Abstract
Specific oncogenes with driver mutations, such as the Epidermal Growth Factor Receptor (EGFR 1) gene can lead to non-small-cell lung cancer formation. Identification of these oncogenes, their driver mutations and downstream effects allow the targeting of these pathways by drugs. Such personalised therapy has become an important strategy in combating lung cancer and highlights the need to test for these mutations. Tyrosine Kinase Inhibitors (TKIs) against EGFR, such as Erlotinib, are able to halt these tumour promoting properties in non-small-cell lung cancers. Third generation EGFR TKIs, such as Osimertinib, are focussing on resulting acquired TKI resistance. Here we report the clinical course of a patient with metastatic non-small-cell lung cancer who has undergone EGFR targeted therapy and been further challenged by TKI acquired resistance. Her extended survival and maintained quality of life are a consequence of these modern, genotype-targeted, personalised metastatic non-small-cell lung cancer therapies.
Keywords: Non-small cell lung cancer, EGFR, Tyrosine kinase inhibitors (TKI), Erlotinib (Tarceva), Osimertinib (Tagrisso), Acquired T790M mutation
1. Introduction
The dependency of a tumour on a specific oncogene, for example the epidermal growth factor receptor type 1 (EGFR), renders it potentially sensitive to inhibitors that preferentially target the altered oncogene [1]. The EGFR, a receptor-tyrosine kinase, can be transactivated by G protein-coupled receptors and controls cell growth and proliferation via a transduction signalling pathway [2]. A mutation of this receptor can lead to over expression of the tyrosine kinase domain in the cell membrane [3], [4]. The result of this is unregulated cell growth and proliferation [3], [4]. Identifying the genotype of a cancer therefore enables a more targeted approach towards treatment. Tyrosine kinase inhibitors (TKIs), such as Erlotinib, are able to halt these tumour promoting properties in EGFR mutated non-small-cell lung cancers [5], [6]. They achieve an average tumour control of 11 months [7]; however, resistance mechanisms such as the T790M mutation within exon 20 can develop with loss of tumour control [6], [8]. Osimertinib is a third generation EGFR TKI that exhibits activity towards the T790M mutation [9]. This patient case study demonstrates the extended ongoing survival (over 4 years) achieved by sequentially targeting the EGFR1 gene mutations as they arose and highlights the importance of looking for such acquired resistance mutations.
2. Case report
A 56-year-old retired midwife and non-smoker, diagnosed with metastatic non-small-cell lung cancer was referred to the Oncology Department, Plymouth Hospitals NHS Trust, in late 2012. Her performance status was 1 with a past medical history of hypothyroidism, hypercholesterolemia, prior breast reduction surgery and hysterectomy. A CT guided biopsy confirmed a left upper lobe adenocarcinoma, T4 N3 M1b, with EGFR gene mutation positive; this was a deletion mutation within exon 19 which was predicted to be a sensitising mutation to EGFR tyrosine kinase inhibitors.
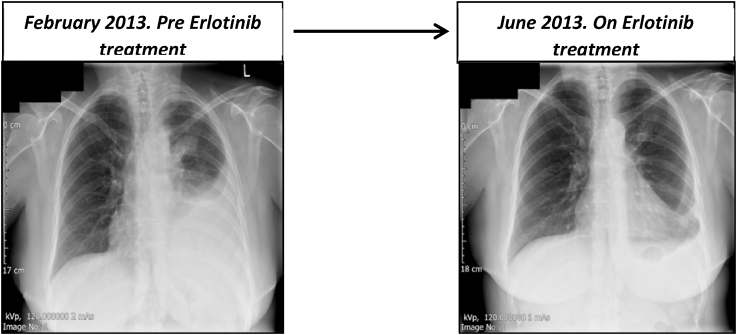
The patient was commenced on Carboplatin and Pemetrexed chemotherapy (as EGFR analysis was not available until March 2013), however due to toxicity this was discontinued after 2 cycles. A first generation TKI, Erlotinib (Tarceva) 150mg once daily was commenced in March 2013. The patient responded and remained well, in remission until February 2015 (Fig. 1). Thereafter, symptoms including tiredness, headaches, back pain and diarrhoea began to increase. This prompted a repeat CT abdomen, thorax and pelvis scan which revealed disease progression in the form of an enlarging left pleural effusion with stable upper lobe mass. The patient then underwent 10 cycles of weekly carboplatin and paclitaxel chemotherapy which was tolerated well with minimal side effects. A further chest x-ray confirmed response prompting a switch to maintenance Pemetrexed chemotherapy in May 2015.
Fig. 1.
Imaging showing treatment response to Erlotinib.
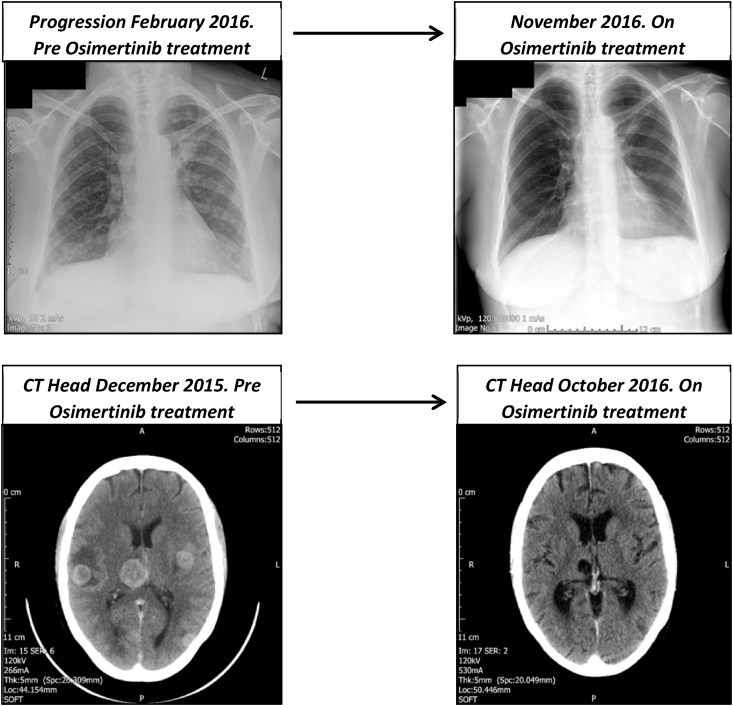
At a routine clinic appointment, new onset pain in the lumbar spine was noted. Spinal x-rays revealed degenerative changes. An urgent nuclear medicine bone scan was undertaken in November 2015. This revealed scattered osteoblastic metastases involving the right scapula, right ninth rib, L2 and L3 pedicle, right iliac crest and left ilium. A subsequent restaging CT scan of the thorax, abdomen, pelvis and brain revealed that the left upper lobe spiculated mass had increased in size in addition to multiple new pulmonary metastases, left supraclavicular lymphadenopathy and a bone metastasis within the L2 vertebral body. The CT brain scan in December 2015 identified multiple brain metastases; treatment was commenced with high dose Dexamethasone, Denosumab for bony metastases, Phenytoin for seizure prophylaxis and whole brain radiation therapy. She continued to deteriorate. An ultrasound guided repeat biopsy of the left supraclavicular fossa lesion was performed. Histopathology results showed an acquired T790M mutation within exon 20 using the Roche Cobas EGFR assay. This is a real time PCR amplification assay of the EGRF1 gene, which will detect mutations at a level >5% in a background of wild-type DNA; it covers 85% of known mutations within the EGFR TK domain exons 18–21 [10]. A third generation EGFR TKI, Osimertinib (Tagrisso), 80mg once daily was commenced in late February 2016 under the extended access programme. From this point forward, investigations show resolution of brain metastases and review is ongoing (Fig. 2).
Fig. 2.
Imaging showing treatment response to Osimertinib.
3. Discussion
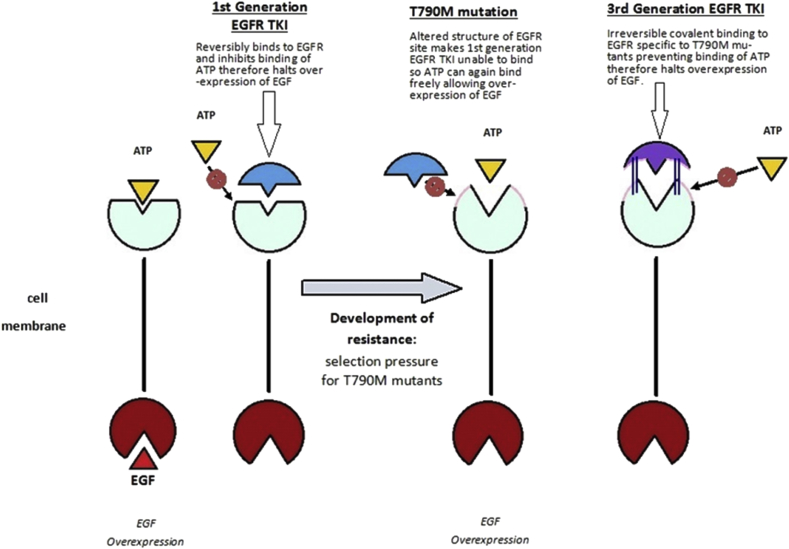
The oncogenes directly associated with non-small-cell lung cancer mutations for which treatment is available are: EGFR - accounting for 10–15% of non-squamous non-small cell lung cancers in Western populations [6], [11], and anaplastic lymphoma kinase (ALK) which accounts for 3–5% [12] of non-squamous non-small cell lung cancers. These driver genotypes determine the corresponding active treatment. First generation TKIs, such as Erlotinib, have been shown to provide substantially better response rates in comparison with standard chemotherapy regimes in patients with a sensitising EGFR mutation [6], [13]. EGFR dimerization stimulates its intrinsic intracellular protein-tyrosine kinase activity; by reversibly binding to EGFR the TKI preferentially inhibits the binding of the ATP to the tyrosine kinase domain thereby halting cell proliferation, leading to cell death [3]. The second-generation EGFR-TKIs such as Afatinib irreversibly bind to the tyrosine kinase of EGFR. Afatinib has also been approved as first-line treatment of advanced NSCLC harbouring activating EGFR mutations [14].
Acquired resistance to first generation TKI may arise through secondary mutation of the EGFR - such as T790M mutation within exon 20, by bypass tracks arising from MET amplification, P13K mutations or small cell transformation [15], [16]. The T790M mutation, as in this case, is found in up to 60% of EGFR TKI- treated tumours [8], [15], [17] and is a point mutation at codon 790 substituting methionine for threonine in the EGFR gene [15]. These T790M mutations arrive either innately, or upon treatment with a TKI as a resistance mutation [15]. The T790M mutation has a higher affinity to binding ATP and therefore activates the transduction signalling pathway and promotes tumour growth (Fig. 3) [8].
Fig. 3.
Mechanism of acquired T790m mutation and action of 3rd generation EGFR TKI.
Third generation TKIs can inhibit EGFR resistance mutations [8]. Osimertinib (Tagrisso) is a 3rd generation TKI which forms an irreversible covalent bond to EGFR, specifically targeting the T790M mutation [9]. Licensed for use in the EU since February 2016 for patients with EGFR T790M mutation positive non-small-cell-lung cancers; it has been found in Phase II studies to have an objective response rate (ORR) in the region of 67% [8]. Progression free survival has been recorded at 9.6 months [8]. Treatment is generally well tolerated. Side effects include diarrhoea, skin and nail changes, stomatitis, or rarely QT interval prolongation, shortness of breath/pneumonitis and bone marrow suppression [9]. Thus far diarrhoea is the only reported side effect in this case.
To conclude, our ability to identify the oncogenes responsible for non-small-cell lung cancers means that we are now able to offer specifically targeted treatments, as in the case of this lady with metastatic non-small-cell lung cancer. Studies to-date suggest that TKI therapies offer significant opportunity. Third generation TKIs have helped to address issues of acquired resistance in T790M mutations. Further studies are ongoing to establish the comparative benefits of Osimertinib versus chemotherapy for patients with EGFR T790M mutation positivity. This patient's extended survival and maintained quality of life are an example of the benefits that are consequent to the use of these modern, genotype-targeted personalised therapies for metastatic non-small-cell lung cancer. Gaining the full benefits of anti EGFR1 strategies requires repeated testing of this oncogene. This is most commonly undertaken by repeat biopsy as in this case, although circulating tumour DNA (ctDNA) technology may help us look for specific mutations such as the T790m mutation [18].
4. Financial disclosure and conflicts of interest
All authors confirm that there are no known conflicts of interest associated with the publication of this case study and that there has been no financial support of any kind for this work that could have influenced the outcome.
References
- 1.Markman M. 2016. Genetics of Non-small Cell Lung Cancer. [online] Medscape.http://emedicine.medscape.com/article/1689988-overview Available at: [Accessed 23rd October 2016] [Google Scholar]
- 2.Wang Z. Transactivation of epidermal growth factor Receptor by G Protein-coupled receptors: recent progress, challenges and future research. Int. J. Mol. Sci. 2016;17(1):95. doi: 10.3390/ijms17010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scaltriti M., Baselga J. The epidermal growth factor receptor pathway: a model for targeted therapy. Clin. Cancer Res. 2006;12(18):5268–5272. doi: 10.1158/1078-0432.CCR-05-1554. [DOI] [PubMed] [Google Scholar]
- 4.Siegelin M., Borczuk A. Epidermal growth factor receptor mutations in lung adenocarcinoma. Lab. Investig. 2014;94:129–137. doi: 10.1038/labinvest.2013.147. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y., Schmid-Bindert G., Zhou C. Erlotinib in the treatment of advanced non-small cell lung cancer: an update for clinicians. Ther. Adv. Med. Oncol. 2012;4(1):19–29. doi: 10.1177/1758834011427927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas A., Rajan A., Giaccone G. Tyrosine kinase inhibitors in lung cancer. Hematol. Oncol. Clin. North Am. 2012;26(3):589–605. doi: 10.1016/j.hoc.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takeda M., Okamoto I., Nakagawa K. Survival outcome assessed according to tumour response and shrinkage pattern in patients with EGFR mutation-positive non-small-cell lung cancer treated with gefitinib or Erlotinib. J. Thorac. Oncol. 2014;9(2):200–204. doi: 10.1097/JTO.0000000000000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang S., Cang S., Liu D. Third-generation inhibitors targeting EGFR T790M mutation in advanced non-small cell lung cancer. J. Hematol. Oncol. 2016;9(34) doi: 10.1186/s13045-016-0268-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Electronic Medicines Compendium . 2016. SPC: TAGRISSO 40 mg and 80 mg Film-coated Tablets.https://www.medicines.org.uk/emc/medicine/31496 [online] Available at: [Accessed 23rd October 2016] [Google Scholar]
- 10.Angulo B., Lopez-Rios F., Gonzalez D. A new generation of companion diagnostics: cobas BRAF, KRAS and EGFR mutation detection tests. Expert Rev. Mol. Diag. 2014;14(5):517–524. doi: 10.1586/14737159.2014.910120. [DOI] [PubMed] [Google Scholar]
- 11.Midha A., Dearden S., McCormack R. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: a systematic review and global map by ethnicity (mutMapll) Am. J. Cancer Res. 2015;5(9):2892–2911. [PMC free article] [PubMed] [Google Scholar]
- 12.Chia P., Mitchell P., Dobrovic A., John T. Prevalence and natural history of ALK positive non-small-cell lung cancer and the clinical impact of targeted therapy with ALK inhibitors. Clin. Epidemiol. 2014;6:423–432. doi: 10.2147/CLEP.S69718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou C. Erlotinib versus chemotherapy as a first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 14.National Institute for Health and Care Excellence (NICE) 2014. Afatinib for Treating Epidermal Growth Factor Receptor Mutation-positive Locally Advanced or Metastatic Non-small-cell Lung Cancer.https://www.nice.org.uk/guidance/ta310/chapter/1-Guidance [online] Available at: [Accessed 23rd October 2016] [Google Scholar]
- 15.Suda K., Onozato R., Yatabe Y., Mitsudomi T. EGFR T790M mutation a double role in lung cancer cell survival? J. Thorac. Oncol. 2009;4(1) doi: 10.1097/JTO.0b013e3181913c9f. [DOI] [PubMed] [Google Scholar]
- 16.Suda K. Small cell lung cancer transformation in T790M mutation; complimentary roles in acquired resistance to kinase inhibitors in lung cancer. Sci. Rep. 2015;5:14447. doi: 10.1038/srep14447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.My Cancer Genome . 2015. EGFR c.2369C>T (T790M) Mutation in Non-small Cell Lung Cancer.https://www.mycancergenome.org/content/disease/lung-cancer/egfr/4/ [online] Available at: [Accessed 23rd October 2016] [Google Scholar]
- 18.Sundaresan T.K. Detection of T790M, the acquired resistance EGFR mutation, by tumor biopsy versus noninvasive blood-based analyses. Clin. Cancer Res. 2016;22(5):1103–1110. doi: 10.1158/1078-0432.CCR-15-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]