interactions continues to be a leading driver for ecology and evolution in general and that these studies can be used to inform conservation but that efforts need to be redoubled to counter the rapidly changing dynamics of the Anthropocene.
Plant defense theory
The study of plant defense against herbivores is one of the cornerstones of ecology and evolution, underpinning the theory of co-evolution 1, the field of chemical ecology 26, and some of the most prominent mechanisms explaining the success of invasive species 27– 29. Recent work, however, has challenged some of the key paradigms in these fields, invigorating and broadening a variety of research perspectives. For example, invasive plants have long been thought to succeed via enemy loss (that is, the enemy release hypothesis), but direct evidence for this idea has been alternately supportive 30, 31, conflicting 32, 33, and ambivalent 34. Similarly, although chemical novelty from native species is one of the key mechanisms thought to drive enemy release 35, limited experimental tests show little relationship between novel chemistry and herbivore deterrence 36. Furthermore, enemy release is often unassociated with either increased invasiveness 37– 39 or competitive effects on neighboring plants 40. In light of these results, recent work has emphasized that we should be examining whether enemy release interacts with other ecological drivers, including resource availability or disturbance 31, 41, 42, and whether the complexity and integrity of the entire recipient food web are better predictors of invasion resistance than plant-herbivore interactions alone 43.
Similarly, plant chemical defenses have long been regarded as a primary source of defense against herbivores 44, and a host of articles have demonstrated the advantages of plant chemical defenses, particularly when examined in a comparative framework across model taxa (for example, 45). Nevertheless, when examined at the community scale across sympatric species, the relationships between chemical defense and herbivory are often weaker 46, 47. Some of this discrepancy might be due to the difficulty of comparing defense levels across disparate species with a diversity of chemical defenses that make direct comparisons problematic 48. However, the ambiguity between comparative and community-level studies highlights a recent re-emphasis of the importance of having mixed defensive strategies against a range of consumer types 49, 50. For example, variation in chemical defenses can interact with life history traits, structural defenses, nutrient quality, and the relative distribution of above-versus-belowground chemical defenses to modulate herbivory 51– 56, highlighting the need to examine the efficacy of chemical defenses within the context of a shifting mosaic of plant traits.
For example, theory predicts that low nutrient quality should lead to linear reductions in herbivory 57, 58, but Wetzel et al. 7 found that variance in nutrient traits (for example, protein and nitrogen content, among others), not low nutrient quality per se, determined the performance of 53 insect herbivore species. Insect performance increased when tissue nutrient levels were low but eventually declined when nutrients were high, likely due to either nutrient toxicity or even nutrient-toxin interactions (for example, 59), whereas increasing defenses nearly always led to linear declines in insect performance. Interestingly, relatively homogenous nutrient levels could provide a mechanistic explanation for why crops and some species-poor natural communities might be more prone to insect outbreaks when compared with diverse systems 60, 61. Moreover, these findings generally mirror those from research focusing on plant traits, as variance in plant traits appears to be just as important as mean trait levels if not more so 62.
Numerous studies have also demonstrated that plant-herbivore interactions are context-dependent, modulated by the surrounding plant community 63– 68, by local nutrient conditions 69– 71, by the local predator community 72, and by plants’ fungal and bacterial microbiomes 73. Taken together, the overall picture that emerges is a highly complex “phytochemical landscape” that dynamically shapes the co-evolution of plants and their herbivores 74– 76. Moving forward, this new appreciation of the integrative nature of trait-mediated plant-herbivore interactions suggests that plant functional traits are the key to understanding food-web interactions and ultimately ecosystem processes 77.
Herbivore diversity and ecosystem function
The importance of species and functional diversity for maintaining healthy, resilient ecosystems is now widely recognized 78– 80. Although the field of biodiversity-ecosystem function is still dominated by plant-specific studies, consumer diversity is also clearly important for ecosystem function 81– 83. Herbivore diversity has strong impacts on many aspects of primary producer communities, such as primary production, plant diversity, and consumption of producer biomass 84– 89. Although it is not clear whether these patterns are due to complementarity among herbivore species or the idiosyncratic importance of individual species 90, the loss of herbivore diversity clearly impacts a variety of ecosystem functions.
More recent work has tried to integrate diet with other aspects of herbivore ecology, including movement, population growth, and predation risk. This has led to a more integrative understanding of herbivore complementarity and often finds higher levels of functional diversity among herbivore species than diet alone suggests. For example, herbivorous fishes on coral reefs often have similar diets but generally live in different habitats, feed from different substrates, and forage at different spatial scales 91– 94. These subtle differences in habitat selection and foraging range may influence how different herbivore species impact reef algal dynamics and competitive interactions between corals and algae, re-emphasizing that functional trait diversity is an important consideration in ecosystem-function studies 95.
Furthermore, both synthetic analyses and empirical work suggest that biodiversity supports multifunctionality in ecosystems 79, 81. In other words, in addition to influencing primary production, diversity influences ecosystem processes such as nutrient retention, nutrient cycling, and decomposition rates, and it is important to consider as many of these interconnected processes as possible 96. For example, Lefcheck et al. 79 used data from 94 biodiversity-ecosystem function experiments to test how species diversity impacts ecosystem multifunctionality across a range of taxa, trophic levels, and habitats. The authors showed that the effects of diversity on ecosystem multifunctionality (that is, the number of ecosystem processes surpassing a critical threshold of function) grew generally stronger the more functions that were considered, with consistent impacts across aquatic and terrestrial habitats. Perhaps most strikingly, the positive effects of plant diversity tapered off and even became negative at higher thresholds, whereas herbivore diversity had strong positive effects on multiple ecosystem processes even at the highest threshold levels. This result may stem from the fact that consumers often use a greater variety of resources and likely exhibit more complex behavior than plants 97 (but see 98). It also emphasizes conceptual predictions that consumer diversity might have stronger impacts on ecosystem function when compared with producer diversity 99.
New technologies for understanding herbivore diversity: DNA metabarcoding
Determining herbivore diet breadth, and thus functional diversity, is challenging and difficult in many systems. Fortunately, this problem is becoming more tractable with recent technological advances. For example, dietary characterizations typically result from careful, time-consuming observations of herbivore feeding and behavior 91, 100, 101. However, visual observations are often limited by the ability to discriminate the actual species being consumed in mixed plant communities, the ability to observe nocturnal foraging, and difficulty in determining whether plant use is related to diet or habitat or both 101. To solve some of these problems, visual identification of plant fragments in either gut contents or dung is a common tool for a range of consumers 102, 103. However, identification of plants and other material is unequal across herbivores with different digestive physiologies, and smaller diet items often are resolvable only to functional group or family, and rare but potentially important diet items are typically missed entirely 23.
The development of DNA metabarcoding of herbivore gut contents may help quantitatively resolve herbivore diets, revealing previously cryptic aspects of functional diversity, complementarity, and niche partitioning 104, 105. DNA metabarcoding outperforms many traditional analyses in resolving diet identification 23, 106 and provides quantitative estimates of relative consumption of different foods and captures rare diet items 23, 107, 108. Furthermore, DNA metabarcoding has revealed previously cryptic functional differences among sympatric herbivore species using largely the same habitat. For example, Kartzinel et al. 22 used metabarcoding to examine diet niche partitioning for seven large mammalian herbivores—the African savanna elephant, impala, two species of zebra, buffalo, domestic cattle, and dik-dik—all co-occurring in a Kenyan savanna with over 100 plant species as potential diet items. Barcoding revealed that diets differed considerably across all levels of comparison. For example, grazers such as zebra consumed more than 99% grasses, mixed-feeding impala consumed about 35% grasses, and strict browsers such as dik-dik consumed less than 1% grass. Barcoding even revealed relatively fine-scale differences across ecologically similar grazer species (for example, the Grevy’s versus Plains zebra and African buffalo versus domestic cattle). Remarkably, although the two species of zebra consumed nearly identical proportions of grasses, they relied on very different suites of grass species, a finding that would have been difficult to detect using traditional methods of diet analysis.
Barcoding technology could be especially important for unpacking the ecology of previously intractable species, including large generalist herbivores with broad home ranges across a variety of habitats (for example, terrestrial ungulates), for herbivores that feed on visually cryptic species (for example, algal-feeding fishes), for morphologically similar herbivores (for example, rolled-leaf beetles 109, 110), and for revealing spatiotemporal patterns in diet. Given the continually shrinking cost of high-throughput, next-generation sequencing, these types of large-scale, long-term projects will hopefully become increasingly common.
Too scared to eat? context-dependent effects of predation risk on plant-herbivore interactions
Risk of predation can alter herbivore foraging behavior and subsequently impact the abundance, distribution, or diversity of primary producers. This cascade is evident across a variety of ecosystems, including rocky intertidal habitats 111, seagrass beds 112, coral reefs 113, 114, freshwater ponds and streams 115, 116, old fields 117, 118, temperate grasslands and forests 72, 119– 121, and African savannas 122. Ultimately, predation risk may even drive patterns of carbon sequestration in heavily vegetated habitats 123, 124, as the mere risk of predation alters levels of plant consumption and the standing stock of carbon trapped in plant biomass.
More recent work has focused on how context-dependent factors such as habitat complexity, predator identity, herbivore identity, body size, and prey hunger level can influence the cascading effects of risk aversion 125, 126. For example, a study of herbivorous fishes on a coral reef showed that decoy predatory fishes suppressed herbivory by parrotfishes and surgeonfishes significantly more in high-complexity areas and at distances farther from the decoy when compared with low-complexity areas ( Figure 2), likely due to decreased visibility and perceived escape ability in more complex areas 127. Furthermore, smaller herbivorous fishes were more willing to forage closer to the decoys than were larger fishes, especially in more complex areas, possibly because they were less of a target for the much larger predators and because complex areas provided more small refuges for smaller individuals. Thus, there may be strong interactions in habitat complexity and body size in shaping patterns of risk-driven herbivory.
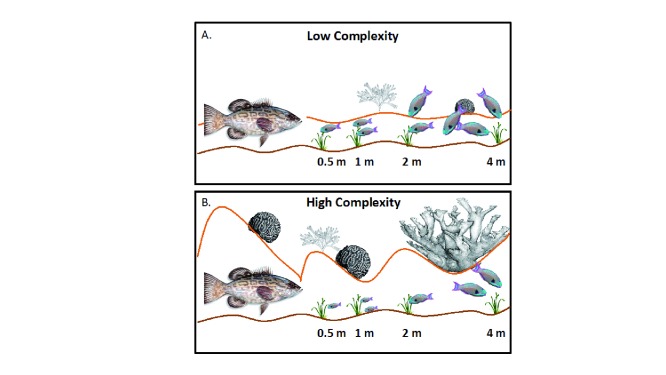
Figure 2. The impact of risk of predation on plant-herbivore interactions often depends on many context-dependent factors such as habitat complexity.
Catano et al. 127 used taxidermy decoy black grouper to manipulate risk in areas of low and high complexity on a Caribbean coral reef. They measured rates of herbivory and quantified bites of common parrotfishes and surgeonfishes at increasing distances from the decoy grouper. In areas of low complexity ( A), overall rates of herbivory by parrotfishes and surgeonfishes are lower than in control areas without grouper decoys (not shown) but are significantly higher than in areas of higher complexity ( B). In areas of higher complexity, rates of herbivory are significantly lower at closer distances to the decoy grouper than in the low-complexity areas. However, feeding by the smallest herbivorous fishes was greatest at closer distances in the higher-complexity areas, potentially due to the smaller fishes being less vulnerable to large grouper and also due to having more refuge from predation in the complex habitats. Illustration courtesy of Laura Catano.
Species identity is likely also an important context-dependent driver of how predation risk impacts patterns of herbivory across the landscape. For example, in the species-rich guild of ungulate herbivores in African savannas, different species such as giraffe, zebra, and impala exhibit different responses to increased density of woody vegetation, typical hiding spots for predators like lions and leopards 128, 129. This differential habitat selection due to risk aversion interacts with forage quality and quantity, resulting in heterogeneous impacts of herbivores across the landscape. Wildebeest and impala, for example, exert strong top-down control on plant communities in different parts of the savanna on the basis of how predation risk and food resources shape their habitat selection 130. Interestingly, increasing climate variability may alter the fear landscape. In normal rainfall years, herbivores such as zebra and gazelles favored areas with fewer trees and higher visibility to detect predators. But, during a drought, these same herbivores frequented areas with more grass regardless of tree density and predation risk 131. Thus, these herbivores were trading safety for food during stressful times, showing that variations in climate and resource levels strongly influence the landscape of fear.
The role of predator foraging behavior (for example, sit-and-wait versus roving) has also emerged as an important topic for understanding the impact on herbivore behavior and ultimately their impact on plant communities. Schmitz 118 used a three-year experiment in grassland mesocosms to show that actively hunting spiders reduced grasshopper abundance, resulting in increased plant species diversity and enhanced aboveground net primary production and nitrogen mineralization rate. In contrast, sit-and-wait ambush spiders mostly impacted grasshopper behavior, not density, which had the opposite effect on plant communities and ecosystem processes. Sit-and-wait predators often result in larger shifts in herbivore behavior than do more mobile, yet more unpredictable, predators, but it is unclear how strongly and how often this effect filters down to influence plant communities 132– 134.
Advances in technology, specifically in telemetry, satellite imagery, and remote sensing, have opened up myriad ways to better document and understand the cascading effects of predation risk 24, 135. For example, in an African savanna, Ford et al. 122 used GPS telemetry, satellite imagery, and elegant small-scale experiments to show that the risk of predation from leopard and wild dog drove impala into areas with lower woody cover and thus a lower probability of encountering predators. In turn, this led to a suppression of the palatable tree Acacia brevispica in areas of high impala abundance while facilitating the abundance of Acacia etbacia, a well-defended, thorny species. Interestingly, the effects of predation risk can even be seen from space. For example, in coral reef ecosystems, the halos of bare space that herbivorous fishes create around patch reefs, in large part due to predation risk in the open water, are clearly evident even in relatively low-resolution Google Earth images 136. These halos tend to be smaller in areas of high predation risk but lower, or even nonexistent, in areas of low predation risk. Further development and cost reductions of these technologies will help reveal aspects of risk-driven trophic cascades that have been hidden to date.
Plant-herbivore interactions in an era of climate change
Recent evidence suggests that climate change is happening 10 times faster than at any time in the last 65 million years 137, having profound consequences for life on earth. The pace of climate change varies considerably across ecosystems 138, but one of the most striking aspects of numerous recent studies is the relatively rapid ecological and evolutionary responses of plant-herbivore interactions to our warming climate. Hundreds of species of crop pests and pathogens, for example, have moved poleward at an average of 2.7 km/year since 1960 in the Northern Hemisphere 139, essentially matching the observed temperature increases 140. In many cases, herbivores appear to be responding faster to climate change than their host plants 141, 142, leading to altered selective pressures and novel ecological interactions in their new ranges. The habitat specialist mangrove crab ( Aratus pisonii), for example, is moving northward on the eastern Atlantic coastline by 6.2 km/year 143, far outpacing estimated mangrove migration rates of 1.3 to 4.5 km/year 144. In mangrove habitats, A. pisonii is an important herbivore and closely tied to mangrove trees 145, but the lack of their hosts in salt marshes leads to altered behavior and habitat selection, diet, size, and reproductive traits 146, 147.
Similarly, climate change has strengthened the flow of ocean currents, leading to “oceanic hotspots” and the expansion of the ranges of many tropical fish species into more temperate regions. The “tropicalization” of these temperate ecosystems has already resulted in overgrazing on temperate macroalgal communities in the Mediterranean, Japan, the Gulf of Mexico, Australia, and South Africa 148. Indeed, the sudden arrival of tropical herbivorous fishes has essentially eliminated kelp recruitment on some temperate Australian reefs, leading to potentially persistent phase-shifts away from fleshy-kelp communities toward algal turf-dominated reefs 149. A similar process of “phenological mismatch” has happened in the temperate boreal zone, where warmer winters have reduced snowpack, leading to increased herbivory on aspen and other woody species 150. Interestingly, warmer winters have the opposite effect in the High Arctic, where increasing amounts of rain during extremely warm winters have hardened the snowpack and reduced availability of winter forage availability for overwintering vertebrate herbivores. As a consequence, these extreme events can cause widespread herbivore population crashes that ripple through to predator populations 151, 152.
In most of these cases, changes in herbivore behavior allow for rapid responses to climate change (for example, 153), but there is some question over whether there is subsequent genetic changes that could allow for adaption to climate change. One example of rapid evolution to climate change is that of the winter moth ( Operophtera brumata), in which egg hatching date has closely tracked phenological changes in budburst of its host, the oak Quercus robur 154. However, herbivores with longer generation times may be less able to respond adaptively to a rapidly changing climate. For example, roe deer in Western Europe are experiencing earlier springs, but because they have relatively inflexible mean birthing dates, fawns are increasingly born when high-quality early-spring vegetation is becoming less abundant 155, having potential long-term consequences on their abundance and distribution.
The main drivers of climate change—increasing carbon dioxide (CO 2) and temperature—also fundamentally alter the physiology and metabolism of both herbivores and plants 156. Metabolic theory predicts that warmer temperatures should lead to elevated metabolism in ectothermic consumers resulting in increased feeding rates 157, 158. Yet experimental work shows that feeding rates of insect herbivores can increase, decrease, or remain unchanged at higher temperatures 159– 162 and that responses vary even for a single herbivore species among different host-plant species 161, 162 ( Figure 3). In other cases, there is a striking interaction with elevated temperatures and the intake of plant secondary compounds, and there is some evidence for enhanced toxicity of compounds at higher temperatures 163, but again with considerable variability among different species 162.
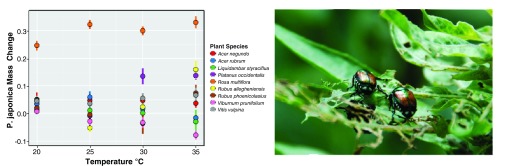
Figure 3. Climate warming may have idiosyncratic effects on plant-herbivore interactions, depending on the species involved.
Lemoine et al. 162 showed that increasing temperature affects growth rates of Japanese beetles ( Popilla japonica, at right skeletonizing an Oenothera biennis leaf) differently depending on the plant species they are feeding on. For example, Japanese beetle growth increased with temperature on plants such as Platanus occidentalis (purple points) but declined on species such as Viburnum prunifolium (pink points). These data highlight the difficulty in predicting the impact of climate warming on plant-herbivore interactions. Data redrawn after Lemoine et al. 162. Image from Dejeanne Doublet.
Climate change, particularly increasing CO 2 levels, may also impact how plants allocate resources to growth, defense, and reproduction, which may profoundly influence herbivore feeding behavior. Elevated CO 2 may lower plant nutritional quality, particularly nitrogen density, slowing herbivore growth and reproduction and forcing herbivores to increase consumption rates or shift diets 164– 166. However, the responses of insect herbivores to elevated CO 2 vary widely, and some taxa like Lepidoptera decline in performance, on average, with increased CO 2 while Homopteran performance increases 166. Increasing CO 2 also alters the production of chemical defenses in plants, although the changes in defensive chemistry are often idiosyncratic depending on the plant taxa and chemical compounds 167. Flavonoids, phenolics, and condensed tannins increase, on average, with elevated CO 2 while terpene production declines 166. For example, elevated CO 2 caused reduction in the production of cardenolide defensive chemicals in the milkweed ( Asclepias syriaca), yet CO 2 stimulated production in physical defenses (for example, leaf toughness and latex production), which could have balanced the effect of reduced chemical defenses on herbivores 168. In the future, work examining interactions between temperature and increased CO 2 on plant-herbivore interactions at meaningful ecological scales is sorely needed, as is work that examines the evolutionary consequences of climate change.
Conclusions: conservation of plant-herbivore interactions in the Anthropocene
Species are being lost from many ecosystems at an alarming rate, and large vertebrates often are the first to go 169, 170. Although predator loss is often emphasized, herbivores are also being continually lost to extinction 171, 172, having cascading impacts on the integrity of entire ecosystems 173– 176. Sadly, parallels can be drawn between the contemporaneous loss of herbivores with the vast changes that occurred following the Pleistocene megafaunal extinction, when many continents lost most of their large consumers, resulting in “no-analog” plant communities with novel suites of interactions and presumably altered ecosystem functionality 177– 179.
In contrast, the opposite effect is also a prevailing problem in many ecosystems. For example, explosive population growth of white-tailed deer ( Odocoileus virginianus) resulting from loss of predators and human-altered habitat has led to widespread overbrowsing and loss of plant diversity in temperate forests in North America 179– 183, a situation that may take decades to reverse 184. White-tailed deer are particularly emblematic of the difficulties inherent in managing plant-herbivore interactions within the context of ecosystem conservation. For example, numerous studies show that the negative effects of deer can be ameliorated and even reversed at densities approaching their historical levels (for example, 180, 185, 186), but there remains significant opposition to implementing meaningful hunting and culling programs aimed at reducing deer densities 187. These intransigent problems demonstrate that in many cases we do not lack the scientific information to exert meaningful differences, only the collective willpower.
Finally, we note that the study of plant-herbivore interactions continues to be a leading light in ecology and evolution, demonstrating the power of applying new technologies and multiple perspectives to resolving long-standing uncertainties. These newfound approaches show that in many cases the world is even more complex than we once thought 22, 109. Thus, our challenge is to find conservation solutions that accurately reflect the pervasive impacts of plant-herbivore interactions across broad temporal and spatial scales, preserving ecosystem multifunctionality and sustainability for future generations.
Acknowledgments
The authors thank Mark Hay for his dedicated mentorship, profound influence in shaping the study of plant-herbivore interactions, rigorous application of the scientific method, and fine spirits. We thank Nathan Lemoine, P.B. Ribbon, Karin Burghardt, and Eric Griffin for feedback on early versions of the manuscript.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Oswald Schmitz, School of Forestry and Environmental Studies, Yale University , New Haven, CT, USA
Walter Carson, Department of Biological Sciences, University of Pittsburgh, Pittsburgh, PA, USA
Funding Statement
DEB’s research on marine and terrestrial plant-herbivore interactions has been funded by U.S. National Science Foundation grants DEB-0841917 and OCE-1130786. JDP’s research has been funded by the U.S. National Aeronautics and Space Administration (NNX-11AO94G), the U.S. National Science Foundation (EF1065821), and the Gates Foundation.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 2 approved]
References
- 1. Ehrlich PR, Raven PH: Butterflies and Plants: A Study in Coevolution. Evolution. 1964;18(4):586–608. 10.2307/2406212 [DOI] [Google Scholar]
- 2. Johnson MT, Campbell SA, Barrett SC: Evolutionary Interactions Between Plant Reproduction and Defense Against Herbivores. Annu Rev Ecol Evol Syst. 2015;46(1):191–213. 10.1146/annurev-ecolsys-112414-054215 [DOI] [Google Scholar]
- 3. Hay ME, Fenical W: Marine Plant-Herbivore Interactions: The Ecology of Chemical Defense. Annu Rev Ecol Syst. 1988;19(1):111–45. 10.1146/annurev.es.19.110188.000551 [DOI] [Google Scholar]
- 4. Rasher DB, Stout EP, Engel S, et al. : Marine and terrestrial herbivores display convergent chemical ecology despite 400 million years of independent evolution. Proc Natl Acad Sci U S A. 2015;112(39):12110–5. 10.1073/pnas.1508133112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schuman MC, Baldwin IT: The Layers of Plant Responses to Insect Herbivores. Annu Rev Entomol. 2016;61:373–94. 10.1146/annurev-ento-010715-023851 [DOI] [PubMed] [Google Scholar]
- 6. Raubenheimer D, Simpson SJ, Mayntz D: Nutrition, ecology and nutritional ecology: Toward an integrated framework. Funct Ecol. 2009;23(1):4–16. 10.1111/j.1365-2435.2009.01522.x [DOI] [Google Scholar]
- 7. Wetzel WC, Kharouba HM, Robinson M, et al. : Variability in plant nutrients reduces insect herbivore performance. Nature. 2016;539(7629):425–7. 10.1038/nature20140 [DOI] [PubMed] [Google Scholar]
- 8. Sterner RW, Elser JJ: Ecological Stoichiometry. Princeton, New Jersey, USA: Princeton University Press;2002 Reference Source [Google Scholar]
- 9. Hillebrand H, Borer ET, Bracken ME, et al. : Herbivore metabolism and stoichiometry each constrain herbivory at different organizational scales across ecosystems. Ecol Lett. 2009;12(6):516–27. 10.1111/j.1461-0248.2009.01304.x [DOI] [PubMed] [Google Scholar]
- 10. Lemoine NP, Giery ST, Burkepile DE: Differing nutritional constraints of consumers across ecosystems. Oecologia. 2014;174(4):1367–76. 10.1007/s00442-013-2860-z [DOI] [PubMed] [Google Scholar]
- 11. Burkepile DE: Comparing aquatic and terrestrial grazing ecosystems: Is the grass really greener? Oikos. 2013;122(2):306–12. 10.1111/j.1600-0706.2012.20716.x [DOI] [Google Scholar]
- 12. Owen-Smith N: Megaherbivores: The Influence of Very Large Body Size on Ecology.Cambridge, UK: Cambridge University Press;1988. Reference Source [Google Scholar]
- 13. Brose U, Jonsson T, Berlow EL, et al. : Consumer-resource body-size relationships in natural food webs. Ecology. 2006;87(10):2411–7. 10.1890/0012-9658(2006)87[2411:CBRINF]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 14. Olff H, Ritchie ME: Effects of herbivores on grassland plant diversity. Trends Ecol Evol. 1998;13(7):261–5. 10.1016/S0169-5347(98)01364-0 [DOI] [PubMed] [Google Scholar]
- 15. Proulx M, Mazumder A: Reversal of Grazing Impact on Plant Species Richness in Nutrient-Poor vs. Nutrient-Rich Ecosystems. Ecology. 1998;79(8):2581–92. 10.1890/0012-9658(1998)079[2581:ROGIOP]2.0.CO;2 [DOI] [Google Scholar]
- 16. Ward CL, McCann KS, Rooney N: HSS revisited: multi-channel processes mediate trophic control across a productivity gradient. Ecol Lett. 2015. 10.1111/ele.12498 [DOI] [PubMed] [Google Scholar]
- 17. Edger PP, Heidel-Fischer HM, Bekaert M, et al. : The butterfly plant arms-race escalated by gene and genome duplications. Proc Natl Acad Sci U S A. 2015;112(27):8362–6. 10.1073/pnas.1503926112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barbour MA, Rodriguez-Cabal MA, Wu ET, et al. : Multiple plant traits shape the genetic basis of herbivore community assembly. Funct Ecol. 2015;29(8):995–1006. 10.1111/1365-2435.12409 [DOI] [Google Scholar]
- 19. Castagneyrol B, Jactel H, Vacher C, et al. : Effects of plant phylogenetic diversity on herbivory depend on herbivore specialization. J Appl Ecol. 2014;51(1):134–41. 10.1111/1365-2664.12175 [DOI] [Google Scholar]
- 20. Parker JD, Burkepile DE, Lajeunesse MJ, et al. : Phylogenetic isolation increases plant success despite increasing susceptibility to generalist herbivores. Diversity and Distributions. 2012;18(1):1–9. 10.1111/j.1472-4642.2011.00806.x [DOI] [Google Scholar]
- 21. Schuldt A, Assmann T, Bruelheide H, et al. : Functional and phylogenetic diversity of woody plants drive herbivory in a highly diverse forest. New Phytol. 2014;202(3):864–73. 10.1111/nph.12695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kartzinel TR, Chen PA, Coverdale TC, et al. : DNA metabarcoding illuminates dietary niche partitioning by African large herbivores. Proc Natl Acad Sci U S A. 2015;112(26):8019–24. 10.1073/pnas.1503283112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pompanon F, Deagle BE, Symondson WO, et al. : Who is eating what: diet assessment using next generation sequencing. Mol Ecol. 2012;21(8):1931–50. 10.1111/j.1365-294X.2011.05403.x [DOI] [PubMed] [Google Scholar]
- 24. Kays R, Crofoot MC, Jetz W, et al. : ECOLOGY. Terrestrial animal tracking as an eye on life and planet. Science. 2015;348(6240):aaa2478. 10.1126/science.aaa2478 [DOI] [PubMed] [Google Scholar]
- 25. Lemoine NP, Burkepile DE, Parker JD: Quantifying Differences Between Native and Introduced Species. Trends Ecol Evol. 2016;31(5):372–81. 10.1016/j.tree.2016.02.008 [DOI] [PubMed] [Google Scholar]
- 26. Feeny P: Plant apparency and chemical defense. In: Biochemical Interaction between Plants and Insects: proceedings of the 15th annual meeting of the Phytochemical Society of North America Edited by Wallace JW, Mansell RL. New York: Plenum Press;1976;1–40. 10.1007/978-1-4684-2646-5_1 [DOI] [Google Scholar]
- 27. Maron JL, Vila M: When do herbivores affect plant invasion?: Evidence for the natural enemies and biotic resistance hypotheses. Oikos. 2001;95(3):361–73. 10.1034/j.1600-0706.2001.950301.x [DOI] [Google Scholar]
- 28. Verhoeven KJ, Biere A, Harvey JA, et al. : Plant invaders and their novel natural enemies: who is naïve? Ecol Lett. 2009;12(2):107–17. 10.1111/j.1461-0248.2008.01248.x [DOI] [PubMed] [Google Scholar]
- 29. Keane R: Exotic plant invasions and the enemy release hypothesis. Trends Ecol Evol. 2002;17(4):164–70. 10.1016/S0169-5347(02)02499-0 [DOI] [Google Scholar]
- 30. Kalisz S, Spigler RB, Horvitz CC: In a long-term experimental demography study, excluding ungulates reversed invader's explosive population growth rate and restored natives. Proc Natl Acad Sci USA. 2014;111(12):4501–6. 10.1073/pnas.1310121111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Heckman RW, Wright JP, Mitchell CE: Joint effects of nutrient addition and enemy exclusion on exotic plant success. Ecology. 2016;97(12):3337–45. 10.1002/ecy.1585 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 32. Heard MJ, Sax DF: Coexistence between native and exotic species is facilitated by asymmetries in competitive ability and susceptibility to herbivores. Ecol Lett. 2013;16(2):206–13. 10.1111/ele.12030 [DOI] [PubMed] [Google Scholar]
- 33. Alofs KM, Jackson DA: Meta-analysis suggests biotic resistance in freshwater environments is driven by consumption rather than competition. Ecology. 2014;95(12):3259–70. 10.1890/14-0060.1 [DOI] [Google Scholar]
- 34. Heger T, Jeschke JM: The enemy release hypothesis as a hierarchy of hypotheses. Oikos. 2014;123(6):741–50. 10.1111/j.1600-0706.2013.01263.x [DOI] [Google Scholar]
- 35. Macel M, de Vos RC, Jansen JJ, et al. : Novel chemistry of invasive plants: exotic species have more unique metabolomic profiles than native congeners. Ecol Evol. 2014;4(13):2777–86. 10.1002/ece3.1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lind EM, Parker JD: Novel weapons testing: are invasive plants more chemically defended than native plants? PLoS One. 2010;5(5):e10429. 10.1371/journal.pone.0010429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schultheis EH, Berardi AE, Lau JA: No release for the wicked: enemy release is dynamic and not associated with invasiveness. Ecology. 2015;96(9):2446–57. 10.1890/14-2158.1 [DOI] [PubMed] [Google Scholar]
- 38. Seabloom EW, Borer ET, Buckley YM, et al. : Plant species' origin predicts dominance and response to nutrient enrichment and herbivores in global grasslands. Nat Commun. 2015;6:7710. 10.1038/ncomms8710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Parker JD, Burkepile DE, Hay ME: Opposing effects of native and exotic herbivores on plant invasions. Science. 2006;311(5766):1459–61. 10.1126/science.1121407 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 40. Felker-Quinn E, Schweitzer JA, Bailey JK: Meta-analysis reveals evolution in invasive plant species but little support for Evolution of Increased Competitive Ability (EICA). Ecol Evol. 2013;3(3):739–51. 10.1002/ece3.488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Blumenthal DM: Interactions between resource availability and enemy release in plant invasion. Ecol Lett. 2006;9(7):887–95. 10.1111/j.1461-0248.2006.00934.x [DOI] [PubMed] [Google Scholar]
- 42. Gruntman M, Segev U, Glauser G, et al. : Evolution of plant defences along an invasion chronosequence: Defence is lost due to enemy release - but not forever. J Ecol. 2017;105(1):255–64. 10.1111/1365-2745.12660 [DOI] [Google Scholar]; F1000 Recommendation
- 43. Smith-Ramesh LM, Moore AC, Schmitz OJ: Global synthesis suggests that food web connectance correlates to invasion resistance. Glob Chang Biol. 2017;23(2):465–473. 10.1111/gcb.13460 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 44. Fraenkel GS: The raison d'ĕtre of secondary plant substances; these odd chemicals arose as a means of protecting plants from insects and now guide insects to food. Science. 1959;129(3361):1466–70. 10.1126/science.129.3361.1466 [DOI] [PubMed] [Google Scholar]
- 45. Agrawal AA, Hastings AP, Bradburd GS, et al. : Evolution of plant growth and defense in a continental introduction. Am Nat. 2015;186(1):E1–E15. 10.1086/681622 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 46. Carmona D, Lajeunesse MJ, Johnson MT: Plant traits that predict resistance to herbivores. Funct Ecol. 2011;25(2):358–67. 10.1111/j.1365-2435.2010.01794.x [DOI] [Google Scholar]
- 47. Schuldt A, Bruelheide H, Durka W, et al. : Plant traits affecting herbivory on tree recruits in highly diverse subtropical forests. Ecol Lett. 2012;15(7):732–9. 10.1111/j.1461-0248.2012.01792.x [DOI] [PubMed] [Google Scholar]
- 48. Agrawal AA, Weber MG: On the study of plant defence and herbivory using comparative approaches: how important are secondary plant compounds. Ecol Lett. 2015;18(10):985–91. 10.1111/ele.12482 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 49. Moles AT, Peco B, Wallis IR, et al. : Correlations between physical and chemical defences in plants: tradeoffs, syndromes, or just many different ways to skin a herbivorous cat? New Phytol. 2013;198(1):252–63. 10.1111/nph.12116 [DOI] [PubMed] [Google Scholar]
- 50. Hay ME, Kappel QE, Fenical W: Synergisms in Plant Defenses against Herbivores: Interactions of Chemistry, Calcification, and Plant Quality. Ecology. 1994;75(6):1714–26. 10.2307/1939631 [DOI] [Google Scholar]
- 51. Agrawal AA, Hastings AP, Johnson MT, et al. : Insect herbivores drive real-time ecological and evolutionary change in plant populations. Science. 2012;338(6103):113–6. 10.1126/science.1225977 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 52. Campbell SA, Thaler JS, Kessler A: Plant chemistry underlies herbivore-mediated inbreeding depression in nature. Ecol Lett. 2013;16(2):252–60. 10.1111/ele.12036 [DOI] [PubMed] [Google Scholar]
- 53. Parker JD, Salminen JP, Agrawal AA: Herbivory enhances positive effects of plant genotypic diversity. Ecol Lett. 2010;13(5):553–63. 10.1111/j.1461-0248.2010.01452.x [DOI] [PubMed] [Google Scholar]
- 54. Parker JD, Salminen JP, Agrawal AA: Evolutionary potential of root chemical defense: genetic correlations with shoot chemistry and plant growth. J Chem Ecol. 2012;38(8):992–5. 10.1007/s10886-012-0163-1 [DOI] [PubMed] [Google Scholar]
- 55. Turley NE, Odell WC, Schaefer H, et al. : Contemporary evolution of plant growth rate following experimental removal of herbivores. Am Nat. 2013;181(Suppl 1):S21–34. 10.1086/668075 [DOI] [PubMed] [Google Scholar]
- 56. Mundim FM, Alborn HT, Vieira-Neto EH, et al. : A whole-plant perspective reveals unexpected impacts of above- and belowground herbivores on plant growth and defense. Ecology. 2017;98(1):70–8. 10.1002/ecy.1619 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 57. Coley PD, Bryant JP, Chapin FS, 3rd: Resource availability and plant antiherbivore defense. Science. 1985;230(4728):895–9. 10.1126/science.230.4728.895 [DOI] [PubMed] [Google Scholar]
- 58. Endara M, Coley PD: The resource availability hypothesis revisited: A meta-analysis. Functional Ecology. 2011;25(2):389–98. 10.1111/j.1365-2435.2010.01803.x [DOI] [Google Scholar]
- 59. Tao L, Berns AR, Hunter MD: Why does a good thing become too much?: Interactions between foliar nutrients and toxins determine performance of an insect herbivore. Funct Ecol. 2014;28(1):190–6. 10.1111/1365-2435.12163 [DOI] [Google Scholar]
- 60. Andow DA: Vegetational Diversity and Arthropod Population Response. Annu Rev Entomol. 1991;36:561–86. 10.1146/annurev.en.36.010191.003021 [DOI] [Google Scholar]
- 61. Dyer LA, Carson WP, Leigh EG: Insect outbreaks in tropical forests: Patterns, mechanisms, and consequences.In: Insect Outbreaks Revisited Edited by Barbosa P, Letourneau DK, Agrawal A. Chichester, UK: John Wiley & Sons, Ltd;2012. 10.1002/9781118295205.ch11 [DOI] [Google Scholar]
- 62. Bolnick DI, Amarasekare P, Araujo MS, et al. : Why intraspecific trait variation matters in community ecology. Trends Ecol Evol. 2011;26(4):183–92. 10.1016/j.tree.2011.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Barabas G, D'Andrea R: The effect of intraspecific variation and heritability on community pattern and robustness. Ecol Lett. 2016;19(8):977–86. 10.1111/ele.12636 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 64. Barbosa P, Hines J, Kaplan I, et al. : Associational Resistance and Associational Susceptibility: Having Right or Wrong Neighbors. Annu Rev Ecol Evol Syst. 2009;40:1–20. 10.1146/annurev.ecolsys.110308.120242 [DOI] [Google Scholar]
- 65. Castagneyrol B, Giffard B, Péré C, et al. : Plant apparency, an overlooked driver of associational resistance to insect herbivory. J Ecol. 2013;101(2):418–29. 10.1111/1365-2745.12055 [DOI] [Google Scholar]
- 66. Morrell K, Kessler A: Plant communication in a widespread goldenrod: Keeping herbivores on the move. Funct Ecol. 2016. 10.1111/1365-2435.12793 [DOI] [Google Scholar]; F1000 Recommendation
- 67. Riolo MA, Rohani P, Hunter MD: Local variation in plant quality influences large-scale population dynamics. Oikos. 2015;124(9):1160–70. 10.1111/oik.01759 [DOI] [Google Scholar]
- 68. Underwood N, Inouye BD, Hamback PA: A conceptual framework for associational effects: when do neighbors matter and how would we know? Q Rev Biol. 2014;89(1):1–19. 10.1086/674991 [DOI] [PubMed] [Google Scholar]
- 69. Burghardt KT: Nutrient supply alters goldenrod's induced response to herbivory. Funct Ecol. 2016;30(11):1769–78. 10.1111/1365-2435.12681 [DOI] [Google Scholar]; F1000 Recommendation
- 70. Schweitzer JA, Juric I, van de Voorde TF, et al. : Are there evolutionary consequences of plant-soil feedbacks along soil gradients? Funct Ecol. 2014;28:55–64. 10.1111/1365-2435.12201 [DOI] [Google Scholar]
- 71. van Nuland ME, Wooliver RC, Pfennigwerth AA, et al. : Plant-soil feedbacks: Connecting ecosystem ecology and evolution. Funct Ecol. 2016;30(7):1032–42. 10.1111/1365-2435.12690 [DOI] [Google Scholar]; F1000 Recommendation
- 72. Flagel DG, Belovsky GE, Beyer DE Jr: Natural and experimental tests of trophic cascades: gray wolves and white-tailed deer in a Great Lakes forest. Oecologia. 2016;180(4):1183–94. 10.1007/s00442-015-3515-z [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 73. Christian N, Whitaker BK, Clay K: Microbiomes: unifying animal and plant systems through the lens of community ecology theory. Front Microbiol. 2015;6:869. 10.3389/fmicb.2015.00869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Glassmire AE, Jeffrey CS, Forister ML, et al. : Intraspecific phytochemical variation shapes community and population structure for specialist caterpillars. New Phytol. 2016;212(1):208–19. 10.1111/nph.14038 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 75. Hunter MD: The Phytochemical Landscape: Linking Trophic Interactions and Nutrient Dynamics.In.: Princeton University Press;2016. Reference Source [Google Scholar]
- 76. Thompson JN: The Geographic Mosaic of Coevolution. University of Chicago Press;2005. Reference Source [Google Scholar]
- 77. Schmitz OJ, Buchkowski RW, Burghardt KT, et al. : Chapter Ten - Functional Traits and Trait-Mediated Interactions: Connecting Community-Level Interactions with Ecosystem Functioning.In: Advances in Ecological Research Edited by Samraat Pawar GW, Anthony ID, Academic Press;2015;52:319–343. 10.1016/bs.aecr.2015.01.003 [DOI] [Google Scholar]
- 78. Isbell F, Craven D, Connolly J, et al. : Biodiversity increases the resistance of ecosystem productivity to climate extremes. Nature. 2015;526(7574):574–7. 10.1038/nature15374 [DOI] [PubMed] [Google Scholar]
- 79. Lefcheck JS, Byrnes JE, Isbell F, et al. : Biodiversity enhances ecosystem multifunctionality across trophic levels and habitats. Nat Commun. 2015;6:6936. 10.1038/ncomms7936 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 80. Tilman D, Isbell F, Cowles JM: Biodiversity and Ecosystem Functioning. Annu Rev Ecol Evol Syst. 2014;45:471–93. 10.1146/annurev-ecolsys-120213-091917 [DOI] [Google Scholar]
- 81. Lefcheck JS, Duffy JE: Multitrophic functional diversity predicts ecosystem functioning in experimental assemblages of estuarine consumers. Ecology. 2015;96(11):2973–83. 10.1890/14-1977.1 [DOI] [PubMed] [Google Scholar]
- 82. Hooper DU, Adair EC, Cardinale BJ, et al. : A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature. 2012;486(7401):105–8. 10.1038/nature11118 [DOI] [PubMed] [Google Scholar]
- 83. Schneider FD, Brose U, Rall BC, et al. : Animal diversity and ecosystem functioning in dynamic food webs. Nat Commun. 2016;7:12718. 10.1038/ncomms12718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Burkepile DE, Hay ME: Herbivore species richness and feeding complementarity affect community structure and function on a coral reef. Proc Natl Acad Sci U S A. 2008;105(42):16201–6. 10.1073/pnas.0801946105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Duffy JE, Macdonald KS, Rhode JM, et al. : Grazer diversity, functional redundancy, and productivity in seagrass beds: An experimental test. Ecology. 2001;82(9):2417–34. 10.1890/0012-9658(2001)082[2417:GDFRAP]2.0.CO;2 [DOI] [Google Scholar]
- 86. Rasher DB, Hoey AS, Hay ME: Consumer diversity interacts with prey defenses to drive ecosystem function. Ecology. 2013;94(6):1347–58. 10.1890/12-0389.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Emmett Duffy J, Paul Richardson J, Canuel EA: Grazer diversity effects on ecosystem functioning in seagrass beds. Ecol Lett. 2003;6(7):637–45. 10.1046/j.1461-0248.2003.00474.x [DOI] [Google Scholar]
- 88. Burkepile DE, Fynn RW, Thompson DI, et al. : Herbivore size matters for productivity-richness relationships in African savannas. J Ecol. 2016. 10.1111/1365-2745.12714 [DOI] [Google Scholar]
- 89. Emmett Duffy J, Paul Richardson J, France KE: Ecosystem consequences of diversity depend on food chain length in estuarine vegetation. Ecol Lett. 2005;8(3):301–9. 10.1111/j.1461-0248.2005.00725.x [DOI] [Google Scholar]
- 90. Cardinale BJ, Srivastava DS, Duffy JE, et al. : Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature. 2006;443(7114):989–92. 10.1038/nature05202 [DOI] [PubMed] [Google Scholar]
- 91. Adam TC, Kelley M, Ruttenberg BI, et al. : Resource partitioning along multiple niche axes drives functional diversity in parrotfishes on Caribbean coral reefs. Oecologia. 2015;179(4):1173–85. 10.1007/s00442-015-3406-3 [DOI] [PubMed] [Google Scholar]
- 92. Brandl SJ, Bellwood DR: Individual-based analyses reveal limited functional overlap in a coral reef fish community. J Anim Ecol. 2014;83(3):661–70. 10.1111/1365-2656.12171 [DOI] [PubMed] [Google Scholar]
- 93. Brandl SJ, Robbins WD, Bellwood DR: Exploring the nature of ecological specialization in a coral reef fish community: morphology, diet and foraging microhabitat use. Proc Biol Sci. 2015;282(1815): pii: 20151147. 10.1098/rspb.2015.1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Nash KL, Graham NA, Jennings S, et al. : Herbivore cross-scale redundancy supports response diversity and promotes coral reef resilience. J Appl Ecol. 2016;53:646–55. 10.1111/1365-2664.12430 [DOI] [Google Scholar]
- 95. Deraison H, Badenhausser I, Loeuille N, et al. : Functional trait diversity across trophic levels determines herbivore impact on plant community biomass. Ecol Lett. 2015;18(12):1346–55. 10.1111/ele.12529 [DOI] [PubMed] [Google Scholar]
- 96. Hector A, Bagchi R: Biodiversity and ecosystem multifunctionality. Nature. 2007;448(7150):188–90. 10.1038/nature05947 [DOI] [PubMed] [Google Scholar]
- 97. Duffy JE, Cardinale BJ, France KE, et al. : The functional role of biodiversity in ecosystems: incorporating trophic complexity. Ecol Lett. 2007;10(6):522–38. 10.1111/j.1461-0248.2007.01037.x [DOI] [PubMed] [Google Scholar]
- 98. Griffin EA, Traw MB, Morin PJ, et al. : Foliar bacteria and soil fertility mediate seedling performance: a new and cryptic dimension of niche differentiation. Ecology. 2016;97(11):2998–3008. 10.1002/ecy.1537 [DOI] [PubMed] [Google Scholar]
- 99. Duffy JE: Biodiversity loss, trophic skew and ecosystem functioning. Ecol Letters. 2003;6(8):680–7. 10.1046/j.1461-0248.2003.00494.x [DOI] [Google Scholar]
- 100. Kleynhans EJ, Jolles AE, Bos MR, et al. : Resource partitioning along multiple niche dimensions in differently sized African savanna grazers. Oikos. 2011;120(4):591–600. 10.1111/j.1600-0706.2010.18712.x [DOI] [Google Scholar]
- 101. Singer MS: Behaviorally plastic host-plant use by larval Lepidoptera in tri-trophic food webs. Curr Opin Insect Sci. 2016;14:56–60. 10.1016/j.cois.2016.01.005 [DOI] [PubMed] [Google Scholar]
- 102. Kelly EL, Eynaud Y, Clements SM, et al. : Investigating functional redundancy versus complementarity in Hawaiian herbivorous coral reef fishes. Oecologia. 2016;182(4):1151–63. 10.1007/s00442-016-3724-0 [DOI] [PubMed] [Google Scholar]
- 103. de Iongh HH, de Jong CB, van Goethem J, et al. : Resource partitioning among African savanna herbivores in North Cameroon: The importance of diet composition, food quality and body mass. J Trop Ecol. 2011;27(5):503–13. 10.1017/S0266467411000307 [DOI] [Google Scholar]
- 104. Valentini A, Pompanon F, Taberlet P: DNA barcoding for ecologists. Trends Ecol Evol. 2009;24(2):110–7. 10.1016/j.tree.2008.09.011 [DOI] [PubMed] [Google Scholar]
- 105. Kress WJ, Garcia-Robledo C, Uriarte M, et al. : DNA barcodes for ecology, evolution, and conservation. Trends Ecol Evol. 2015;30(1):25–35. 10.1016/j.tree.2014.10.008 [DOI] [PubMed] [Google Scholar]
- 106. Jurado-Rivera JA, Vogler AP, Reid CA, et al. : DNA barcoding insect-host plant associations. Proc Biol Sci. 2009;276(1657):639–48. 10.1098/rspb.2008.1264 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 107. Soininen EM, Valentini A, Coissac E, et al. : Analysing diet of small herbivores: the efficiency of DNA barcoding coupled with high-throughput pyrosequencing for deciphering the composition of complex plant mixtures. Front Zool. 2009;6:16. 10.1186/1742-9994-6-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Willerslev E, Davison J, Moora M, et al. : Fifty thousand years of Arctic vegetation and megafaunal diet. Nature. 2014;506(7486):47–51. 10.1038/nature12921 [DOI] [PubMed] [Google Scholar]
- 109. Garcia-Robledo C, Kuprewicz EK, Staines CL, et al. : Limited tolerance by insects to high temperatures across tropical elevational gradients and the implications of global warming for extinction. Proc Natl Acad Sci U S A. 2016;113(3):680–5. 10.1073/pnas.1507681113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Garcia-Robledo C, Erickson DL, Staines CL, et al. : Tropical plant-herbivore networks: reconstructing species interactions using DNA barcodes. PLoS One. 2013;8(1):e52967. 10.1371/journal.pone.0052967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Trussell GC, Ewanchuk PJ, Bertness MD, et al. : Trophic cascades in rocky shore tide pools: distinguishing lethal and nonlethal effects. Oecologia. 2004;139(3):427–32. 10.1007/s00442-004-1512-8 [DOI] [PubMed] [Google Scholar]
- 112. Burkholder DA, Heithaus MR, Fourqurean JW, et al. : Patterns of top-down control in a seagrass ecosystem: could a roving apex predator induce a behaviour-mediated trophic cascade? J Anim Ecol. 2013;82(6):1192–202. 10.1111/1365-2656.12097 [DOI] [PubMed] [Google Scholar]
- 113. Madin EM, Gaines SD, Madin JS, et al. : Fishing indirectly structures macroalgal assemblages by altering herbivore behavior. Am Nat. 2010;176(6):785–801. 10.1086/657039 [DOI] [PubMed] [Google Scholar]
- 114. Rizzari JR, Frisch AJ, Hoey AS, et al. : Not worth the risk: Apex predators suppress herbivory on coral reefs. Oikos. 2014;123(7):829–36. 10.1111/oik.01318 [DOI] [Google Scholar]
- 115. Power ME, Matthews WJ, Stewart AJ: Grazing Minnows, Piscivorous Bass, and Stream Algae: Dynamics of a Strong Interaction. Ecology. 1985;66:1448–56. 10.2307/1938007 [DOI] [Google Scholar]
- 116. Peckarsky BL, McIntosh AR: Fitness and community consequences of avoiding multiple predators. Oecologia. 1998;113:565–76. 10.1007/s004420050410 [DOI] [PubMed] [Google Scholar]
- 117. Beckerman AP, Uriarte M, Schmitz OJ: Experimental evidence for a behavior-mediated trophic cascade in a terrestrial food chain. Proc Natl Acad Sci U S A. 1997;94(20):10735–8. 10.1073/pnas.94.20.10735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Schmitz OJ: Effects of predator hunting mode on grassland ecosystem function. Science. 2008;319(5865):952–4. 10.1126/science.1152355 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 119. Frank DA: Evidence for top predator control of a grazing ecosystem. Oikos. 2008;117(11):1718–24. 10.1111/j.1600-0706.2008.16846.x [DOI] [Google Scholar]
- 120. Christianson D, Creel S: Risk effects in elk: Sex-specific responses in grazing and browsing due to predation risk from wolves. Behav Ecol. 2008;19(6):1258–66. 10.1093/beheco/arn079 [DOI] [Google Scholar]
- 121. Kuijper DPJ, de Kleine C, Churski M, et al. : Landscape of fear in Europe: Wolves affect spatial patterns of ungulate browsing in Białowieża Primeval Forest, Poland. Ecography. 2013;36(12):1263–75. 10.1111/j.1600-0587.2013.00266.x [DOI] [Google Scholar]
- 122. Ford AT, Goheen JR, Otieno TO, et al. : Large carnivores make savanna tree communities less thorny. Science. 2014;346(6207):346–9. 10.1126/science.1252753 [DOI] [PubMed] [Google Scholar]
- 123. Atwood TB, Connolly RM, Ritchie EG, et al. : Predators help protect carbon stocks in blue carbon ecosystems. Nat Clim Chang. 2015;5:1038–45. 10.1038/nclimate2763 [DOI] [Google Scholar]; F1000 Recommendation
- 124. Wilmers CC, Schmitz OJ: Effects of gray wolf-induced trophic cascades on ecosystem carbon cycling. Ecosphere. 2016;7(10):e01501 10.1002/ecs2.1501 [DOI] [Google Scholar]; F1000 Recommendation
- 125. Preisser EL, Orrock JL, Schmitz OJ: Predator hunting mode and habitat domain alter nonconsumptive effects in predator-prey interactions. Ecology. 2007;88(11):2744–51. 10.1890/07-0260.1 [DOI] [PubMed] [Google Scholar]
- 126. Preisser EL, Orrock JL: The allometry of fear: Interspecific relationships between body size and response to predation risk. Ecosphere. 2012;3(9):1–27, art77. 10.1890/ES12-00084.1 [DOI] [Google Scholar]
- 127. Catano LB, Rojas MC, Malossi RJ, et al. : Reefscapes of fear: predation risk and reef hetero-geneity interact to shape herbivore foraging behaviour. J Anim Ecol. 2016;85(1):146–56. 10.1111/1365-2656.12440 [DOI] [PubMed] [Google Scholar]
- 128. Burkepile DE, Burns CE, Tambling CJ, et al. : Habitat selection by large herbivores in a southern African savanna: The relative roles of bottom-up and top-down forces. Ecosphere. 2013;4(11):1–19, art139. 10.1890/ES13-00078.1 [DOI] [Google Scholar]
- 129. Riginos C, Grace JB: Savanna tree density, herbivores, and the herbaceous community: bottom-up vs. top-down effects. Ecology. 2008;89(8):2228–38. 10.1890/07-1250.1 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 130. Burkepile DE, Thompson DI, Fynn RWS, et al. : Fire frequency drives habitat selection by a diverse herbivore guild impacting top-down control of plant communities in an African savanna. Oikos. 2016;125(11):1636–46. 10.1111/oik.02987 [DOI] [Google Scholar]
- 131. Riginos C: Climate and the landscape of fear in an African savanna. J Anim Ecol. 2015;84(1):124–33. 10.1111/1365-2656.12262 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 132. Thaker M, Vanak AT, Owen CR, et al. : Minimizing predation risk in a landscape of multiple predators: effects on the spatial distribution of African ungulates. Ecology. 2011;92(2):398–407. 10.1890/10-0126.1 [DOI] [PubMed] [Google Scholar]
- 133. Creel S, Schuette P, Christianson D: Effects of predation risk on group size, vigilance, and foraging behavior in an African ungulate community. Behav Ecol. 2014;25(4):773–84. 10.1093/beheco/aru050 [DOI] [Google Scholar]
- 134. Catano LB, Barton MB, Boswell KM, et al. : Predator identity and time of day interact to shape the risk-reward trade-off for herbivorous coral reef fishes. Oecologia. 2016;1–11. 10.1007/s00442-016-3794-z [DOI] [PubMed] [Google Scholar]
- 135. Loarie SR, Tambling CJ, Asner GP: Lion hunting behaviour and vegetation structure in an African savanna. Anim Behav. 2013;85(5):899–906. 10.1016/j.anbehav.2013.01.018 [DOI] [Google Scholar]
- 136. Madin EM, Madin JS, Booth DJ: Landscape of fear visible from space. Sci Rep. 2011;1:14. 10.1038/srep00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Diffenbaugh NS, Field CB: Changes in ecologically critical terrestrial climate conditions. Science. 2013;341(6145):486–92. 10.1126/science.1237123 [DOI] [PubMed] [Google Scholar]
- 138. Loarie SR, Duffy PB, Hamilton H, et al. : The velocity of climate change. Nature. 2009;462(7276):1052–5. 10.1038/nature08649 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 139. Bebber DP, Ramotowski MA, Gurr SJ: Crop pests and pathogens move polewards in a warming world. Nat Clim Chang. 2013;3:985–8. 10.1038/nclimate1990 [DOI] [Google Scholar]
- 140. Burrows MT, Schoeman DS, Buckley LB, et al. : The pace of shifting climate in marine and terrestrial ecosystems. Science. 2011;334(6056):652–5. 10.1126/science.1210288 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 141. de Sassi C, Tylianakis JM: Climate change disproportionately increases herbivore over plant or parasitoid biomass. PLoS One. 2012;7(7):e40557. 10.1371/journal.pone.0040557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Lu X, Siemann E, Shao X, et al. : Climate warming affects biological invasions by shifting interactions of plants and herbivores. Glob Chang Biol. 2013;19(8):2339–47. 10.1111/gcb.12244 [DOI] [PubMed] [Google Scholar]
- 143. Riley ME, Johnston CA, Feller IC, et al. : Range Expansion of Aratus pisonii (Mangrove Tree Crab) into Novel Vegetative Habitats. Southeast Nat. 2014;13(4):N43–N48. 10.1656/058.013.0405 [DOI] [Google Scholar]
- 144. Williams AA, Eastman SF, Eash-Loucks WE, et al. : Record Northernmost Endemic Mangroves on the United States Atlantic Coast with a Note on Latitudinal Migration. Southeast Nat. 2014;13(1):56–63. 10.1656/058.013.0104 [DOI] [Google Scholar]
- 145. Feller IC, Chamberlain A: Herbivore responses to nutrient enrichment and landscape heterogeneity in a mangrove ecosystem. Oecologia. 2007;153(3):607–16. 10.1007/s00442-007-0760-9 [DOI] [PubMed] [Google Scholar]
- 146. Cannizzo ZJ, Griffen BD: Changes in spatial behaviour patterns by mangrove tree crabs following climate-induced range shift into novel habitat. Anim Behav. 2016;121:79–86. 10.1016/j.anbehav.2016.08.025 [DOI] [Google Scholar]
- 147. Riley ME, Griffen BD: Climate-change induced range expansion alters traditional ecogeographic patterns of life history. in review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Verges A, Steinberg PD, Hay ME, et al. : The tropicalization of temperate marine ecosystems: climate-mediated changes in herbivory and community phase shifts. Proc Biol Sci. 2014;281(1789):20140846. 10.1098/rspb.2014.0846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Bennett S, Wernberg T, Harvey ES, et al. : Tropical herbivores provide resilience to a climate-mediated phase shift on temperate reefs. Ecol Lett. 2015;18(7):714–23. 10.1111/ele.12450 [DOI] [PubMed] [Google Scholar]
- 150. Brodie J, Post E, Watson F, et al. : Climate change intensification of herbivore impacts on tree recruitment. Proc Biol Sci. 2012;279(1732):1366–70. 10.1098/rspb.2011.1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Hansen BB, Isaksen K, Benestad RE, et al. : Warmer and wetter winters: Characteristics and implications of an extreme weather event in the High Arctic. Environ Res Lett. 2014;9(11):114021 10.1088/1748-9326/9/11/114021 [DOI] [Google Scholar]
- 152. Hansen BB, Grøtan V, Aanes R, et al. : Climate events synchronize the dynamics of a resident vertebrate community in the high Arctic. Science. 2013;339(6117):313–5. 10.1126/science.1226766 [DOI] [PubMed] [Google Scholar]
- 153. Rivrud IM, Heurich M, Krupczynski P, et al. : Green wave tracking by large herbivores: an experimental approach. Ecology. 2016;97(12):3547–53. 10.1002/ecy.1596 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 154. van Asch M, Salis L, Holleman LJ, et al. : Evolutionary response of the egg hatching date of a herbivorous insect under climate change. Nat Clim Chang. 2012;3:244–8. 10.1038/nclimate1717 [DOI] [Google Scholar]; F1000 Recommendation
- 155. Gaillard JM, Hewison AJ, Klein F, et al. : How does climate change influence demographic processes of widespread species? Lessons from the comparative analysis of contrasted populations of roe deer. Ecol Lett. 2013;16 Suppl 1:48–57. 10.1111/ele.12059 [DOI] [PubMed] [Google Scholar]
- 156. DeLucia EH, Nabity PD, Zavala JA, et al. : Climate change: resetting plant-insect interactions. Plant Physiol. 2012;160(4):1677–85. 10.1104/pp.112.204750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Brown JH, Gillooly JF, Allen AP, et al. : Toward a Metabolic Theory of Ecology. Ecology. 2004;85(7):1771–89. 10.1890/03-9000 [DOI] [Google Scholar]
- 158. O'Connor MI, Gilbert B, Brown CJ: Theoretical predictions for how temperature affects the dynamics of interacting herbivores and plants. Am Nat. 2011;178(5):626–38. 10.1086/662171 [DOI] [PubMed] [Google Scholar]
- 159. O'Connor MI: Warming strengthens an herbivore-plant interaction. Ecology. 2009;90(2):388–98. 10.1890/08-0034.1 [DOI] [PubMed] [Google Scholar]
- 160. Lemoine NP, Burkepile DE: Temperature-induced mismatches between consumption and metabolism reduce consumer fitness. Ecology. 2012;93(11):2483–9. 10.1890/12-0375.1 [DOI] [PubMed] [Google Scholar]
- 161. Lemoine NP, Burkepile DE, Parker JD: Variable effects of temperature on insect herbivory. PeerJ. 2014;2:e376. 10.7717/peerj.376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Lemoine NP, Drews WA, Burkepile DE, et al. : Increased temperature alters feeding behavior of a generalist herbivore. Oikos. 2013;122(12):1669–78. 10.1111/j.1600-0706.2013.00457.x [DOI] [Google Scholar]
- 163. Kurnath P, Merz ND, Dearing MD: Ambient temperature influences tolerance to plant secondary compounds in a mammalian herbivore. Proc Biol Sci. 2016;283(1822): pii: 20152387. 10.1098/rspb.2015.2387 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 164. Fajer ED, Bowers MD, Bazzaz FA: The effects of enriched carbon dioxide atmospheres on plant--insect herbivore interactions. Science. 1989;243(4895):1198–200. 10.1126/science.243.4895.1198 [DOI] [PubMed] [Google Scholar]
- 165. Veteli TO, Kuokkanen K, Julkunen-Tiitto R, et al. : Effects of elevated CO 2 and temperature on plant growth and herbivore defensive chemistry. Glob Chang Biol. 2002;8(12):1240–52. 10.1046/j.1365-2486.2002.00553.x [DOI] [Google Scholar]
- 166. Robinson EA, Ryan GD, Newman JA: A meta-analytical review of the effects of elevated CO 2 on plant-arthropod interactions highlights the importance of interacting environmental and biological variables. New Phytol. 2012;194(2):321–36. 10.1111/j.1469-8137.2012.04074.x [DOI] [PubMed] [Google Scholar]
- 167. Zavala JA, Nabity PD, DeLucia EH: An emerging understanding of mechanisms governing insect herbivory under elevated CO 2. Annu Rev Entomol. 2013;58:79–97. 10.1146/annurev-ento-120811-153544 [DOI] [PubMed] [Google Scholar]
- 168. Vannette RL, Hunter MD: Genetic variation in expression of defense phenotype may mediate evolutionary adaptation of Asclepias syriaca to elevated CO 2. Glob Chang Biol. 2011;17(3):1277–88. 10.1111/j.1365-2486.2010.02316.x [DOI] [Google Scholar]
- 169. Dirzo R, Young HS, Galetti M, et al. : Defaunation in the Anthropocene. Science. 2014;345(6195):401–6. 10.1126/science.1251817 [DOI] [PubMed] [Google Scholar]
- 170. McCauley DJ, Pinsky ML, Palumbi SR, et al. : Marine defaunation: animal loss in the global ocean. Science. 2015;347(6219):1255641. 10.1126/science.1255641 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 171. Ripple WJ, Newsome TM, Wolf C, et al. : Collapse of the world‘s largest herbivores. Sci Adv. 2015;1(4):e1400103. 10.1126/sciadv.1400103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Edwards CB, Friedlander AM, Green AG, et al. : Global assessment of the status of coral reef herbivorous fishes: evidence for fishing effects. Proc Biol Sci. 2014;281(1774):20131835. 10.1098/rspb.2013.1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Zaneveld JR, Burkepile DE, Shantz AA, et al. : Overfishing and nutrient pollution interact with temperature to disrupt coral reefs down to microbial scales. Nat Commun. 2016;7:11833. 10.1038/ncomms11833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Young HS, Dirzo R, Helgen KM, et al. : Declines in large wildlife increase landscape-level prevalence of rodent-borne disease in Africa. Proc Natl Acad Sci U S A. 2014;111(19):7036–41. 10.1073/pnas.1404958111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Graham NA, Jennings S, MacNeil MA, et al. : Predicting climate-driven regime shifts versus rebound potential in coral reefs. Nature. 2015;518(7537):94–7. 10.1038/nature14140 [DOI] [PubMed] [Google Scholar]
- 176. Pringle RM, Young TP, Rubenstein DI, et al. : Herbivore-initiated interaction cascades and their modulation by productivity in an African savanna. Proc Natl Acad Sci U S A. 2007;104(1):193–7. 10.1073/pnas.0609840104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Bakker ES, Gill JL, Johnson CN, et al. : Combining paleo-data and modern exclosure experiments to assess the impact of megafauna extinctions on woody vegetation. Proc Natl Acad Sci U S A. 2016;113(4):847–55. 10.1073/pnas.1502545112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Gill JL: Ecological impacts of the late Quaternary megaherbivore extinctions. New Phytol. 2014;201(4):1163–9. 10.1111/nph.12576 [DOI] [PubMed] [Google Scholar]
- 179. Gill JL, Williams JW, Jackson ST, et al. : Pleistocene megafaunal collapse, novel plant communities, and enhanced fire regimes in North America. Science. 2009;326(5956):1100–3. 10.1126/science.1179504 [DOI] [PubMed] [Google Scholar]
- 180. Nuttle T, Royo AA, Adams MB, et al. : Historic disturbance regimes promote tree diversity only under low browsing regimes in eastern deciduous forest. Ecol Monogr. 2013;83(1):3–17. 10.1890/11-2263.1 [DOI] [Google Scholar]
- 181. Royo AA, Collins R, Adams MB, et al. : Pervasive interactions between ungulate browsers and disturbance regimes promote temperate forest herbaceous diversity. Ecology. 2010;91(1):93–105. 10.1890/08-1680.1 [DOI] [PubMed] [Google Scholar]
- 182. Heckel CD, Bourg NA, McShea WJ, et al. : Nonconsumptive effects of a generalist ungulate herbivore drive decline of unpalatable forest herbs. Ecology. 2010;91(2):319–26. 10.1890/09-0628.1 [DOI] [PubMed] [Google Scholar]
- 183. DiTommaso A, Morris SH, Parker JD, et al. : Deer browsing delays succession by altering aboveground vegetation and belowground seed banks. PLoS One. 2014;9(3):e91155. 10.1371/journal.pone.0091155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Pendergast TH, Hanlon SM, Long ZM, et al. : The legacy of deer overabundance: Long-term delays in herbaceous understory recovery. Can J For Res. 2016;46(3):362–9. 10.1139/cjfr-2015-0280 [DOI] [Google Scholar]; F1000 Recommendation
- 185. Cook-Patton SC, LaForgia M, Parker JD: Positive interactions between herbivores and plant diversity shape forest regeneration. Proc Biol Sci. 2014;281(1783): 20140261. 10.1098/rspb.2014.0261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Horsley SB, Stout SL, deCalesta DS: White-Tailed Deer Impact on the Vegetation Dynamics of A Northern Hardwood Forest. Ecol Appl. 2003;13(1):98–118. 10.1890/1051-0761(2003)013[0098:WTDIOT]2.0.CO;2 [DOI] [Google Scholar]
- 187. Cambronne A: Deerland: America‘s Hunt for Ecological Balance and the Essence of Wildness.Lyons Press;2013. Reference Source [Google Scholar]