Abstract
Background
Low adherence to oral bisphosphonates is a common problem that jeopardizes the efficacy of treatment of osteoporosis. No clear screening strategy for the assessment of compliance is widely accepted in these patients.
Methods
The International Osteoporosis Foundation and the European Calcified Tissue Society have convened a working group to propose a screening strategy to detect a lack of adherence to these drugs. The question to answer was whether the bone turnover markers (BTMs) PINP and CTX can be used to identify low adherence in patients with postmenopausal osteoporosis initiating oral bisphosphonates for osteoporosis. The findings of the TRIO study specifically address this question and were used as the basis for testing the hypothesis.
Results
Based on the findings of the TRIO study, specifically addressing this question, the working group recommends measuring PINP and CTX at baseline and three months after starting therapy to check for a decrease above the least significant change (decrease of more than 38% for PINP and 56% for CTX). Detection rate for the measurement of PINP is 84%, for CTX 87% and, if variation in at least one is considered when measuring both, the level of detection is 94.5%.
Conclusions
If a significant decrease is observed the treatment can continue but if no decrease occurs the clinician should reassess to identify problems with the treatment, mainly low adherence
Keywords: Adherence, Bisphosphonates, Osteoporosis treatment, Screening, Position paper
Introduction
Oral bisphosphonates are a first line treatment for osteoporosis. However, as in any other chronic diseases, low adherence is a common clinical problem. It has been observed that the adherence to oral bisphosphonates is as low as 59 (1) or 43% (2) at one year and appears to be worse with generic medications (3). This problem significantly jeopardizes the anti-fracture efficacy and cost effectiveness (4). Few interventions to improve adherence have been tested, and the ones that may be effective include education programs (5, 6).
Monitoring adherence is the first step in managing this problem since it detects those patients with problems with the medication. The response of bone turnover markers to therapy, in the individual patient, is one of the methods suggested for treatment monitoring (7). Their advantage is that they are widely available, affordable and clinicians are familiar with their interpretation. They reflect the early effect of the drug on bone tissue (8). Low response may be detected shortly after treatment has been started and may indicate low adherence, low bioavailability, interactions with other drugs or the presence of secondary osteoporosis (9). Moreover, bone turnover marker response has been used as an intervention for improving treatment adherence(5) (10).
Serum PINP (Procollagen type I N- terminal propeptide) and CTX (Collagen type I C-terminal telopeptide) have been recommended as reference markers by a committee of the International Osteoporosis Foundation (IOF) and the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) (8). Oral bisphosphonates decrease levels of PINP and CTX rapidly in most patients, beyond the least significant change (LSC), the margin of change as demonstrated from the results of a recent controlled trial (11). Therefore they are an excellent candidate for screening treatment effect early after starting the drug and may detect any problem with adherence.
The aim of the present work is to establish a clinically feasible and practical strategy, based on bone turnover marker measurement, to detect a lack of response which may indicate a problem with adherence to oral osteoporosis drugs, specifically amino bisphosphonates, and give a recommendation for their use in clinical practice in the individual patient. For this the International Osteoporosis Foundation and the European Calcified Tissue Society have convened a working group to answer this question.
Methods
The Working Group (WG) proposed the following question: Can the bone turnover markers (BTMs) PINP and CTX be used to identify low adherence in patients with postmenopausal osteoporosis initiating oral bisphosphonates for osteoporosis?
Bone turnover markers
Measurement of bone turnover markers is considered as the most specific early method for measuring the biological effect of bisphosphonates. The WG focused on the two markers prioritized by the IOF, namely serum CTX and PINP.
It is necessary to know what proportion of patients with osteoporosis has changes in these markers that exceed the least significant change when taking oral bisphosphonate therapy. This proportion provides the detection rate of the test (i.e., the sensitivity). The least significant change is defined here as the 95% confidence bounds for change in patients treated with bisphosphonates for 12 weeks; thus, only 2.5% of untreated patients would exceed the least significant change (the false positive rate). Responders are considered as those patients who show changes in BTMs that exceed the LSC. The levels established for LSC were 56% decrease for CTX and 38% decrease for PINP (11). Two measurements of bone turnover markers, at baseline and three months after the prescription, are recommended. This 3 month interval is long enough to be able to detect the change, is acceptable to the patient and represents the period during which treatment is often discontinued (12). Where baseline levels were not available, ‘response’ was also considered if the BTM were below the premenopausal mean (32 ng/L for CTX and 28 ug/L for PINP).
Analysis of the TRIO Study
The primary source of information to establish the reference values for response rate is the TRIO study (11, 13). The TRIO study was so named as it was a study of three drugs (a trio of drugs). Under conditions of a controlled clinical trial, the results of the TRIO study can be assumed to be a benchmark for response of turnover markers.
The TRIO study was a single-center randomized controlled trial of three oral bisphosphonates (alendronate, ibandronate and risedronate) at their licensed doses to study their effect on bone turnover markers and bone mineral density in postmenopausal osteoporosis. The study specifically addresses the proportion of patients initiating oral bisphosphonates that show decreases in BTMs beyond the LSC after three months of therapy. The least significant change used was 2-tailed, p<0.05, thus we can be 95% sure that a change (up or down) as large as this, or larger, is significant. Furthermore, to minimize variability standardized sample collection, appropriate instructions to the patient and exclusion of individuals with fracture in the preceding 12 months were applied. We have focused on 3 months for simplicity, but the window could be widened, especially for PINP, and in an unpublished analysis we found that a later measurement did not affect the results.
The TRIO study included 172 women with postmenopausal osteoporosis ages 53-84 who were treated for up to 2 years. There was a concurrent (non-randomized) control group of 87 premenopausal women ages 35 to 40 years. Women with osteoporosis were entered into an open label, parallel, randomized intervention with the three commonly-used oral bisphosphonates, alendronate, ibandronate and risedronate (11) along with calcium and vitamin D supplementation.
Blood was taken in the fasting state between 0800 and 1000 before any supplement was given (-1 week), one week on supplement (0 week) and after starting bisphosphonates (1, 2, 4, 12, 13, 48 and 96 weeks). This analysis will use the -1 week as baseline and the average of the 12 and 13-week samples for on treatment. Biochemical measurements included serum CTX and PINP using the IDS iSYS automated immunoassay platform. All specimens were collected fasting, frozen at -80aC and were measured in one analytical batch. Many other measurements were made of biochemical variables as well as bone density, but these are not included here.
Adherence was evaluated by the Medication Event Monitoring System (MEMS) caps. Good adherence was defined as taking more than 80% of tablets over 48 weeks.
Detection rate
An additional analysis on the TRIO results was performed to identify the proportion of patients receiving oral bisphosphonates that show decreases in each of the two proposed markers, CTX and PINP, as well as the proportion of cases that showed this change with either one of the other. From this, we calculated the detection rate for a single marker and for both markers, considering decrease in at least one of the markers beyond the LSC as a positive test.
The formula for calculating detection rate was:
‘positives’ describe the cases showing a decrease > LSC in one or both markers; ‘negatives’ describe the cases with no change or a change less than the LSC.
Results
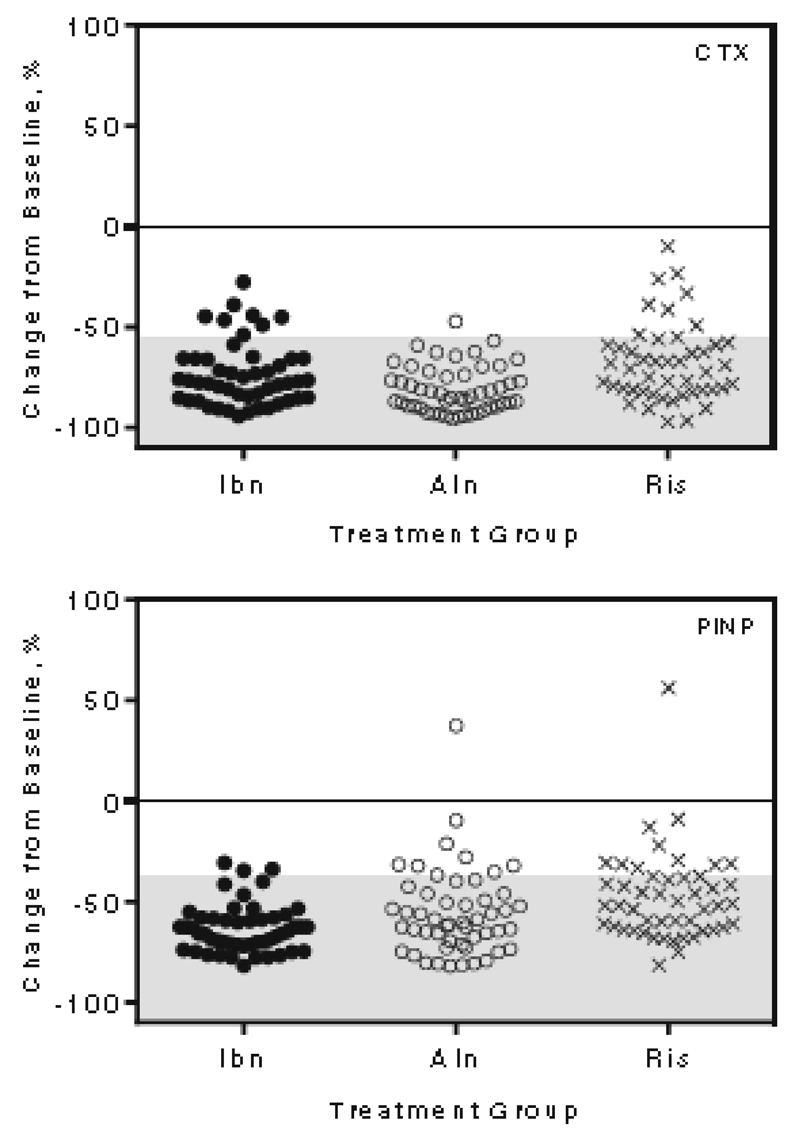
The characteristics of the TRIO population are described in detail in the original publication [13]. In Table 1 are summarized the baseline values for each of the treatment groups, the values after three months of treatment and the percentage of decrease in CTX and PINP each for each drug (see also Figure 1).
Table 1.
Values of biochemical markers at baseline and after three months of treatment and percentage of decrease for each of the treatment groups
| Ibandronate | Alendronate | Risedronate | Young controls | |
|---|---|---|---|---|
| N | 57 | 57 | 58 | 87 |
| CTX, ng/mL, mean baseline | 0.68 | 0.64 | 0.59 | 0.32 |
| CTX response, 3 months, % decrease | 73 | 81 | 68 | -- |
| % of CTX responders, 3 months* | 84 | 98 | 78 | -- |
| PINP, ng/mL, mean baseline | 49.9 | 46.2 | 44.0 | 29.0 |
| PINP response, 3 months, % decrease | 63 | 56 | 48 | -- |
| % of PINP responders, 3 months* | 94 | 82 | 75 | -- |
% of patients with a decrease at three months > least significant change (LSC). LSC based on CTX values was defined as a decrease of 56%, based on PINP by 38%
Thus, with serum CTX 78-98% of women, and with serum PINP 75-94% of women are considered 'responders' after 3 months of treatment, depending on the bisphosphonate used.
Figure 1.
1 Change from baseline after three months of treatment with the three tested bisphosphonates (Ibandronate, Alendronate and Risedronate) in CTX (upper panel) and PINP (lower panel). Shadowed zone indicates change > least significant change for the marker
Ibn= Ibandronate; Aln = Alendronate; Ris = Risedronate
Good adherence, defined as taking more than 80% of tablets over 48 weeks, was found in 104/135 subjects who completed 48 weeks. This was associated with a 79% decrease in CTX as compared to low adherence, which was associated with a 64% decrease. Also it was associated with a 67% decrease in PINP as compared to low adherence, which was associated with a 51% decrease. However, it has to be pointed out that the relationship between adherence and response to BPs is not a simple linear one and very low adherence is necessary to have no response (Table 2).
Table 2.
| Tablets taken | Mean % change | Responders, % |
|---|---|---|
| <26 | -36 | 56 |
| 26-52 | -42 | 59 |
| 52-78 | -49 | 72 |
| 78-104 | -62 | 64 |
| >104 | -63 | 87 |
Adherence measured at 48 weeks. Responders are considered the individual patients that show changes in BTMs that exceed the LSC.
Responders were considered as those cases with a decrease in markers more than the LSC, based on a decrease in CTX of 56% and a decrease in PINP of 38%. The lumbar spine BMD (bone mineral density) increase at 96 weeks in CTX responders was 6.0% and 1.3% in non-responders. The total hip BMD increase at 96 weeks in CTX responders was 3.2% and 1.0% in non-responders. The lumbar spine BMD increase at 96 weeks in PINP responders was 6.2 and 2.3% in non-responders. The total hip BMD increase at 96 weeks in PINP responders was not different to non-responders. All these changes were independent on baseline values of turnover markers. Responders were considered those cases with a decrease in markers more than the LSC.
The average decrease for CTX ranged from 68 to 81% and for PINP between 48 and 63% for the different treatment arms. For the different bisphosphonates, the percentage of individuals with a decrease beyond the LSC ranged from 78 to 98% for CTX and between 75 and 94% for PINP (Table 1).
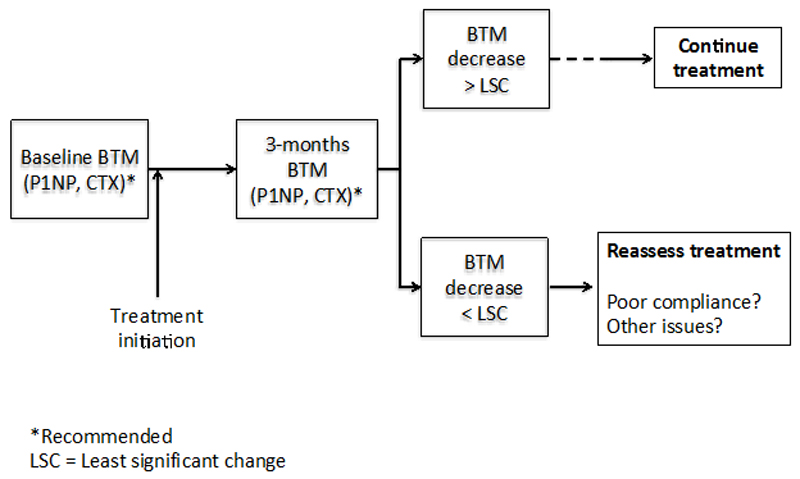
Table 3 summarizes the detection rate for the overall cohort, that is, the percentage of individuals in the overall cohort that show a decrease in CTX, PINP, beyond the LSC, or at least in one of them if both are measured, after three months of initiating therapy. For CTX the detection rate reaches a 87%, for PINP 84% and, if decrease in at least one is considered when measuring both, the level of detection is 94.5% (Table 3). This means that the patients are taking and responding to the medication. Based on these results, a screening strategy is proposed as summarized in Figure 2. In brief, after measuring bone turnover markers before initiating medication, a second measurement is performed at three months. If the decrease does not exceed the LSC the clinician should reassess the treatment, mainly the adherence and, eventually, if an underlying cause of secondary osteoporosis or low response to the drug has not been previously detected.
Table 3.
Detection rate as percent of cases in the overall cohort with the prespecified decrease>LSC in CTX, PINP or both after the initiation of treatment
| BTM | N | N with decrease>LSC | N with decrease<LSC | Detection rate (%) |
|---|---|---|---|---|
| CTX | 146 | 127 | 19 | 86.9 |
| PINP | 149 | 125 | 24 | 83.9 |
| CTX+PINP | 146 | 138 | 8 | 94.5 |
Figure 2.
Algorithm for the assessment of adherence based on the measurement of CTX and/or PINP
A ‘response’ was also considered if the BTM were below the premenopausal mean (which was 32 ng/L for CTX and 28 ug/L for PINP). The baseline CTX and PINP were above these thresholds in 91 and 89% of women, respectively. The CTX values after 12 weeks of treatment were below these thresholds in 86%, 96% and 83% for ibandronate, alendronate and risedronate, respectively. . The PINP values after 12 weeks of treatment were below these thresholds in 96%, 82% and 75% for ibandronate, alendronate and risedronate, respectively. This approach is useful if there is no baseline BTM available.
In practice, we wouldn’t usually repeat the measurement of CTX and PINP at both 12 and 13 weeks on treatment. In an unpublished analysis, we checked whether the responder rates were the same with just the 12-week measurement and whether we defined response by the least significant change or by being below the premenopausal mean, the number responding was only 1 to 3% fewer.
Discussion
The working group recommends a screening policy for assessing adherence to oral bisphosphonates given as treatment for osteoporosis, by measuring PINP and CTX three months after starting therapy. At this time-point we would only expect 2.5% of untreated patients to exceed the least significant changes of 56% for CTX and 38% for PINP, yet with the oral bisphosphonate therapy overall, we found that 75-98% of women responded.
This strategy fulfills the requirements for a screening procedure. A screening test needs to offer a high detection rate and perform well when the incidence of a condition in a given population is high. Accordingly, it is well known that patients treated with oral bisphosphonates have a good response in turnover markers. Moreover, a screening test has to be easy to perform, widely available, cheap, common practice and needs to show a high sensitivity (detection rate). In other words, the detection rate for the decrease of BTMs (beyond the LSC) should be very high to minimize the proportion of false negative tests. Therefore, the present recommendations are in accordance with all these principles and can be translated to clinical practice.
We use the statistic ‘detection rate’. Detection rate has been considered as synonymous with sensitivity (14) although these concepts are not identical. For calculating sensitivity a gold standard test is needed and, from that, a 2x2 table can be built and sensitivity estimated. For the clinical assessment of the exposure of bone tissue to a drug, biochemical markers are the gold standard since obtaining bone biopsies is not acceptable and, moreover, in the largest series the correlation between markers and histomorphometry is moderately strong (15). Moreover, bone histomorphometry predicts fracture in children poorly (16) and biochemical and histomorphometric indexes correlate moderately well in adults treated with denosumab (17) or teriparatide (18).
The objective of the TRIO study used here as the basis for our analysis was perfectly in accordance with the required information needed for developing the current recommendations. The data comprises the three first-line (and most prescribed) oral bisphosphonates used for treating osteoporosis, at their licensed doses. In another publication, Sebba et al. used the biochemical marker changes as response indicators (19) from the FACT (Fosamax Actonel Comparison Trial) study. They defined a CTX response of 60% and a PINP response of 50% as significant although they didn't provide any evidence for these cut-offs. The response rate detected by CTX at 3 months was 70% for alendronate and 40% for risedronate whereas for PINP was 77 and 50%. The lower responder rate in FACT is likely to be due to less use of calcium and vitamin D supplements in that study as well as the greater estimates of LSC.
Indeed the required variation in the individual patient should exceed the least significant change value, and this is the basis for making the clinical judgment about low adherence or underlying causes of impaired response such as undiagnosed secondary osteoporosis or medications that interfere with the effect of the drug (20). The LSC thresholds of the TRIO study here used (11) are comparable with those previously reported in the literature as summarized in Table 4 (21–23). In the field of bone densitometry, it is regarded as good practice by the Internataion Society for Clinical Densitometry for each clinical centre to establish its own least significant change. This may be too onerous in practice to obtain for bone turnover markers.
Table 4.
LSC thresholds of the TRIO study compared with those previously reported
The results of the TRIO study also offer strength to the clinical recommendations because they represent the benchmark of what can be achieved in clinical practice from a controlled trial where the monitoring and adherence are the best that can be obtained. The timing for assessment, three months after prescription (decision to treat) and in most cases acceptance (start of the therapy), is optimal because the changes in the markers are already complete although the same performance of the screening can be expected if for some reason the second measurement is made later on, at six or even 12 months. The three month measurement is early enough to assess how the patient accepts and tolerates the treatment and also covers the critical period of primary non-adherence, in the first weeks after the prescription is given when patients may have discontinued treatment or may never have started (12). Some limitations should be also mentioned. The results of the TRIO study refer to postmenopausal women in a given geographical area and, therefore, their translation to men and premenopausal patients as well as to other areas has to be extrapolated. Furthermore, the small number of participants is another limitation although TRIO is a study that specifically addresses our research question. We found no systematic study on men and young women and so we could speculate that they would have the same response rate. However, more research on this aspect is needed and further validation of the results obtained in the TRIO study in different clinical trials and/or different drugs must be obtained to fully certify the proposed strategy.
Moreover, the results in real world practice are always expected to be worse, in terms of adherence, than what is observed in a clinical trial. However, this is why the TRIO results should be considered as a benchmark of the best possible scenario under ideal conditions of practice. The cost of the test, in settings where are not covered by reimbursement policies, may be another practical limitation. Finally, the biological variability of CTX is quite large when it is evaluated in a sufficiently large population.
There is an alternative way to evaluating treatment response that was described for the TRIO study. Sometimes we don't have a baseline BTMs result. It would appear that most patients before treatment are above the average value for young women and most women on treatment are below this value. Thus, a second approach to identifying response could be to measure BTMs on treatment and if the value is below the young normal mean (e.g. PINP 35 ug/L, CTX 25 ng/L) then consider that adequate suppression of bone turnover that wouldn't happen if treatment was not taken. However, we believe that not having baseline determinations of bone markers adds uncertainty and makes hard to apply this screening strategy.
Can other commonly used assays for CTX and PINP (such as the assays offered by Roche Diagnostics) be used in the samem way as the IDS assays used in TRIO? We don’t know of any side by side comparison of serum CTX and PINP by the IDS and other assays for treatment response. However, the antibody used by the two suppliers of CTX assays are the same, so it is likely that the percentage response and probably the LSC will be similar. The PINP assays from IDS and Roche do differ in that the IDS measures only intact PINP whereas Roche measures both the intact and the monomer forms. However, this doesn’t seem to affect the absolute value of the PINP and only seems to be a problem in end-stage renal disease.
The working group does not suggest that this screening strategy will have a direct impact on adherence. It may be interesting to further evaluate whether these recommendations have an impact on medication adherence in a real-life setting, considering also the cost-effectiveness balance. Adherence is a complex issue and interventions address the specific perceptions (e.g. patient’s beliefs about osteoporosis and its treatment) and practicalities (e.g. capability and resources) influencing the motivation and ability to start and continue with the treatment. (24) However, assessing adherence is a crucial first step to any intervention and the feedback to the patient has to ensure a no-blame approach and be made in the context of their behavior and beliefs characteristics.
Bone densitometry is the most commonly used method for measuring the effect of treatments in clinical practice and its value for defining the goal of therapies is currently one of the hot topics in the field. However the time required for detecting a significant variation is considerably longer than for biochemical markers. This fact limits the clinical utility of BMD monitoring for an early assessment of the effect of oral bisphosphonates, precisely when most of the adherence problems occur.
In summary, the Working Group proposes measurement of PINP and CTX levels at baseline and after three months of initiating treatment. In those individuals where the decrease does not exceed the least significant change (38 and 56%, respectively) assessment of adherence or, eventually, investigation of secondary osteoporosis (9), should be carried out.
Mini Abstract.
Adherence to oral bisphosphonates is low. A screening strategy is proposed based on the response of biochemical markers of bone turnover after three months of therapy. If no change is observed, the clinician should reassess the adherence to the treatment and also other potential issues with the drug.
Footnotes
Disclosures
A Diez-Perez is speaker or advisor for Amgen, Lilly, Merck, UCB and Active Life Sci. Bo Abrahamsen reports current institutional research grants and contracts with Novartis and UCB, past institutional research contracts with Amgen and NPS Pharmaceuticals. D. Agnusdei has a consultancy contract with Eli Lilly. Erik F Eriksen is a speaker and advisor for Amgen, Lilly, Merck, IDS and Shire. Núria Guañabens is speaker or advisor for Amgen, Lilly and Alexion Pharmaceuticals. Robert Josse is medical advisory board member and speaker honoraria and research grant: Merck,Amgen,Lilly. Daniel Prieto-Alhambra’s research group has received unrestricted grants from AMGEN S.A. Jean-Yves Reginster has consulting fees or paid advisory boards in Servier, Novartis, Negma, Lilly, Wyeth, Amgen, GlaxoSmithKline, Roche, Merckle, Nycomed-Takeda, NPS, IBSA-Genevrier, Theramex, UCB, Asahi Kasei, Endocyte, Radius Health also has lecture fees when speaking at the invitation of the commercial sponsors: Merck Sharp and Dohme, Lilly, Rottapharm, IBSA, Genevrier, Novartis, Servier, Roche, GlaxoSmithKline, Merckle, Teijin, Teva, Analis, Theramex, Nycomed, NovoNordisk, Ebewee Pharma, Zodiac, Danone, Will Pharma, Amgen, PharmEvo. He has grant support from industry: Bristol Myers Squibb, Merck Sharp & Dohme, Rottapharm, Teva, Roche, Amgen, Lilly, Novartis, GlaxoSmithKline, Servier, Pfizer, Theramex, Danone, Organon, Therabel, Boehringer, Chiltern, Galapagos. M. Carola Zillikens has received fee for speaking and advice from Amgen, Eli Lilly and MSD. R. Eastell has consulting fees from Amgen, AstraZeneca, Chronos, GSK, Immunodiagnostic Systems, Fonterra Brands, Ono Pharma, Lilly, Bayer, Janssen Research, Alere, CL Biosystems, Teijin Pharm, D-Star, Roche Diagnostics, Inverness Medical; grant support from Amgen, Alexion, Immunodiagnostic Systems, Roche, AstraZeneca. Kim E Naylor, Maria Luisa Brandi, Cyrus Cooper, Elaine Dennison, Deborah Gold, Peyman Hadji, Mickael Hiligsmann, Robert Horne, John A Kanis, Barbara Obermayer-Pietsch, René Rizzoli, and Stuart Silverman have no conflict of interest.
Reference List
- 1.Cramer JA, Gold DT, Silverman SL, Lewiecki EM. A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos Int. 2007;18(8):1023–31. doi: 10.1007/s00198-006-0322-8. [DOI] [PubMed] [Google Scholar]
- 2.Kothawala P, Badamgarav E, Ryu S, Miller RM, Halbert RJ. Systematic review and meta-analysis of real-world adherence to drug therapy for osteoporosis. Mayo Clin Proc. 2007;82(12):1493–501. doi: 10.1016/S0025-6196(11)61093-8. [DOI] [PubMed] [Google Scholar]
- 3.Kanis JA, Reginster JY, Kaufman JM, Ringe JD, Adachi JD, Hiligsmann M, et al. A reappraisal of generic bisphosphonates in osteoporosis. Osteoporos Int. 2012;23(1):213–21. doi: 10.1007/s00198-011-1796-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hiligsmann M, Rabenda V, Gathon HJ, Ethgen O, Reginster JY. Potential clinical and economic impact of nonadherence with osteoporosis medications. Calcif Tissue Int. 2010;86(3):202–10. doi: 10.1007/s00223-009-9329-4. [DOI] [PubMed] [Google Scholar]
- 5.Hiligsmann M, Salas M, Hughes DA, Manias E, Gwadry-Sridhar FH, Linck P, et al. Interventions to improve osteoporosis medication adherence and persistence: a systematic review and literature appraisal by the ISPOR Medication Adherence & Persistence Special Interest Group. Osteoporos Int. 2013;24(12):2907–18. doi: 10.1007/s00198-013-2364-z. [DOI] [PubMed] [Google Scholar]
- 6.Kanis JA, Cooper C, Hiligsmann M, Rabenda V, Reginster JY, Rizzoli R. Partial adherence: a new perspective on health economic assessment in osteoporosis. Osteoporos Int. 2011;22(10):2565–73. doi: 10.1007/s00198-011-1668-0. [DOI] [PubMed] [Google Scholar]
- 7.Kanis JA, McCloskey EV, Johansson H, Cooper C, Rizzoli R, Reginster JY, et al. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2013;24(1):23–57. doi: 10.1007/s00198-012-2074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vasikaran S, Eastell R, Bruyere O, Foldes AJ, Garnero P, Griesmacher A, et al. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int. 2011;22(2):391–420. doi: 10.1007/s00198-010-1501-1. [DOI] [PubMed] [Google Scholar]
- 9.Diez-Perez A, Adachi JD, Agnusdei D, Bilezikian JP, Compston JE, Cummings SR, et al. Treatment failure in osteoporosis. Osteoporos Int. 2012;23(12):2769–74. doi: 10.1007/s00198-012-2093-8. [DOI] [PubMed] [Google Scholar]
- 10.Delmas PD, Vrijens B, Eastell R, Roux C, Pols HA, Ringe JD, et al. Effect of monitoring bone turnover markers on persistence with risedronate treatment of postmenopausal osteoporosis. J Clin Endocrinol Metab. 2007;92(4):1296–304. doi: 10.1210/jc.2006-1526. [DOI] [PubMed] [Google Scholar]
- 11.Naylor KE, Jacques RM, Paggiosi M, Gossiel F, Peel NF, McCloskey EV, et al. Response of bone turnover markers to three oral bisphosphonate therapies in postmenopausal osteoporosis: the TRIO study. Osteoporos Int. 2016;27(1):21–31. doi: 10.1007/s00198-015-3145-7. [DOI] [PubMed] [Google Scholar]
- 12.Carbonell-Abella C, Pages-Castella A, Javaid MK, Nogues X, Farmer AJ, Cooper C, et al. Early (1-year) Discontinuation of Different Anti-osteoporosis Medications Compared: A Population-Based Cohort Study. Calcif Tissue Int. 2015;97(6):535–41. doi: 10.1007/s00223-015-0040-3. [DOI] [PubMed] [Google Scholar]
- 13.Paggiosi MA, Peel N, McCloskey E, Walsh JS, Eastell R. Comparison of the effects of three oral bisphosphonate therapies on the peripheral skeleton in postmenopausal osteoporosis: the TRIO study. Osteoporos Int. 2014;25(12):2729–41. doi: 10.1007/s00198-014-2817-z. [DOI] [PubMed] [Google Scholar]
- 14.Wald NJ. Guidance on terminology. J Med Screen. 2008;15(1):50. doi: 10.1258/jms.2008.008got. [DOI] [PubMed] [Google Scholar]
- 15.Chavassieux P, Portero-Muzy N, Roux JP, Garnero P, Chapurlat R. Are Biochemical Markers of Bone Turnover Representative of Bone Histomorphometry in 370 Postmenopausal Women? J Clin Endocrinol Metab. 2015;100(12):4662–8. doi: 10.1210/jc.2015-2957. [DOI] [PubMed] [Google Scholar]
- 16.Mayranpaa MK, Tamminen IS, Kroger H, Makitie O. Bone biopsy findings and correlation with clinical, radiological, and biochemical parameters in children with fractures. J Bone Miner Res. 2011;26(8):1748–58. doi: 10.1002/jbmr.373. [DOI] [PubMed] [Google Scholar]
- 17.Reid IR, Miller PD, Brown JP, Kendler DL, Fahrleitner-Pammer A, Valter I, et al. Effects of denosumab on bone histomorphometry: the FREEDOM and STAND studies. J Bone Miner Res. 2010;25(10):2256–65. doi: 10.1002/jbmr.149. [DOI] [PubMed] [Google Scholar]
- 18.Dobnig H, Sipos A, Jiang Y, Fahrleitner-Pammer A, Ste-Marie LG, Gallagher JC, et al. Early changes in biochemical markers of bone formation correlate with improvements in bone structure during teriparatide therapy. The Journal of clinical endocrinology and metabolism. 2005;90(7):3970–7. doi: 10.1210/jc.2003-1703. Epub 2005/04/21. [DOI] [PubMed] [Google Scholar]
- 19.Sebba AI, Bonnick SL, Kagan R, Thompson DE, Skalky CS, Chen E, et al. Response to therapy with once-weekly alendronate 70 mg compared to once-weekly risedronate 35 mg in the treatment of postmenopausal osteoporosis. Curr Med Res Opin. 2004;20(12):2031–41. doi: 10.1185/030079904x16768. [DOI] [PubMed] [Google Scholar]
- 20.Diez-Perez A, Olmos JM, Nogues X, Sosa M, Diaz-Curiel M, Perez-Castrillon JL, et al. Risk factors for prediction of inadequate response to antiresorptives. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2012;27(4):817–24. doi: 10.1002/jbmr.1496. Epub 2011/12/14. [DOI] [PubMed] [Google Scholar]
- 21.Fink E, Cormier C, Steinmetz P, Kindermans C, Le Bouc Y, Souberbielle JC. Differences in the capacity of several biochemical bone markers to assess high bone turnover in early menopause and response to alendronate therapy. Osteoporos Int. 2000;11(4):295–303. doi: 10.1007/PL00004183. [DOI] [PubMed] [Google Scholar]
- 22.Hannon R, Blumsohn A, Naylor K, Eastell R. Response of biochemical markers of bone turnover to hormone replacement therapy: impact of biological variability. J Bone Miner Res. 1998;13(7):1124–33. doi: 10.1359/jbmr.1998.13.7.1124. [DOI] [PubMed] [Google Scholar]
- 23.Rogers A, Glover SJ, Eastell R. A randomised, double-blinded, placebo-controlled, trial to determine the individual response in bone turnover markers to lasofoxifene therapy. Bone. 2009;45(6):1044–52. doi: 10.1016/j.bone.2009.07.089. Epub 2009/08/12. [DOI] [PubMed] [Google Scholar]
- 24.Horne R, Chapman SC, Parham R, Freemantle N, Forbes A, Cooper V. Understanding patients' adherence-related beliefs about medicines prescribed for long-term conditions: a meta-analytic review of the Necessity-Concerns Framework. PloS one. 2013;8(12):e80633. doi: 10.1371/journal.pone.0080633. Epub 2013/12/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eastell R, Vrijens B, Cahall DL, Ringe JD, Garnero P, Watts NB. Bone turnover markers and bone mineral density response with risedronate therapy: relationship with fracture risk and patient adherence. J Bone Miner Res. 2011;26(7):1662–9. doi: 10.1002/jbmr.342. [DOI] [PubMed] [Google Scholar]
- 26.Fink E, Cormier C, Steinmetz P, Kindermans C, Le Bouc Y, Souberbielle, et al. Differences in the capacity of several biochemical bone markers to assess high bone turnover in early menopause and response to alendronate therapy. Osteoporosis International. 2000;11(4):295–303. doi: 10.1007/PL00004183. [DOI] [PubMed] [Google Scholar]
- 27.Garnero P, Borel O, Delmas PD. Evaluation of a fully automated serum assay for C-terminal cross-linking telopeptide of type I collagen in osteoporosis. Clin Chem. 2001;47(4):694–702. [PubMed] [Google Scholar]
- 28.Garnero P, Vergnaud P, Hoyle N. Evaluation of a fully automated serum assay for total N-terminal propeptide of type I collagen in postmenopausal osteoporosis. Clinical chemistry. 2008;54(1):188–96. doi: 10.1373/clinchem.2007.094953. Epub 2007/11/14. [DOI] [PubMed] [Google Scholar]