Abstract
A case-control study was conducted to investigate potential risk factors for toe tip necrosis syndrome (TTNS) in western Canadian feedlot cattle. Feedlot veterinarians provided hooves from 222 animals that died of either TTNS (“cases”) or from all other causes (“controls”). The claws were sectioned by researchers to confirm the diagnoses; there was very good agreement between the practitioners’ field diagnosis and that of the researchers (Cohen’s kappa = 0.81; P < 0.001). The sole thickness of the apical white line region was thinner (P < 0.001) in the cases (3.74 mm) than the controls (4.72 mm). Claws from cases were 5.0 [95% confidence interval (CI): 1.5 to 8.6; P < 0.001] and 7.3 times (95% CI: 1.5 to 69.3; P < 0.01) more likely than those of controls to yield a heavy growth of Escherichia coli and Trueperella pyogenes, respectively. Cases were 4.4 times (95% CI: 4.4 to 22.9; P < 0.001) more likely to be acutely/transiently infected with bovine viral diarrhea virus than were controls. The findings support the hypothesis that TTNS is initiated by excessive wear along the white line, leading to separation and bacterial colonization of the 3rd phalangeal bone (P3) and associated soft tissues.
Résumé
Étude prospective de cas-témoins du syndrome de la nécrose du bout des orteils dans un parc d’engraissement de l’Ouest canadien. Une étude de cas-témoins a été réalisée pour investiguer les facteurs de risques potentiels pour le syndrome de la nécrose du bout des orteils (SNBO) chez le bétail des parcs d’engraissement de l’Ouest canadien. Les vétérinaires des parcs d’engraissement ont fourni des sabots provenant de 222 animaux qui sont morts soit du SNBO («cas») ou d’autres causes («témoins»). Les ongles ont été sectionnés par les chercheurs pour confirmer les diagnostics; il y avait une très bonne concordance entre le diagnostic sur le terrain des praticiens et celui des chercheurs (Kappa de Cohen = 0,81; P < 0,001). L’épaisseur de la sole dans la région de la ligne blanche atypique était plus mince (P < 0,001) dans les cas (3,74 mm) que dans les témoins (4,72 mm). Il était 5,0 fois (IC de 95 % de 1,5 à 8,6; P < 0,001) et 7,3 fois (IC de 95 % de 1,5 à 69,3; P < 0,01) plus probable que les ongles des cas donnent une croissance importante d’Escherichia coli et de Trueperella pyogenes, respectivement. Il était 4,4 fois (IC de 95 % de 4,4 à 22,9; P < 0,001) plus probable que les cas soient infectés de manière aiguë ou transitoire par le virus de la diarrhée virale des bovins comparativement aux témoins. Les résultats appuient l’hypothèse que le SNBO est amorcé par une usure excessive le long de la ligne blanche, ce qui entraîne une séparation et la colonisation bactérienne de l’os de la troisième phalange (P3) et des tissus mous connexes.
(Traduit par Isabelle Vallières)
Introduction
The mean (median) incidence of lameness in American feedlots has been estimated to be at 3.8% (2.0%), with the 3 most common causes of lameness being interdigital necrobacillosis (footrot), traumatic injury, and toe abscesses (1). One of the earliest reports of toe abscesses of feedlot cattle came from Sick et al in 1982 (2), wherein they described the clinical signs, treatment options, and postmortem findings of the disease. Furthermore, they speculated that toe abscesses were more common in high-spirited animals; animals shipped in aluminum trailers without sufficient bedding; and animals exposed to concrete surfaces. The disease also tended to affect the lateral digit of the hind limb and it was posited that excessive wear to the apex of the toe resulted in white line separation, bacterial invasion, and abscess formation.
Sick et al’s report (2) was followed by a second in 1994, wherein Miskimins (3) described an unusual occurrence of toe abscesses in mid-western US feedlots during the winter of 1992 to 1993. Most cases developed within 3 wk after arrival at the feedlot and the affected animals had excessive wear along the apex of the hind toes, which was associated with white line separation and toe abscess formation. It was speculated that wet weather conditions may have led to softening of the horn tissue, resulting in excessive wear of the sole. Prolonged standing and exposure to concrete surfaces were also considered to be risk factors for the disease.
Outbreaks of toe abscesses, however, are not unique to North American feedlot cattle. In 1979, Dewes (4) described an outbreak of “transit-related lameness” in a group of Jersey replacement heifers in New Zealand. Significantly, the toe lesions developed within 3 wk of transport, only involved the hind toes, and toe abscesses were associated with white line separation. Dewes attributed the disease to the os pedis being forced through the sole during transport. Van Amstel and Shearer (5) have also described the occurrence of toe abscesses in North American dairy cattle and have suggested that the distance to the milk parlour, exposure to new (“green”) concrete, and social factors such as commingling are potential risk factors for the disease. More recently, Mason et al (6) described an outbreak of toe abscesses in New Zealand dairy heifers; however, lameness was not associated with transport, but rather with the heifers being exposed to wet coarse concrete and being commingled with cows.
Common to all these outbreaks is the finding of white line separation, toe abscesses, and sequelae that include osteitis of the 3rd phalanx (P3); osteomyelitis of the 2nd phalanx (P2); ascending tenosynovitis and cellulitis; septicemia; and embolic events involving the lungs, liver, and kidneys. Although feedlot producers and veterinarians often refer to the disease as toe abscesses, more recently, researchers have suggested a more encompassing term — toe tip necrosis syndrome (7–9). This terminology not only describes the main clinical findings (toe tip necrosis), but the term “syndrome” includes the sequelae associated with the inciting lesion.
A number of theories have been postulated for the cause of the disease; however, the “abrasion theory” is perhaps the mainstream theory. This theory proposes that excessive wear along the apical white line leads to white line separation and subsequent bacterial invasion of the claw. Lameness is probably manifested when the infection reaches the corium (coriitis), and becomes intractable once P3 becomes infected. In addition to the abrasion theory, others have suggested that excessive standing during processing and transport may lead to vascular disturbances that result in ischemic necrosis of the P3 bone (10). However, current research suggests that the lesion begins with white line separation and progresses into the claw versus moving from P3 outwards (8). Veterinary pathologists have also noted that there is anecdotal evidence to suggest that bovine viral diarrhea virus (BVDV) may be a risk factor for the disease.
This is a companion paper to a previous study in which the epidemiology of TTNS was described (9). The descriptive epidemiological study identified a number of potential risk factors for TTNS, which were investigated in the present prospective case-control study.
Materials and methods
Sample collection
Three veterinary practices in Alberta, Canada participated in this prospective case-control study, which extended over a 2-year period, 2012 to 2013 and 2013 to 2014. The cases and controls were identified during field postmortem examinations. The case definition for toe tip necrosis syndrome (TTNS) was evidence of white line separation and gross pathological involvement of P3. A control animal was the next animal to be necropsied, but without having gross pathology of P3. Control animals could be from a different cohort of cattle, but had to be similar in the number of days on feed (DOF), body weight, and age class (calf or yearling).
Figure 1 is a sagittal section of a claw taken from a control animal. Note the uniform thickness of the sole, particularly at the tip of the toe. Figure 2 shows the plantar surface of the hoof of an animal with TTNS; the toe is rounded and there is white line separation. Figure 3 illustrates an early case of TTNS with necrotic debris and organic material located along the white line. The tip of the toe is also rounded and the apex of P3 has a darkened mottled appearance.
Figure 1.
Sagittal section of a claw taken from a control animal.
Figure 2.
Plantar surface of a claw showing white line separation and excessive wear at the apex of the toe.
Figure 3.
Sagittal section of a toe taken from an animal with early stages of toe tip necrosis syndrome. Note the separation of the white line at the apex of the toe along with the necrotic material and organic debris located in this same area. The toe tip laminae have a frayed appearance and all visible laminae are congested.
Veterinary practitioners were instructed to collect the following samples from each case and control animal: hooves, heart, and skin. The hooves were stored under refrigeration, and then shipped by courier in an insulated cooler to researchers at the Western College of Veterinary Medicine (WCVM) where they were stored at −20°C until processing. The heart (papillary muscle and septum) and skin samples were preserved in 10% neutral buffered formalin.
Claw morphometric parameters
At the WCVM the field diagnosis was confirmed by sectioning the hoof samples with a band saw. A thin sagittal section, 1.0 to 2.0 cm thick, was sectioned and used for the morphometric analyses. The cut surfaces were swabbed for microbiological testing.
Digital photographs (Nikon CoolPix L810; Nikon Canada, Mississauga, Ontario) were taken of the thin sections and uploaded onto image processing software (Fiji continuous release open source software) (http://fiji.sc/Fiji), which was used to calculate 9 morphometric claw measurements: 5 sole and hoof wall thickness measurements and 4 hoof angle measurements (Figure 4). The angle measurements were used to assess for P3 rotation (11).
Figure 4.

Claw morphometric parameters: A — thickness of dorsal hoof capsule at midpoint between periople and apex; B — thickness of dorsal hoof wall capsule at solar corium; C — thickness of sole at the white line; D — thickness of sole at sole-heel junction; E — thickness of sole at plantar aspect of pedal bone; a = angle of outer dorsal hoof wall and sole; b = angle of inner dorsal hoof wall and sole; c = angle of dorsal aspect of pedal bone and sole; d = angle of dorsal and ventral pedal bone.
Bacterial culture
Aerobic and anaerobic culture swabs (BBL CultureSwab; Becton, Dickinson and Company; Sparks, Maryland, USA) were used to sample the sectioned surfaces of the pedal bone. Care was taken to avoid contaminating the cut surface prior to swabbing. These samples were submitted to Prairie Diagnostic Services (PDS; Saskatoon, Saskatchewan) and cultured on blood agar and MacConkey agar plates (Becton Dickinson and Company). Agar plates were incubated at 37°C in 5% CO2 and also under anaerobic conditions for a maximum of 48 h. Bacterial cultures were identified using standardized biochemical tests (12). The dilution streak method was used as a semi-quantitative method for bacterial growth, with growth being categorized as: “few,” 1+, 2+, 3+, and 4+ (13).
Immunohistochemistry for BVDV and Histophilus somni
Heart samples were submitted to PDS for immunohistochemical (IHC) testing for the presence of bovine viral diarrhea virus (BVDV) and Histophilus somni specific antigens. Tests for BVDV were also conducted on skin samples. These 2 antigens were chosen because BVDV is known to cause immunosuppression, whereas H. somni is associated with causing a vasculitis. Personnel from PDS were blinded with respect to which samples were cases versus controls.
Immunohistochemical (IHC) staining for BVDV was conducted using a commercial staining platform (Benchmark staining platform; Ventana Medical Systems, Tuscon, Arizona, USA) and an HRP-labelled multimer detection system (BMK Ultraview DAB Paraffin detection kit; Ventana Medical Systems). Enzymatic epitope retrieval was performed (Protease 3; Ventana Medical Systems) and the primary antibody (Mouse anti-BVD clone 15c5) was applied for 32 min at a dilution of 1:500 (14). Weak staining for BVDV in the heart, without a strong positive reaction from the skin was interpreted as an acute (transient) BVDV infection, while strong positive reactions from the skin and heart were interpreted as the animal being persistently infected (PI).
Immunohistochemical staining of the heart tissues for H. somni was conducted with an automated slide stainer (CodeOn Histomatic Stainer; Fisher Scientific, Edmonton, Alberta). Following pretreatment and enzymatic epitope retrieval, sections were incubated overnight at 4°C with a rabbit poly-clonal antibody (Vaccine and Infectious Disease Organization, Saskatoon, Saskatchewan) (15). Binding of the primary antibody was detected using biotinylated immunoglobulins (Vector Labs, Burlington, Ontario) and an avidin-biotin immunoperoxidase complex reagent (Vector Labs), with 3,3′-diaminobenzidine tetrahydrochloride (Electron Microscopy Science, Fort Washington, Pennsylvania, USA) as the chromogen.
Examination for vasculitis
The formalized heart samples were sectioned, embedded in paraffin, stained with hematoxylin and eosin (H&E), and a veterinary anatomic pathologist used light microscopy to examine the heart samples for vasculitis, perivasculitis, and periarteritis. This testing was undertaken because it was hypothesized that vasculitis of the corium may contribute to TTNS. Furthermore, because the corium is difficult to process for histological testing, it was assumed that if the heart had vasculitis, then there may also be a generalized vasculitis involving the corium.
Data analyses
Data were entered into a relational database (Microsoft Access v. 14; Microsoft Corporation, Redmond, Washington, USA) and exported to a commercial spreadsheet program (Microsoft Excel v. 12; Microsoft Corporation) for generating the descriptive statistics. The data were subsequently exported to a statistical program, SPSS (Version 20, SPSS, Chicago, Illinois, USA) for further analyses.
The Cohen’s kappa test statistic compared the inter-rater agreement between the practitioners’ diagnoses to those made by researchers at the WCVM. The positive predictive value (PPV) and negative predictive value (NPV) were calculated for the field diagnoses.
The microbiological results were provided as semi-quantitative data (no growth, “few,” 1+, 2+, 3+, and 4+) and then transformed into a dichotomous variable: “light growth” (few, 1+ and 2+) and “heavy growth” (3+ and 4+). Separate Chi-square analyses were used to determine whether the cases and controls were associated with a particular dichotomous outcome: light or heavy bacterial growth; positive or negative IHC results from the skin and heart for BVDV (acute and persistently infected); positive or negative IHC results for H. somni; and microscopic evidence of a cardiac vasculitis. Fisher’s exact test was used if 1 or more cells in the contingency table had an expected frequency of ≤ 5. The two-sample t-test was used to assess for differences between the cases and controls with respect to each of the 9 morphometric claw measurements. The level of significance for all statistical analyses was P < 0.05 (two-tailed).
Results
Three veterinary practices provided 222 submissions from 16 feedlots. Practitioners provided a diagnosis for 201 of the submissions, while WCVM researchers were able to make a definitive diagnosis on 199 submissions. There were 187 submissions that had a diagnosis from both practitioners and researchers (Table 1). Assuming the WCVM diagnoses to be the “gold standard,” the positive (PPV) and negative predictive values (NPV) were 86.4% and 95.2%, respectively. There was very good agreement between the practitioners and researchers with respect to categorizing the submissions as cases versus controls (κ = 0.81; P < 0.001).
Table 1.
Number (%) of samples identified as cases and controls by the field veterinarians versus the WCVM researchers
| WCVM | Total | ||
|---|---|---|---|
|
| |||
| Control (%) | Case (%) | ||
| Field | |||
| Control | 80 (95.2) | 4 (4.8) | 84 |
| Case | 14 (13.6) | 89 (86.4) | 103 |
| Total | 94 (50.3) | 93 (49.7) | 187 |
Claw morphometric measurements
Table 2 is a summary of the descriptive statistics for the claw morphometric measurements. The sole thickness at site “C” (apical white line) was thinner in the cases than the controls (P < 0.001), with a similar trend (P = 0.06) at site “D.” There were no differences in the mean angle measurements taken from the cases and controls.
Table 2.
Descriptive statistics for claw morphometric measurements
| Variable | Mean | Median | Standard deviation | Maximum | Minimum | P-value |
|---|---|---|---|---|---|---|
| Site A (mm) | ||||||
| Case (n = 64) | 4.65 | 4.60 | 1.12 | 8.20 | 2.56 | 0.62 |
| Control (n = 63) | 4.75 | 4.69 | 0.93 | 6.79 | 2.50 | — |
| Site B (mm) | ||||||
| Case (n = 62) | 5.04 | 5.24 | 1.32 | 8.71 | 2.50 | 0.31 |
| Control (n = 61) | 5.27 | 5.22 | 1.26 | 8.79 | 2.84 | — |
| Site C (mm) | ||||||
| Case (n = 64) | 3.74 | 3.66 | 1.14 | 7.42 | 1.14 | <0.001 |
| Control (n = 60) | 4.72 | 4.77 | 1.25 | 8.05 | 1.91 | — |
| Site D | ||||||
| Case (n = 63) | 3.44 | 3.28 | 0.78 | 5.69 | 2.11 | 0.06 |
| Control (n = 60) | 3.78 | 3.47 | 1.22 | 8.13 | 2.04 | — |
| Site E | ||||||
| Case (n = 63) | 5.71 | 5.71 | 1.22 | 8.65 | 3.65 | 0.76 |
| Control (n = 62) | 5.64 | 5.60 | 1.32 | 9.37 | 3.29 | — |
| Angle a (°) | ||||||
| Case (n = 64) | 56.86 | 56.43 | 4.75 | 68.83 | 44.39 | 0.14 |
| Control (n = 62) | 58.12 | 57.83 | 5.19 | 72.45 | 47.83 | — |
| Angle b (°) | ||||||
| Case (n = 64) | 58.26 | 57.54 | 5.03 | 69.97 | 43.77 | 0.54 |
| Control (n = 62) | 58.78 | 57.74 | 4.87 | 71.46 | 49.62 | — |
| Angle c (°) | ||||||
| Case (n = 63) | 58.20 | 58.54 | 5.99 | 72.92 | 39.76 | 0.88 |
| Control (n = 62) | 58.35 | 57.48 | 4.90 | 69.38 | 48.09 | — |
| Angle d (°) | ||||||
| Case (n = 64) | 52.10 | 51.71 | 6.46 | 69.96 | 36.77 | 0.10 |
| Control (n = 62) | 53.68 | 53.21 | 4.74 | 64.50 | 43.09 | — |
Bacterial testing
A total of 230 swabs (122 cases and 108 controls) were submitted for microbiological testing. The number of swabs exceeds the number of cases and controls because aerobic swabs were taken from all the cut claw surfaces, and a smaller subset of anaerobic culture swabs was also submitted. In addition, in some instances more than one claw was affected, and hence all affected claws were swabbed. Overall, across all isolates, the samples from cases were 2.5 times (95% CI: 1.4 to 4.3; P = 0.001) more likely than those from the controls to result in a heavy growth of bacteria.
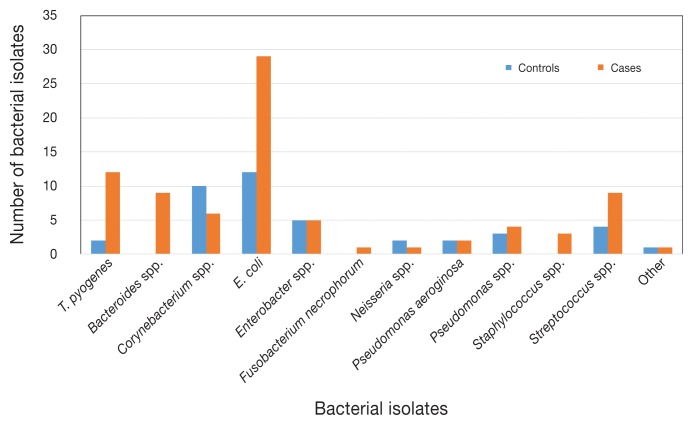
Figure 5 shows the number of heavy growth isolates, by bacterial species, recovered from the case and control claws. Cases were 5.0 times (95% CI: 1.5 to 8.6; P < 0.001) more likely than the controls to have a heavy growth of Escherichia coli and 7.3 times (95% CI: 1.5 to 69.3; P < 0.01) more likely to have a heavy growth of Trueperella pyogenes.
Figure 5.
Number of bacterial isolates, by species, that were cultured from the claws of the cases and controls and that yielded a “heavy” growth of bacteria (N = 123).
Immunohistochemistry and microscopic examination
Table 3 provides the breakdown of the number (percent) of cases and controls that were either acutely or persistently infected (PI) with BVDV. There were 188 heart samples tested for BVDV, but only 151 skin samples were collected and submitted. However, the 37 heart samples for which there was no corresponding skin sample all tested negative for BVDV. Cases were 4.4 times more likely to have an acute BVDV infection than were the controls (P < 0.001); however, there was no statistical difference with respect to being PI (Fisher Exact Test P = 0.12). There was a strong trend for the cases to have evidence of a cardiac vasculitis (P = 0.07). All BVDV positive animals, both acutely infected and PI animals, were 10.0 times (95% CI: 4.4 to 22.9; P < 0.001) more likely to have a vasculitis than were the BVDV negative animals.
Table 3.
Samples from the cases and controls that were either acutely or persistently infected (PI) with bovine viral diarrhea virus (BVDV)
| Test | Result | Case (%) | Control (%) | OR | 95% CI | P-value | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Lower | Upper | ||||||
| BVDV Acute | Positive | 43 (45.7) | 15 (16.0) | 4.4 | 2.2 | 8.8 | < 0.001 |
| (n = 188) | Negative | 51 (54.3) | 79 (84.0) | — | — | — | — |
| BVDV PI | Positive | 7 (8.6) | 2 (2.9) | 3.2 | 0.65 | 16.1 | 0.12 |
| (n = 151) | Negative | 74 (91.4) | 68 (97.1) | — | — | — | — |
| Vasculitis | Positive | 22 (23.4) | 13 (13.8) | 1.9 | 0.89 | 4.05 | 0.07 |
| (n = 188) | Negative | 72 (76.6) | 81 (86.2) | — | — | — | — |
Immunohistochemistry for H. somni was performed on the first 42 cardiac muscle samples; however, only 1/11 (9.1%) controls and 1/31 cases (3.2%) tested positive for H. somni (P = 0.46). Both positive animals had a vasculitis, but both were BVDV negative. Due to the very low prevalence of H. somni, further tests were not conducted.
Discussion
There was very good agreement between the practitioners’ diagnoses and that of the researchers; cases and controls were correctly identified 86% and 95% of the time, respectively. Some discrepancies in the diagnoses may be related to simple mis-labeling of the field samples by the field veterinarians. However, there were also instances in which claws identified as cases by the field veterinarians had evidence of white line separation, but no P3 involvement and hence they did not meet the case definition. In other instances, the foot lesions initially appeared similar to toe tip necrosis syndrome (TTNS) lesions; however, upon closer inspection there was no white line separation and pathological changes affecting P3 were attributed to a penetrating foreign body. There was, however, excellent agreement when it came to the classification of the controls; only 4 claws submitted as controls were in fact cases. The findings highlights that most cases and controls can be reliably diagnosed under field conditions.
There was no evidence from the hoof angle measurements of P3 rotation. However, the sole in the apical white line region was thinner in the TTNS cases than the controls, and there was a very strong trend for thinning in the area of the sole-heel junction. These findings are in agreement with previous reports of excessive wear occurring along the leading edge of the toes (2,3). The significance of this finding is that it supports the “abrasion theory,” wherein the disease is initiated when cattle develop excessive wear along the apical and abaxial regions of the white line. As a consequence, the integrity of the white line is reduced, allowing bacteria to penetrate the hoof capsule and infect the underlying structures.
Because the disease is assumed to be initiated by excessive wear along the apex of the toes, this part of the theory deserves further elaboration. The white line is composed of 3 parts or zones: outer, intermediate, and inner (16). This is salient because the horn tissue of each zone is produced by different laminae (dermis), resulting in horn with distinctly different structural properties. Therefore, the white line is not a homogenous tissue, but rather a composite of tissues. As a result, the white line is an inherent weakness in the hoof capsule, the “Achilles’ heel,” so to speak. Referring to Figure 1, the white line resides at the intersection of the sole and the hoof wall, and it is not too difficult to appreciate that abrasion of the solar horn at the apex of the toe would reduce the length of the white line, leading to a loss in its structural integrity. Once compromised, it is hypothesized that the repetitive cycling of weight-bearing forces, during the act of ambulation, would lead to the propagation of micro-fissures along the outer zone of the white line. These fissures would lead to further weakening along the white line, eventually culminating in complete separation of the outer zone from the intermediate zone.
Organic materials such as shards of macerated straw were occasionally found embedded within the white line of the claws obtained from TTNS animals. This begs the question as to how this material penetrated the white line. The most likely explanation is that the white line gapes open during the load-bearing phase of ambulation. This opening and closing action allows bacteria-laden organic debris to become impacted in the apex of the claw, explaining the heavy growth of E. coli and T. pyogenes. Significantly, T. pyogenes is a facultative anaerobe that thrives in both aerobic and anaerobic conditions and is associated with abscess formation. Presumably, following colonization, the bacteria migrate along the white line, breach the highly vascularized corium, and then infect the poorly vascularized P3 bone and soft tissues of the hoof.
The other finding of interest was the association between BVDV and TTNS, and hence some additional discussion is warranted with respect to how the testing was conducted as well as the interpretation of the results. The utility of immunohistochemical (IHC) testing for detecting persistently infected (PI) BVDV animals has been well-established (15,17,18). While the sensitivity of IHC testing for identifying PI animals is nearly 100%, its specificity is slightly lower because acutely (transiently) infected animals may appear indistinguishable from PI animals (19). This is less of an issue when testing live animals because retesting at a later date can distinguish false positives from the true positive PI animals (20). Unfortunately this option does not exist when testing dead animals. Therefore, in the current study, an animal was considered to be acutely (transiently) infected if BVDV antigen was present in the heart, but not in the skin. On the other hand, a strong reaction to the antigen in both the heart and skin samples was interpreted as the animal being PI. Heart tissues were chosen because BVDV is known to infect the heart tissue of experimentally (acutely infected) challenged calves (21,22). It was also convenient to use heart tissue because the field veterinarians were collecting heart tissue for H. somni testing and histopathological examination.
With respect to the BVDV findings, 2.9% of the control calves in the current study were PI animals, which is similar to a previous feedlot study wherein 2.5% of all dead animals were PI (23). Although 8.6% of the TTNS animals were PI, the prevalence of PI animals in the cases and controls was not statistically significant (P = 0.12). However, this deserves additional research since the lack of association may be related to a lack of statistical power.
While the association between PI status and TTNS was equivocal, TTNS cases were 4.4 times more likely to be acutely infected with BVDV than were the controls. Furthermore, the BVDV positive animals were 10 times more likely than the BVDV negative animals to have histological evidence of a cardiac vasculitis. This latter association is of particular interest because researchers have speculated that BVDV may evoke a vasculitis (24). If so, then an acute BVDV infection may have initiated a generalized vasculitis involving the highly vascularized corium (dermis). Nutrients and hormones diffuse from the highly vascularized dermis into the non-vascularized epidermis, and hence homeostasis of this diffusion gradient is critical to the development of healthy horn tissue (25,26). Conceivably, a BVDV-induced vasculitis of the dermal vasculature could disrupt normal perfusion, leading to a loss of integrity along the white line, contributing to white line separation. This pathogenesis would be similar to laminitis, which is also thought to arise from impaired perfusion of the corium (27).
An alternate explanation for the association between BVDV and TTNS may be related to immunosuppression. Bovine viral diarrhea virus is known to cause immunosuppression (28,29) and is considered a risk factor for feedlot diseases such as bovine respiratory disease (30). Therefore, bacterial invasion and colonization of the white line may be more likely to occur in immunocompromised animals, which may lead to a fulminant infection culminating in death. It must, however, also be recognized that association is not synonymous with causation. Perhaps the TTNS animals were more likely to be exposed in the hospital pens to other sick animals who were shedding BVDV. In this scenario, the transient BVDV infections may have developed after TTNS. Finally, although there was a strong association between acute BVDV infections and TTNS, BVDV was not a necessary cause for the disease; ~50% of TTNS cases were BVDV negative.
While TTNS has been described in dairy animals (4,6), this disease is primarily a disease of feedlot cattle. Care must be taken not to confuse TTNS with the commonly encountered thin sole toe ulcers (TSTU) of dairy cattle (31). Both TSTU and TTNS are associated with thinning of the soles, the lesions occur in the same general regions of the claw, both involve white line separation, and in advanced cases the lesions progress to abscess formation and P3 osteitis. However, in TTNS the separation of the white line appears to be occurring along the outer zone of the white line, whereas in TSTU the sole is separating away from the inner zone of the white line. At this point, it is difficult to determine whether the 2 diseases are distinct and have different etiologies and pathogeneses, or whether it is the same disease but manifested in 2 different manners.
If the 2 diseases are in fact related, then the different clinical presentations of the disease may be related to temperament, facilities, and cattle handling. Dairy cattle are relatively docile, but are also exposed to more concrete and wetter environmental conditions, risk factors that lead to increased wear over a period of weeks to months. In contrast, the more temperamental feedlot cattle are raised in earthen-floored pens and hence exposure of their hind feet to concrete and other hard abrasive surfaces occurs over a relatively short period at the time of transport, at auctions, and during processing at feedlots. Although feedlot cattle have less contact with abrasive surfaces than do dairy cattle, both their temperament and how they are handled may lead to abrasion of the apex of the toe. It is not uncommon to see feedlot cattle being overcrowded and agitated while being mustered in pens, cattle liners, and as they move through chutes. During these scenarios the cattle frequently push and strain against the animals ahead of them. If forces generated by the muscular hind limbs exceed the coefficient of friction, then the claws lose traction and the course abrasive flooring acts like a rasp to remove the horn tissue; torsional forces may also lead to tearing or separation along the white line. Therefore, the inciting event is probably short in duration, extremely forceful, and the abrasion of the solar horn is primarily localized to the apical region of the toe.
If the disease is left unattended in both dairy and feedlot cattle, then the endpoint is probably the same: toe abscesses, infection along the white line, P3 osteitis, and other sequalae such as tenosynovitis and embolic pneumonia. However, dairy animals are under constant surveillance and lame animals are often identified and treated early in the course of the disease. In contrast, TTNS occurs early in the feeding period and the disease clusters in the fall of the year when feedlots are extremely busy processing new arrivals and dealing with other diseases such as bovine respiratory disease. Therefore it is more challenging to identify cattle in the early stages of lameness, and even if they are identified, most operations are ill-equipped to perform a proper lameness examination. Lame animals would typically receive antimicrobials, but no other ancillary treatment such as debriding the ulcer and bandaging the foot. Animals that fail to respond to antimicrobial therapy either die naturally or are euthanized due to intractable lameness. As a result, it is this population of TTNS cases that feedlot veterinarians are most likely to see, which bear no resemblance to the early developing TSTU condition of dairy cattle.
Although the two diseases have a number of features in common, it would be presumptuous to conclude that they are in fact the same disease. While characterizing the white line separation as either separation along the outer as opposed to the inner zone may seem overly specific, this detail must not be overlooked, and hence more research is required to gain a better understanding of the pathogenesis of TTNS. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Terrell SP, Thomson DU, Reinhardt CD, Apley MD, Larson CK, Stackhouse-Lawson KR. Perception of lameness management, education, and effects on animal welfare of feedlot cattle by consulting nutritionists, veterinarians, and feedlot managers. Bovine Practitioner. 2014;48:53–60. [Google Scholar]
- 2.Sick FL, Bleeker CM, Mouw JK, Thompson WS. Toe abscesses in recently shipped feeder cattle. Vet Med Small Anim Clin. 1982;77:1385–1387. [Google Scholar]
- 3.Miskimins DW. Bovine Toe Abscesses. Proc 8th International Symposium on Disorders of the Ruminant Digit; Banff, Alberta. 1994. pp. 54–57. [Google Scholar]
- 4.Dewes HF. Transit-related lameness in a group of Jersey heifers. N Z Vet J. 1979;27:45. doi: 10.1080/00480169.1979.34598. [DOI] [PubMed] [Google Scholar]
- 5.Van Amstel SR, Shearer JK. Toe abscess: A serious cause of lameness in the US dairy industry. Proc. XI Interantional Symposium on Disorders of the Ruminant Digit; Parma, Italy. 2000. pp. 212–214. [Google Scholar]
- 6.Mason WA, Laven LJ, Laven RA. An outbreak of toe ulcers, sole ulcers and white line disease in a group of dairy heifers immediately after calving. N Z Vet J. 2012;60(1):76–81. doi: 10.1080/00480169.2011.634783. [DOI] [PubMed] [Google Scholar]
- 7.Paetsch CD, Jelinski MD. Toe-tip necrosis syndrome in feedlot cattle in western Canada. Proc 17th International Symposium and 9th International Conference on Lameness in Ruminants; Bristol, UK. 2013. pp. 152–153. [Google Scholar]
- 8.Gyan LA, Paetsch CD, Jelinski MD, Allen AL. The lesions of toe tip necrosis in southern Alberta feedlot cattle provide insight into the pathogenesis of the disease. Can Vet J. 2015;56:1134–1139. [PMC free article] [PubMed] [Google Scholar]
- 9.Jelinski MD, Fenton K, Perrett T, Paetsch CD. Epidemiology of toe tip necrosis syndrome (TNNS) involving North American feedlot cattle. Can Vet J. 2016;57:829–834. [PMC free article] [PubMed] [Google Scholar]
- 10.Greenough PR, editor. Bovine Laminitis and Lameness: A Hands-On Approach. 1st ed. Philadelphia, Pennsylvania: Saunders Elsevier; 2007. pp. 100–102. [Google Scholar]
- 11.Sloet van Oldruitenborgh-Oosterbaan MM. Laminitis in the horse: A review. Vet Quart. 1999;21:121–127. doi: 10.1080/01652176.1999.9695006. [DOI] [PubMed] [Google Scholar]
- 12.Quinn PJ, Markey B, Leonard FC, Fitzpatrick ES, Fanning S, Hartigan PJ. Veterinary Microbiology and Microbial Disease. 2nd ed. West Sussex, UK: Wiley-Blackwell; 2011. pp. 179–405. [Google Scholar]
- 13.Carter GR. Clinical Veterinary Microbiology. 9th ed. St. Louis, Missouri: Mosby; 1993. [Google Scholar]
- 14.Barry AL. Clinical specimen for microbiologic examination. In: Hoeprich P, editor. Infectious Disease: A Guide to the Understanding and Management of Infectious Process. New York, New York: Harper and Ron Publishing; 1972. pp. 103–107. [Google Scholar]
- 15.Haines DM, Moline KM, Sargent RA, Campbell JR, Myers DJ, Doig PA. Immunohistochemical study of Hemophilus somnus, Mycoplasma bovis, Mannheimia hemolytica, and bovine viral diarrhea virus in death losses due to myocarditis in feedlot cattle. Can Vet J. 2004;45:231–234. [PMC free article] [PubMed] [Google Scholar]
- 16.Shearer JK, Plummer PJ, Schleining JA. Perspectives on the treatment of claw lesions in cattle. Vet Med Res Reports. 2015;6:273–292. doi: 10.2147/VMRR.S62071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Njaa BL, Clark EG, Janzen E, Ellis JA, Haines DM. Diagnosis of persistent bovine viral diarrhea virus infection by immunohistochemical staining of formalin-fixed skin biopsy specimens. J Vet Diagn Invest. 2000;12:393–399. doi: 10.1177/104063870001200501. [DOI] [PubMed] [Google Scholar]
- 18.Brodersen BW. Immunohistochemistry used as a screening method for persistent bovine viral diarrhea virus infection. Vet Clin Food Anim. 2004;20:85–93. doi: 10.1016/j.cvfa.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Dubovi EJ. Laboratory diagnosis of bovine viral diarrhea virus. Biologicals. 2013;41:8–13. doi: 10.1016/j.biologicals.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Cornish TE, van Olphen AL, Cavender JL, et al. Comparison of ear notch immunohistochemistry, ear notch antigen-capture ELISA, and buffy coat virus isolation for detection of calves persistently infected with bovine viral diarrhea. J Vet Diagn Invest. 2005;17:110–117. doi: 10.1177/104063870501700203. [DOI] [PubMed] [Google Scholar]
- 21.Spagnuolo-Weaver M, Allan GM, Kennedy S, Foster JC, Adair BM. Distribution of cytopathic and noncytopathic bovine viral diarrhea virus antigens in tissues of calves following acute experimental infection. J Vet Diagn Invest. 1997;9:287–297. doi: 10.1177/104063879700900310. [DOI] [PubMed] [Google Scholar]
- 22.Liebler-Tenorio EM, Ridpath JF, Neill JD. Distribution of viral antigen and development of lesions after experimental infection with highly virulent bovine viral diarrhea virus type 2 in calves. Am J Vet Res. 2002;63:1575–1584. doi: 10.2460/ajvr.2002.63.1575. [DOI] [PubMed] [Google Scholar]
- 23.Loneragan GH, Thomson DU, Montgomery DL, Mason GL, Larson RL. Prevalence, outcome, and health consequences associated with persistent infection with bovine viral diarrhea virus in feedlot cattle. J Am Vet Med Assoc. 2005;226:595–601. doi: 10.2460/javma.2005.226.595. [DOI] [PubMed] [Google Scholar]
- 24.Désilets A, Montpetit C, Trépanier H, Archambault D. BVDV Infection: Five possible pathogenesis mechanisms related to the development of Type III hypersensitivity lesions in BVDV infected animals. Proceedings International Symposium, Bovine Viral Diarrhea Virus: A 50 year review; Cornell, New York: Cornell University; 1996. p. 192. [Google Scholar]
- 25.Tomlinson DJ, Mülling CH, Fakler TM. Invited review: Formation of keratins in the bovine claw: Roles of hormones, minerals, and vitamins in functional claw integrity. J Dairy Sci. 2004;87:797–809. doi: 10.3168/jds.S0022-0302(04)73223-3. [DOI] [PubMed] [Google Scholar]
- 26.Mülling CH, Bragulla HH, Reese S, Budras K-D, Steinberg W. How structures in bovine hoof epidermis are influenced by nutritional factors. Anat Histol Embryol. 1999;28:103–108. doi: 10.1046/j.1439-0264.1999.00180.x. [DOI] [PubMed] [Google Scholar]
- 27.Hendry KAK, MacCallum AJ, Knight CH, Wilde CJ. Review article: Laminitis in the dairy cow: A cell biological approach. J Dairy Res. 1997;64:475–486. [PubMed] [Google Scholar]
- 28.Chase CCL. The impact of BVDV infection on adaptive immunity. Biologicals. 2013;41:52–60. doi: 10.1016/j.biologicals.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 29.Lanyon SR, Hill FI, Reichel MP, Brownlie J. Bovine viral diarrhea: Pathogenesis and diagnosis. Vet J. 2014;199:201–209. doi: 10.1016/j.tvjl.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 30.Ridpath J. The contribution of infections with bovine viral diarrhea viruses to bovine respiratory disease. Vet Clin Food Anim. 2010;26:335–348. doi: 10.1016/j.cvfa.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 31.Shearer JK, van Amstel SR. Toe lesions in dairy cattle. Proceedings 46th Florida Dairy Production Conference; Gainsville, Florida. 2009. pp. 47–55. [Google Scholar]