Abstract
Background
Periodontitis is an inflammatory disease accompanied by alveolar bone loss and progressive inflammation without pain. However, the potential contributors eliminating pain associated with gingival inflammation are unknown.
Results
we examined the involvement of CXC chemokine receptor type 4 (CXCR4) on the mechanical sensitivity of inflamed periodontal tissue, using a mouse model of periodontitis established by the ligation of the tooth cervix of a maxillary second molar and inoculation with Porphyromonas gingivalis (P. gingivalis). Infiltration of inflammatory cells into gingival tissue was not observed following the inoculation. Under light anesthesia, the mechanical head withdrawal threshold (MHWT) on the buccal gingiva was measured using an electronic von Frey anesthesiometer. No significant changes in MHWT were observed in the mice with P. gingivalis-induced periodontitis during the experimental period. Continuous administration of CXCR4 neutralizing antibody to the gingival tissue significantly decreased MHWT and increased the number of gingival CXCR4 immunoreactive macrophages in the periodontitis group. Nitric oxide metabolites in the gingival tissue were significantly increased after the inoculation of P. gingivalis and were reduced by gingival CXCR4 neutralization. Gingival L-arginine administration induced gingival mechanical allodynia in naive animals. Moreover, the decrease in MHWT after treatment with P. gingivalis and CXCR4 neutralization was partially reversed by nitric oxide synthase inhibition in the gingival tissue. Nuclear factor-kappa B was expressed in infiltrating macrophages after inoculation of P. gingivalis and administration of the nuclear factor-kappa B activator betulinic acid induced gingival mechanical allodynia in naive mice.
Conclusions
These findings suggest that CXCR4 signaling inhibits nitric oxide release from infiltrating macrophages and is involved in modulation of the mechanical sensitivity in the periodontal tissue in P. gingivalis-induced periodontitis.
Keywords: Mechanical sensitivity, periodontitis, CXC chemokine receptor type 4, nuclear factor-kappa B, nitric oxide, macrophage
Background
Periodontal diseases, which includes gingivitis and periodontitis, are progressive inflammatory diseases caused by bacterial infection and are accompanied by periodontal tissue destruction and alveolar bone loss.1,2 Though most inflammatory diseases are known to proceed with chronic painful states, periodontal disease is unusual as there is no obvious pain.3 Therefore, numerous patients with periodontitis are unaware of the progression, resulting in delays in detection and treatment of the pathological condition, which leads to severe periodontal tissue damage.4 However, the potential contributors to the absence of periodontal pain during periodontitis remain unknown.
Porphyromonas gingivalis (P. gingivalis) is the dominant pathogen associated with human periodontitis, and infection causes a chronic inflammatory disease of the periodontal tissue.5 Many studies indicate that the accumulation of microbial complexes including P. gingivalis in the dental biofilm initiates and maintains periodontitis, and the fimbriae of P. gingivalis (P. gingivalis-fimbriae), which are comprised of polymerized fimbrillin, are believed to serve as a major colonizing factor.6–8 In macrophages, it is known that P. gingivalis-fimbriae initiate intracellular signaling via toll-like receptor 2 (TLR2).9 Macrophages are also known to express CXC chemokine receptor type 4 (CXCR4), which serves as a TLR2-associated receptor, and TLR2 and CXCR4 interaction with P. gingivalis-fimbriae plays a significant role in the mechanisms underlying immune evasion.10 However, it is not known if TLR2 and CXCR4 interaction in infiltrated macrophages can modulate the excitability of peripheral nociceptors during periodontitis.
In the present study, we examined the involvement of CXCR4 signaling in infiltrating macrophages in periodontal mechanical sensitivity associated with periodontitis, in order to evaluate the mechanisms underlying the indolent condition of the inflamed periodontal tissue in a mouse model of periodontitis.
Materials and methods
Animals
A total of 126 male C57BL/6 mice (20–30 g; Japan SLC, Shizuoka, Japan) were used in this study. Mice were maintained in a temperature-controlled room (23℃) on a semidiurnal light–dark cycle (light on 7:00 to 19:00), with ad libitum access to food and water. This study was approved by the Animal Experimentation Committee at Nihon University (AP15D010-1) and conducted according to the guidelines of the International Association for the Study of Pain.11 All efforts were made to minimize animal suffering and reduce the number of animals utilized.
Periodontitis model
Mice were deeply anesthetized with sodium pentobarbital (50 mg/ kg, intraperitoneally) and placed on a warm mat (37℃). On day 0, a 5-0 silk ligature was tied around the maxillary right second molar avoiding the nearby gingival tissue,12 or complete Freund’s adjuvant (CFA) was injected into the gingival tissue adjacent to cervical regions of the maxillary right second molar, while the mouth was held open by a mouth opener. Under light anesthesia with 1.5% isoflurane (Mylan, Canonsburg, PA), the FDC381 strain of P. gingivalis (1010 colony-forming units/mL) seeded in 200 µl of 2% carboxymethyl cellulose was inoculated into the tied silk ligature on days 0, 1, and 2 after the ligation (P.g.-L). On day 12 after P.g.-L treament, mRNA of P. gingivalis was confirmed in the tied silk ligature by reverse transcription polymerase chain reaction techniques (data not shown). In the sham control mice, the placement of the mouth-opening device and 2% carboxymethyl cellulose inoculation were performed under deep anesthesia, which was identical to the P.g.-L treatment except for the ligation and P. gingivalis seeding (sham).
Gingival mechanical sensitivity
Mice were lightly anesthetized with 2% isoflurane (Mylan, Canonsburg, PA) and the mouth was gently opened by mouth opener. After stopping the supply of 2% isoflurane, graded mechanical stimulation was applied to the gingival tissue adjacent to cervical regions of the maxillary right second molar using an electronic von Frey anesthesiometer (0 to 100 g (cutoff, 100 g), 10 g/s; Bioseb, Chaville, France), once an identical weak flexion reflex of the hindlimb, which ensures an adequate level of maintenance of anesthesia, was induced by noxious pressure applied to the hindpaw. Mice could escape freely from the gingival mechanical stimulation. The lowest mechanical intensity to evoke a nocifensive reflex (head withdrawal) by mechanical stimulation of the gingival tissue was defined as the mechanical head-withdrawal threshold (MHWT). The graded mechanical stimuli were applied at 1 min intervals for each stimulus; the MHWT was determined as the average mechanical intensity that evoked head withdrawal in response to three applications of stimulus. All measurements of mechanical sensitivity in the gingival tissue were conducted under blinded conditions.
Immunohistochemistry
On day 2 after the ligation, mice were anesthetized with sodium pentobarbital (50 mg/kg, intraperitoneally) and transcardially perfused with saline followed by a fixative containing 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Ipsilateral maxillae, containing the maxillary right second molar with periodontal tissues, were dissected out and immersed in the same fixative for 4 h at 4℃. The maxillae were decalcified with 50% K-CX (Falma, Tokyo, Japan) for 24 h, and neutralized in 5% sodium sulphate overnight. The postfixed maxillae were placed in 0.01 M phosphate buffered saline (PBS) containing 20% sucrose for 12 h for cryoprotection and embedded in TissueTek (Sakura Finetek, Tokyo, Japan) at −20℃. The maxillae were cut in the buccolingual plane on a cryostat at a thickness of 16 µm. Sections were thaw-mounted onto MAS-coated Superfrost Plus microscope slides (Matsunami, Osaka, Japan) and dried in the dark at room temperature.
The sections were incubated overnight at 4℃ with anti-CXCR4 monoclonal rat antibody (1:50, cat. MAB21651, R&D system, Minneapolis, MN), anti-F4/80 monoclonal rabbit antibody (1:100, cat. ab111101, Abcam, Cambridge, UK), or anti-nuclear factor-kappa B (NF-κB) p65 monoclonal mouse antibody (1:200, cat. sc-8008, Santa Cruz, Santa Cruz, CA) diluted in 0.01 M PBS containing 4% normal goat serum and 0.3% Triton X-100 (Sigma-Aldrich, St. Louis, MO). After rinsing with 0.01 M PBS, the sections were incubated with Alexa Fluor 488-conjugated goat anti-rabbit IgG (1:200 in 0.01 M PBS; Thermo Fisher Scientific, Waltham, MA) and Alexa Fluor 568-conjugated goat anti-rat IgG (1:200 in 0.01 M PBS; Thermo Fisher Scientific) or Alexa Fluor 568-conjugated goat anti-mouse IgG (1:200 in 0.01 M PBS; Thermo Fisher Scientific) for 2 h at room temperature. After rinsing with 0.01 M PBS, sections were coverslipped in mounting medium (Thermo Fisher Scientific, Waltham, MA) and examined under a fluorescence microscope. The cells showing an intensity twofold greater than the average background were considered positive for immunoreactivity. The number of CXCR4-immunoreactive (IR) and F4/80-IR cells in an area (500 µm downward from the gingival margin) in gingival tissue was counted for each animal. No specific labeling was observed in the absence of primary antibody. Sections of the gingival tissues were analyzed by hematoxylin and eosin staining to visualize the pathological changes.
Drug administration
Under light anesthesia with 2% isoflurane, anti-CXCR4 neutralizing antibody (1 µL, 50 µg/mL; cat. MAB21651; R&D Systems) dissolved in 0.01 M PBS was administered subcutaneously into the gingival tissue for 12 successive days (day 0 to day 11) in mice subjected to P.g.-L treatment. The MHWTs were determined for 12 days (day 0 to day 12), and the number of CXCR4-IR and F4/80-IR cells in the gingival tissue was counted on day 2 in sham mice and in mice subjected to P.g.-L treatment with or without the administration of anti-CXCR4 neutralizing antibody as described above.
To examine the role of nitric oxide (NO) in gingival mechanical hypersensitivity in mice subjected to P.g.-L treatment with the administration of anti-CXCR4 neutralizing antibody, 0.2 mL of the nitric oxide synthase (NOS) inhibitor L-NG-Nitroarginine methyl ester (L-NAME; 40 mg/kg, Cayman, Ann Arbor, MI) dissolved in vehicle (saline) or vehicle alone was intraperitoneally administered to mice subjected to P.g.-L treatment and administration of anti-CXCR4 neutralizing antibody or vehicle. The MHWT was determined for 12 days after treatment as described above. Two µL of betulinic acid, a NF-κB activator (50 mM, R&D Systems), dissolved in vehicle (saline), or vehicle alone was administered to the gingival tissue for 12 successive days (day 0 to day 11) in P.g.-L-treated mice. The MHWTs were determined for 12 days after treatment as described above. In naive mice, the substrate of NO, L-arginine (1 µL, 5 μM, Sigma-Aldrich), dissolved in vehicle (saline) or vehicle alone was administered into the gingival tissue, and the MHWT was determined for 12 h after administration as described above.
Measurement of nitrate and nitrite
Because NO itself is unstable under in vivo physiological conditions, the amount of serum NO metabolites (nitrate/nitrite) was measured as an indicator of NO level.13 On day 2, in mice subjected to sham or P.g.-L. treatment with the administration of anti-CXCR4 neutralizing antibody or vehicle, the mice were transcardially perfused with saline under deep anesthesia. The gingival tissue was removed, homogenized, and centrifuged at 4℃ in 0.01 M PBS. Total nitrate and nitrite in the extracted supernatants from the homogenized gingival tissue were measured spectrophotometrically using a nitrate/nitrite colorimetric assay kit (Cayman Chemical, Ann Arbor, MI, USA) as previously described.14 Absorbance values of standards and samples were corrected by subtraction of the background value to correct for absorbance attributable to non-specific binding. Absorbance was measured using an iMark microplate absorbance reader (Bio-Rad, Hercules, CA, USA).
Statistical analysis
Data were expressed as means ± SEM. Statistical analyses were performed by one-way, two-way analysis of variance (ANOVA), or two-way repeated-measures ANOVA followed by Bonferroni’s, Tukey’s, or Newman-Keuls's multiple comparison tests where appropriate. A value of p < 0.05 was considered significant.
Results
Changes in gingival mechanical sensitivity
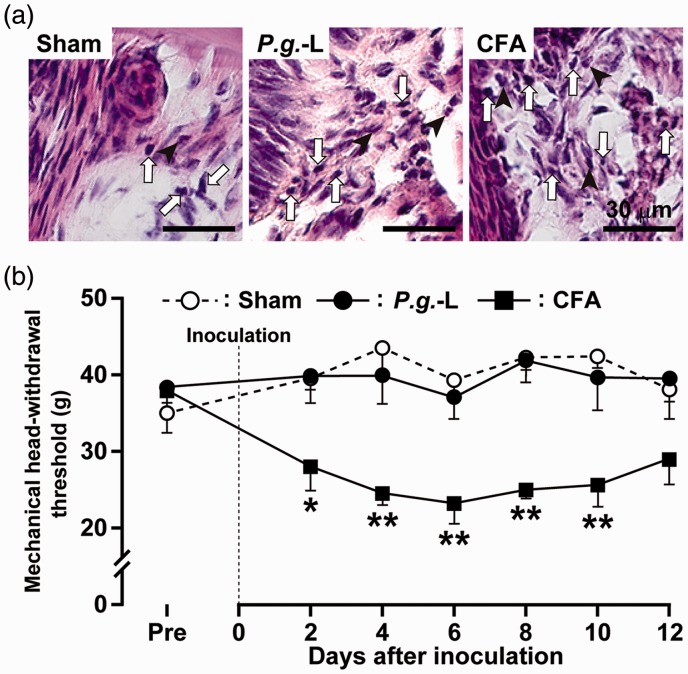
The gingival tissue was inflamed and a dramatic tissue infiltration of inflammatory cells such as lymphocytes, neutrophils, and macrophages was observed on day 2 in mice injected with CFA and P.g.-L-treated mice, but not in sham (Figure 1(a)). There was no significant difference in the MHWT between P.g.-L treatment and sham during the experimental period (Figure 1(b)). The MHWT was significantly decreased on days 2 to 10 after CFA injection into the gingival tissue when compared with that of sham and P.g.-L treatment. Motor deficits and sedation were not observed during the experimental period (data not shown).
Figure 1.
Changes in histology and mechanical sensitivity in the gingival tissue following P.g.-L treatment. (a) Histology of the gingival tissue on day 2 after sham, P.g.-L, or CFA treatment. Arrows indicate lymphocytes. Arrowheads indicate neutrophils. (b) Changes in mechanical sensitivity measured in the gingival tissue following sham, P.g.-L, or CFA treatment for 12 days. Data represent mean ± SEM. Pre: three days before inoculation. *p < 0.05, **p < 0.01 versus sham (n = 10 in sham, n = 10 in P.g.-L, n = 8 in CFA; two-way ANOVA followed by Bonferroni’s multiple-comparison test).
CXCR4-IR macrophages in gingival tissue
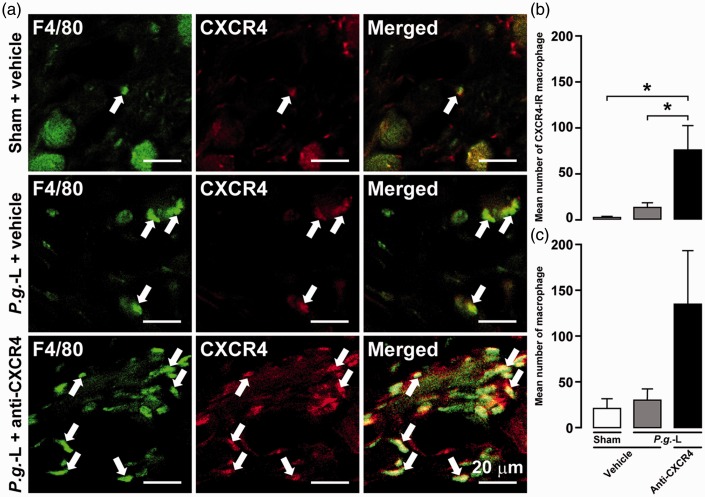
On day 2 in sham and P.g.-L-treated mice administered anti-CXCR4 neutralizing antibody or vehicle, expression of CXCR4-IR and F4/80-IR (a widely used mouse macrophage antigen marker) cells was detected in gingival tissue (Figure 2(a)). The mean number of CXCR4-IR macrophages in mice subjected to P.g.-L treatment along with administration of anti-CXCR4 neutralizing antibody (76.4 ± 26.5) was significantly higher than that of sham (2.2 ± 1.0) and P.g.-L-treated mice administered vehicle alone (13.2 ± 4.8) (Figure 2(b)). Moreover, the mean number of macrophages in mice subjected to P.g.-L treatment and anti-CXCR4 neutralizing antibody (135.8 ± 57.5) tended to be higher than that of the sham group (21.8 ± 9.3) and P.g.-L-treated mice administered vehicle (24.5 ± 2.3) (Figure 2(c)).
Figure 2.
Involvement of CXCR4 signaling in infiltration of macrophages in gingival tissue following P.g.-L treatment. (a) CXCR4-IR and F4/80-IR cells in gingival tissue on day 2 following sham or P.g.-L treatment with vehicle or anti-CXCR4 neutralizing antibody. The arrows indicate CXCR4-IR and F4/80-IR cells in gingival tissue. The mean number of CXCR4-IR macrophages (b) and macrophages (c) in gingival tissue on day 2 after sham or P.g.-L treatment with vehicle or anti-CXCR4 neutralizing antibody. Data represent mean ± SEM. *p < 0.05 (n = 5 in each; one-way ANOVA followed by Tukey’s multiple-comparison test).
Effect of CXCR4 neutralization on mechanical sensitivity and NO production
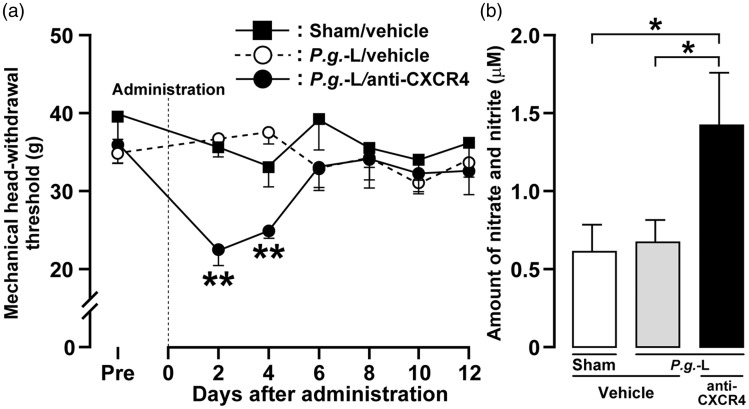
On days 2 and 4, the MHWT in mice subjected to P.g.-L treatment and administration of anti-CXCR4 neutralizing antibody was significantly decreased when compared with mice receiving P.g.-L treatment and vehicle (Day 2, P.g.-L + anti-CXCR4; 22.4 ± 2.1 g, P.g.-L + vehicle; 36.7 ± 1.4 g) (Figure 3(a)). The amount of nitrate and nitrite in the gingival tissue was significantly increased on day 2 after P.g.-L treatment with CXCR4 neutralization (1.4 ± 0.3 µM) when compared to P.g.-L treatment with vehicle (0.7 ± 0.1 µM) or sham (0.6 ± 0.2 µM) (Figure 3(b)).
Figure 3.
Involvement of CXCR4 signaling in mechanical sensitivity and NO production in gingival tissue following P.g.-L treatment. (a) Changes in mechanical sensitivity measured in the gingival tissue following sham or P.g.-L treatment with vehicle or anti-CXCR4 neutralizing antibody. Data represent mean ± SEM. Pre: three days before inoculation. **p < 0.01 versus sham with vehicle (two-way ANOVA followed by Bonferroni’s multiple-comparison test). (b) Quantitative analysis of nitrate and nitrite on day 2 after sham or P.g.-L treatment with vehicle or anti-CXCR4 neutralizing antibody. Data represent mean ± SEM. *p < 0.05 (n = 6 or 7 in each; one-way ANOVA followed by Newman-Keuls’s multiple-comparison test).
NO signaling mediates mechanical sensitivity
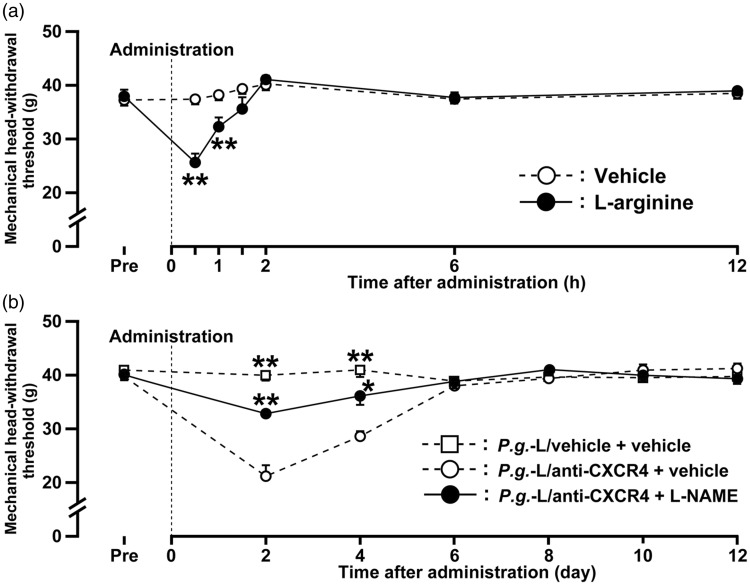
The MHWT was significantly decreased for 1 h after administration of L-arginine to the gingival tissue of naive mice (Figure 4(a)). Reduction of the MHWT peaked at 30 min after L-arginine treatment (25.6 ±1.7 g). L-NAME administration to the gingival tissue resulted in a marked recovery of the reduced MHWT observed in mice subjected to P.g.-L treatment and administration of anti-CXCR4 neutralizing antibody, which peaked at 2 days after administration (21.2 ± 2.0 g) (Figure 4(b)).
Figure 4.
Effect of NO on mechanical sensitivity in the gingival tissue following P.g.-L treatment. (a) Time course of change in mechanical sensitivity of gingival tissue after administration of L-arginine in naive mice. Data represent mean ± SEM. Pre: 3 hours before administration. **p < 0.01 versus vehicle (n = 6 in each; two-way repeated-measures ANOVA followed by Bonferroni’s multiple-comparison test). (b) Time course of change in mechanical sensitivity in gingival tissue after administration of vehicle or L-NAME in mice treated with P.g.-L with vehicle or anti-CXCR4 neutralizing antibody. Data represent mean ± SEM. Pre: three days before inoculation. **p < 0.01 versus P.g.-L with vehicle + vehicle (n = 7 in each; two-way repeated-measures ANOVA followed by Bonferroni’s multiple-comparison test).
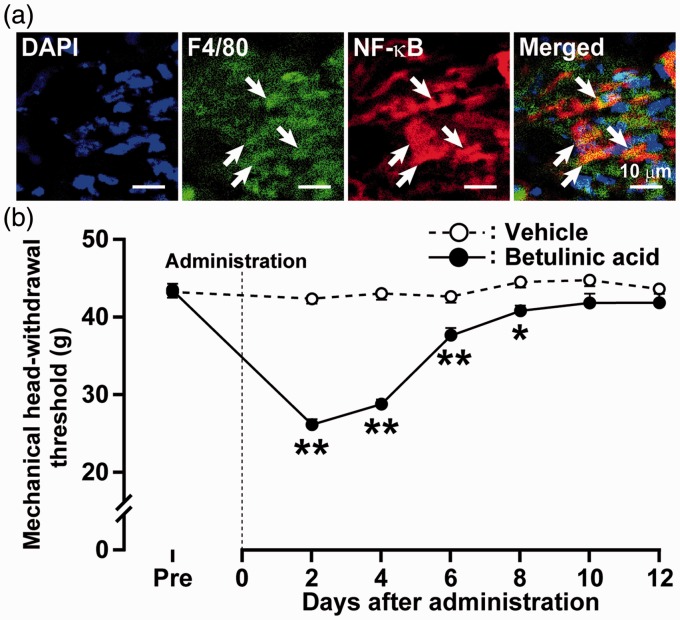
NF-κB-IR macrophages were observed in the gingival tissue on day 2 after P.g.-L treatment (Figure 5(a)). Successive betulinic acid administration to the gingival tissue (from day 0 to 11) decreased the MHWT on days 2 to 8 after P.g.-L treatment (Figure 5(b)). Reduction of the MHWT peaked on day 2 after administration (26.2 ± 0.4 g). Motor deficits or sedation were not observed during the experimental period (data not shown).
Figure 5.
Effect of NF-κB signaling on mechanical sensitivity in gingival tissue. (a) Photomicrographs of DAPI-labeled F4/80-IR and NF-κB-IR cells in gingival tissue on day 2 after P.g.-L treatment. The arrows indicate double-IR cells. (b) Time course of change in mechanical sensitivity in gingival tissue after administration of betulinic acid in P.g.-L-treated mice. Data represent mean ± SEM. Pre: three days before inoculation. *p < 0.05, **p < 0.01 versus vehicle (n = 5 or 6 in each; two-way ANOVA followed by Bonferroni’s multiple-comparison test).
Discussion
Local inflammation induces the release of various chemical mediators from immune cells or peripheral nerve endings, which act on nociceptive nerve endings to lower the neuronal excitation threshold or increase afferent firing leading to the development of pain hypersensitivity.15 For instance, the increase of P2X3 receptors induced by enhanced glutamate or calcitonin gene-related peptide signaling in locally inflamed sites sensitizes primary afferent neurons, resulting in orofacial pain hypersensitivity.16,17 However, periodontal disease such as gingivitis and periodontitis, which is characteristic of orofacial inflammatory conditions, is not accompanied by pain hypersensitivity in most patients.3 In the present study, little to no change was observed in the mechanical sensitivity of the inflamed gingival tissue in mice treated with P.g.-L, while gingival CFA injection induced mechanical allodynia during the experimental period. P.g.-L treatment induced marked recruitment of inflammatory cells into the gingival tissue and alveolar bone resorption similar to CFA treatment; however, no changes in gingival mechanical sensitivity were observed despite the apparent evidence of periodontal tissue inflammation. These results indicate that this is a model of periodontitis with the complete absence of periodontal pain. Clinically, periodontitis develops in the absence of apparent periodontal pain; thus, this model provides a means of assessing the modulation of gingival nociception in patients with periodontitis.
Inducible NOS (iNOS) in macrophages was shown to be upregulated in response to inflammatory stimuli and peripheral NO signaling enhanced the nociceptive response, which was depressed by iNOS inhibition.18 The expression of iNOS mRNA in macrophages was enhanced in inflamed tooth pulp, and iNOS inhibition reduced pulpal upregulation of cyclooxygenase 2 and the release of interleukin-1 beta and tumor necrosis factor alpha (TNF-α).19 In sensory ganglion neurons, prostaglandin E2 synthesized by cyclooxygenase 2, one of two isoforms of the rate-limiting enzyme COX, caused the enhancement of the tetrodotoxin-resistant sodium current, resulting in the sensitization of cutaneous C-fibers to mechanical stimuli.20,21 Additionally, interleukin-1 beta and TNF-α released from resident and infiltrating immune cells enhanced the mechanical sensitivity of peripheral nociceptors.22,23 In this study, CXCR4-IR infiltrated macrophages, and nitrate and nitrite, which are considered indicators of NO level in gingival tissue, were significantly increased following P.g.-L treatment with CXCR4 neutralization, indicating that CXCR4 signaling in infiltrated macrophages inhibits the release and production of NO. Administration of the NO synthase inhibitor L-NAME to the gingival tissue produced a marked reversal of mechanical allodynia following P.g.-L treatment with CXCR4 neutralization. Furthermore, treatment with the NO substrate L-arginine significantly decreased the gingival mechanical sensitivity in naive mice. Taken together, present findings suggest that the decrease in NO production in macrophages caused by CXCR4 signaling inhibits sensitization of gingival nociceptors, resulting in the reduction of gingival mechanical hypersensitivity observed here in P.g.-L-induced periodontitis.
Macrophage migration inhibitory factor (MIF), a pro-inflammatory cytokine, plays a critical role in innate immunity and has been shown to be a key mediator of inflammatory diseases.24 MIF is stored in the cytoplasm of macrophages and is rapidly released in response to various stimuli.25 MIF binds and signals through CXCR4, which mainly regulates inflammatory cell recruitment.26 Macrophages express CXCR4 which regulates cell migration, and inhibition of CXCR4 signaling leads to macrophage infiltration and retention at the site of inflammation,27 where they release various molecules including TNF-α and C-C motif ligand 2.28,29 By extension, the peripheral application of TNF-α and C-C motif ligand 2 can induce pain hypersensitivity by exciting primary nociceptive neurons.30,31 In this study, although the CXCR4-IR macrophages were detected in gingival tissue after P.g.-L treatment, there was no significant difference in the number of infiltrating CXCR4-IR macrophages between sham and P.g.-L-treated mice. However, the mean number of infiltrating CXCR4-IR macrophages in mice treated with P.g.-L and anti-CXCR4 neutralizing antibody was significantly higher than that of sham and P.g.-L treatment alone. These findings suggest that the suppression of macrophage infiltration and retention at the inflamed site by CXCR4 signaling also leads to the absence of periodontal pain in periodontitis following P.g.-L treatment.
The P. gingivalis-fimbriae binds CXCR4 coexpressed with TLR2 in infiltrating macrophages.7,32 P. gingivalis-fimbriae induce protein kinase A activation via cyclic CXCR4-mediated adenosine monophosphate signaling, which inhibits TLR2-induced NF-κB activation.10 The activation of NF-κB in resident macrophages leads to intracellular signaling pathways that facilitate the production of pro-inflammatory molecules closely related to nociceptive modulation.33 In this study, the NF-κB-IR macrophages were observed in the gingival tissue, and NF-κB activation induced gingival mechanical allodynia in naive mice. Therefore, it is possible that TLR2/CXCR4 co-association induced by P. gingivalis-fimbriae signaling inhibits NO production via NF-κB activation in macrophages, leading to the gingival anti-nociceptive action in periodontitis following P.g.-L treatment.
In conclusion, we have established a mouse model of periodontal inflammation with the complete absence of periodontal pain, which mimics the clinical condition in patients suffering from periodontitis. During periodontitis, P. gingivalis-fimbriae-CXCR4 signaling in macrophages in the gingival tissue inhibits the release and production of NO via the NF-κB pathway, and the resulting decrease in NO is likely to result in the absence of periodontal pain in periodontitis following P.g.-L treatment.
Author’s note
All authors discussed the results and commented on the manuscript. H.N. performed experiments, results analysis, and manuscript editing. M.S. and K.I. wrote the manuscript and assisted in analysis. K.H. performed results analysis and generation of figures. N.K., M.W., T.S., N.S., and S.S. contributed to conception of the study and performed experiments. M.S. was responsible for the study concept and design, results analysis, and manuscript writing. All authors read and approved the final manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported in part by research grants from Sato and Uemura Funds from the Nihon University School of Dentistry, a grant from the Dental Research Center in Nihon University School of Dentistry, Nihon University Multidisciplinary Research Grant for (2016) and from KAKENHI (Grant-in-Aid for Challenging Exploratory Research 15K15748) and MEXT-Supported Program for the Strategic Research Foundation at Private Universities 2013–2017.
References
- 1.Lakhssassi N, Elhajoui N, Lodter JP, et al. Antimicrobial susceptibility variation of 50 anaerobic periopathogens in aggressive periodontitis: an interindividual variability study. Oral Microbiol Immunol 2005; 20: 244–252. [DOI] [PubMed] [Google Scholar]
- 2.Mariotti A. Dental plaque-induced gingival diseases. Ann Periodontol 1999; 4: 7–19. [DOI] [PubMed] [Google Scholar]
- 3.Gaurilcikaite E, Renton T and Grant AD. The paradox of painless periodontal disease. Oral Dis 2016. DOI: 10.1111/odi.12537. [DOI] [PubMed]
- 4.Shaddox LM, Walker CB. Treating chronic periodontitis: current status, challenges, and future directions. Clin Cosmet Investig Dent 2010; 2: 79–91. [PMC free article] [PubMed] [Google Scholar]
- 5.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet 2005; 366: 1809–1820. [DOI] [PubMed] [Google Scholar]
- 6.Socransky SS, Haffajee AD, Cugini MA, et al. Microbial complexes in subgingival plaque. J Clin Periodontol 1998; 25: 134–144. [DOI] [PubMed] [Google Scholar]
- 7.Lamont RJ, Jenkinson HF. Life below the gum line: pathogenic mechanisms of Porphyromonas gingivalis. Microbiol Mol Biol Rev 1998; 62: 1244–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang M, Shakhatreh MA, James D, et al. Fimbrial proteins of Porphyromonas gingivalis mediate in vivo virulence and exploit TLR2 and complement receptor 3 to persist in macrophages. J Immunol 2007; 179: 2349–2358. [DOI] [PubMed] [Google Scholar]
- 9.Hajishengallis G, Tapping RI, Harokopakis E, et al. Differential interactions of fimbriae and lipopolysaccharide from Porphyromonas gingivalis with the toll-like receptor 2-centred pattern recognition apparatus. Cell Microbiol 2006; 8: 1557–1570. [DOI] [PubMed] [Google Scholar]
- 10.Hajishengallis G, Wang M, Liang S, et al. Pathogen induction of CXCR4/TLR2 cross-talk impairs host defense function. Proc Natl Acad Sci U S A 2008; 105: 13532–13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983; 16: 109–110. [DOI] [PubMed] [Google Scholar]
- 12.Abe T, Hajishengallis G. Optimization of the ligature-induced periodontitis model in mice. J Immunol Methods 2013; 394: 49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chapman ME, Wideman RF., Jr Evaluation of total plasma nitric oxide concentrations in broilers infused intravenously with sodium nitrite, lipopolysaccharide, aminoguanidine, and sodium nitroprusside. Poult Sci 2006; 85: 312–320. [DOI] [PubMed] [Google Scholar]
- 14.Sugiyama T, Shinoda M, Watase T, et al. Nitric oxide signaling contributes to ectopic orofacial neuropathic pain. J Dent Res 2013; 92: 1113–1117. [DOI] [PubMed] [Google Scholar]
- 15.Muley MM, Krustev E, McDougall JJ. Preclinical assessment of inflammatory pain. CNS Neurosci Ther 2016; 22: 88–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yasuda M, Shinoda M, Kiyomoto M, et al. P2X3 receptor mediates ectopic mechanical allodynia with inflamed lower lip in mice. Neurosci Lett 2012; 528: 67–72. [DOI] [PubMed] [Google Scholar]
- 17.Honda K, Noma N, Shinoda M, et al. Involvement of peripheral ionotropic glutamate receptors in orofacial thermal hyperalgesia in rats. Mol Pain 2011; 7: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guhring H, Tegeder I, Lotsch J, et al. Role of nitric oxide in zymosan induced paw inflammation and thermal hyperalgesia. Inflamm Res 2001; 50: 83–88. [DOI] [PubMed] [Google Scholar]
- 19.Kawashima N, Nakano-Kawanishi H, Suzuki N, et al. Effect of NOS inhibitor on cytokine and COX2 expression in rat pulpitis. J Dent Res 2005; 84: 762–767. [DOI] [PubMed] [Google Scholar]
- 20.Ma W, Quirion R. Does COX2-dependent P.G.-LE2 play a role in neuropathic pain? Neurosci Lett 2008; 437: 165–169. [DOI] [PubMed] [Google Scholar]
- 21.Petho G, Reeh PW. Sensory and signaling mechanisms of bradykinin, eicosanoids, platelet-activating factor, and nitric oxide in peripheral nociceptors. Physiol Rev 2012; 92: 1699–1775. [DOI] [PubMed] [Google Scholar]
- 22.Zhang XC, Kainz V, Burstein R, et al. Tumor necrosis factor-alpha induces sensitization of meningeal nociceptors mediated via local COX and p38 MAP kinase actions. Pain 2011; 152: 140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X, Burstein R, Levy D. Local action of the proinflammatory cytokines IL-1beta and IL-6 on intracranial meningeal nociceptors. Cephalalgia 2012; 32: 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooke G, Armstrong ME, Donnelly SC. Macrophage migration inhibitory factor (MIF), enzymatic activity and the inflammatory response. Biofactors 2009; 35: 165–168. [DOI] [PubMed] [Google Scholar]
- 25.Kim KW, Kim HR. Macrophage migration inhibitory factor: a potential therapeutic target for rheumatoid arthritis. Korean J Intern Med 2016; 31: 634–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernhagen J, Krohn R, Lue H, et al. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat Med 2007; 13: 587–596. [DOI] [PubMed] [Google Scholar]
- 27.Angsana J, Chen J, Liu L, et al. Efferocytosis as a regulator of macrophage chemokine receptor expression and polarization. Eur J Immunol 2016; 46: 1592–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turner MD, Nedjai B, Hurst T, et al. Cytokines and chemokines: at the crossroads of cell signalling and inflammatory disease. Biochim Biophys Acta 2014; 1843: 2563–2582. [DOI] [PubMed] [Google Scholar]
- 29.Han YL, Li YL, Jia LX, et al. Reciprocal interaction between macrophages and T cells stimulates IFN-gamma and MCP-1 production in Ang II-induced cardiac inflammation and fibrosis. PLoS One 2012; 7: e35506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qin X, Wan Y, Wang X. CCL2 and CXCL1 trigger calcitonin gene-related peptide release by exciting primary nociceptive neurons. J Neurosci Res 2005; 82: 51–62. [DOI] [PubMed] [Google Scholar]
- 31.Woolf CJ, Allchorne A, Safieh-Garabedian B, et al. Cytokines, nerve growth factor and inflammatory hyperalgesia: the contribution of tumour necrosis factor alpha. Br J Pharmacol 1997; 121: 417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hajishengallis G, McIntosh ML, Nishiyama SI, et al. Mechanism and implications of CXCR4-mediated integrin activation by Porphyromonas gingivalis. Mol Oral Microbiol 2013; 28: 239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruiz-Miyazawa KW, Pinho-Ribeiro FA, Zarpelon AC, et al. Vinpocetine reduces lipopolysaccharide-induced inflammatory pain and neutrophil recruitment in mice by targeting oxidative stress, cytokines and NF-kappaB. Chem Biol Interact 2015; 237: 9–17. [DOI] [PubMed] [Google Scholar]