Abstract
Background
Although pain is one of the most distressing non-motor symptoms among patients with Parkinson’s disease, the underlying mechanisms of pain in Parkinson’s disease remain elusive. The aim of the present study was to investigate the role of serotonin (5-hydroxytryptamine) in the rostral ventromedial medulla (RVM) and spinal cord in pain sensory abnormalities in a 6-hydroxydopamine-treated rat model of Parkinson’s disease.
Methods
The rotarod test was used to evaluate motor function. The radiant heat test and von Frey test were conducted to evaluate thermal and mechanical pain thresholds, respectively. Immunofluorescence was used to examine 5-hydroxytryptamine neurons and fibers in the rostral ventromedial medulla and spinal cord. High-performance liquid chromatography was used to determine 5-hydroxytryptamine and 5-hydroxyindoleacetic acid levels.
Results
The duration of running time on the rotarod test was significantly reduced in 6-hydroxydopamine-treated rats. Nociceptive thresholds of both mechanical and heat pain were reduced compared to sham-treated rats. In addition to the degeneration of cell bodies and fibers in the substantia nigra pars compacta, the number of rostral ventromedial medulla 5-hydroxytryptamine neurons and 5-hydroxytryptamine fibers in the spinal dorsal horn was dramatically decreased. 5-Hydroxytryptamine concentrations in both the rostral ventromedial medulla and spinal cord were reduced. Furthermore, the administration of citalopram significantly attenuated pain hypersensitivity. Interestingly, Intra-rostral ventromedial medulla (intra-RVM) microinjection of 5,7-dihydroxytryptamine partially reversed pain hypersensitivity of 6-hydroxydopamine-treated rats.
Conclusions
These results suggest that the decreased 5-hydroxytryptamine contents in the rostral ventromedial medulla and spinal dorsal horn may be involved in hyperalgesia in the 6-hydroxydopamine-induced rat model of Parkinson’s disease.
Keywords: Parkinson’s disease, rostral ventromedial medulla, spinal cord, 5-hydroxytryptamine, pain
Background
Parkinson’s disease (PD) is a common neurodegenerative disease in the elderly. Although its cardinal symptoms are tremor, rigidity, akinesia, and postural instability, several non-motor symptoms such as depression, constipation, and sleep disturbances significantly reduce quality of life in patients with PD.1,2 Pain is one of the most distressing non-motor symptoms of PD.3–6 Its prevalence in PD has been reported to range from 40% to 85%.7
Despite its impact, pain in PD is often treated inadequately because the underlying mechanisms remain elusive. A relationship between the dopaminergic system and pain in both healthy subjects and patients suffering from some pain conditions has been reported.8,9 Moreover, an effect of L-3,4-dihydroxyphenylalanine on pain threshold has also been demonstrated in patients with PD-related pain.10–12 However, not all studies have found an association between pain threshold and L-3,4-dihydroxyphenylalanine.13,14 In addition, Dellapina et al.15 have demonstrated that apomorphine has no influence on the pain threshold in patients with PD. Therefore, it remains unclear whether impairment of the dopaminergic system is associated with PD-related pain. Although subthalamic nucleus deep brain stimulation has been reported to relieve PD-related pain, new types of pain develop in patients with PD-related pain postoperatively,16–18 suggesting the possibility that other anatomic structures besides the basal ganglia are involved in the pathophysiology of pain in PD.
According to Braak’s staging of PD, the rostral ventromedial medulla (RVM), which comprises the nucleus raphe magnus and gigantocellular reticular nucleus, can be affected early in PD.19 This anatomic structure has been suggested to be the last relay station for top-down descending control of pain20 and can exert both inhibitory and facilitatory actions on pain modulation via various transmitter-receptor pathways.21–26 Among these pathways, the descending 5-hydroxytryptamine (HT) pathway originating from the RVM has been extensively investigated, and a facilitatory role of this pathway in pain under pathological conditions has been increasingly recognized.27,28 In addition, 5-HT within the RVM has been reported to regulate the activity of pain-modulating neurons in the RVM and participate in the analgesic effect of morphine.29–31 The aim of the present study, therefore, was to determine the roles and possible mechanism of 5-HT in the RVM in the occurrence of pain in a rat model of PD. Here, we showed that 5-HT contents in the RVM and spinal dorsal horn were decreased in 6-hydroxydopamine (OHDA)-induced PD rat model, and these alterations are correlated with hyperalgesia in this rat model. Our study might provide a novel mechanism or therapeutic strategy for pain in patients with PD.
Methods
Animals
Adult male Sprague Dawley rats (200 ± 10 g) were housed (five rats per cage) in a temperature-controlled room (22℃–25℃) illuminated from 08:00 a.m. to 20:00 p.m. Food and water were available ad libitum. All animal care and procedures were approved by and performed according to the guidelines of the Animal Use and Care Committee of Soochow University. The rats were provided with care and handling to ameliorate suffering in accordance with the guidelines of the International Association for the Study of Pain.
6-OHDA-induced PD rat model and rotarod test
Rats were deeply anesthetized with 4% chloral hydrate (400 mg/kg, i.p.) and placed in a Stoelting stereotaxic apparatus (Stoelting Co., Kiel, WI, USA). According to a rat brain atlas, 6-OHDA (Sigma, St. Louis, MO, USA), which was dissolved in saline (16 µg/8 µl two side) was injected into the bilateral SNpc at a rate of 0.5 µl/min using a 10 -µl Hamilton syringe. The coordinates for the bilateral SNpc were −5.3 mm anteroposterior, ±1.0 mm mediolateral, and −7.8 mm dorsoventral (from the dura) from the bregma.32 The syringe was left in the SNpc for 4 min and then slowly retracted. Two weeks after surgery, the rotarod test was performed using the Rotarod system (ZH-300, Zhenghua, Anhui Province, China) to test motor function. Each rat was trained for three consecutive days (three times per day) with the speed of the rotor accelerated from 4 to 25 r/min at a rate of 0.5 r/min. After the last training session, rats were tested three times at a speed of 25 r/min, and the longest duration of running time was recorded.
Drug treatments
5,7-DHT at two different volumes (100 µg, 10 or 100 µg/µl) was microinjected into the RVM to induce 5-HT neuronal lesions. Rats were randomly assigned to one of four groups for these experiments: (1) sham with vehicle injection; (2) sham with 5,7-DHT injection; (3) 6-OHDA with vehicle injection; and (4) 6-OHDA with 5,7-DHT injection. Microinjections were administered four weeks after surgery; rats receiving saline (10 or 1 µl) were used as control. To avoid noradrenergic (NE) neuronal lesions, we pre-treated rats with desipramine (20 mg/kg, i.p.), an inhibitor of neuronal NE reuptake, 30 min before 5,7-DHT or saline microinjection.
Citalopram (20 µg, 4 or 20 µg/µl dissolved in saline) was microinjected into the RVM to enhance extracellular 5-HT levels. Rats were randomly assigned to one of four groups for these experiments: (1) sham with vehicle injection, (2) sham with citalopram injection, (3) 6-OHDA with vehicle injection, and (4) 6-OHDA with citalopram injection. Microinjections were administered four weeks after surgery; rats receiving saline (5 or 1 µl) were used as control. Similarly, citalopram (100 µg, 20 µg/µl dissolved in saline) was intrathecally injected into the spinal cord to enhance 5-HT levels. Rats were randomly assigned to one of four groups for these experiments: (1) sham with vehicle injection (n = 4), (2) sham with citalopram injection (n = 4), (3) 6-OHDA with vehicle injection (n = 4), and (4) 6-OHDA with citalopram injection (n = 4). Intrathecal injections were administered four weeks after surgery; rats receiving saline (5 µl) were used as control.
Immunofluorescence
Rats were anesthetized with 4% chloral hydrate and perfused with saline and 4% paraformaldehyde. Brains were removed and post-fixed overnight in 4% paraformaldehyde. After dehydration with 15% sucrose and 30% sucrose, midbrain sections containing the SNpc were sectioned at 20 µm on a cryostat (Leica, Wetzlar, Germany). The RVM as well as the L4–L6 lumbar spinal cord were sectioned at 35 µm. Endogenous peroxidase activity was inactivated with 0.3% H2O2 for 30 min. After washing with phosphate-buffered saline, sections were incubated in 2% bovine serum albumin/0.1% Triton X-100 in 0.1 M phosphate-buffered saline for 1 h, followed by incubation with primary antibodies against proteins of interest: nigra substance with mouse anti-TH antibody (1:1000, Sigma, St. Louis, USA); RVM and spinal cord with rabbit anti-5-HT antibody (1:1000, Sigma) at 4℃ overnight. Slides then were incubated with anti-mouse Alexa488 secondary antibody or anti-rabbit Alexa488 secondary antibody for 1 h at room temperature. In addition to routine immunofluorescence staining, TdT-mediated dUTP-biotin nick end labeling was performed to identify apoptotic cells in the RVM with a commercially available kit (Beyotime, Nantong, China). Sections were observed and photographed using a Zeiss microscope (AXIO SCOPE A1, Zeiss Corp, Goettingen, Germany). The number of RVM 5-HT neurons was counted in one out of every neighboring three sections by two researchers blind to the treatment condition.
RVM in vivo microdialysis
Rats were anesthetized with 4% chloral hydrate (400 mg/kg, i.p.) and placed in a Stoelting stereotaxic apparatus. A microdialysis guide cannula (CMA, Kista, Sweden) was implanted into the RVM at −10.5 mm anteroposterior, 0 mm mediolateral, and 9.0 mm dorsoventral from the bregma.28 Twenty-four hours after surgery, a microdialysis probe (2-mm sensor tip, CMA, Kista, Sweden) inserted into the cannula, and the RVM was perfused with artificial cerebrospinal fluid (aCSF, 125 mM NaCl, 2.5 mM KCl, 2 mM CaCl2, 1.3 mM MgCl2, 1.3 mM NaH2PO4, 25 mM NaHCO3, and 10 mM glucose) at a constant flow rate of 2 μl/min via microinjection pump (CMA). Samples were collected from freely moving rats in 20-min intervals for 120 min. Collected dialysate samples were measured by HPLC.
HPLC analysis of 5-HT and its metabolites 5-HIAA, DA and its metabolites DOPAC
To detect the level of 5-HT and its metabolite 5-HIAA, DA and its metabolites DOPAC in spinal cord, spinal cord was homogenized with 4% perchloric acid and centrifuged. The supernatant liquid was filtered. HPLC with an electrochemical detector (Antec, Zoeterwoude, Netherlands) was used. Samples of RVM or spinal cord were measured with a mobile phase containing: 100 mM NaH2PO4, 0.74 mM sodium octanesulfonate, 0.027 mM EDTA-Na2, 2 mM KCl, and 15% (vol/vol) methanol (pH = 3.32). A C18 chromatographic column (2.1 mm × 100 mm, 3 µm, Antec) was used to analyze the qualitative and quantitative content of neurotransmitter, and the column temperature was kept at 30℃. Solvent was delivered at 0.20 ml/min using an HPLC pump.
Western blotting
Protein from striatal tissues was homogenized in lysis buffer (150 mM NaCl, 25 mM Tris, 5 mM EDTA, 1% Nonidet P-40; pH 7.5) with protease inhibitor cocktail tablets (Roche Diagnostics, Penzberg, Germany). The lysates were centrifuged at 13,200 g for 15 min at 4℃, and the concentration of protein in each supernatant was determined using a bicinchoninic acid assay (Pierce, Rackford, IL, USA). Forty microgram aliquots were separated by 10% sodium dodecyl sulfate-polyacrylamide gels and transferred onto polyvinylidene fluoride membranes (Millipore, Bedford, MA, USA). The membranes were blocked in 5% (w/v) fat-free dry milk in 0.1% Tris buffered saline/Tween 20 for 1 h and incubated with primary mouse anti-TH antibody (1:5000, Sigma T1299) and mouse anti-β-actin antibody (1:8000, Sigma) at 4℃ overnight. Membranes were briefly washed and incubated with IRDye 680 CS-conjugated anti-mouse secondary antibody for 1 h. Immunoreactive bands were obtained by Odyssey CLx (LI-COR Biosciences, Lincoln, NE, USA). Densitometric analysis was performed using ImageJ software (National Institute of Health, USA).
Pain behavior test
Mechanical allodynia
Rats were acclimatized in an individual animal enclosure chamber for at least 1 h. Von Frey filaments (Aesthesio, DanMic Global, San Jose, CA, USA) were used to test hind paw mechanical withdrawal threshold (PWT) changes. A series of calibrated von Frey filaments (1, 1.4, 2, 4, 6, 8, 10, 15, 26, and 60 g) were applied perpendicularly to the plantar surface of the rat hind paw with sufficient force to bend the filaments for 30 s or until the rat withdrew. In the presence of a response, the next lower filament force was applied. In the absence of a response, the next greater filament force was applied. To avoid potential injury, the cutoff strength of the von Frey filaments was set at 60 g. The filament force was recorded when the same force induces withdrawal of the paw three consecutive times as described previously in literature.33
Thermal hyperalgesia test
Thermal hyperalgesia of the rat paw was measured as previously described in literature.34 The intensity of thermal stimulus applied to the left paw was adjusted to 50% light intensity for the radiant heat source (IITC/Life Science Instruments, Woodland Hills, CA, USA). The heat beam was turned off once the animal started to withdraw its paw. The time between the start of the beam and a functional response was defined as the response latency.
Retrograde labeling of the SNpc
Five rats were deeply anesthetized with 4% chloral hydrate (400 mg/kg, i.p.), placed in a Stoelting stereotaxic apparatus, and retrogradely labeled with FluoroGold™ (Fluorochrome, LLC, Denver, CO, USA), which was dissolved in physiological saline solution to a final concentration of 4% w/v. FG solution (4% w/v, 0.2 µl) was injected into the bilateral SNpc at a rate of 0.05 µl/min via a 10 -µl Hamilton syringe. The syringe was left in the SNpc for 4 min before slowly retracting the syringe to allow for toxin diffusion and to prevent toxin reflux.
Statistical analysis
Data were analyzed using GraphPad Prism 5 (GraphPad Software, La Jolla, CA, USA). The results are presented as the mean ± standard error of the mean (SEM). Differences between two groups were determined by student’s t test. Differences across multiple groups were determined by one-way analysis of variance followed by Tukey’s post-hoc test. P < 0.05 was considered statistically significant for all tests.
Results
Decreased thermal and mechanical pain thresholds in 6-OHDA-induced PD rat model
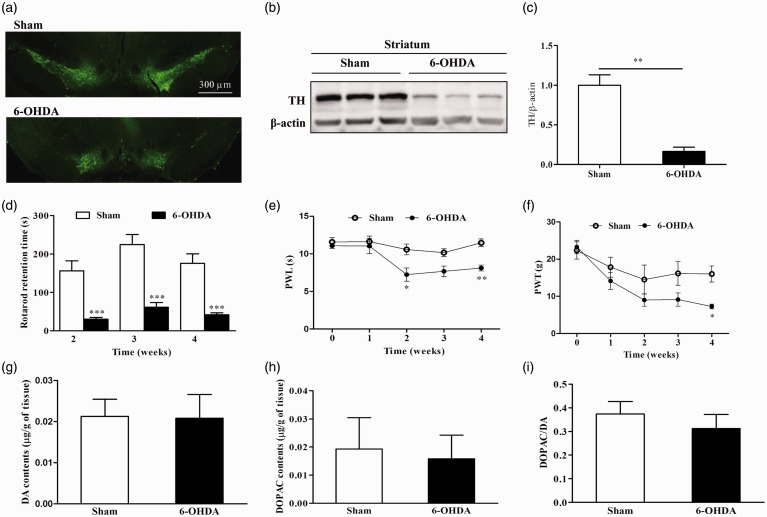
In the present study, we first confirmed the 6-OHDA-induced PD model by immunohistochemistry, Western blotting, and behavioral testing. Bilateral injection of 6-OHDA caused significant degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNpc) (Figure 1(a)). The protein level of tyrosine hydroxylase (TH) was also decreased in the striatum of 6-OHDA-treated rats compared to sham rats (Figure 1(b) and (c)). Compared to sham rats, rotarod retention time was significantly reduced in 6-OHDA-treated rats started from the second week after injection of 6-OHDA (Figure 1(d)). These results indicate that a 6-OHDA-induced PD rat model was successfully established.
Figure 1.
Decrease in thermal and mechanical thresholds in 6-OHDA-treated rats. (a) Visualization of loss of dopaminergic neurons by tyrosine hydroxylase (TH) immunostaining in the substantia nigra (SN). (b) and (c) A representative western blot image using a TH antibody (n = 3 for each group, **P < 0.01 unpaired t test). (d) Motor performance was significantly decreased (***P < 0.001, unpaired t test) in 6-OHDA-treated rats (n = 18) compared to sham-treated rats (n = 17). (e) Hind paw withdrawal latency (PWL) to thermal stimulation was significantly reduced (*P < 0.05, **P < 0.01, two-way analysis of variance with Bonferroni’s post-hoc test) in 6-OHDA-treated rats (n = 8) compared to sham-treated rats (n = 7) two weeks after surgery. (f) Paw withdrawal threshold (PWT) in the von Frey test was significantly decreased (*P < 0.05, two-way analysis of variance with Bonferroni’s post-hoc test) in 6-OHDA-treated rats (n = 8) compared to sham-treated rats (n = 7) four weeks after surgery. (g) to (i) The dopaminergic system in spinal cord was not altered in 6-OHDA-treated rats (n = 4) compared to sham rats (n = 4).
Next, we used two nociceptive behavioral tests to measure thermal and mechanical pain thresholds in sham and 6-OHDA-treated rats. Compared to sham rats, thermal pain threshold (paw withdrawal latency) was significantly reduced in 6-OHDA-treated rats at time points of two and four weeks after surgery (Figure 1(e)). The mechanical pain threshold (PWT) was reduced in 6-OHDA-treated rats four weeks after surgery (Figure 1(f)). These results suggest that the PD rat model induced by bilateral lesions of the SNpc displays thermal and mechanical pain hypersensitivity.
Since recent evidence showed the contribution of brain dopaminergic system to central mechanisms of anti-nociception and the involvement of its deficit in persistent pain in animal models,35,36 we therefore detected the level of dopamine (DA) and its metabolite 3,4-dihydroxyphenylacetic acid (DOPAC) in the spinal cord dorsal horn. The results showed that there was no significant difference between sham and 6-OHDA group (Figure 1(g) and (h)). Furthermore, the ratio of DOPAC to DA, which means the DA turnover, also appeared no significant difference between sham and 6-OHDA group (Figure 1(i)). These results suggest that dopaminergic system in spinal cord may not contribute to the thermal and mechanical hypersensitivity in the rat model of PD.
Impaired 5-HT content in the raphe nuclei in the 6-OHDA-induced PD rat model
To determine the alterations of 5-HT neurons in the RVM in the 6-OHDA-induced PD rat model, we examined the number of 5-HT neurons in the RVM via immunofluorescence. 5-HT neurons were found throughout the RVM in both sham and 6-OHDA-treated rats. However, the number of 5-HT neurons in the RVM was significantly reduced from 45.6 ± 1.9 in sham group to 30.8 ± 2.3 in 6-OHDA-treated rats (Figure 2(a) and (b)). In addition, changes of 5-HT neurons in dorsal raphe nucleus (DR) were also examined. The number of 5-HT neurons in the DR was decreased in 6-OHDA-treated rats (Figure 2(c) and (d)). To focus on the role of 5-HT in the RVM in the hyperalgesia of 6-OHDA-treated rats, microdialysis was used to determine the extracellular concentrations of 5-HT and 5-hydroxyindoleacetic acid (HIAA) in the RVM. Both extracellular 5-HT and 5-HIAA concentrations in the RVM were significantly lower in 6-OHDA-treated rats compared to sham rats (Figure 2(e) and (f)). These results indicate that the 5-HT content in the RVM is decreased in the 6-OHDA-induced PD rats.
Figure 2.
Impaired 5-HT content in the raphe nuclei in 6-OHDA-treated rats. (a) and (b) 5-HT neuronal loss in the RVM of 6-OHDA-treated rats (n = 6 for each group, ***P < 0.001, unpaired t test). (c) and (d) 5-HT neuronal loss in the DR of 6-OHDA-treated rats (n = 3 for each group, *P < 0.05, unpaired t test). (e) and (f) Extracellular 5-HT and 5-HIAA concentration in the RVM was significantly lower in 6-OHDA-treated rats compared to sham-treated rats (n = 3 for each group, *P < 0.05, unpaired t test).
In order to detect whether only 5-HT-containing neurons are lost in the RVM after 6-OHDA treatment, the immunostainings of GABA-immunoreactive (GABA-IR), mu-opioid receptor-immunoreactive (MOR-IR), and neuronal death marker TdT-mediated dUTP-biotin nick end labeling in the RVM of sham and PD rats were performed. The number of apoptosis neurons in the RVM of PD rats was similar to that of sham rats (Figure 3(a) and (b)). The number of GABA-IR and MOR neurons in the RVM of naïve rats was comparable to that of PD rats (Figure 3(c) to (f)). These results suggest that the microinjection of 6-OHDA into SNpc may not lead to the neuronal loss of GABA-IR and MOR neurons in the RVM. The loss of 5-HT-containing neurons may be due to its ability to take in DA precursor by serotonin transporter.37
Figure 3.
Immunostaining of neuronal death marker, MOR-IR, and GABA-IR in the RVM of sham and PD rats. (a) and (b) The neuronal death marker detected by TUNEL Apoptosis Assay Kit (n = 3 for each group, unpaired t test). (c) and (d) The number of GABA-IR neurons in the RVM of sham rats was comparable to that of PD rats (n = 5 for each group, unpaired t test). (e) and (f) The number of MOR-IR neurons in the RVM of sham rats was comparable to that of PD rats (n = 3 for each group, unpaired t test).
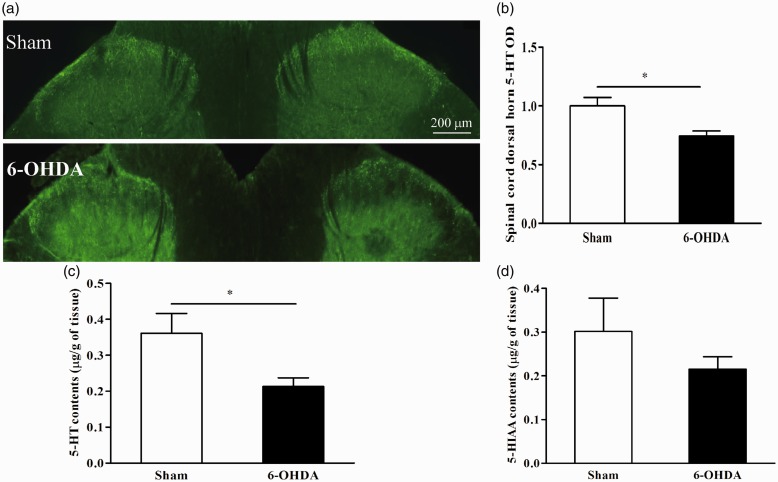
Decreased 5-HT content in the spinal dorsal horn in the 6-OHDA-induced PD rat model
Since 5-HT neurons in the RVM project to dorsal spinal cord, we next examined whether spinal 5-HT nerve fibers were decreased in 6-OHDA-induced PD rats. The density of 5-HT nerve fibers was significantly lower at spinal cord at levels L4–L6 in 6-OHDA-treated rats when compared to sham-treated rats (Figure 4(a) and (b)). We detected 5-HT content in spinal cord at L4–L6 levels via high-performance liquid chromatography (HPLC). 5-HT concentration was significantly lower in 6-OHDA-treated rats than sham-treated rats (Figure 4(c)). However, 5-HIAA concentration was not significantly decreased (Figure 4(d)). Taken together, these results suggest that 5-HT content in the spinal dorsal horn is decreased in the 6-OHDA-induced PD rats.
Figure 4.
Decreased 5-HT content in the spinal dorsal horn in 6-OHDA-treated rats. (a) and (b) The density of 5-HT-immunoresponsive nerve fibers at spinal levels L4–L6 was significantly lower in 6-OHDA-treated rats (n = 3) compared to sham-treated rats (n = 4, *P < 0.05, unpaired t test). (c) 5-HT concentration was significantly lower (*P < 0.05, unpaired t test) at spinal levels L4–L6 in 6-OHDA-treated rats (n = 3) compared to sham-treated rats (n = 4). (d) 5-HIAA concentration did not alter in 6-OHDA-treated rats (n = 3) compared to sham-treated rats (n = 4).
Administration of citalopram attenuated hyperalgesia in the 6-OHDA-induced PD rats
To verify whether decreased 5-HT content within the RVM was correlated with hyperalgesia in the 6-OHDA-induced PD rats, intra-RVM microinjection of citalopram was performed. Citalopram was used to increase extracellular 5-HT concentrations. At the very beginning, we adopted 5 µl as the injection volume for citalopram (thermal pain test: n = 4 for each group; mechanical pain test: n = 8 for sham, 6-OHDA and 6-OHDA + citalopram group, respectively, n = 5 for sham + citalopram). Considering this dose may be so large that the drug may spread out of the RVM, we then changed the injection volume to 1 µl (thermal pain test: n = 4 for sham, n = 3 for 6-OHDA, 6-OHDA + citalopram and sham + citalopram group, respectively; mechanical pain test: n = 4 for each group). The results of two doses are similar so we pooled these data together and analyzed. The thermal pain threshold was significantly reversed at day 3 after citalopram injection in 6-OHDA-treated rats (Figure 5(a)), whereas the mechanical pain threshold was not significantly altered (Figure 5(b)). These results suggest that decreased 5-HT content within the RVM is correlated with hyperalgesia in the 6-OHDA-induced PD rats.
Figure 5.
Administration of citalopram attenuated hyperalgesia in 6-OHDA-treated rats. (a) Intra-RVM microinjection of citalopram ameliorated 6-OHDA-induced thermal hyperalgesia (n = 8 for sham, n = 7 for 6-OHDA, sham + citalopram and 6-OHDA + citalopram group, respectively, ***P < 0.001 versus sham; ###P < 0.001 versus 6-OHDA, one-way analysis of variance and Tukey’s post-hoc test). (b) 6-OHDA-induced mechanical hyperalgesia was not altered after intra-RVM microinjection of citalopram (n = 12 for sham, 6-OHDA and 6-OHDA + citalopram, resepectively, n = 9 for sham + citalopram, **P < 0.01 versus sham, one-way analysis of variance and Tukey’s post-hoc test). (c) and (d) Intrathecal injection of citalopram ameliorated 6-OHDA-induced thermal and mechanical hyperalgesia (n = 4 for each group, *P < 0.05, **P < 0.01 versus sham-treated rats; #P < 0.05 versus 6-OHDA + citalopram-treated rats, two-way analysis of variance and Tukey’s post-hoc test).
To determine the role of decreased 5-HT level in the spinal dorsal horn in hyperalgesia in the 6-OHDA-induced PD rat model, intrathecally injection of citalopram was carried out. As expected, thermal pain threshold was significantly enhanced starting 3 h after injection, and this effect lasted approximately 9 h (Figure 5(c)). Similarly, mechanical pain threshold was significantly elevated starting 12 h after injection (Figure 5(d)). These results confirm that the decrease 5-HT level at the spinal dorsal horn is involved in the hyperalgesia in the 6-OHDA-induced PD rats.
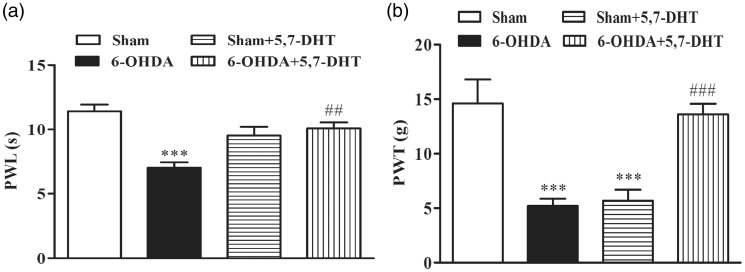
Intra-RVM microinjection of 5,7-dihydroxytryptamine partially reversed pain hypersensitivity
Since the descending 5-HT pathway originating from the RVM has been extensively investigated, and a facilitatory role of this pathway in pain under pathological conditions has been increasingly recognized,27,28 we then microinjected 5,7-dihydroxytryptamine (5,7-DHT) into the RVM to induce 5-HT neuronal lesions. At the very beginning, we adopted 10 µl as the injection volume for 5,7-DHT (thermal pain test: n = 7 for each group; mechanical pain test: n = 6 for each group). Considering this dose may be so large that the drug may spread out of the RVM, we then changed the injection volume to 1 µl (thermal pain test: n = 3 for sham and 6-OHDA group, respectively, n = 4 for sham + 5,7-DHT and 6-OHDA + 5,7-DHT group, respectively; mechanical pain test: n = 4 for each group). Since the results of two doses are similar so we pooled the data together and analyzed. Thermal and mechanical pain thresholds were significantly reversed on day 9 after 5,7-DHT injection into 6-OHDA-treated rats (Figure 6(a) and (b)). Although RVM injection did not produce significant effect on paw withdrawal latency of control rats, it greatly reduced the PWT of control rats. These results indicate that the impaired descending 5-HT pathway from the RVM is involved in hyperalgesia in the PD rats.
Figure 6.
Intra-RVM microinjection of 5,7-dihydroxytryptamine (5,7-DHT) partially reversed pain hypersensitivity. (a) Intra-RVM microinjection of 5,7-DHT ameliorated 6-OHDA-induced thermal hyperalgesia (n = 10 for sham and 6-OHDA, respectively; n = 11 for sham + 5,7-DHT and 6-OHDA + 5,7-DHT, respectively, ***P < 0.001 versus sham; ##P < 0.01 versus 6-OHDA, one-way analysis of variance and Tukey’s post-hoc test). (b) 6-OHDA-induced hyperalgesia to mechanical stimuli was ameliorated by intra-RVM microinjection of 5,7-DHT (n = 10 for each group, ***P < 0.001 versus sham; ### P < 0.001 versus 6-OHDA, one-way analysis of variance and Tukey’s post-hoc test). Intra-RVM microinjection of 5,7-DHT also reduced the PWT of control rats.
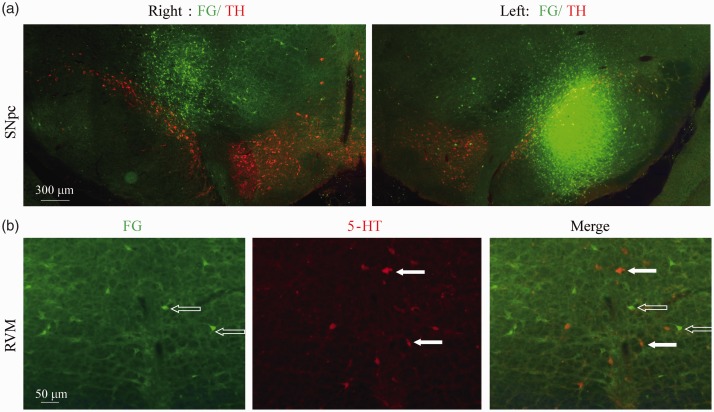
RVM neurons receiving input from SNpc were not 5-HT positive neurons
To verify whether 5-HT neuronal loss in the RVM was caused directly by 6-OHDA toxicity, we examined the connections between RVM and SNpc of control rats. FluoroGold was injected into the bilateral SNpc of control rats (Figure 7(a)). Although FluoroGold retrograde labelings were observed in RVM cells (Figure 7(b) left), no labeled cells demonstrated 5-HT immunoreactivity (Figure 7(b) right). These results are suggest that neurons in RVM receiving SNpc input not 5-HT positive neurons.
Figure 7.
Retrogradely labeling of RVM neurons by injection of FluoroGold into the substantia nigra pars compacta (SNpc). (a) The injection site of FluoroGold in the right side and left side of SNpc. Green labelings are FG. Red labelings were TH-positive staining. (b) 5-HT immunostaining in the RVM showed that 5-HT neurons were not labeled by FG.
Discussion
In this study, we showed that the rat model of PD induced by lesions of dopaminergic neurons in the SNpc manifested more sensitive nociceptive behavior when thermal or mechanical stimulation was introduced. 6-OHDA-treated rats exhibited lower PWTs in the von Frey test compared to sham-treated rats. This result indicates that hyperalgesia to mechanical stimulation was elicited by 6-OHDA injection, which is consistent with a previous report in which the administration of 6-OHDA increased nociceptive response to mechanical stimulation.38 6-OHDA-treated rats also exhibited a shorter pain latencies to thermal stimulation compared to sham-treated rats, suggesting altered behavioral responses to thermal stimulation, which is consistent with a previous study in which a unilateral lesion of the SNpc was induced by 6-OHDA.39
The mechanism underlying pain in PD remains elusive, and thus no effective therapies for PD-related pain exist. Although the loss of DA neurons in the SNpc is the classic pathological characteristic of PD, studies investigating the effects of DA replacement therapy on pain threshold have yielded conflicting results.11–14 In addition, a clinical study showed that apomorphine had no effect on pain thresholds or pain-induced cerebral activities in patients with PD.15 Together, these findings indicate that the mechanisms underlying PD-related pain may involve substrates other than DA. In support of this hypothesis, a clinical study showed that duloxetine, a serotonin-norepinephrine reuptake inhibitor, relieved pain in patients with PD.40 Consistent with this study, another recent clinical study showed that reductions of plasma 5-HT and 5-HIAA levels, which may partially reflect 5-HTergic activity in the brain, were associated with pain in PD.41
The basal ganglion is an important region with influences on sensory perception, suggesting an importance of this structure for pain. In support of this hypothesis, recent clinical studies have demonstrated the therapeutic efficacy of subthalamic nucleus deep brain stimulation on pain in PD.18 However, new types of pain, especially musculoskeletal pain, develop in most patients postoperatively,16,17 indicating that other anatomic structures besides the basal ganglia are involved in the pathophysiology of pain in PD. Notably, according to Braak’s staging of PD, synuclein pathologies initially occur in the olfactory bulb and the inferior brain stem area before becoming widely distributed in the brain,19 which suggests that pathologic changes occur in the pain-modulating brain stem and cerebral structures such as periaqueductal gray42 and insular cortex.43 Therefore, some pain-modulating structures besides the basal ganglia are likely to be involved in the occurrence of pain in PD.
The RVM, which comprises the nucleus raphe magnus and nucleus reticularis gigantocellularis pars alpha, is a key brainstem structure that relays information about pain modulation from higher brain sites and exerts bidirectional pain modulation of spinal pain transmission.23,25 Indeed, there are three types of neurons, “on-cells,” “off-cells,” and “neutral-cells” in the RVM. On-cells exhibit a burst of activity in response to nociceptive input and are thought to contribute to the facilitatory drive, whereas off-cells exhibit a transitory decrease in firing in response to nociceptive input and are thought to be inhibitory. Neutral-cells appear to exhibit no response to nociceptive input.44 Evidence suggests that some 5-HT-containing neurons belong to neutral-cells.45 Furthermore, some on-cells use 5-HT as a neurotransmitter.46 However, all these cells have been reported to be innervated by serotoninergic nerve fibers.31,47 Taken together, such observations lead to the reasonable assumption that the 5-HT system of the RVM may play an important role in pain modulation. In support of this hypothesis, one study has shown that 5-HT modulates the activity of tonically discharging neurons in the RVM.29 Furthermore, systemic administration of morphine induces the release of serotonin in the RVM,30 suggesting that 5-HT in the RVM exerts an inhibitory influence on pain modulation. Given that the 5-HT system is impaired in PD,48 it is reasonable to assume that reduction of 5-HT in the RVM may be involved in the occurrence of pain in PD.
In the present study, we found that 5-HT concentration in the RVM was decreased in the RVM of 6-OHDA-treated rats, which is consistent with our knowledge of an impaired 5-HT system in PD.41,48 Furthermore, the administration of citalopram attenuated hyperalgesia in the 6-OHDA-induced PD rat model. 5-HT within the RVM has been reported to regulate the activity of pain-modulating neurons in the RVM via 5-HT1 and 5-HT2 receptors, and the total effect is antinociceptive.49 However, it has been well recognized from clinical trial and animal research that only 5-HT/NA reuptake inhibitors but not selective serotonin reuptake inhibitors are anti-nociceptive,50 which means that some anti-depressant drugs used for chronic pain therapy are not predominately dependent on their actions on blocking 5-HT reuptake. Citalopram-induced effects may not only result from increasing 5-HT level in the RVM or the spinal cord. At the moment, we cannot exclude other mechanisms.
In addition to the pain modulation of the extracellular 5-HT in the RVM, 5-HT neurons in the RVM can also exert a facilitatory influence on spinal pain transmission.27,28,51 Our data showed that the descending 5-HT pathway originating from the RVM was impaired but still involved in the hypersensitivity to thermal and mechanical stimuli, which is consistent with a previous report on the effect of the impaired descending 5-HT pathway in a model of neuropathic pain.52 However, it remains unclear why the descending 5-HT facilitatory pathway is activated. Given the loss of 5-HT neurons in the RVM, we infer that the factors that lead to the death of some 5-HT neurons in the RVM or the death of these 5-HT neurons themselves triggered the activation of the descending 5-HT facilitatory pathway or reduction in descending inhibition. Notably, hyperalgesia to thermal and mechanical stimuli was significantly attenuated rather than exacerbated when we intrathecally injected citalopram in the 6-OHDA-treated rat. The conflicting results may be explained by two possible reasons: First, several studies have reported that 5-HT at the spinal dorsal horn can cause biphasic modulation. The reduced amount of 5-HT may contribute to hyperalgesia by reduction in descending inhibition by subtypes of 5-HT receptors on one hand or by altered facilitation by regulation of inhibitory transmission in the spinal cord on the other. Since some neurotransmitters bind functional diversity of the receptor subtypes, thus their effects should depend on an integration of all subtype activations. Descending 5-HT from the RVM induces facilitatory or inhibitory influences on pain behaviors based on its activation of excitatory or inhibitory subtype receptors and its targeted excitatory or inhibitory neurons in the spinal dorsal horn. Pharmacological and electrophysiological studies showed that effect of 5-HT is dose-dependent: higher dose produces pain inhibition; lower dose facilitates pain responses.53–55 Therefore, we may not exclude that intrathecal citalopram-induced attenuation of hyperalgesia is related to its increase of 5-HT. Second, although citalopram is traditionally recognized as a selective serotonin reuptake inhibitor, recent studies has reported its role in immune suppression and its effect on Ca2+ channel.56,57 As a result, we cannot exclude that the antinociceptive effect of citalopram in our PD rat model is due to other mechanism in addition to its effect on impaired 5-HT function. The differences in onset of behavioral changes (at three days or 3 h) and mechanical hypersensitivity (no effect or significant effect) after intra-RVM or intrathecal application of citalopram may be caused by the different 5-HT-dependent pathways or just the pharmacological effect of citalopram itself.
Since 5,7-DHT and citalopram are not selective, intra-RVM injection of 5,7-DHT could damage both RVM 5-HT-containing neuronal soma and inputting 5-HT fibers. Also, 5-HT reuptake proteins exist in both 5-HT terminals and soma, intra-RVM injection of citalopram could inhibit reuptake of 5-HT both from neuronal soma and inputting fibers. Therefore, effects of these drugs after injection into the RVM on pain behaviors cannot simply boil down to only change in descending 5-HT functions induced by 5,7-DHT or ascending 5-HT fibers induced by citalopram. Further investigation into the detailed mechanisms is definitely needed.
To exclude a direct toxic effect of 6-OHDA on 5-HT neuronal loss in the RVM, we used FluoroGold retrograde tracer and showed that no labeled cells in the RVM demonstrated 5-HT immunoreactivity. This finding indicates that 5-HT neuronal loss in the RVM is not caused by a direct toxic effect of 6-OHDA. Although the primary cause of neuron loss remains largely unknown, it is thought that PD affects several neurotransmitter systems including dopaminergic and serotonergic system according to Braak’s staging of PD. Lots of pathogenic factors including oxidative stress, mitochondrial dysfunction, inflammatory reaction, and signaling cascades involved in apoptosis may be related to the changes in 5-HT systems in PD.
Although we investigated the role of 5-HT in pain sensory abnormalities in a PD rat model induced by bilateral lesions of the SNpc, we did not explore the role of the loss of the dopaminergic neurons in the SNpc in hyperalgesia. Since intra-RVM injection of 10 µl 5,7-DHT or 5 µl citalopram may spread these drugs out of the RVM, the neighboring structures might be involved as well. Therefore, further studies are needed to confirm the effects of small volume of these drugs and the role of DA in the SNpc in pain sensory abnormalities in the PD rats. As the fact that 5-HT may regulate spinal motor neurons, at the moment, we did not rule out this possibility that animal pain behavioral responses were affected by altered motor function. However, since the running time on the rotarod performed by 6-OHDA-treated was significantly decreased while the pain behavioral responses were enhanced, it is obvious that the decrease in motor performance did not give rise to the enhanced pain behaviors. Instead, it would dampen the pain hypersensitivity.
In summary, our data indicate that a PD rat model induced by bilateral lesions of the SNpc manifests hyperalgesia to thermal and mechanical stimuli. This hypersensitivity can be attributed at least partially to the decreased 5-HT contents in the RVM and spinal dorsal horn.
Author Contributions
CTW and CJM performed experiments, analyzed data, prepared figures, and drafted the manuscript. XQZ, CYZ, DJL, YPY, KLX, JYL, and FW performed experiments and analyzed data. LFH, GYX, and CFL revised the manuscript. CFL designed and supervised the experiments and edited the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from Suzhou Clinical Research Center of Neurological Disease (Szzx201503); Jiangsu Provincial Special Program of Medical Science (BL2014042); Suzhou Clinical Key Disease Diagnosis and Treatment Technology Foundation (LCZX201304); Suzhou Science and Technology Development Program (SYS201620); National Natural Science Pre-Research Foundation of China (SDFEYGJ1605); and also partly supported by Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
References
- 1.Santos-Garcia D, de la Fuente-Fernandez R. Impact of non-motor symptoms on health-related and perceived quality of life in Parkinson’s disease. J Neurol Sci 2013; 332: 136–140. [DOI] [PubMed] [Google Scholar]
- 2.Martinez-Martin P, Rodriguez-Blazquez C, Kurtis MM, et al. The impact of non-motor symptoms on health-related quality of life of patients with Parkinson’s disease. Mov Disord 2011; 26: 399–406. [DOI] [PubMed] [Google Scholar]
- 3.Politis M, Wu K, Molloy S, et al. Parkinson’s disease symptoms: the patient’s perspective. Mov Disord 2010; 25: 1646–1651. [DOI] [PubMed] [Google Scholar]
- 4.Ford B. Pain in Parkinson’s disease. Mov Disord 2010; 25: S98–S103. [DOI] [PubMed] [Google Scholar]
- 5.Lee MA, Walker R, Hildreth TJ, et al. A survey of pain in idiopathic Parkinson’s disease. J Pain Symptom Manage 2006; 32: 462–469. [DOI] [PubMed] [Google Scholar]
- 6.Quittenbaum BH, Grahn B. Quality of life and pain in Parkinson’s disease: a controlled cross-sectional study. Parkinsonism Relat Disord 2004; 10: 129–136. [DOI] [PubMed] [Google Scholar]
- 7.Broen MP, Braaksma MM, Patijn J, et al. Prevalence of pain in Parkinson’s disease: a systematic review using the modified QUADAS tool. Mov Disord 2012; 27: 480–484. [DOI] [PubMed] [Google Scholar]
- 8.Leknes S, Tracey I. A common neurobiology for pain and pleasure. Nat Rev Neurosci 2008; 9: 314–320. [DOI] [PubMed] [Google Scholar]
- 9.Jaaskelainen SK, Rinne JO, Forssell H, et al. Role of the dopaminergic system in chronic pain – a fluorodopa-PET study. Pain 2001; 90: 257–260. [DOI] [PubMed] [Google Scholar]
- 10.Schestatsky P, Kumru H, Valls-Sole J, et al. Neurophysiologic study of central pain in patients with Parkinson disease. Neurology 2007; 69: 2162–2169. [DOI] [PubMed] [Google Scholar]
- 11.Gerdelat-Mas A, Simonetta-Moreau M, Thalamas C, et al. Levodopa raises objective pain threshold in Parkinson’s disease: a RIII reflex study. J Neurol Neurosurg Psychiatry 2007; 78: 1140–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brefel-Courbon C, Payoux P, Thalamas C, et al. Effect of levodopa on pain threshold in Parkinson’s disease: a clinical and positron emission tomography study. Mov Disord 2005; 20: 1557–1563. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y, Mao CJ, Li SJ, et al. Quantitative and fiber-selective evaluation of pain and sensory dysfunction in patients with Parkinson’s disease. Parkinsonism Relat Disord 2015; 21: 361–365. [DOI] [PubMed] [Google Scholar]
- 14.Djaldetti R, Shifrin A, Rogowski Z, et al. Quantitative measurement of pain sensation in patients with Parkinson disease. Neurology 2004; 62: 2171–2175. [DOI] [PubMed] [Google Scholar]
- 15.Dellapina E, Gerdelat-Mas A, Ory-Magne F, et al. Apomorphine effect on pain threshold in Parkinson’s disease: a clinical and positron emission tomography study. Mov Disord 2011; 26: 153–157. [DOI] [PubMed] [Google Scholar]
- 16.Jung YJ, Kim HJ, Jeon BS, et al. An 8-year follow-up on the effect of subthalamic nucleus deep brain stimulation on pain in Parkinson disease. JAMA Neurol 2015; 72: 504–510. [DOI] [PubMed] [Google Scholar]
- 17.Kim HJ, Jeon BS, Lee JY, et al. The benefit of subthalamic deep brain stimulation for pain in Parkinson disease: a 2-year follow-up study. Neurosurgery 2012; 70: 18–23. [DOI] [PubMed] [Google Scholar]
- 18.Kim HJ, Paek SH, Kim JY, et al. Chronic subthalamic deep brain stimulation improves pain in Parkinson disease. J Neurol 2008; 255: 1889–1894. [DOI] [PubMed] [Google Scholar]
- 19.Braak H, Tredici KD, Rüb U, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 2003; 24: 197–211. [DOI] [PubMed] [Google Scholar]
- 20.Gebhart GF. Descending modulation of pain. Neurosci Biobehav Rev 2004; 27: 729–737. [DOI] [PubMed] [Google Scholar]
- 21.Vanegas H, Schaible HG. Descending control of persistent pain: inhibitory or facilitatory? Brain Res Rev 2004; 46: 295–309. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki R, Rahman W, Hunt SP, et al. Descending facilitatory control of mechanically evoked responses is enhanced in deep dorsal horn neurones following peripheral nerve injury. Brain Res 2004; 1019: 68–76. [DOI] [PubMed] [Google Scholar]
- 23.Millan MJ. Descending control of pain. Progress Neurobiol 2002; 66: 355–474. [DOI] [PubMed] [Google Scholar]
- 24.Calejesan AA, Kim SJ, Zhuo M. Descending facilitatory modulation of a behavioral nociceptive response by stimulation in the adult rat anterior cingulate cortex. Eur J Pain-London 2000; 4: 83–96. [DOI] [PubMed] [Google Scholar]
- 25.Zhuo M, Gebhart GF. Biphasic modulation of spinal nociceptive transmission from the medullary raphe nuclei in the rat. J Neurophysiol 1997; 78: 746–758. [DOI] [PubMed] [Google Scholar]
- 26.Pertovaara A, Wei H, Hamalainen MM. Lidocaine in the rostroventromedial medulla and the periaqueductal gray attenuates allodynia in neuropathic rats. Neurosci Lett 1996; 218: 127–130. [DOI] [PubMed] [Google Scholar]
- 27.Kim YS, Chu Y, Han L, et al. Central terminal sensitization of TRPV1 by descending serotonergic facilitation modulates chronic pain. Neuron 2014; 81: 873–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei F, Dubner R, Zou S, et al. Molecular depletion of descending serotonin unmasks its novel facilitatory role in the development of persistent pain. J Neurosci 2010; 30: 8624–8636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piguet P, Stoeckel ME, Schlichter R. Synaptically released 5-HT modulates the activity of tonically discharging neuronal populations in the rostral ventral medulla (RVM). European J Neurosci 2000; 12: 2662–2675. [DOI] [PubMed] [Google Scholar]
- 30.Taylor BK, Basbaum AI. Systemic morphine-induced release of serotonin in the rostroventral medulla is not mimicked by morphine microinjection into the periaqueductal gray. J Neurochem 2003; 86: 1129–1141. [DOI] [PubMed] [Google Scholar]
- 31.Roychowdhury SM, Heinricher MM. Effects of iontophoretically applied serotonin on three classes of physiologically characterized putative pain modulating neurons in the rostral ventromedial medulla of lightly anesthetized rats. Neurosci Lett 1997; 226: 136–138. [DOI] [PubMed] [Google Scholar]
- 32.Wang S, Hu LF, Yang Y, et al. Studies of ATP-sensitive potassium channels on 6-hydroxydopamine and haloperidol rat models of Parkinson’s disease: implications for treating Parkinson’s disease? Neuropharmacology 2005; 48: 984–992. [DOI] [PubMed] [Google Scholar]
- 33.Wang Q, Zhu H, Zou K, et al. Sensitization of P2X3 receptors by cystathionine beta-synthetase mediates persistent pain hypersensitivity in a rat model of lumbar disc herniation. Mol Pain 2015; 11: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yan J, Zou K, Liu X, et al. Hyperexcitability and sensitization of sodium channels of dorsal root ganglion neurons in a rat model of lumber disc herniation. Eur Spine J 2016; 25: 177–185. [DOI] [PubMed] [Google Scholar]
- 35.Rosland JH, Hunskaar S, Broch OJ, et al. Acute and long term effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in tests of nociception in mice. Pharmacol Toxicol 1992; 70: 31–37. [DOI] [PubMed] [Google Scholar]
- 36.Barnes CD, Fung SJ, Adams WL. Inhibitory effects of substantia nigra on impulse transmission from nociceptors. Pain 1979; 6: 207–215. [DOI] [PubMed] [Google Scholar]
- 37.Navailles S, Bioulac B, Gross C, et al. Serotonergic neurons mediate ectopic release of dopamine induced by l-DOPA in a rat model of Parkinson’s disease. Neurobiol Dis 2010; 38: 136–143. [DOI] [PubMed] [Google Scholar]
- 38.Zengin-Toktas Y, Ferrier J, Durif F, et al. Bilateral lesions of the nigrostriatal pathways are associated with chronic mechanical pain hypersensitivity in rats. Neurosci Res 2013; 76: 261–264. [DOI] [PubMed] [Google Scholar]
- 39.Chen CC, Shih YY, Chang C. Dopaminergic imaging of nonmotor manifestations in a rat model of Parkinson’s disease by fMRI. Neurobiol Dis 2013; 49: 99–106. [DOI] [PubMed] [Google Scholar]
- 40.Djaldetti R, Yust-Katz S, Kolianov V, et al. The effect of duloxetine on primary pain symptoms in Parkinson disease. Clin Neuropharmacol 2007; 30: 201–205. [DOI] [PubMed] [Google Scholar]
- 41.Tong Q, Zhang L, Yuan Y, et al. Reduced plasma serotonin and 5-hydroxyindoleacetic acid levels in Parkinson’s disease are associated with nonmotor symptoms. Parkinsonism Relat Disord 2015; 21: 882–887. [DOI] [PubMed] [Google Scholar]
- 42.Liu TY, Cheng Y, Qin XY, et al. Pharmacologically inhibiting GluR2 internalization alleviates neuropathic pain. Neurosci Bull 2015; 31: 611–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu C, Yang T, Zhao H, et al. Insular cortex is critical for the perception, modulation, and chronification of pain. Neurosci Bull 2016; 32: 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Urban MO, Gebhart GF. Supraspinal contributions to hyperalgesia. Proc Natl Acad Sci U S A 1999; 96: 7687–7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Potrebic SB, Fields HL, Mason P. Serotonin immunoreactivity is contained in one physiological cell class in the rat rostral ventromedial medulla. J Neurosci 1994; 14: 1655–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bardin L. The complex role of serotonin and 5-HT receptors in chronic pain. Behav Pharmacol 2011; 22: 390–404. [DOI] [PubMed] [Google Scholar]
- 47.Potrebic SB, Mason P, Fields HL. The density and distribution of serotonergic appositions onto identified neurons in the rat rostral ventromedial medulla. J Neurosci 1995; 15: 3273–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huot P, Fox SH, Brotchie JM. The serotonergic system in Parkinson’s disease. Prog Neurobiol 2011; 95: 163–212. [DOI] [PubMed] [Google Scholar]
- 49.Fields HL, Heinricher MM, Mason P. Neurotransmitters in nociceptive modulatory circuits. Annu Rev Neurosci 1991; 14: 219–245. [DOI] [PubMed] [Google Scholar]
- 50.Nakajima K, Obata H, Iriuchijima N, et al. An increase in spinal cord noradrenaline is a major contributor to the antihyperalgesic effect of antidepressants after peripheral nerve injury in the rat. Pain 2012; 153: 990–997. [DOI] [PubMed] [Google Scholar]
- 51.Cai YQ, Wang W, Hou YY, et al. Optogenetic activation of brainstem serotonergic neurons induces persistent pain sensitization. Mol Pain 2014; 10: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leong ML, Gu M, Speltz-Paiz R, et al. Neuronal loss in the rostral ventromedial medulla in a rat model of neuropathic pain. J Neurosci 2011; 31: 17028–17039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hori Y, Endo K, Takahashi T. Long-lasting synaptic facilitation induced by serotonin in superficial dorsal horn neurones of the rat spinal cord. J Physiol 1996; 492: 867–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li P, Zhuo M. Silent glutamatergic synapses and nociception in mammalian spinal cord. Nature 1998; 393: 695–698. [DOI] [PubMed] [Google Scholar]
- 55.Li P, Kerchner GA, Sala C, et al. AMPA receptor-PDZ interactions in facilitation of spinal sensory synapses. Nat Neurosci 1999; 2: 972–977. [DOI] [PubMed] [Google Scholar]
- 56.Sacre S, Medghalchi M, Gregory B, et al. Fluoxetine and citalopram exhibit potent antiinflammatory activity in human and murine models of rheumatoid arthritis and inhibit toll-like receptors. Arthritis Rheum 2010; 62: 683–693. [DOI] [PubMed] [Google Scholar]
- 57.Zahradnik I, Minarovic I, Zahradnikova A. Inhibition of the cardiac l-type calcium channel current by antidepressant drugs. J Pharmacol Exp Ther 2007; 324: 977–984. [DOI] [PubMed] [Google Scholar]