Abstract
Objective
Non-invasive estimation of arterial–ventricular coupling has been extensively used for the evaluation of cardiovascular performance, however, a relative small amount of data is available regarding arterial–ventricular coupling and its components in hypertension. The present study was designed to investigate the relationship between left ventricular elastance, arterial elastance, parameters of vascular stiffness and the influence of gender in a population of hypertensive individuals.
Methods
In 102 patients, trans-thoracic cardiac ultrasound, parameters of aortic stiffness (carotid-femoral pulse wave velocity) and wave reflection (augmentation index) were recorded. Ultrasound images of common carotid arteries were acquired for the assessment of intima-media thickness as well as carotid compliance and distensibility coefficient.
Results
Mean age was 61 years, 32% diabetes, 56% dyslipidemia, 9% previous cardiovascular events; women (n = 32) and men were superimposable for cardiovascular risk factors prevalence. In the population, ventricular elastance was significantly correlated with arterial elastance (r = 0.887), age (r = 0.334), gender (r = −0.494), BMI (r = −0.313), augmentation index (r = 0.479) (all p < 0.001); and with carotid compliance and distensibility coefficient (r = 0.229 and r = − 0.250, respectively, both p < 0.05); however, only arterial elastance and gender were independently associated with ventricular elastance in multiple regression models adjusted for confounding factors. Gender-specific analysis revealed that arterial elastance and augmentation index remained statistically significant associated with ventricular elastance in men (r = 0.275, p = 0.04); instead augmentation index was no longer significant (r = 0.052, p = 0.77) in the female sex.
Conclusions
In hypertensive patients, main determinants of ventricular elastance are arterial elastance, as an integrated index of arterial vascular load, and gender; however, pressure augmentation might play an additional role in men.
Keywords: Other hypertension, hypertension, cardiology, echocardiography, diagnostic testing, cardiology, Doppler ultrasound, Transcranial Doppler etc, imaging of the brain and arteries, cardiology
Introduction
Heart is anatomically and functionally connected with the vascular tree and their interplay, described as arterial–ventricular coupling (AVC), is a major determinant of cardiovascular (CV) performance, which influences the efficiency of the system and the magnitude of energy transmitted from heart to peripheral circulation.1
AVC can be expressed as the ratio of arterial elastance (Ea) to left ventricular elastance (Elv),2 where Ea is an integrated index of net arterial load that is imposed to left ventricle work and Elv is an index of contractility and systolic stiffness of myocardium.3 Mechanical energy transferred from heart to vessels is maximal when the two elastances are approximately equal.4 In hypertensive individuals, increased arterial stiffness coupled with increased ventricular stiffness have been reported, and despite an optimal coupling (Ea/Elv ≈ 1), these alterations might lead to hemodynamic consequences, because a stiffer heart–arterial system generates a greater systolic pressure change for a given stroke volume (SV).5
Moreover, there are known sex differences in arterial and ventricular properties: (women tend to have higher values of Ea and Elv compared to men) which might play an important role in the well established increased prevalence of heart failure with preserved ejection fraction (HFpEF) in the female sex6,7
However, little is known about influence of arterial stiffness on AVC and in particular, the influence of vascular properties on Elv, which can be considered an index of contractility partially independent to load conditions.
Based on these concepts, the present study investigated the relation between Elv, Ea, parameters of vascular stiffness and the influence of gender in a population of hypertensive individuals.
Materials and methods
The study included 102 patients, enrolled at the Hypertension Outpatient clinic of the Department of Clinical and Experimental Medicine of the University of Pisa (Italy); hypertension was diagnosed based on a history of this condition and/or high blood pressure (BP) values according to 2013 ESH/ESC Guidelines for the management of arterial hypertension.8 Clinical evaluation, BP measurements and blood tests were performed at the time when ventricular and arterial properties were assessed: all patients were hypertensive individuals in sinus rhythm.
Office BP was measured at the brachial level in the sitting position by a trained physician, with the patients resting for at least 10 min under quiet environmental conditions. BP measurement was repeated at least three times at 2-min intervals using an automatic oscillometric device (OMRON-705IT, Omron Corporation, Kyoto, Japan).
The exclusion criteria of the study were: diagnosis of heart failure, coronary heart disease, valvular heart disease, arrhythmia, arm circumference too large or small to allow accurate BP measurement, severe chronic kidney disease (eGFR < 30 mL/min/1.73 mq).
The study conformed to the Declaration of Helsinki and all patients provided written informed consent prior to entering the study.
Echocardiography study
Trans-thoracic cardiac ultrasound was performed in all patients using a GE Vivid 7 Ultrasound system in line with published recommendation of the American Society of Echocardiography and the European Association of Cardiovascular Imaging.9 All measurements were performed and analyzed by a single expert operator and acquisitions were individually optimized for depth, gain and frame rate to maximize image quality and minimize inconsistency in acoustic windows. Standard M-mode and 2D imaging were undertaken at rest. Images were saved in raw data format for offline analysis.
LV mass (LVM) was calculated according to the Recommendations of the American Society of Echocardiography and the European Association of Cardiovascular Imaging9 and it was indexed both to BSA, to obtain the LV mass index (LVMI), and to height 2.7, to obtain LVMI/h to avoid a systematic misclassification of CV risk in overweight and obese patients.
LV end systolic volume (ESV) and LV end diastolic volumes (EDV) were measured using Simpson’s method and their values were used for the evaluation of ejection fraction (EF). Stroke volume was calculated using LV outflow track (LVOT) diameter and velocity time integral.9
LV diastolic function was estimated by aid of conventional Doppler mitral inflow and tissue Doppler of mitral annulus, in line with published recommendations10 and the ratio E/e′ was used for the evaluation of LV filling pressure.
Integrated assessment of arterial function
Images of right and left common carotid arteries were acquired and analyzed with high-resolution echo-tracking system (MyLab25, Esaote, Florence, Italy). Intima-media thickness (IMT) was determined according to the established standards11 (and the mean value between right and left artery was used for statistical analysis). After clear visualization of the IMT of both anterior and posterior arterial wall in its longitudinal axis with maximal internal diameter, the echo-tracking sample was used for continuous detection of carotid diameter changes. Carotid distensibility coefficient (DC) and carotid cross-sectional compliance (CC) were calculated using validated formula12,13 (Box 1).
Table 1.
Clinical characteristics of the study population according to gender.
| Parameter | Overall population (n = 102) | Male (n = 70) | Female (n = 32) | p value |
|---|---|---|---|---|
| Age (years) | 61 ± 11 | 60 ± 12 | 63 ± 9 | 0.158 |
| BMI (kg/m2) | 28.5 ± 4.5 | 28.6 ± 4.3 | 28.4 ± 5 | 0.855 |
| Obesitya,b | 34 (33.3) | 23 (32.8) | 11 (34.4) | 0.693 |
| Waist circumference (cm) | 102.3 ± 13.4 | 105 ± 12 | 97 ± 14.2 | 0.005 |
| Smokinga | 27 (26.5) | 17 (24.3) | 10 (31.3) | 0.708 |
| Previous CV eventa | 9 (8.8) | 6 (8.6) | 3 (9.3) | 0.156 |
| Diabetesa,c | 33 (32.4) | 23 (32.1) | 10 (31.25) | 0.135 |
| Dyslipidemiaa,d | 57 (55.8) | 36 (51.4) | 21 (65.6) | 0.132 |
| Systolic BP (mmHg) | 141.6 ± 17.3 | 141.4 ± 15.2 | 142.1 ± 21.4 | 0.827 |
| Diastolic BP (mmHg) | 79.1 ± 11 | 79.3 ± 11.3 | 78.6 ± 10.1 | 0.811 |
| Heart rate (bpm) | 71.5 ± 11.5 | 70 ± 11.1 | 74.9 ± 11.7 | 0.036 |
| Fasting plasma glucose (mg/dL) | 108.5 ± 25.5 | 108.8 ± 29.1 | 107.9 ± 27.8 | 0.909 |
| Total cholesterol (mg/dL) | 207.3 ± 44.9 | 204.7 ± 47.5 | 213.40 ± 38.4 | 0.472 |
| LDL cholesterol (mg/dL) | 128.1 ± 41.1 | 127.3 ± 43.8 | 127.7 ± 35.2 | 0.959 |
| HDL cholesterol (mg/dL) | 51.8 ± 15.8 | 46.4 ± 12.8 | 62.8 ± 17.1 | <0.005 |
| Triglycerides (mg/dL) | 149.2 ± 85 | 159.1 ± 93.6 | 126.3 ± 55.9 | 0.150 |
| Creatinine (mg/dL) | 0.9 ± 0.2 | 1 ± 0.2 | 0.7 ± 0.1 | <0.005 |
Values are expressed as mean ± SEM or aabsolute value (percentage).
Obesity is defined by BMI > 30 kg/mq.
Diabetes was diagnosed according to medical history.
Dyslipidemia was diagnosed according to medical history.
Applanation tonometry of the radial artery was used for the evaluation of central BP, augmentation index (AIx) and end-systolic pressure (ESP), using a generalized validated transfer function (SphygmoCor software).14 An in-device quality rating of ≥80% was required for all recordings used in analysis.
Pulse wave velocity (PWV) was measured using a previously described technique.12 Distances from the suprasternal notch to the femoral artery and from the carotid artery to the suprasternal notch were measured as straight lines with a tape measure, recorded to the nearest mm and subtracted; pressure waveforms were taken at the right common carotid artery and then at the right femoral artery for the evaluation of transit time and calculation of carotid-femoral PWV.
Arterial ventricular coupling
Non-invasive assessment of Ea and Elv (Box 1) was determined using end systolic pressure (ESP) derived from the arterial waveform using applanation tonometry, as this method is more reliable compared to the estimation from brachial systolic BP.15 Subsequently their ratio of Ea/Elv was calculated in every patient and it was used as index of AVC (Box 1).
Statistical analysis
Statistical analysis was performed using NCSS 2008 (NCSS: Kaysville, Utah, USA). Results were expressed as mean ± SD. Differences in means among gender groups were analyzed using t test for normally distributed variables, or Wilcoxon Rank Sum Test for not normally distributed variables. Categorical variables were analyzed by χ2 test. Pearson’s Product was used to explore correlations among variables. Multiple linear regression analysis was performed including parameters correlated with the dependent variable with p < 0.05.
Results
Clinical characteristics of the population are shown in Table 1. Mean age was 61 years old; 32% had diabetes, 56% dyslipidemia, 9% previous CV events. Women (n = 32) and men were superimposable for CV risk factor prevalence.
Arterial function parameters, cardiac ultrasound findings and AVC characteristics are presented in Table 2.
Table 2.
Vascular and cardiac parameters of the study population according to gender.
| Overall population (n = 102) | Male (n = 70) | Female (n = 32) | p value | |
|---|---|---|---|---|
| IMTb (mm) | 0.757 ± 0.176 | 0.748 ± 0.191 | 0.775 ± 0.139 | 0.486 |
| CCc (10−3 kPa) | 9.77 ± 4.15 | 10.27 ± 3.91 | 8.65 ± 4.48 | 0.074 |
| DCd (10−3 kPa) | 23.06 ± 9.93 | 23.79 ± 9.67 | 21.39 ± 10.47 | 0.270 |
| aSBPe (mmHg) | 128.8 ± 17.3 | 127.2 ± 16 | 132.5 ± 19.8 | 0.153 |
| aDBPf (mmHg) | 80.5 ± 10.8 | 80.4 ± 11.3 | 80.7 ± 10 | 0.910 |
| AIx (%) | 24.9 ± 11.7 | 21.9 ± 11.4 | 31.4 ± 9.6 | <0.001 |
| PWV (m/s) | 9.2 ± 2.2 | 8.8 ± 1.9 | 9.8 ± 2.6 | 0.028 |
| LAVIg (ml/m2) | 31.5 ± 11.3 | 31.7 ± 11.5 | 30.9 ± 11.1 | 0.730 |
| LVMI (g/m2) | 120.3 ± 18.1 | 124.8 ± 16.7 | 108.9 ± 16.9 | <0.001 |
| LVMI/h (g/m2.7) | 54.8 ± 9.3 | 55.9 ± 9.1 | 52.1 ± 9.6 | 0.080 |
| RWTh | 0.39 ± 0.04 | 0.40 ± 0.04 | 0.39 ± 0.03 | 0.288 |
| E/Ai | 0.86 ± 0.23 | 0.89 ± 0.25 | 0.81 ± 0.19 | 0.135 |
| E/E′j | 8.99 ± 2.65 | 8.90 ± 2.55 | 9.16 ± 2.87 | 0.645 |
| S′k (cm/s) | 8.23 ± 1.15 | 8.38 ± 1.25 | 7.90 ± 0.85 | 0.051 |
| EF (%) | 56.29 ± 3.12 | 56.20 ± 3.20 | 56.47 ± 2.30 | 0.683 |
| SV (ml/min) | 62.05 ± 14.62 | 65.37 ± 15.07 | 55.10 ± 10.80 | <0.001 |
| Elv (mmHg/mL) | 1.45 ± 0.42 | 1.31 ± 0.34 | 1.73 ± 0.43 | <0.001 |
| Ea (mmHg/mL) | 1.13 ± 0.38 | 1.02 ± 0.29 | 1.36 ± 0.42 | <0.005 |
| Ea/Elv | 0.78 ± 0.10 | 0.78 ± 0.10 | 0.77 ± 0.09 | 0.650 |
AIx: augmentation index; PWV: pulse wave velocity; LVMI: LV mass index; EF: ejection fraction; SV: stroke volume; Elv: ventricular elastance; Ea: arterial elastance.
Values are mean ± SEM.
Intimal-media-thickness.
Carotid compliance.
Carotid distensibility coefficient.
Aortic systolic BP.
Aortic diastolic BP.
Left atrium volume index.
Relative wall thickness.
Ratio of the early (E) to late (A) ventricular filling velocities.
Ratio of tissue Doppler imaging of early diastolic velocity of the mitral annulus (E′) and the early (E) ventricular filling velocity.
Tissue Doppler Imaging of peak systolic velocity at the mitral annulus.
In the overall population, Elv was significantly correlated with Ea (r = 0.887, p < 0.001), age (r = 0.334, p < 0.001), gender (r = −0.494, p < 0.001) and BMI (r = −0.313, p < 0.001).
Independent significant associations between Elv and different clinical parameters at the univariate analysis were identified with multivariate linear regression analysis (including EA, BMI, age, BP and gender) and only Ea (r = 0.860, p < 0.001) and gender (r = −0.024, p = 0.011) were independently associated with Elv.
Focusing on parameters of vascular stiffness, direct correlation of Elv with AIx (r = 0.479, p < 0.001) and CC (r = 0.229, p < 0.05): and inverse correlation with DC (r = −0.250, p < 0.05) were found. However these associations were not statistically significant in multiple regression models adjusted for confounding factors (age, sex, BMI, BP). Univariate analysis showed the lack of correlation between Elv and PWV (r = 0.140, p = 0.166).
When vascular characteristics were analyzed separately according to gender, Elv and Ea were higher in women than in men (1.73 ± 0.43 vs 1.31 ± 0.34 mmHg/mL and 1.36 ± 0.42 vs 1.02 ± 0.29 mmHg/mL, p < 0.001), while their ratio was similar (0.77 ± 0.09 and 0.78 ± 0.10, p = 0.650). Women showed also greater AIx (31.42 ± 9.62% vs 21.88 ± 11.36%, p < 0.001) and PWV (9.85 ± 2.57 vs 8.85 ± 1.92 m/s, p = 0.028) values compared to men, while CC, DC and IMT were similar (Table 2).
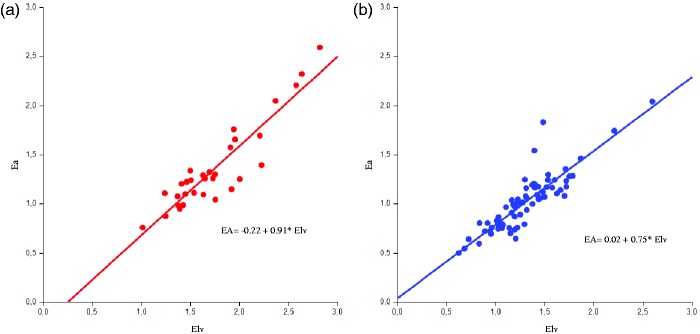
Analyzing correlations of Elv, a significant association with Ea (women: r = 0.833; men: r = 0.880, all p < 0.001) (Figure 1) and BMI (women: r = −0.458; men r = −0.352, all p < 0.01) was found in both sexes. Regarding parameters of vascular stiffness, AIx showed a positive correlation with Elv in both sexes, though significance was reached only in men (men: r = 0.372, p = 0.002; women r = 0.339, p = 0.06) while DC was associated with Elv only in women (r = −0.384, p = 0.036; men: r = −0.203, p = 0.097). No statistically significant correlation was found for PWV.
Figure 1.
Regression between arterial elastance (Ea, mmHg/ml) and ventricular elastance (Elv, mmHg/ml) in the study population according to gender: male (A) and female (B).
Multiple regression models adjusted for age, BMI, BP-lowering therapy, BP and including the variables significantly associated with Elv in the univariate analysis showed that Ea and AIx remained statistically significantly associated in men; in contrast in women only Ea remained correlated with Elv (Table 3).
Table 3.
Multiple regression models in male and female participants in the study, considering Elv as dependent variable and adjusted for age, BMI, BP-lowering therapy, BP.
| Male |
Female |
|||||
|---|---|---|---|---|---|---|
| Standardized coefficient | R2 | p value | Standardized coefficient | R2 | p value | |
| Ea | 0.897 | 0.540 | <0.001 | 0.883 | 0.392 | <0.001 |
| AIx | 0.275 | 0.043 | 0.040 | 0.052 | 0.002 | 0.767 |
| DC | −0.062 | 0.003 | 0.645 | −0.185 | 0.027 | 0.276 |
Elv: ventricular elastance; BP: blood pressure; Ea: arterial elastance; AIx: augmentation index; DC: carotid distensibility coefficient.
Discussion
Data regarding AVC in hypertension are yet available, however, little is still known on the influence of clinical and vascular parameters on ventricular stiffness estimated non-invasively as Elv. The main finding of our research was that, in a population of hypertensive patients, major determinants of Elv were only gender and Ea, while indices of vascular properties and functions were no longer significant in multivariate models adjusted for confounding factors.
Data regarding Ea and Elv in literature are not homogeneous mainly due to different techniques used for their evaluation, however, our results regarding elastances are in line with other published observations and the population of Asklepios study (n = 1755): Ea 1.42 (1.19–1.68) mmHg/mL, Elv 1.73 (1.44–2.12) mmHg/mL, AVC 0.82 (0.72–0.92).16
The reciprocal influence of arterial and Elv can be explained by the anatomical and the functional connection of the heart with the arterial tree. It has been already shown that LV systolic and diastolic stiffness increase in parallel with arterial stiffness in various conditions, such as age and hypertension5,17,18 in order to preserve the maximum efficiency of CV system.
That is not surprising since arterial hypertension is responsible for several features of vascular and cardiac remodeling, such as increased carotid wall thickness, central arterial wall stiffness, early reflected waves, LV hypertrophy and myocardial fibrosis, which are tightly interconnected.19 In presence of hypertension, Ea increases and Elv rises in order to respond to the increased afterload and maintain optimal systolic blood ejection. The rise in Elv is accomplished through increased contractility and cardiac remodeling, allowing the normalization of LV systolic wall stress and the preservation of LV systolic function.19 Some studies have already examined the impact of hypertension on AVC and its components: Ea and Elv are reported to be increased, between 15–60 and 16–95%, respectively, in hypertensive patients compared with normotensive controls, while the coupling ratio remained similar20,21 However, higher values of Ea and Elv (even with a preserved ratio), may be not without a cost: a stiffer heart-arterial system generates a greater systolic pressure change for a given change in SV, resulting in greater BP liability and increased sensitivity to fluid changes.16,22 An altered coupling also influences myocardial perfusion by elevating the proportion of coronary flow during systole (up to 50%), thus worsening the impact of regional coronary ischemia. Furthermore, remodeling process (associated with the progression of hypertensive disease) causes loss of LV contractile and diastolic reserve, resulting in a higher risk of development of HFpEF, especially in women.6,7,23
Indeed, gender differences have been reported: women have a higher value of resting Ea and Elv compared to men at any age.18,21 Chantler and Lakatta17 also noticed in an untreated population free from CV disease, Elv was disproportionately greater in hypertensive women compared to Ea, resulting in lover AVC. In our population, similarly to other published works, Elv and Ea were confirmed to be higher in women compared to men (while their ratio was similar) suggesting that in hypertensive patients AVC is tightly controlled, within a narrow range to optimize energetic efficiency. Furthermore, though Elv is widely regarded as a load independent index of LV contractility, it is also influenced by the geometric and biochemical properties that underlie left ventricular stiffness24 indeed women, which show higher Elv than men, have also been reported to develop greater LV hypertrophy and more maintained systolic function compared to men in response to pressure overload.25
Another important finding is that AIx, which was higher in women as compared to men, appeared to be related to increased ventricular stiffness only in men. AIx has already been independently associated with LV hypertrophy,26,27 though these results were not statistically powered to detect a sex-specific effect (but in both studies, more than 70% of the participants were men). Interestingly, in a sample of 808 subjects of black African descent28 AIx was associated with LVMI in men, but not in women. Therefore, assuming a “gender-related effect” of AIx on LVM and the influence of LVM and geometry on Elv, it seems possible that AIx may affect Elv in men but not in women. According to this hypothesis, in our population the association between AIx and LVM was borderline statistically significant for men (r = 0.23, p = 0.05) but not in women (r = 0.122, p = 0.52), supporting the concept that the gender difference may be mediated by cardiac remodeling and hypertrophy.
Box 1.
Formulas.
| Carotid compliance (CC) | (2Δd/ds)/Δp |
| Carotid distensibility coefficient (DC) | (Δd/ds)/2Δp/πds2 |
| Ventricular elastance (Elv) | ESP/ESV-V0 |
| Arterial elastance (Ea) | ESP/SV |
| Arterial–ventricular coupling (AVC) | Ea/Elv |
Δd: change in arterial diameter; ds: systolic diameter; Δp: difference between the average systolic and the average diastolic blood pressures; ESP: end systolic pressure; ESV: end systolic volume; V0: theoretical volume when no pressure is generated; SV: stroke volume.
Because of the nature of our study, we cannot exclude a completely different point of view. AIx can be considered not a genuine index of wave reflection because aortic reservoir function and left ventricular systolic function have an influence on it as well: moreover augmentation pressure can be directly altered by ventricular contraction/relaxation dynamics rather than vascular properties.29 Therefore, gender differences highlighted by our results could be, at least partially, explained, by confounding factor related to other LV characteristics which cannot be fully assessed by conventional standard two dimensional echocardiography.
Other limitations of the study should also be acknowledged: cross-sectional design does not allow a causality examination and the small sample size might have led to misleading results in gender-specific analysis.
Elv calculation was estimated non-invasively considering negligible the value of V0 (the theoretical ventricular volume at zero pressure) and Ea has been used as genuine index of arterial load even if this assumption is a matter of debate.30
Finally, no significant correlation between Elv and arterial stiffness was found. Although carotid-femoral PWV measured with applanation tonometry technique is considered as the “gold-standard” measurement of vascular stiffness, some controversies which might lead to misleading results should be mentioned; particularly the potential error in measuring the distance between femoral and carotid site (since small inaccuracies may influence the absolute value of PWV) as well as the “validity” of generalized transferred function.
Hence, further studies are needed in order to evaluate extensively the relationship between vascular stiffness parameters and Elv and the influence of gender.
In conclusion, in hypertensive patients, main determinants of Elv are Ea, as an integrated index of arterial vascular load, while large artery stiffness per se seems to play a marginal role. Furthermore we highlighted a gender-specific association between Elv and pressure augmentation, which might play an additional role in men.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval
The study conformed to the Declaration of Helsinki and all patients provided written informed consent prior to entering the study.
Guarantor
Prof. Stefano Taddei.
Contributorship
Not applicable
References
- 1.Starling MR. Left ventricular-arterial coupling relations in the normal human heart. Am Heart J 1993; 125: 1659–1666. [DOI] [PubMed] [Google Scholar]
- 2.Sunagawa K, Maughan WL, Burkhoff D, et al. Left ventricular interaction with arterial load studied in isolated canine ventricle. Am J Physiol 1983; 245: H773–H780. [DOI] [PubMed] [Google Scholar]
- 3.Chantler PD, Lakatta EG, Najjar SS. Arterial–ventricular coupling: Mechanistic insights into cardiovascular performance at rest and during exercise. J Appl Physiol 2008; 105: 1342–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Tombe PP, Jones S, Burkhoff D, et al. Ventricular stroke work and efficiency both remain nearly optimal despite altered vascular loading. Am J Physiol 1993; 264: H1817–H1824. [DOI] [PubMed] [Google Scholar]
- 5.Faconti L, Bruno RM, Ghiadoni L, et al. Ventricular and vascular stiffening in aging and hypertension. Curr Hypertension Rev 2015; 11: 100–109. [DOI] [PubMed] [Google Scholar]
- 6.Coutinho T, Borlaug BA, Pellikka PA, et al. Sex differences in arterial stiffness and ventricular–arterial interactions. J Am Coll Cardiol 2013; 61: 96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chantler PD, Melenovsky V, Schulman SP, et al. The sex-specific impact of systolic hypertension and systolic blood pressure on arterial–ventricular coupling at rest and during exercise. Am J Physiol Heart Circ Physiol 2008; 295: H145–H153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mancia G, Fagard R, Narkiewicz K, et al. Task Force for the Management of Arterial Hypertension of the European Society of Hypertension, the European Society of Cardiology. 2013 ESH/ESC practice guidelines for the management of arterial hypertension. Blood Press 2014; 23: 3–16. [DOI] [PubMed] [Google Scholar]
- 9.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015; 16: 233–270. [DOI] [PubMed] [Google Scholar]
- 10.Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016; 29(4): 277–314. [DOI] [PubMed] [Google Scholar]
- 11.Salonen R, Salonen JT. Determinants of carotid intima-media thickness: A population-based ultrasonography study in eastern Finnish men. J Intern Med 1991; 229: 225–231. [DOI] [PubMed] [Google Scholar]
- 12.Van Bortel LM, Duprez D, Starmans-Kool MJ, et al. Clinical applications of arterial stiffness, task force III: Recommendations for user procedures. Am J Hypertens 2002; 15: 445–452. [DOI] [PubMed] [Google Scholar]
- 13.Bruno RM, Bianchini E, Faita F, et al. Intima media thickness, pulse wave velocity, and flow mediated dilation. Cardiovasc Ultrasound 2014; 12: 34–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharman JE, Lim R, Qasem AM, et al. Validation of a generalized transfer function to noninvasively derive central blood pressure during exercise. Hypertension 2006; 47: 1203–1208. [DOI] [PubMed] [Google Scholar]
- 15.Kappus RM, Ranadive SM, Yan H, et al. Validity of predicting left ventricular end systolic pressure changes following an acute bout of exercise. J Sci Med Sport/Sports Med Aust 2013; 16: 71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chirinos JA, Rietzschel ER, De Buyzere ML, et al. Arterial load and ventricular–arterial coupling: Physiologic relations with body size and effect of obesity. Hypertension 2009; 54: 558–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chantler PD, Lakatta EG. Arterial–ventricular coupling with aging and disease. Front Physiol 2012; 3: 90–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Redfield MM, Jacobsen SJ, Borlaug BA, et al. Age- and gender-related ventricular-vascular stiffening: A community-based study. Circulation 2005; 112: 2254–2262. [DOI] [PubMed] [Google Scholar]
- 19.Virdis A, Bruno RM, Neves MF, et al. Hypertension in the elderly: An evidence-based review. Curr Pharm Des 2011; 17: 3020–3031. [DOI] [PubMed] [Google Scholar]
- 20.Cohen-Solal A, Caviezel B, Himbert D, et al. Left ventricular-arterial coupling in systemic hypertension: Analysis by means of arterial effective and left ventricular elastances. J Hypertens 1994; 12: 591–600. [PubMed] [Google Scholar]
- 21.Saba PS, Ganau A, Devereux RB, et al. Impact of arterial elastance as a measure of vascular load on left ventricular geometry in hypertension. J Hypertens 1999; 17: 1007–1015. [DOI] [PubMed] [Google Scholar]
- 22.Chen CH, Nakayama M, Nevo E, et al. Coupled systolic-ventricular and vascular stiffening with age: Implications for pressure regulation and cardiac reserve in the elderly. J Am Coll Cardiol 1998; 32: 1221–1227. [DOI] [PubMed] [Google Scholar]
- 23.Chirinos JA, Segers P, Gillebert TC, et al. Arterial properties as determinants of time-varying myocardial stress in humans. Hypertension 2012; 60: 64–70. [DOI] [PubMed] [Google Scholar]
- 24.Claessens TE, Rietzschel ER, De Buyzere ML, et al. Noninvasive assessment of left ventricular and myocardial contractility in middle-aged men and women: Disparate evolution above the age of 50? Am J Physiol Heart Circ Physiol 2007; 292: H856–H865. [DOI] [PubMed] [Google Scholar]
- 25.Krumholz HM, Larson M, Levy D. Sex differences in cardiac adaptation to isolated systolic hypertension. Am J Cardiol 1993; 72: 310–313. [DOI] [PubMed] [Google Scholar]
- 26.Hashimoto J, Imai Y, O’Rourke MF. Indices of pulse wave analysis are better predictors of left ventricular mass reduction than cuff pressure. Am J Hypertens 2007; 20: 378–384. [DOI] [PubMed] [Google Scholar]
- 27.Hashimoto J, Watabe D, Hatanaka R, et al. Enhanced radial late systolic pressure augmentation in hypertensive patients with left ventricular hypertrophy. Am J Hypertens 2006; 19: 27–32. [DOI] [PubMed] [Google Scholar]
- 28.Sibiya MJ, Norton GR, Hodson B, et al. Gender-specific contribution of aortic augmentation index to variations in left ventricular mass index in a community sample of African ancestry. Hypertens Res 2014; 37: 1021–1027. [DOI] [PubMed] [Google Scholar]
- 29.Fok H, Guilcher A, Li Y, et al. Augmentation pressure is influenced by ventricular contractility/relaxation dynamics: Novel mechanism of reduction of pulse pressure by nitrates. Hypertension 2014; 63: 1050–1055. [DOI] [PubMed] [Google Scholar]
- 30.Chirinos JA, Rietzschel ER, Shiva-Kumar P, et al. Effective arterial elastance is insensitive to pulsatile arterial load. Hypertension 2014; 64: 1022–1031. [DOI] [PubMed] [Google Scholar]