Efforts at elucidating genetic mechanisms, as well as cellular perturbations that influence plaque stability of atherosclerotic lesions have gained momentum in recent years, and aided understanding of pathophysiological events associated with the disease. In this edition of Hypertension, Liu et al. provide new insight of previously unknown roles for Interferon Regulatory Factor 3 (IRF3) as a proatherogenic mediator via direct modulation of endothelial intracellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) (pages xxx-xxx).
While lifestyle modifiable risk-factors (e.g. smoking, obesity, etc.), as well as lipid lowering drugs, such as statins provide partial solutions at mitigating cardiovascular complications, the most common cause of death relative to other human pathologies is due to cardiovascular-related events. Decades-long research on atherosclerosis has delineated various factors that contribute to the development of necrotic core, including the roles of immune response system, chemokines, as well as toxic lipid build-up in the arteries.1–4 However, atherosclerosis and the processes associated with the condition remain confounding. Simply stated, atherogenesis results from defective endothelial lipid clearance system. Build-up of modified serum lipids within the arteries (such as oxidized low-density lipoprotein; oxLDL) provoke endothelial immune response that drive recruitment of macrophage cells typically required for efficient degradation and “clean-up” of the toxic endothelial-anchored lipids. However, alternative mechanisms propose that repetitive macrophage-mediated lipotoxic clearance may be responsible for the development of necrotic core.1 Endothelial cells within the interior surface of blood vessels have been implicated in early-stage development of atherosclerosis, as well as later phases of plaque stability.1,2 Additionally, proinflammatory cytokines and cellular adhesion molecules appear intimately linked to atherogenesis.1–3
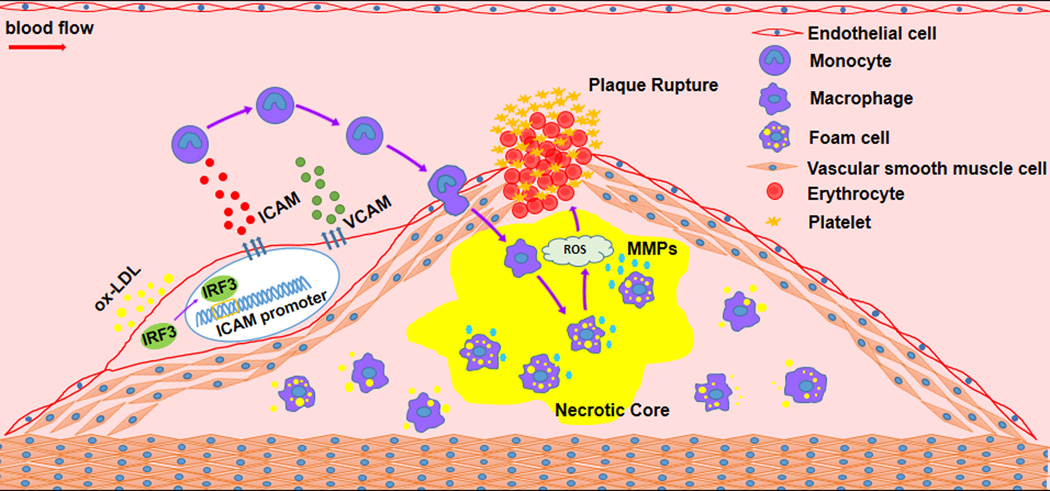
In this edition of Hypertension, Liu et al. provide intriguing insight on how ablation of endothelial IRF3 protects against atherosclerosis via attenuated secretion of adhesion molecules, and concomitant macrophage infiltration (Fig. 1).5 IRF3 is a member of the interferon regulatory factor family which comprises of transcriptional regulators of the interferon (IFN)-induced signaling pathway.5 Mammalian IRF consists of 9 members that possess various biological functions, and IRF3 is somewhat unique in that inactive forms are predominantly localized in the cytosol. In response to stimuli, IRF3 may become activated via phosphorylation, and translocate into the nucleus.5 In this study, the authors observed that IRF3 was increased in patients with coronary heart disease (CHD). Upregulated IRF3 level was confirmed further using ApoE-deficient mice, a commonly used atherosclerotic mice model relative to IRF3−/−ApoE−/−. It was further observed that hyperlipidimic IRF3−/−ApoE−/− mice were resistant to high-fat diet-induced atherogenesis, while displaying decreased plaque inflammation and improved plaque stability.5 Another highpoint of the article was the observation that several defining hallmarks of stable plaques, including thick fibrous cap cover, increased collagen deposition and smooth muscle cell (SMC) content, as well as decreased macrophage infiltration were evident upon ablation of IRF3 expression.5,6 Cross-group bone marrow transplantation demonstrated that IRF3 deficiency in endothelial cells was critical for decreased plaque development rather than bone marrow-derived macrophages.5
Figure 1.
IRF3 promotes proatherogenesis and atherosclerotic plaque instability. Illustration of findings by Liu et al. depict how under hyperlipidemia condition, IRF3 transcriptionally upregulates ICAM in the endothelium, and subsequently promote macrophage migration and inflammation. (oxLDL: oxidized low-density lipoprotein; MMPs: matrix metalloprotineases).
Mechanistically, the authors demonstrated that IRF3 promoter binds to ICAM-1, thereby suppressing gene transcription, and to a lesser extent VCAM-1 expression. Based on bioinformatics analysis, a series of putative ISRE-binding sites in the ICAM-1 promoter were identified (designated as, P1–P4). Utilizing chromatin immunoprecipitation (Chip) assay in HUVECs, followed by PCR of ICAM-1 and VCAM-1 promoters, a specific and direct binding of IRF3 to region P1 of ICAM promoter was confirmed.5 Depletion of IRF3 using specific siRNA in HUVECs and subsequent treatment with TNF-α, correlated with significant decrease in ICAM-1 and VCAM-1 expression at both the mRNA and protein levels.5 Together, these findings highlight an established network of inflammatory trigger on endothelial integrity, and subsequent pro-atherogenic events via altered secretion of adhesion molecules.
While the study provides exciting findings on previously unknown functions of endothelial and macrophage IRF3 on plaque stability, some questions persist. Given well-described roles of ICAM-1 and VCAM-1 in promoting endothelial permeability,5 whether additional IRF isoforms can target these cell adhesion molecules remain unknown. Furthermore, blood pressure measurements, as well as effects of additional inflammatory chemokines in the presence or absence of IRF3 may shed more light on atherogenic events mediated by IRF3.
Multiple processes contribute to the pathogenesis or exacerbation of vulnerable plaques, including endothelial dysfunction, inflammation, VSMC proliferation and alteration of membrane matrix.5,7 It is against this backdrop that this study provides exciting mechanistic insight of how endothelial IRF3 promotes plaque vulnerability via altered secretions of adhesion molecules, ICAM-1 and VCAM-1. In future studies, it would be interesting to elucidate the function of IRF3 in lipotoxic signaling pathways, and potential cross-talk with canonical pro-inflammatory chemokines.
Acknowledgments
Sources of Funding
Dr. M. H. Zou’s laboratory is supported by grants (HL079584, HL080499, HL089920, HL110488, HL128014, HL132500, CA213022, and AG047776) from the National Institutes of Health.
Footnotes
Disclosures
None
References
- 1.Webb NR. Getting to the core of atherosclerosis. Nat Med. 2008;14:1015–1016. doi: 10.1038/nm1008-1015. [DOI] [PubMed] [Google Scholar]
- 2.Liu X, Ntambi JN. Atherosclerosis: keep your macrophages in shape. Nat Med. 2009;15:1357–1358. doi: 10.1038/nm1209-1357. [DOI] [PubMed] [Google Scholar]
- 3.Koenen RR, Weber C. Therapeutic targeting of chemokine interactions in atherosclerosis. Nat Rev Drug Discov. 2010;9:141–153. doi: 10.1038/nrd3048. [DOI] [PubMed] [Google Scholar]
- 4.Dzau VJ, Braun-Dullaeus RC, Seeding DG. Vascular proliferation and atherosclerosis: New perspectives and therapeutic strategies. Nat Med. 2002;8:1249–1256. doi: 10.1038/nm1102-1249. [DOI] [PubMed] [Google Scholar]
- 5.Liu H, Cheng W, Jiang X, et al. Ablation of Interferon Regulatory Factor 3 protects against atherosclerosis in apolipoprotein E-deficient mice. Hypertension. 2017 doi: 10.1161/HYPERTENSIONAHA.116.08395. In Press. [DOI] [PubMed] [Google Scholar]
- 6.Erbay E, Babev VR, Mayers JR, et al. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat Med. 2009;15:1383–1391. doi: 10.1038/nm.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding Y, Zhang M, Zhang W, et al. AMP-Activated Protein Kinase Alpha 2 Deletion Induces VSMC Phenotypic Switching and Reduces Features of Atherosclerotic Plaque Stability. Circ Res. 2016;119:718–730. doi: 10.1161/CIRCRESAHA.116.308689. [DOI] [PMC free article] [PubMed] [Google Scholar]