Abstract
Objective
Alterations in the normal developmental trajectory of amygdala resting state-functional connectivity (rs-FC) have been associated with atypical emotional processes and psychopathology. Little is known, however, regarding amygdala rs-FC at birth or its relevance to outcomes. This study examined amygdala rs-FC in healthy, full-term (FT) infants and in very preterm (VPT) infants and tested whether variability of neonatal amygdala rs-FC predicted internalizing symptoms at age 2 years.
Method
Resting state-fMRI data were obtained shortly after birth from 65 FT infants (gestational age [GA] ≥36 weeks) and 57 VPT infants (GA <30 weeks) at term-equivalent. Voxel-wise correlation analyses were performed using individual-specific bilateral amygdala regions of interest. Total internalizing symptoms and the behavioral inhibition, depression/withdrawal, general anxiety, and separation distress subdomains were assessed in a subset (n=44) at age 2 years using the Infant Toddler Social Emotional Assessment.
Results
In FT and VPT infants, the amygdala demonstrated positive correlations with subcortical and limbic structures and negative correlations with cortical regions, though magnitudes were decreased in VPT infants. Neonatal amygdala rs-FC predicted internalizing symptoms at age 2 with regional specificity consistent with known pathophysiology in older populations: connectivity with the anterior insula related to depressive symptoms, with the dorsal anterior cingulate related to generalized anxiety, and with the medial prefrontal cortex related to behavioral inhibition.
Conclusion
Amygdala rs-FC is well-established in neonates. Variability in regional neonatal amygdala rs-FC predicted internalizing symptoms at 2 years, suggesting that risk for internalizing symptoms may be established in neonatal amygdala functional connectivity patterns.
Keywords: infant, internalizing, amygdala, functional connectivity
INTRODUCTION
The amygdala plays a critical role in the expression and processing of emotion and in determining stimuli’s emotional significance.1–3 Amygdala activity increases in response to emotional stimuli in healthy children and adults,4,5 and variations in activation are associated with affective disorders at both ages.6,7 Amygdala activation is putatively regulated through connections with numerous subcortical and cortical structures,2,8,9 and amygdala structural connections undergo substantial modification during the first years of life.10–13 Critical unresolved issues addressed in this study include defining the normative patterns of neonatal amygdala functional connectivity and the relationship between neonatal amygdala functional connectivity and risk for development of early childhood affective symptoms.
Resting state functional magnetic resonance imaging (rs-fMRI) has been utilized to investigate functional connectivity (rs-FC) in the developing brain.14,15 Infants and children demonstrate rs-FC patterns, including those involving the amygdala,14,16,17 that evolve with age towards those of adults.18 One relationship that has been well-characterized during early development is between the amygdala and medial prefrontal cortex (mPFC).19,20 The mPFC is an important regulator of amygdala activation, 2,8,9 and variation in rs-FC measures between these regions in older children and adults has been linked to anxiety,21–23 behavioral inhibition,24 and harm avoidance.25 Additionally, altered rs-FC between the amygdala and other cortical and subcortical regions was reported in 6-month-old infants at high risk for developing internalizing symptoms17 and is associated with infant fear at age 6 months,26 atypical childhood emotional and cognitive processes,16,27 and adult psychiatric disorders.28,29
While some preliminary evidence exists linking amygdala rs-FC to internalizing symptoms even in infancy, there is almost no information about factors that might moderate the nature of such relationships. One potential modifying factor is prematurity. Core features of the “preterm behavioral phenotype” include social difficulties and internalizing symptoms,30 and very preterm children (VPT; gestational age [GA] <32 weeks) have increased rates of behavioral inhibition and introversion.31,32 Premature infants provide a unique opportunity to study variability in amygdala rs-FC relative to both typical development and emergence of affective symptoms given prior evidence of altered rs-FC in preterm infants in other regions.14 Prior studies have linked prematurity-associated neonatal brain abnormalities to subsequent social-emotional development.33–35 However, it is unclear if the etiology of internalizing symptoms for preterm children differs from that leading to internalizing symptoms in full-term (FT) children.
We investigated patterns of amygdala rs-FC in healthy, FT infants and VPT infants at term-equivalent postmenstrual age (PMA) and determined whether variations in neonatal connectivity predicted internalizing symptoms at age 2 years. The goals of this study were three-fold: (1) characterize regions demonstrating synchronous, spontaneous neuronal activity with the amygdala during infancy (i.e., rs-FC); (2) assess whether variation in amygdala rs-FC relates to development of early-onset internalizing symptoms; and (3) evaluate whether prematurity alters amygdala rs-FC and/or modifies relationships between amygdala rs-FC and early-onset internalizing symptoms.
METHOD
Participants
VPT infants were recruited from St. Louis Children’s Hospital Neonatal Intensive Care Unit. FT infants (GA ≥36 weeks) were recruited from the adjoining mother-baby unit at Barnes-Jewish Hospital from a contemporaneous, companion prospective study evaluating the association between electronic fetal heart rate recordings and neonatal brain development.36 FT infants had no recorded history of in utero illicit substance exposure nor evidence of acidosis (pH <7.20) on cord blood gas. Infants with chromosomal abnormalities or suspected/proven congenital infection were excluded. Parental written informed consent was obtained prior to participation. The study was approved by the Washington University Human Studies Committee.
Anatomic MR images were reviewed by a neuroradiologist (J.S.S.) and pediatric neurologist (C.D.S.). Exclusion criteria included: grade III–IV intraventricular hemorrhage, cystic periventricular leukomalacia, moderate–severe cerebellar hemorrhage, and/or cortical/deep nuclear gray matter lesions.37
Data Acquisition
FT infants underwent MRI within the first four days of life and were scanned at a mean PMA of 39.4 weeks (±1.2 weeks). VPT infants underwent MRI at term equivalent PMA (38.0 weeks, ±1.5 weeks). All infants were imaged without sedation during sleep or while resting quietly38 when clinically stable to travel to the MRI scanner. Identical scanning procedures were used for all infants. All imaging was performed on a Siemens Trio 3T scanner (Erlangen, Germany) utilizing an infant-specific, quadrature head coil (Advanced Imaging Research, Cleveland, OH). Structural images were collected using a T2-weighted sequence (TR 8600ms; TE 161ms; voxel size 1×1×1mm). rs-fMRI data were collected utilizing a gradient echo, echo-planar-image (EPI) sequence sensitized to T2* blood oxygen level dependent (BOLD) contrast (TR 2910ms; TE 28ms; voxel size 2.4×2.4×2.4mm; flip angle 90°; FOV 151 mm; and matrix size was of 64 x 64). Each fMRI run included 200 volumes (frames). A minimum of one run (9.6 minutes) was obtained in each infant, with additional runs acquired in a subset of participants depending upon tolerance.
Data Analysis
rs-fMRI Preprocessing
rs-fMRI data were preprocessed as previously described utilizing in-house software (ftp://imaging.wustl.edu/pub/raichlab/4dfp_tools/).39 Magnetization inhomogeneity-related distortions were corrected using a mean field map technique.40 Atlas transformation was computed using infant templates. Volumetric time series in adult Talairach atlas space (3×3×3 mm voxels) were generated, combining motion correction and atlas transformation in a single resampling step. Additional preprocessing included regression of nuisance waveforms derived from rigid body motion correction, cerebrospinal fluid, and white matter regions, plus whole brain global signal. The data were low-pass filtered and spatially smoothed (see Supplementary Methods, available online).
Frames affected by sudden change in head position (volume-to-volume head displacement ≥0.5 mm) or root-mean-squared BOLD signal intensity change (DVARS ≥0.5%) were excluded from the rs-fMRI computations (“scrubbing”).41 Data passing more rigorous censoring criteria (volume-to-volume head displacement ≥0.25 mm or DVARS ≥0.3%) were also analyzed (see Figure S1, available online). A minimum of 5 minutes of rs-fMRI data, excluding censored frames, was required for inclusion in the analysis. FT (n=65) infants provided an average of 156 frames (±42, range 100–357 frames, ~7.8 minutes, mean FD .17mm), with 36% of acquired frames censored. VPT infants (n=57) provided an average of 182 frames (±53, range 102–381 frames, ~9.1 min, mean FD .15), with 24% of acquired frames censored.
Amygdala Regions of Interest
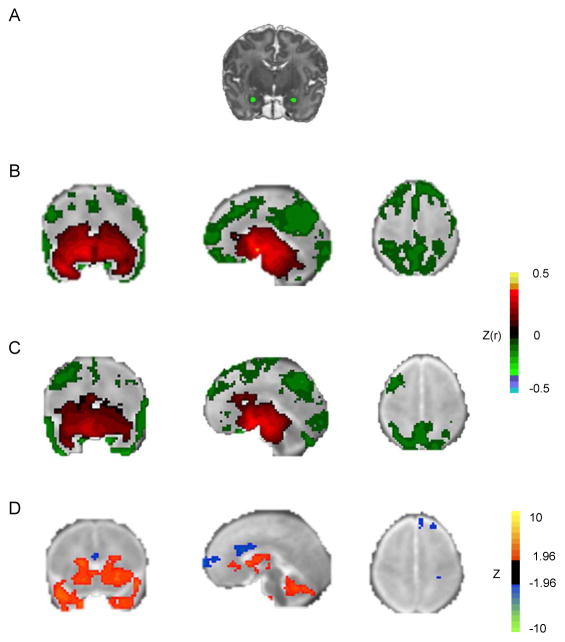
Individualized bilateral amygdala regions of interest (ROIs) were created for each participant (Figure 1A). T2-weighted images were loaded into ANALYZE version 10.0 (Mayo Foundation, Rochester, MN). The amygdala was identified in the coronal plane using adjacent landmarks, including the temporal horn and hippocampus. The ROI was then manually drawn in 1×1×1 mm voxel space; ROIs were cross-checked in sagittal and axial orientations. These ROIs were reviewed and manually adjusted as needed by a neuroradiologist (J.S.S.) and resampled to 3×3×3 mm voxel atlas space for extraction of the BOLD timeseries by averaging over all included voxels. There were no significant differences in ROI sizes between groups (left amygdala ROI: term 5.6 voxels and preterm 5.9 voxels, p=.60; right amygdala ROI: term 5.7 voxels and preterm 6.8 voxels, p=.14). Peak coordinates were extracted (Talairach x, y, z: left amygdala −23, −7, −18; right amygdala 24, −8, −14).
Figure 1.
Amygdala functional connectivity in full-term and preterm neonates. Note: A) Bilateral amygdala regions of interest (ROIs) in representative subject, overlaid on subject-specific T2-weighted image. B) Group mean amygdala resting state-functional connectivity (rs-FC) correlation maps for full-term infants. C) Group mean amygdala rs-FC correlation maps for very preterm infants. Both results obtained using left amygdala ROI. Group-level significance estimated using individual Fisher z transformed correlation maps and computing voxelwise one-sample t-test (comparing z[r] against zero across the group), corrected for multiple comparisons. Results overlaid on neonatal atlas target. D) Z scores demonstrating group differences in left amygdala rs-FC obtained from voxelwise t-test. Blue voxels denote areas with greater negative correlations, and orange voxels denote areas with greater positive correlations in term infants. Results thresholded using |Z|>2.25 and 53 contiguous voxels achieving whole-brain false positive rate of .05.
Functional Connectivity Analyses
Amygdala rs-FC was investigated using a whole-brain voxel-wise approach. Using individual-specific bilateral amygdala ROIs for each participant, correlation maps were computed using Pearson correlations. Correlation coefficients were Fisher z-transformed (generating z[r] correlation maps).
Internalizing Symptoms
Forty-one of the 57 VPT participants were enrolled in our longitudinal study, which included age 2 neurodevelopmental follow-up. Of the 41, 9 were lost to follow-up, four had incomplete data, and one died. Twenty-five FT children with high-quality imaging data were selected from the companion study for age 2 assessments to closely match the VPT cohort in sex and socioeconomic status (SES). Those with low motion rs-fMRI data were included in this analysis (VPT n=27; FT n=17). Age 2 assessments included the Bayley Scales of Infant and Toddler Development, 3rd edition (Bayley-III)43 to assess cognitive, language, and motor skills and the Infant Toddler Social Emotional Assessment (ITSEA)44, a 166-item parent-report measure assessing four social-emotional domains (internalizing, externalizing, dysregulation, and competence). The ITSEA has good psychometric properties44,45 and has been shown to predict anxiety symptoms in later childhood in FT and VPT children.46,47 This analysis focused on the internalizing domain, which contains subscales assessing behavioral inhibition, depression/withdrawal, general anxiety, and separation distress.
Maternal Factors
Maternal factors with reported associations with offspring internalizing symptoms, including social disadvantage48 and a maternal history of affective symptoms,49 were examined as potential confounds. Maternal social risk was defined by a composite index modeled after prior studies.50,51 At 2-year follow-up, the following five characteristics were coded as 1 (present) or 0 (absent) and summed to yield an index: 1) Not a high school graduate, 2) African-American, 3) public insurance at birth, 4) gave birth at age ≤18, and 5) single-parent household. When data were missing, the mean of the remaining components was substituted for the missing one(s) in calculating the sum as done previously.51 Maternal anxiety and depressive symptoms were assessed concurrently at the age 2 evaluation via the Hospital Anxiety and Depression scale (HADS) anxiety subscale52 and the Beck Depression Inventory (BDI)-II, respectively. HADS and BDI scores were dichotomized into none/mild or moderate/severe based on published cutoffs. Maternal report of history of depression and anxiety disorders was assessed via the Family History Assessment Module.53 Mothers who reported a past history of depression or anxiety symptoms (n=5) and/or scored in the moderate/severe range (n=20) on the HADS or BDI were classified as having a history of affective symptoms.
Statistical Analyses
Differences in demographic and outcome factors were analyzed using t-tests for continuous variables and chi-square analysis for categorical variables. Relationships between maternal HADS and BDI scores and infant internalizing symptoms were tested via correlations. Analyses were conducted with SPSS version 23 (Armonk, NY, USA). Whole-brain voxel-wise analyses were performed using in-house software (www.nil.wustl.edu/labs/fidl/). Voxel-wise one sample t-tests evaluated amygdala rs-FC across the entire brain. We focused on the left amygdala, because left and right amygdala rs-FC is similar18, and left amygdala-mPFC rs-FC has been more consistently related to affective symptoms in young children.17,23 Left amygdala rs-FC results were compared between FT and VPT participants using voxel-wise two-tailed two-sample t-tests (assuming unequal variance). All whole-brain analyses were corrected for multiple comparisons to achieve a whole-brain false positive rate of .05 (see Supplementary Methods, available online).
To examine the relationship between neonatal amygdala rs-FC and outcome at age 2 years, we first computed whole-brain correlation maps between rs-FC with the left amygdala and the ITSEA internalizing domain. Subsequent whole brain regression models evaluated the potential impact of moderating and confounding factors on the link between amygdala rs-FC and internalizing symptoms by adding prematurity, child sex, social risk, and maternal history of affective symptoms along with their interaction with internalizing symptoms to separate models. Whole brain regression models between left amygdala rs-FC and each ITSEA internalizing domain subscale were computed. Initially, correlations were computed separately for each subscale to minimize over-modeling. To test specificity, a post hoc analysis examined the relationships between left amygdala rs-FC and all four internalizing subscales in a single linear model to confirm unique relationships to particular subscales.
RESULTS
Participant Characteristics
Demographic characteristics of the FT and VPT infants are presented in Table 1. As expected, FT and VPT infants differed in GA and birth weight, but not other demographics or maternal variables. Likewise, there were no differences between children with and without outcome data at 2 years in sex, GA, birth weight, or PMA at MRI scan. However, a higher proportion of Caucasians had outcome data at 2 years relative to African-Americans (chi-square=11.1, p=.01). Because Caucasians and African-Americans did not differ in outcomes of interest (amygdala rs-FC and ITSEA scores), subsequent analyses were not adjusted for race. No differences were detected in internalizing symptoms based upon prematurity. There were also no significant associations between child ITSEA internalizing scores and maternal HADS scores (r=.16, p=.3) or BDI scores (r=0.05, p=.77).
Table 1.
Group Characteristics
| Neonatal Characteristic | Term n=65 | Preterm n=57 |
|---|---|---|
| Birth weight, g, mean (SD) | 3302.8 (430) | 890.9 (232.2)*** |
| GA, weeks, mean (SD) | 39.2 (1.2) | 26.4 (1.7)*** |
| Male, n (%) | 27 (42) | 24 (42) |
| Ethnicity, n (%) | ||
| African American | 43 (66) | 30 (53) |
| Caucasian | 19 (29) | 24 (42) |
| Asian | 0 (0) | 3 (5) |
| Hispanic | 3 (5) | 0 (0) |
| Intraventricular hemorrhage, n (%) | ||
| Grade I | - | 9 (16) |
| Grade II | - | 7 (12) |
| Postnatal steroids, n (%) | - | 15 (26) |
| Sepsis, n (%) | - | 13 (28) |
| Duration of ventilation, hours, median (IQR) | - | 72 (24–504) |
| Age 2 Characteristic | Term n=17 | Preterm n=27 |
| Birth weight, g, mean (SD) | 3421.4 (406.7) | 920.1 (255.8)*** |
| GA, weeks, mean (SD) | 39.6 (0.9) | 26.7 (1.8)*** |
| Male, n (%) | 8 (47) | 13 (48) |
| Ethnicity, n (%) | ||
| African American | 11 (65) | 11 (41) |
| Caucasian | 6 (35) | 12 (44) |
| Asian | 0 (0) | 3 (11) |
| Hispanic | 0 (0) | 1 (4) |
| Intraventricular hemorrhage, n (%) | ||
| Grade I | - | 3 (11) |
| Grade II | - | 4 (15) |
| Postnatal steroids, n (%) | - | 6 (22) |
| Sepsis, n (%) | - | 6 (22) |
| Duration of ventilation, hours (median, IQR) | - | 48 (24–432) |
| Maternal social risk, no. of factors present, mean (SD) | 1.8 (1.4) | 1.3 (1.4) |
| % with ≥3 social risk factors | 41 | 30 |
| Medicaid), n (%) | 11 (65) | 14 (52) |
| ITSEA | ||
| Internalizing T-score, mean (SD) | 53.4 (9.4) | 48.9 (11.5) |
| Inhibition to novelty, mean (SD) | 1.0 (.46) | 1.0 (.65) |
| Depression withdrawal, mean (SD) | 0.13 (.18) | 0.07 (.10) |
| General anxiety, mean (SD) | 0.42( .31) | 0.26 (.22) |
| Separation distress, mean (SD) | 0.96 (.38) | 0.78 (.42) |
| Externalizing T-score, mean (SD) | 58.6 (16.5) | 52.5 (13.0) |
| Dysregulation T-score, mean (SD) | 50.2 (16.7) | 46.4 (12.8) |
| Competence T-score, mean (SD) | 48.7 (16.1) | 44.3 (12.8) |
| Bayley-III | ||
| Cognitive composite, mean (SD) | 93.5 (14.3) | 89.3 (9.5) |
| Language composite, mean (SD) | 103.7 (18.7) | 91.4 (12.2)* |
| Motor composite, mean (SD) | 101.8 (15.4) | 87.8 (8.9)** |
| Maternal affective symptoms | ||
| HADS anxiety scale, mean (SD) | 7.8 (4.5) | 9.0 (5.1) |
| Beck Depression Inventory, mean (SD) | 8.9 (8.2) | 5.8 (8.3) |
Note: GA = gestational age; HADS = Hospital Anxiety and Depression Scale; IQR = interquartile range; ITSEA = The Infant Toddler Social Emotional Assessment.
p<.001,
p<.01,
p<.05
Amygdala Functional Connectivity in FT and VPT Infants
The left amygdala demonstrated widespread positive correlations with subcortical and temporal regions and negative correlations with prefrontal, parietal, and occipital regions (Figure 1B and Table S1, available online). Similar patterns in amygdala rs-FC were obtained using even more stringent motion correction procedures (see Figure S1, available online), using an identical amount of data for each participant (see Figure S2, available online) or when using a right amygdala seed (see Figure S3, available online). VPT infants displayed largely similar topography of amygdala rs-FC to FT infants, though FT infants displayed significantly greater positive correlations between the amygdala and subcortical regions (see Table S2, available online) and more negative correlations in dorsal frontal and medial prefrontal regions (Figure 1D).
Neonatal Amygdala rs-FC and Age 2 Child Internalizing Symptoms
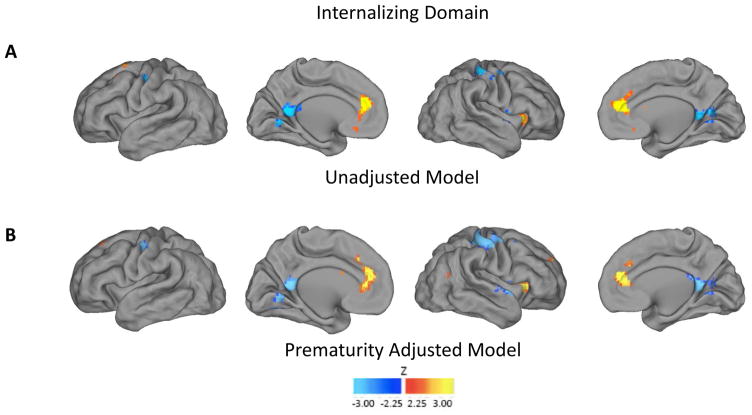
Whole-brain voxel-wise maps in which neonatal left amygdala rs-FC predicted internalizing symptoms at age 2 years, as assessed by the ITSEA internalizing domain, are depicted for all children in Figure 2A and for VPT children separately in Figure S4, available online. Neonatal rs-FC between the left amygdala and several regions was positively associated with age 2 internalizing domain scores (Table S3, available online), including the medial prefrontal cortex, right anterior insula, superior frontal cortex, caudate, and cerebellum. Neonatal rs-FC between the left amygdala and posterior cingulate cortex and pre- and postcentral gyri was negatively associated with internalizing domain scores at age 2. These relationships were largely maintained when prematurity and the interaction between prematurity and internalizing symptoms were added to the model (Figure 2B). The (1) regions in which amygdala rs-FC differed between FT and VPT infants and (2) regions in which amygdala rs-FC was predictive of age 2 internalizing domain scores were largely non-overlapping (Figure S5, available online). Further, there were few regions that differentially predicted internalizing symptoms in the VPT vs. FT groups (i.e., significant interactions), and these regions were almost entirely non-overlapping with the regions related to internalizing symptoms in the entire group (Figure S6 and Table S4, available online). Post hoc analyses on ROIs detected in these analyses revealed that amygdala rs-FC with these ROIs was similar for both FT and VPT infants (Figure S7, available online). Similarly, relationships remained significant after adjusting for the potential confounding effects of sex, maternal social risk, and history of maternal affective in separate models (Figure S8, available online). Similar relationships were not detected between right amygdala rs-FC and internalizing symptoms when correcting for multiple comparisons (Figure S9, available online).
Figure 2.
Relationship between internalizing scores and functional connectivity of left amygdala. Note: Whole-brain analyses correlating internalizing domain with left amygdala resting state-functional connectivity (rs-FC). A) Unadjusted model demonstrating higher total internalizing domain scores were positively correlated with rs-FC between the left amygdala and the medial prefrontal cortex, right anterior insula, and superior frontal cortex. B) Model adjusted for prematurity and interaction between prematurity and internalizing scores. Note that covariate inclusion had limited impact on results. Results thresholded using |Z|>2.25 and 53 contiguous voxels, achieving whole-brain false positive rate of .05.
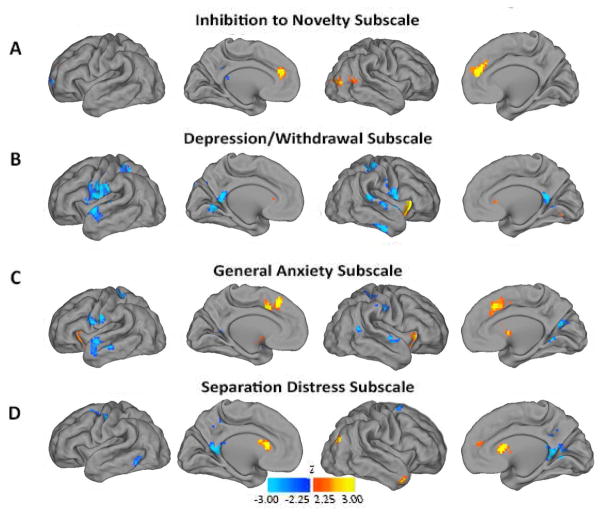
Follow-up analyses examined associations between neonatal left amygdala rs-FC and each of the subscales within the ITSEA internalizing domain at age 2 years. These subscales were only weakly or modestly correlated with each other (Table 2). Consistent with known pathophysiology in older age groups, neonatal left amygdala rs-FC with the following regions was positively related to specific age 2 ITSEA subscale scores (Figure 3): mPFC and higher inhibition to novelty; right anterior insula and higher depression/withdrawal and general anxiety; and dorsal anterior cingulate and higher general anxiety. Neonatal left amygdala rs-FC with the bilateral posterior cingulate cortices was negatively related to depression/withdrawal and separation distress; and with the anterior temporal lobe was negatively related to depression/withdrawal and general anxiety. A follow-up analysis including all four ITSEA internalizing subscale domains in a single model recapitulated the results in Figure 3, consistent with subscale specificity (Figure S10, available online).
Table 2.
Correlations Between Total Internalizing and Subscale Scores
| ITSEA internalizing domain and subscales | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 1. Inhibition to novelty | ||||
| 2. Depression/withdrawal | .10 | |||
| 3. General anxiety | .03 | .42** | ||
| 4. Separation distress | .16 | .41** | .14 |
Note: ITSEA = The Infant-Toddler Social and Emotional Assessment
p<.01
Figure 3.
Relationship between internalizing subscale scores and left amygdala functional connectivity. Note: Whole-brain analyses correlating left amygdala resting state-functional connectivity with internalizing subscale scores. A) Inhibition to novelty scores; B) Depression/withdrawal scores; C) General anxiety scores; D) Separation distress scores. Warm colors denote positive correlations, and cool colors denote negative correlations All results thresholded using |Z|>2.25 and 53 contiguous voxels, achieving whole-brain false positive rate of .05.
DISCUSSION
Summary of Findings
In healthy FT infants, amygdala rs-FC patterns were similar to those of older children and adults – positive correlations with subcortical and limbic regions and negative correlations with many cortical regions. VPT infants demonstrated similar results but with decreased magnitude. Neonatal amygdala rs-FC with regions implicated in the pathophysiology of internalizing disorders such as the mPFC, posterior cinculate cortex (PCC), and anterior insula predicted internalizing symptoms at age 2 years in all infants. Additionally, there was regional specificity of amygdala rs-FC with specific internalizing subscales in a manner consistent with pathophysiology in older age groups. Overall, results demonstrate that amygdala rs-FC is well-established in neonates, diminished in VPT infants, and predictive of internalizing symptoms in the first years of life.
Comparison to Older Populations
Amygdala functional connections in FT neonates were similar to those reported in older infants, children, and adults. Specifically, the amygdala was positively correlated with the insula, thalamus, striatum, and cerebellum,17–20 regions that assign emotional salience to stimuli;1,54,55 and negatively correlated with areas across the frontal and parietal cortices, regions involved in effortful control of affective states.18,20
Role of Neonatal Amygdala Functional Connectivity in Internalizing Symptoms
Most intriguingly, the current study found that variability in neonatal rs-FC between the left amygdala and the mPFC, PCC, and right anterior insula predicted greater internalizing symptoms at age 2 years. Functional connectivity between the amygdala and these regions has been implicated in the pathophysiology of anxiety disorders and depression in older children and adults.16,17,21,22 This study critically extends these prior findings to neonates. The regional specificity of neonatal left amygdala rs-FC with specific symptom domains at 2 years is strikingly similar to associations detected in older populations, suggesting rs-FC patterns during infancy are relevant to later development of psychopathology. For example, left amygdala-right anterior insula rs-FC predicted depression/withdrawal and general anxiety symptoms at age 2 years, consistent with rs-FC/symptom relationships detected in older infants, children, and adults.16,17,22,23,56,57 Similarly, age 2 depression/withdrawal symptoms related to neonatal left amygdala-PCC rs-FC, consistent with prior work in older samples.58 The relationship between amygdala-dorsal anterior cingulate connectivity and later general anxiety symptoms parallels similar findings in older adolescents with generalized anxiety disorder.22 Finally, left amygdala-mPFC rs-FC was associated with behavioral inhibition. The mPFC is frequently implicated in anxiety disorders,22,59 and behavioral inhibition is one of the most potent known risk factors for development of anxiety disorders.60,61
Prematurity and Amygdala Functional Connectivity
FT and VPT infants had similar patterns of amygdala rs-FC, though correlations were decreased in magnitude in VPT participants, consistent with prior investigations.14,62 Decreased correlation strength between the amygdala and thalamus (Figure S3, available online) may play a role in the decreased rs-FC between the amygdala and cortex through the amygdala’s relationship with the mediodorsal thalamic nucleus, which has widespread cortical projections.2 Nevertheless, the cortical regions where the VPT infants had decreased magnitude in left amygdala rs-FC were generally not the regions where left amygdala rs-FC predicted internalizing symptoms. Thus, our results suggest that variability in regional amygdala rs-FC underlies the variability in early childhood internalizing symptoms for both FT and VPT children. Further study is needed to determine if neonatal differences in amygdala rs-FC relate to internalizing symptoms at older ages or to other domains of social-emotional development for which VPT children are at increased risk.
The sample size of 122 infants for the amygdala rs-FC analysis is somewhat modest compared to investigations in older populations, but larger than most neonatal investigations.14,15,62,63 Our sample size was reduced in part due to implementation of strict criteria regarding participant motion during MRI data acquisition. Nevertheless, this method of motion correction is considered best practice in the field to ensure reliable and robust results and reduce spurious findings. Additionally, the small size of the neonatal amygdala limits the ability to assess distinct amygdala subdivisions that may demonstrate differing patterns,1,2 though there is evidence these amygdala subregions have similar cortical connectivity20 and greater regional overlap in rs-FC at younger ages.19 We also cannot fully evaluate the impact of sleep on amygdala rs-FC in studied participants.64 Additionally, the interpretation of negative connectivity in resting state fMRI analyses remains an active area of investigation. Further, the ITSEA relies solely on parental report for identification of internalizing symptoms, rather than direct behavioral observation. Finally, while evidence suggests that children with early internalizing symptoms remain symptomatic as they age,47,65 extending the findings of amygdala rs-FC to evaluations of anxiety symptoms during later childhood is required. Future work in this cohort will allow these investigations as they are being evaluated longitudinally for psychopathology.
Contrary to a prior report,66 VPT and FT children did not differ on internalizing symptoms at age 2 years, and thus prematurity did not mediate relationships between amygdala connectivity and internalizing symptoms. Direct comparison reveals that our FT children had higher internalizing scores than the FT cohort from this prior work, while scores for VPT infants were equivalent across studies. Notably, FT and VPT infants in the prior study differed significantly in social risk factors, whereas groups in the current study did not. Both our FT and VPT cohorts had a large proportion with children with 3 or more maternal social risk factors (41% and 30%, respectively) and had a large proportion of children on Medicaid (65% and 48%, respectively). We suggest that our groups did not differ in outcome because of the high risk of our comparison (FT) group.
This study demonstrates that patterns of neonatal amygdala rs-FC are similar to those in older children and adults. VPT infants have decreased magnitude of amygdala rs-FC, which could relate to differential risk profiles in term versus preterm children. Finally, rs-FC of the amygdala during the neonatal period predicts internalizing symptoms at age 2 years, with regional specificity for specific symptom domains consistent with pathophysiology detected at older ages. These results suggest the seeds of future anxious and depressive symptoms for some individuals may be detectable at birth using amygdala connectivity patterns.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (R01 HD057098, R01 HD061619, UL1 TR000448, KL2 TR000250, K23 MH105179, and K02 NS089852), McDonnell Center for Systems Neuroscience, Intellectual and Developmental Disabilities Research Center at Washington University (P30 HD062171), Child Neurology Foundation, Cerebral Palsy International Research Foundation, Dana Foundation, and Doris Duke Foundation.
The authors wish to thank the following individuals who assisted with this project: Karen Lukas, RN, Anthony Barton, Jessica Conners, Rachel Paul, BA, Jim Alexopoulous, MA, Joe Ackermann, Jr., BA, and Tara Smyser, MSE, all from Washington University School of Medicine. The authors also thank the families who participated.
Footnotes
Disclosure: Dr. Barch has served as a consultant for Amgen, Pfizer, Takeda, and Roche and has a contract to analyze imaging data for Pfizer. Drs. Rogers, Sylvester, Mintz, Shimony, Smyser, and Ms. Kenley report no biomedical financial interests or potential conflicts of interest.
Supplemental material cited in this article is available online
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Dr. Cynthia E. Rogers, Washington University in St. Louis.
Dr. Chad M. Sylvester, Washington University in St. Louis.
Dr. Carrie Mintz, Washington University in St. Louis.
Ms. Jeanette K. Kenley, Washington University in St. Louis.
Dr. Joshua S. Shimony, Mallinckrodt Institute of Radiology, Washington University in St. Louis.
Dr. Deanna M. Barch, Washington University in St. Louis. Mallinckrodt Institute of Radiology, Washington University in St. Louis. Program in Neuroscience at Washington University in St. Louis.
Dr. Christopher D. Smyser, Washington University in St. Louis. Mallinckrodt Institute of Radiology, Washington University in St. Louis.
References
- 1.LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23:727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Price JL. Comparative aspects of amygdala connectivity. Ann N Y Acad Sci. 2003;985:50–58. doi: 10.1111/j.1749-6632.2003.tb07070.x. [DOI] [PubMed] [Google Scholar]
- 3.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 4.Gee DG, Gabard-Durnam LJ, Flannery J, et al. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc Natl Acad Sci U S A. 2013;110:15638–15643. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fusar-Poli P, Placentino A, Carletti F, et al. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci JPN. 2009;34:418–432. [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas KM, Drevets WC, Dahl RE, et al. Amygdala response to fearful faces in anxious and depressed children. Arch Gen Psychiatry. 2001;58:1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- 7.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 9.Ochsner KN, Ray RD, Cooper JC, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 10.Bouwmeester H, Wolterink G, van Ree JM. Neonatal development of projections from the basolateral amygdala to prefrontal, striatal, and thalamic structures in the rat. J Comp Neurol. 2002;442:239–249. doi: 10.1002/cne.10084. [DOI] [PubMed] [Google Scholar]
- 11.Bouwmeester H, Smits K, Van Ree JM. Neonatal development of projections to the basolateral amygdala from prefrontal and thalamic structures in rat. J Comp Neurol. 2002;450:241–255. doi: 10.1002/cne.10321. [DOI] [PubMed] [Google Scholar]
- 12.Verwer RW, Van Vulpen EH, Van Uum JF. Postnatal development of amygdaloid projections to the prefrontal cortex in the rat studied with retrograde and anterograde tracers. J Comp Neurol. 1996;376:75–96. doi: 10.1002/(SICI)1096-9861(19961202)376:1<75::AID-CNE5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 13.Ulfig N, Setzer M, Bohl J. Ontogeny of the Human Amygdala. Ann N Y Acad Sci. 2006;985:22–33. doi: 10.1111/j.1749-6632.2003.tb07068.x. [DOI] [PubMed] [Google Scholar]
- 14.Smyser CD, Snyder AZ, Shimony JS, Mitra A, Inder TE, Neil JJ. Resting-state network complexity and magnitude are reduced in prematurely born infants. Cereb Cortex. 2016;26:322–33. doi: 10.1093/cercor/bhu251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fransson P, Skiöld B, Horsch S, et al. Resting-state networks in the infant brain. Proc Natl Acad Sci U S A. 2007;104:15531–15536. doi: 10.1073/pnas.0704380104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamm LL, Jacobs RH, Johnson MW, et al. Aberrant amygdala functional connectivity at rest in pediatric anxiety disorders. Biol Mood Anxiety Disord. 2014;4:15. doi: 10.1186/s13587-014-0015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qiu A, Anh TT, Li Y, et al. Prenatal maternal depression alters amygdala functional connectivity in 6- month-old infants. Transl Psychiatry. 2015;5:e508. doi: 10.1038/tp.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roy AK, Shehzad Z, Margulies DS, et al. Functional connectivity of the human amygdala using resting state fMRI. NeuroImage. 2009;45:614–626. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qin S, Young CB, Supekar K, Uddin LQ, Menon V. Immature integration and segregation of emotion-related brain circuitry in young children. Proc Natl Acad Sci U S A. 2012;109:7941–7946. doi: 10.1073/pnas.1120408109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gabard-Durnam LJ, Flannery J, Goff B, et al. The development of human amygdala functional connectivity at rest from 4 to 23years: A cross-sectional study. NeuroImage. 2014;95:193–207. doi: 10.1016/j.neuroimage.2014.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim MJ, Gee DG, Loucks RA, Davis FC, Whalen PJ. Anxiety Dissociates Dorsal and Ventral Medial Prefrontal Cortex Functional Connectivity with the Amygdala at Rest. Cereb Cortex. 2011;21:1667–73. doi: 10.1093/cercor/bhq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roy AK, Fudge JL, Kelly C, et al. Intrinsic functional connectivity of amygdala-based networks in adolescent generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry. 2013;52:290–299. e2. doi: 10.1016/j.jaac.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qin S, Young CB, Duan X, Chen T, Supekar K, Menon V. Amygdala subregional structure and intrinsic functional connectivity predicts individual differences in anxiety during early childhood. Biol Psychiatry. 2014;75:892–900. doi: 10.1016/j.biopsych.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roy AK, Benson BE, Degnan KA, et al. Alterations in amygdala functional connectivity reflect early temperament. Biol Psychol. 2014;103:248–254. doi: 10.1016/j.biopsycho.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baeken C, Marinazzo D, Van Schuerbeek P, et al. Left and Right Amygdala - Mediofrontal Cortical Functional Connectivity Is Differentially Modulated by Harm Avoidance. Soriano-Mas C, ed. PLoS ONE. 2014;9(4):e95740. doi: 10.1371/journal.pone.0095740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graham AM, Buss C, Rasmussen JM, et al. Implications of newborn amygdala connectivity for fear and cognitive development at 6-months-of-age. Dev Cogn Neurosci. 2016;18:12–25. doi: 10.1016/j.dcn.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bebko G, Bertocci M, Chase H, et al. Decreased amygdala-insula resting state connectivity in behaviorally and emotionally dysregulated youth. Psychiatry Res. 2015;231:77–86. doi: 10.1016/j.pscychresns.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stegmayer K, Usher J, Trost S, et al. Disturbed cortico-amygdalar functional connectivity as pathophysiological correlate of working memory deficits in bipolar affective disorder. Eur Arch Psychiatry Clin Neurosci. 2015;265:303–11. doi: 10.1007/s00406-014-0517-5. [DOI] [PubMed] [Google Scholar]
- 29.Hahn A, Stein P, Windischberger C, et al. Reduced resting-state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. NeuroImage. 2011;56:881–889. doi: 10.1016/j.neuroimage.2011.02.064. [DOI] [PubMed] [Google Scholar]
- 30.Johnson S, Marlow N. Preterm birth and childhood psychiatric disorders. Pediatr Res. 2011;69:11R–8R. doi: 10.1203/PDR.0b013e318212faa0. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt LA, Miskovic V, Boyle MH, Saigal S. Shyness and timidity in young adults who were born at extremely low birth weight. Pediatrics. 2008;122:e181–187. doi: 10.1542/peds.2007-3747. [DOI] [PubMed] [Google Scholar]
- 32.Allin M. Personality in Young Adults Who Are Born Preterm. Pediatrics. 2006;117:309–316. doi: 10.1542/peds.2005-0539. [DOI] [PubMed] [Google Scholar]
- 33.Rogers CE, Anderson PJ, Thompson DK, et al. Regional cerebral development at term relates to school-age social-emotional development in very preterm children. J Am Acad Child Adolesc Psychiatry. 2012;51:181–191. doi: 10.1016/j.jaac.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rogers CE, Smyser T, Smyser CD, Shimony J, Inder TE, Neil JJ. Regional white matter development in very preterm infants: perinatal predictors and early developmental outcomes. Pediatr Res. 2016;79:87–95. doi: 10.1038/pr.2015.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ure AM, Treyvaud K, Thompson DK, et al. Neonatal brain abnormalities associated with autism spectrum disorder in children born very preterm. Autism Res. 2016;9:543–52. doi: 10.1002/aur.1558. [DOI] [PubMed] [Google Scholar]
- 36.Tuuli MG, Stout MJ, Macones GA, Cahill AG. Umbilical Cord Venous Lactate for Predicting Arterial Lactic Acidemia and Neonatal Morbidity at Term. Obstet Gynecol. 2016;127:674–680. doi: 10.1097/AOG.0000000000001339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kidokoro H, Neil JJ, Inder TE. New MR Imaging Assessment Tool to Define Brain Abnormalities in Very Preterm Infants at Term. AJNR Am J Neuroradiol. 2013;34:2208–14. doi: 10.3174/ajnr.A3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mathur AM, Neil JJ, McKinstry RC, Inder TE. Transport, monitoring, and successful brain MR imaging in unsedated neonates. Pediatr Radiol. 2008;38:260–264. doi: 10.1007/s00247-007-0705-9. [DOI] [PubMed] [Google Scholar]
- 39.Smyser CD, Inder TE, Shimony JS, et al. Longitudinal Analysis of Neural Network Development in Preterm Infants. Cereb Cortex. 2010;20:2852–2862. doi: 10.1093/cercor/bhq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gholipour A, Kehtarnavaz N, Gopinath K, Briggs R, Panahi I. Average field map image template for Echo-Planar image analysis. Conf Proc IEEE Eng Med Biol Soc. 2008;2008:94–7. doi: 10.1109/IEMBS.2008.4649099. [DOI] [PubMed] [Google Scholar]
- 41.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smyser CD, Dosenbach NUF, Smyser TA, et al. Prediction of brain maturity in infants using machine-learning algorithms. NeuroImage. 2016;136:1–9. doi: 10.1016/j.neuroimage.2016.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bayley N. Bayley Scales of Infant and Toddler Development. 3. San Antonio, TX: Pearson Education, Inc; 2006. [Google Scholar]
- 44.Carter AS, Briggs-Gowan MJ. Infant Toddler Social and Emotional Assessment (ITSEA) San Antonio, TX: Psychological Corporation, Harcourt Assessment; 2006. [Google Scholar]
- 45.Carter AS, Briggs-Gowan MJ, Jones SM, Little TD. The Infant-Toddler Social and Emotional Assessment (ITSEA): factor structure, reliability, and validity. J Abnorm Child Psychol. 2003;31:495–514. doi: 10.1023/a:1025449031360. [DOI] [PubMed] [Google Scholar]
- 46.Treyvaud K, Doyle LW, Lee KJ, et al. Social-emotional difficulties in very preterm and term 2 year olds predict specific social-emotional problems at the age of 5 years. J Pediatr Psychol. 2012;37:779–85. doi: 10.1093/jpepsy/jss042. [DOI] [PubMed] [Google Scholar]
- 47.Mian ND, Wainwright L, Briggs-Gowan MJ, Carter AS. An Ecological Risk Model for Early Childhood Anxiety: The Importance of Early Child Symptoms and Temperament. J Abnorm Child Psychol. 2011;39:501–512. doi: 10.1007/s10802-010-9476-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Slopen N, Fitzmaurice G, Williams DR, Gilman SE. Poverty, Food Insecurity, and the Behavior for Childhood Internalizing and Externalizing Disorders. J Am Acad Child Adolesc Psychiatry. 2010;49:444–452. doi: 10.1097/00004583-201005000-00005. [DOI] [PubMed] [Google Scholar]
- 49.Côté SM, Boivin M, Liu X, Nagin DS, Zoccolillo M, Tremblay RE. Depression and anxiety symptoms: onset, developmental course and risk factors during early childhood. J Child Psychol Psychiatry. 2009;50:1201–1208. doi: 10.1111/j.1469-7610.2009.02099.x. [DOI] [PubMed] [Google Scholar]
- 50.Hack M, Taylor HG, Klein N, Eiben R, Schatschneider C, Mercuri-Minich N. School-age outcomes in children with birth weights under 750 g. N Engl J Med. 1994;331:753–759. doi: 10.1056/NEJM199409223311201. [DOI] [PubMed] [Google Scholar]
- 51.Whitaker AH, Feldman JF, Van Rossem R, et al. Neonatal cranial ultrasound abnormalities in low birth weight infants: relation to cognitive outcomes at six years of age. Pediatrics. 1996;98:719–729. [PubMed] [Google Scholar]
- 52.Snaith R, Zigmond A. The Hospital Anxiety and Depression Scale Manual. London: InferNelson Publishing; 1994. [Google Scholar]
- 53.Rice JP, Reich T, Bucholz KK, et al. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol Clin Exp Res. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- 54.Stein JL, Wiedholz LM, Bassett DS, et al. A validated network of effective amygdala connectivity. NeuroImage. 2007;36:736–745. doi: 10.1016/j.neuroimage.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 55.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- 56.Sripada R, King A, Garfinkel S, et al. Altered resting-state amygdala functional connectivity in men with posttraumatic stress disorder. J Psychiatry Neurosci. 2012;37:241–249. doi: 10.1503/jpn.110069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baur V, Hänggi J, Langer N, Jäncke L. Resting-State Functional and Structural Connectivity Within an Insula–Amygdala Route Specifically Index State and Trait Anxiety. Biol Psychiatry. 2013;73:85–92. doi: 10.1016/j.biopsych.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 58.Chase HW, Moses-Kolko EL, Zevallos C, Wisner KL, Phillips ML. Disrupted posterior cingulate-amygdala connectivity in postpartum depressed women as measured with resting BOLD fMRI. Soc Cogn Affect Neurosci. 2014;9:1069–1075. doi: 10.1093/scan/nst083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sylvester CM, Corbetta M, Raichle ME, et al. Functional network dysfunction in anxiety and anxiety disorders. Trends Neurosci. 2012;35:527–535. doi: 10.1016/j.tins.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clauss JA, Blackford JU. Behavioral inhibition and risk for developing social anxiety disorder: a meta-analytic study. J Am Acad Child Adolesc Psychiatry. 2012;51:1066–1075. e1. doi: 10.1016/j.jaac.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chronis-Tuscano A, Degnan KA, Pine DS, et al. Stable early maternal report of behavioral inhibition predicts lifetime social anxiety disorder in adolescence. J Am Acad Child Adolesc Psychiatry. 2009;48:928–935. doi: 10.1097/CHI.0b013e3181ae09df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Doria V, Beckmann CF, Arichi T, et al. Emergence of resting state networks in the preterm human brain. Proc Natl Acad Sci U S A. 2010;107:20015–20020. doi: 10.1073/pnas.1007921107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fransson P, Aden U, Blennow M, Lagercrantz H. The functional architecture of the infant brain as revealed by resting-state fMRI. Cereb Cortex N Y N 1991. 2011;21:145–154. doi: 10.1093/cercor/bhq071. [DOI] [PubMed] [Google Scholar]
- 64.Graham AM, Pfeifer JH, Fisher PA, Lin W, Gao W, Fair DA. The potential of infant fMRI research and the study of early life stress as a promising exemplar. Dev Cogn Neurosci. 2015;12:12–39. doi: 10.1016/j.dcn.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Treyvaud K, Doyle LW, Lee KJ, et al. Social-emotional difficulties in very preterm and term born twoyear olds predict specific social-emotional problems at five years. doi: 10.1093/jpepsy/jss042. under review. [DOI] [PubMed] [Google Scholar]
- 66.Spittle AJ, Treyvaud K, Doyle LW, et al. Early emergence of behavior and social-emotional problems in very preterm infants. J Am Acad Child Adolesc Psychiatry. 2009;48:909–918. doi: 10.1097/CHI.0b013e3181af8235. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.