Abstract
Androgen receptor (AR) splice variants (SV) have been implicated in the development of metastatic castration-resistant prostate cancer and resistance to AR targeting therapies, including abiraterone and enzalutamide. Agents targeting AR-SV are urgently needed to test this hypothesis and further improve the outcome of patients suffering from this lethal disease.
In this issue of Clinical Cancer Research, Yang and colleagues report that targeting the androgen receptor (AR) activation function-1 (AF-1) within the AR N-terminal domain (NTD) using EPI-002 overcomes molecular aberrations implicated in the development of metastatic castration-resistant prostate cancer (mCRPC; ref. 1).
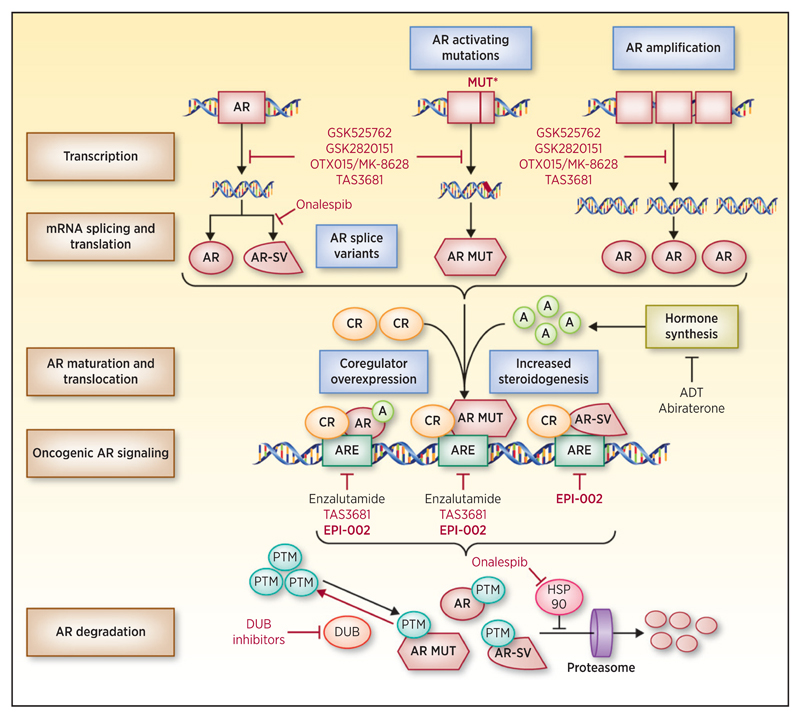
Prostate cancer is the second leading cause of cancer-related death in U.S. men. The mainstay of treatment is androgen deprivation therapy, and despite robust responses, nearly all patients progress to fatal mCRPC. There is now incontrovertible evidence of ongoing AR signaling as patients progress to mCRPC with rising PSA, increased expression of AR coregulators, AR amplification, AR-activating mutations, and increased steroidogenesis (Fig. 1; ref. 2). This led to the development of abiraterone acetate (AA) and enzalutamide, which inhibit AR signaling in mCRPC and improve patient outcome. Despite these advances, responses can be short lived, with resistance to both agents being common.
Figure 1.
Novel therapeutic strategies to block persistent AR signaling in mCRPC. AR gene amplification and mutation leads to increased AR protein expression and gain-of-function AR aberrations, including activating protein mutants (AR MUT). Changes in mRNA splicing generate constitutively active AR splice variants (AR-SV). Androgens (A) and coregulators (CR) regulate AR protein activity. AR, AR MUT, and AR-SV bind androgen response elements (ARE) and regulate oncogenic AR signaling, driving progression to mCRPC and therapeutic resistance. AR protein stability is regulated by post-translational modification (PTM) and proteasomal degradation that can be modulated by the HSP90 chaperone machinery and deubiquitinating enzymes (DUB). Mechanisms implicated in the development of mCRPC are shown (blue fill boxes). Current therapeutic strategies, including androgen deprivation therapy (ADT), abiraterone, and enzalutamide, are shown. Novel therapeutic strategies in preclinical development and early-phase clinical trials to overcome aberrant AR signaling in mCRPC are shown (red).
Expression of constitutively active AR splice variants (AR-SV), of which AR splice variant 7 (AR-V7) has been reported to be the most abundant, has been postulated as important for mCRPC biology and a key mechanism of resistance to current AR-targeting therapies (Fig. 1; refs. 3, 4). Original studies suggested that AR-V7 heterodimerized with full-length AR (AR-FL) to signal. Importantly, AR-V7 can homodimerize with itself and heterodimerize with other AR-SV to signal independently of AR-FL (5). AR-V7 lacks the C-terminal ligand-binding domain (LBD) but maintains an intact AF-1 domain and AR-V7 overexpression in vitro confers resistance to therapies targeting the AR LBD. AR-V7 levels increase as patients progress from hormone-sensitive prostate cancer to mCRPC, and AR-V7 expression correlates with shorter overall survival, suggesting a key role in mCRPC biology (3). Moreover, AR-V7 expression increases further after castration-resistant disease progresses on AA or enzalutamide, associating with treatment resistance (3, 4). Currently, all licensed therapies used in the treatment of mCRPC that modulate AR signaling do so through the AR LBD and have little or no activity against AR-V7.
There are a number of novel (beyond targeting the AR LBD) therapeutic strategies in preclinical development or early clinical trials in mCRPC (Fig. 1). Briefly, these include (i) inhibition of bromodomain and extraterminal domain proteins to downregulate AR-FL/AR-V7 expression (GSK525762, GSK2820151, and OTX015/MK-8628; ref. 6); (ii) targeting HSP90 to deplete AR-FL through proteasomal degradation and decreasing AR-V7 through the blockade of mRNA splicing (onalespib; ref. 7); (iii) inhibiting AR-FL transactivation and reducing AR-FL/AR-V7 protein expression (TAS3681; ref. 8); and (iv) targeting deubiquitinating enzymes to promote AR-FL/AR-V7 degradation.
Importantly, most of the transactivation function of AR stems from the AR AF-1 domain, present in AR-FL and most AR-SV; strategies to target this, although challenging due to the AR NTD having an intrinsically unstructured nature and lack of enzymatic activity, are exciting and have the potential to overcome aberrant AR signaling in mCRPC (9). EPI-001 and its analogues bind to Tau5 of AR AF-1 and block protein-to-protein interactions critical for AR transcriptional activity, inhibiting the growth of CRPC xenografts in mice (10). In this report, Yang and colleagues report the ability of EPI-002, the single (2R, 20S) diastereoisomer of EPI-001, to overcome ongoing AR signaling in mCRPC (1). AR transcriptional activity is modulated by AR coregulators, including the p160 steroid receptor coactivator (SRC) proteins and CBP/p300 that are elevated in prostate cancer and associated with poorer prognosis. Enzalutamide, and to a lesser extent EPI-002, inhibits hormone-dependent SRC family and CBP/p300 coregulator stimulation of AR transactivation, albeit at relatively high concentrations. Moreover, enzalutamide and EPI-002 inhibited hormone-dependent transactivation of AR NTD and AR LBD gain-of-function point mutants (1). The authors should be commended on their rigorous study of clinically relevant aberrations, although EPI-002 had similar activity on AR signaling to currently licensed, well-tolerated, therapies that improve survival of patients with mCRPC. The question remains whether targeting the AR AF-1 will provide clinically significant gains over current AR LBD–targeting therapies for AR aberrations that do not confer resistance to these treatments.
In contrast, AR-SV and, in particular, AR-V7 have been implicated in resistance to current therapies, including AA and enzalutamide (4). AR-FL and AR-V7 have distinct transcriptional activities in mCRPC. In this study, Yang and colleagues (1) used enzalutamide-resistant LNCaP95 cells that express both AR-FL and AR-V7 to demonstrate that EPI-002 and enzalutamide both reduce the expression of genes regulated by AR-FL (PSA and FKBP5). Unlike enzalutamide, which did not do this, EPI-002 reduced the expression of genes reported to be distinctly regulated by AR-V7 (UBE2C and CDC20). Furthermore, in enzalutamide-resistant LNCaP95 mouse xenografts, EPI-002 significantly delayed the growth of tumors (without causing growth regression) when compared with enzalutamide. Yang and colleagues have presented convincing preclinical evidence that targeting the AR NTD AF-1 with EPI-002 decreases AR-V7 transcriptional activity and delays growth in prostate cancer models resistant to current therapies supporting the evaluation of this agent in clinical trials in patients with AR-SV–expressing mCRPC.
Concerns remain, nevertheless, with regards to the high concentrations of EPI-002 required to modulate AR activity in these studies and whether this can be recapitulated in clinical studies at tolerable doses. This has been raised by studies indicating that EPI compounds covalently bind not only to the AF-1 but also to proteins other than AR and that EPI-001 alkylates thiols under basic or neutral conditions (11). Notably, EPI-002 contains a chemically reactive chlorohydrin that is essential for biological activity; indeed, EPI analogues comprising the chlorohydrin moiety covalently bind to the AF-1 domain of AR in cells, whereas analogues lacking the chlorohydrin do not (10). Although these cell-based studies did not reveal an abundance of other protein targets, application of unbiased mass spectrometry–based proteomics could further inform on the specificity of EPI-002 and its analogues. Interestingly, many successful marketed drugs rely upon covalent binding to their target proteins (12). Commonly, minimization of off-target effects for such agents relies upon initial reversible, high affinity, and selective binding to the desired protein target, followed by irreversible covalent binding; furthermore, low efficacious exposure is likely to reduce off-target tolerability issues by avoiding saturation of the endogenous protective glutathione-mediated trapping mechanism for chemically reactive species. Whether EPI-506 (prodrug of EPI-002) will benefit from factors such as these remains to be demonstrated.
The current phase I/II trial (NCT02606123) of EPI-506 in patients with mCRPC who have progressed on AA and/or enzalutamide will be critical in alleviating these concerns. This study will determine whether EPI-506 is well tolerated with a favorable pharmacokinetic profile. Furthermore, it will be important that biomarker-driven pharmacodynamics studies recapitulate the preclinical data presented by Yang and colleagues (1) demonstrating modulation of AR-V7 oncogenic signaling. A majority of patients resistant to AA and enzalutamide demonstrate increased AR-V7 expression; biomarker studies embedded in this trial evaluating AR-V7 mRNA and protein expression will be key to identify patients most likely to respond to EPI-506 (3, 4). Patients whose cancers have decreased AR-FL/AR-V7 expression may have alternative AR-independent mechanisms of resistance. Such patient selection biomarkers will be key to future registration studies. Taken together, if drugs targeting AR AF-1, such as EPI-506, show AR-SV signaling blockade at tolerable doses with durable antitumor activity in patients whose tumors express AR-SV, these agents have the potential to transform the treatment of advanced prostate cancer, not only in the late-stage setting but also in earlier stages of this disease as studies indicate that AR-SV expression can be induced soon after very short durations of androgen deprivation (3).
Grant Support
This work was supported by the U.S. Department of Defense (grant nr. UWSC7395; to J.S. de Bono), Movember/Prostate Cancer UK (grant nr. CEO013-2-002; to J.S. de Bono), the Prostate Cancer Foundation, an Experimental Cancer Medical Centre (ECMC) grant from Cancer Research UK and the Department of Health (grant nr. C51/A7401; to J.S. de Bono), Cancer Research UK core funding to the Cancer Therapeutics Unit (grant nr. C309/A11566), the Medical Research Council (to A. Sharp), and NHS funding to the NIHR Biomedical Research Centre at the Royal Marsden and The Institute of Cancer Research.
Footnotes
Disclosure of Potential Conflicts of Interest
J.S. de Bono is an employee of The Institute of Cancer Research, which has a commercial interest in abiraterone, and is a consultant/advisory board member for Astellas Pharma, AstraZeneca, Bayer, Genentech, GlaxoSmithKline, and Orion Pharma. No potential conflicts of interest were disclosed by the other authors.
Authors' Contributions
Writing, review, and/or revision of the manuscript: A. Sharp, J. Welti, J. Blagg, J.S. de Bono
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): A. Sharp
Other (figure creation): A. Sharp
References
- 1.Yang YC, Banuelos CA, Mawji NR, Wang J, Kato M, Haile S, et al. Targeting androgen receptor activation function-1 with EPI to overcome resistance mechanisms in castration-resistant prostate cancer. Clin Cancer Res. 2016;22:4466–77. doi: 10.1158/1078-0432.CCR-15-2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferraldeschi R, Welti J, Luo J, Attard G, de Bono JS. Targeting the androgen receptor pathway in castration-resistant prostate cancer: progresses and prospects. Oncogene. 2015;34:1745–57. doi: 10.1038/onc.2014.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Welti J, Rodrigues DN, Sharp A, Sun S, Lorente D, Riisnaes R, et al. Analytical validation and clinical qualification of a new immunohistochemical assay for androgen receptor splice variant-7 protein expression in metastatic castration-resistant prostate cancer. Eur Urol. 2016 Apr 22; doi: 10.1016/j.eururo.2016.03.049. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371:1028–38. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu D, Zhan Y, Qi Y, Cao B, Bai S, Xu W, et al. Androgen receptor splice variants dimerize to transactivate target genes. Cancer Res. 2015;75:3663–71. doi: 10.1158/0008-5472.CAN-15-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asangani IA, Wilder-Romans K, Dommeti VL, Krishnamurthy PM, Apel IJ, Escara-Wilke J, et al. BET bromodomain inhibitors enhance efficacy and disrupt resistance to AR antagonists in the treatment of prostate cancer. Mol Cancer Res. 2016;14:324–31. doi: 10.1158/1541-7786.MCR-15-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferraldeschi R, Welti J, Powers MV, Yuan W, Smyth T, Seed G, et al. Second-generation HSP90 inhibitor onalespib blocks mRNA splicing of androgen receptor variant 7 in prostate cancer cells. Cancer Res. 2016;76:2731–42. doi: 10.1158/0008-5472.CAN-15-2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minamiguchi K, Seki M, Aoyagi H, Kajiwara D, Mori T, Masuko N, et al. TAS3681: new class of androgen receptor antagonist with androgen receptor downregulating activity. J Clin Oncol. 2015;33 (suppl 7; abstr 266) [Google Scholar]
- 9.Lavery DN, McEwan IJ. Structural characterization of the native NH2-terminal transactivation domain of the human androgen receptor: a collapsed disordered conformation underlies structural plasticity and protein-induced folding. Biochemistry. 2008;47:3360–9. doi: 10.1021/bi702221e. [DOI] [PubMed] [Google Scholar]
- 10.Myung JK, Banuelos CA, Fernandez JG, Mawji NR, Wang J, Tien AH, et al. An androgen receptor N-terminal domain antagonist for treating prostate cancer. J Clin Invest. 2013;123:2948–60. doi: 10.1172/JCI66398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brand LJ, Olson ME, Ravindranathan P, Guo H, Kempema AM, Andrews TE, et al. EPI-001 is a selective peroxisome proliferator-activated receptor-gamma modulator with inhibitory effects on androgen receptor expression and activity in prostate cancer. Oncotarget. 2015;6:3811–24. doi: 10.18632/oncotarget.2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh J, Petter RC, Baillie TA, Whitty A. The resurgence of covalent drugs. Nat Rev Drug Discov. 2011;10:307–17. doi: 10.1038/nrd3410. [DOI] [PubMed] [Google Scholar]