Abstract
Volatile composition of essential oils from dill, parsley, coriander, and mint were investigated at different harvest dates to determine the most suitable harvest time for each these herbs. Hydrodistillation (HD), using a Deryng system, was used for isolating the essential oils. Isolation and identification of the volatile compounds were performed using gas chromatography-mass spectrometry (GC-MS) instrument. The results of gas chromatography-flame ionization detector (GC-FID) analysis (quantification) showed that the main components in the essential oil of dill shoots were α-phellandrene, dill ether, and β-phellandrene, and the optimal harvest date was D2 (second harvest, fourth week of February 2015). For parsley shoots, the main compounds were 1,3,8-p-menthatriene, β-phellandrene, and P1 (first harvest, third week of November 2014) was the sample with the highest essential oil. For coriander, the main compounds were E-2-dodecenal, dodecanal, and octane and the highest contents were found at C2 (second harvest, 5 February 2015); while, the main two components of mint essential oil were carvone and limonene, and the highest contents were found at M1 (first harvest, second week of December 2014). The present study was the first one reporting data on descriptive sensory analysis of aromatic herbs at this optimal harvest date according to the content of volatile compounds of their essential oils.
Keywords: dill, parsley, coriander, mint, GC-MS, descriptive sensory analysis
1. Introduction
The production of aromatic herbs, such as oregano, marjoram, rosemary, thyme, lavender, or peppermint, is a rising sector in the Mediterranean countries. These plants have many applications, such as ornamental cropping, perfumery, or food and pharmaceutical industries; these later applications are linked to the beneficial health effects of their essential oils, which have antimicrobial, antifungal, insecticidal, and antioxidant properties [1,2].
Dill (Anethum graveolens L.) is an important aromatic herb, which is used as flavoring and seasoning of various foods, such as salads, sauces, soups, sea foods, and especially pickled vegetables [3]. Parsley (Petroselinum crispum) and coriander (Coriandrum sativum) are two culinary herbs commonly used to enhance the flavor of many dishes of the cuisines of China, Mexico, South America, India, and South East Asia [4]. Peppermint (Mentha piperita L.) is a famous aromatic and medicinal herb used in traditional and folk medicines in the world for its antimicrobial and antioxidant properties [5].
In addition, culinary herbal extracts and essential oils have become increasingly popular as alternative sources of natural preservative agents, largely because herbs are widely cultivated, effective, and safe for consumption [5]. Essential oils are extracted from various aromatic plants generally localized in temperate to warm countries, such as the Mediterranean countries [1]. Peppermint (M. piperita L.) essential oils have been obtained by steam distillation in a Clevenger-type apparatus [5], while microwave extraction technique of mint essential oil was used by Costa et al. [6]. Besides, Huopalahti and Linko [3] isolated aroma compounds of dill (Anethum graveolens L.) by using solvent extraction technique.
It is well-known that the presence of essential oils and their composition determine the specific aroma of plants and the flavor of the resulting condiments [7,8]. The main chemical families present in aromatic herbs are: monoterpenes, monoterpenoids, and phenylpropanoids. In lower amount alcohols, sesquiterpenes, sesquiterpenoids, aldehydes, and esters were also found [9,10,11,12]. The composition and concentrations of essential oils from aromatic herbs depend on many factors, including geographical source, climatic and soil conditions, stage of vegetative cycle, seasonal variation, etc. [13,14,15].
Therefore, the aim of this study was to analyze the volatile composition of the essential oils and the sensory quality of different aromatic herbs (parsley, dill, mint, and coriander) grown in different Mediterranean regions of Spain in order to stablish the best harvest time according to the highest content of essential oils and the optimum sensory quality.
2. Materials and Methods
2.1. Plant Material
Four aromatic herbs (dill, parsley, coriander, and mint) were grown under conventional agricultural practices and conditions according to the recommendations of farmers located at Mediterranean regions of Spain according to their experience. These conditions are described as follows:
Dill seeds (Anethum graveolens L. Cv. ELLA) were sown on the 7 September 2014 in expanded polystyrene (EPS) trays and placed in a greenhouse located at Santomera (Murcia, Spain) until 4 October 2014. Then, plantlets were transplanted into a commercial orchard placed at Sucina (Murcia, Spain). Dill samples at the commercial stage were harvested at two different dates within the same plant on the 26 November 2014 (D1: 80 days after sowing) and 28 February 2015 (D2: 174 days after sowing).
Parsley seeds (Petroselinum crispum Cv. Gigante Italiano Darkness) were sown on the 2 September 2014 in expanded polystyrene (EPS) trays and placed in a greenhouse located at Santomera (Murcia, Spain) until 24 September 2014. Then, plantlets were transplanted into a commercial orchard placed at Sucina (Murcia, Spain). Parsley samples at commercial stage were harvested at three different dates within the same plant on the 19 November 2014 (P1: 78 days after sowing), 5 January 2015 (P2: 144 days after sowing), and 25 February 2015 (P3: 175 days after sowing).
Coriander seeds (Coriander sativum Cv. MARINO) were directly sown on the commercial orchard placed at Sucina (Murcia, Spain). Coriander has only one harvest for each plant and in these regions three crops per year are sown, grown, and harvested. The first crop (C1) was planted on 30 September and harvested on 19 November 2014; the second crop (C2) was planted on 22 October 2014 and harvested on 5 February 2015; and, the third crop (C3) was planted on 29 December and harvested on 25 February 2015.
Mint cuttings (Mentha piperita L.) of 5–7 cm were sown on 7 August 2014 in polyethylene trays and placed in a greenhouse located at Santomera (Murcia, Spain) until 6 October 2014. Then, plantlets were transplanted to a commercial orchard placed at Sucina (Murcia, Spain). Mint samples at commercial stages were harvested at two different dates from the same plant on the 11 December 2014 (M1: 133 days after sowing) and 5 February 2015 (M2: 189 days after sowing).
Aromatic herbs were grown using high-frequency drip irrigation systems. The water contribution was carried out using polyethylene pipes of 16 mm of diameter. The emitters were adjusted at 32 cm of distance with a total flow of 1.6 L·h−1. The total volume of water for each crop was as following: 3208 m3·ha−1 for dill, 3849 m3·ha−1 for parsley, 3445 m3·ha−1 for coriander, and 2566 m3·ha−1 for mint.
The irrigation water was of good quality, highlighting its slightly basic pH (7.91), and its proper electrical conductivity (1.26 mS·cm−1), which is suitable for aromatic herbs crops. Soil was uniformly silty-loam in texture, with a low content in organic matter (1.22%), medium salinity conditions (3.35 mS·cm−1), and good levels of sulfates (37.83 meq·L−1) for aromatic herb development.
Along the development of crops, fertilization was carried out with a total amount of N of 130 kg·ha−1, P (P2O5) of 60 kg·ha−1, and K (K2O) of 160 kg·ha−1 for dill development. For parsley, the total amount of fertilizers was 300 kg·N·ha−1, 190 kg·P·ha−1 (P2O5), and 350 kg·K·ha−1 (K2O). Regarding coriander, the fertilization was carried out with a total amount N of 275 kg·ha−1, P (P2O5) of 170 kg·ha−1, and K (K2O) of 310 kg·ha−1. Finally, the total amount of fertilizers was 200 kg·N·ha−1, 130 kg·P·ha−1 (P2O5), and 230 kg·K·ha−1 (K2O).
2.2. Extraction of Essential Oils
Hydrodistillation (HD), using a Deryng system (the Polish version of the Clevenger apparatus), was used for isolating the essential oil in fresh herbs (dill, parsley, coriander, and mint). About 15.0 g of freshly chopped herbs shoots (aerial part of the plant, including stems and leaves) were put in a 500 mL round bottom flask, together with 1.0 g sodium chloride (NaCl), 150 mL of distilled water, and 50 µL of benzyl acetate as an internal standard (987 mg·L−1). After the mixture started boiling, heating was maintained for 1 h. A cold refrigerant was used to condense the vapors, and 1 mL of cyclohexane was added to the Deryng apparatus at the beginning of the hydrodistillation process to retain the essential oil distilled from the samples of herbs shoots. After 60 min of extraction, the solvent, enriched with the volatile compounds, was transferred into a 2.5 mL vial, after drying it over anhydrous sodium sulfate (Na2SO4), and kept at −15 °C until the gas chromatography-mass spectrometry (GC-MS) analyses were conducted. The extractions were conducted in triplicate.
2.3. Chromatographic Analyses
Analysis and identification of the volatile compounds were performed using a Shimadzu GC-17A gas chromatograph coupled with a Shimadzu QP-5050A mass spectrometer detector (Shimadzu Corporation, Kyoto, Japan). The GC-MS system was equipped with a TRACSIL Meta.X5 (95% dimethylpolysiloxane and 5% diphenylpolysiloxane) column (60 m × 0.25 mm, 0.25 μm film thickness; Teknokroma S. Coop. C. Ltd, Barcelona, Spain). Analyses were carried out using helium as carrier gas at a column flow rate of 0.3 mL·min−1 and a total flow of 3.9 mL·min−1 in a split ratio of 1:11 and the following program: (a) 80 °C for 0 min; (b) increase of 3 °C·min−1 from 80 °C to 210 °C and hold for 1 min; (c) increase of 25 °C·min−1 from 210 °C to 300 °C and hold for 3 min. The temperatures of the injector and detector were 230 °C and 300 °C, respectively.
All compounds were identified using three different analytical methods: (1) comparison of experimental retention indexes (RI) with those of the literature; (2) GC-MS retention times (authentic standards of “all” compounds reported in Table 1, Table 2, Table 3, Table 4 and Table 5 were used for identification purposes); and, (3) mass spectra (authentic chemicals and NIST05 spectral library collection). Only fully identified compounds have been reported in this study.
Table 1.
Identification of essential oils found in dill, parsley, coriander, and mint samples.
| Compound | Herb | RT (min) | Retention Indexes (RI) | Descriptor ¶ | |
|---|---|---|---|---|---|
| Exp. † | Lit. † | ||||
| trans-2-Hexenal ‡ | dill | 11.09 | 806 | 800 | Green, banana, aldehydic ‡ |
| Octane | coriander | 12.12 | 808 | 800 | |
| α-Thujene | dill | 13.19 | 873 | 905 | Woody, green, herb |
| Santene | mint | 13.29 | 879 | 880 | |
| α-Pinene | dill, parsley, mint | 13.58 | 896 | 909 | Fresh, camphor, sweet, pine, earthy, woody |
| Camphene | mint | 14.38 | 944 | 945 | Fresh, woody, fir, terpene |
| Sabinene | dill, parsley | 14.89 | 975 | 975 | Woody, terpene, citrus, pine, spice |
| Myrcene | dill, parsley, mint | 15.15 | 991 | 991 | Peppery, terpene, spicy |
| β-Pinene | dill, parsley | 15.25 | 997 | 990 | Dry, woody, pine, hay, green |
| cis-3-Hexenyl acetate | parsley, coriander, mint | 15.70 | 1008 | 1009 | Fresh, green, sweet, fruity, banana, apple |
| α-Phellandrene | dill, parsley | 16.20 | 1020 | 1013 | Citrus, herbal, terpene, green, woody, peppery |
| α-Terpinene | mint | 16.68 | 1031 | 1018 | Woody, terpene, lemon, herbal, citrus |
| p-Cymene | dill, parsley, mint | 16.88 | 1036 | 1034 | Fresh, citrus, terpene, woody, spice |
| Limonene | dill, parsley, coriander, mint | 17.08 | 1040 | 1039 | Terpene, pine, herbal, peppery |
| β-Phellandrene | dill, parsley, coriander | 17.25 | 1044 | 1036 | Mint, terpentine |
| trans-β-Ocimene | dill, parsley, mint | 17.38 | 1047 | 1047 | Citrus, tropical, green, terpene, woody |
| γ-Terpinene | parsley, mint | 18.20 | 1066 | 1066 | Woody, terpene, lemon, lime, tropical, herbal |
| trans-Sabinene hydrate | mint | 19.00 | 1084 | 1087 | Warm, balsamic, woody |
| Terpinolene | dill, parsley | 19.47 | 1095 | 1097 | Fresh, woody, sweet, pine, citrus. |
| Undecane | dill, coriander | 19.63 | 1098 | 1099 | Fusel-like |
| Linalool | coriander, mint | 19.82 | 1103 | 1103 | Citrus, orange, floral, terpy, rose |
| Nonanal | coriander, mint | 20.03 | 1107 | 1107 | Aldehydic, rose, fresh, orris, orange, peel |
| 1,3,8-p-Menthatriene | parsley | 20.74 | 1125 | 1115 | Turpentine, camphor, herbal, woody |
| cis-Limonene oxide | mint | 21.87 | 1149 | 1140 | Fresh, citrus |
| trans-Limonene oxide | mint | 22.04 | 1153 | 1147 | Fresh, citrus, mild, green |
| cis-p-Mentha-2.8-dien-1-ol | mint | 23.74 | 1192 | 1193 | |
| trans-p-Mentha-2.8-dien-1-ol | mint | 24.07 | 1199 | 1196 | Fresh, minty |
| Dill ether | dill | 24.40 | 1206 | 1187 | Herbal, dill, spicy |
| α-Terpineol | parsley | 24.74 | 1213 | 1200 | Pine, terpene, lilac, citrus, woody, floral |
| Decanal | coriander | 24.79 | 1214 | 1207 | Sweet, aldehydic, orange, waxy, citrus rind |
| cis-Carveol | mint | 25.05 | 1220 | 1221 | Caraway, spicy, citrus, fruity |
| trans-Carveol | mint | 25.43 | 1228 | 1217 | Caraway, green, oily |
| Carvone | dill, coriander, mint | 27.13 | 1264 | 1262 | Herbaceous, grapefruit, pepper, spicy, woody |
| E-2-Decenal | coriander | 27.54 | 1273 | 1278 | Earthy, coriander green, mushroom, aldehydic |
| 1-Decanol | coriander | 28.45 | 1292 | 1287 | Floral, orange, sweet, clean watery |
| Tridecane | dill | 29.06 | 1297 | 1299 | Citrus, fruity, Fusel-like |
| Bornyl acetate | mint | 29.10 | 1305 | 1291 | Woody, camphor, mentholic, spicy |
| Undecanal | coriander | 29.63 | 1317 | 1310 | Fresh, citrus, waxy, aldehydic |
| Carvomenthyl acetate | mint | 30.70 | 1339 | 1344 | |
| E-2-Undecenal | coriander | 32.40 | 1375 | 1371 | Aldehydic, citrus |
| 1-Undecanol | coriander | 33.88 | 1407 | 1386 | Earthy, soapy, waxy, fatty, honey, coconut |
| β-Bourbonene | mint | 34.08 | 1412 | 1407 | Herbal, Woody |
| Decyl acetate | coriander | 34.13 | 1412 | 1410 | Waxy, sweet, fatty, creamy |
| β-Caryophyllene | mint | 34.26 | 1416 | 1418 | Sweet, woody, spice clove dry |
| Dodecanal | coriander | 34.39 | 1419 | 1420 | Orange, fatty, herbaceous |
| trans-β-Caryophyllene | parsley, mint | 35.68 | 1448 | 1455 | Woody, spicy |
| Z-2-Dodecenal | coriander | 36.37 | 1463 | 1467 | Green, citrus, fruity, mandarin orange, herbal |
| E-2-Dodecenal | coriander | 37.12 | 1480 | 1468 | Citrus, mandarin orange, aldehydic |
| α-Humulene | mint | 37.48 | 1489 | 1489 | |
| E-2-Dodecen-1-ol | coriander | 37.78 | 1495 | 1483 | Oily, fatty |
| 1-Dodecanol | coriander | 38.08 | 1502 | 1485 | Earthy, soapy, waxy, fatty, honey, coconut |
| Germacrene-D | dill, parsley, mint | 38.42 | 1475 | 1477 | Woody, spice |
| Tridecanal | coriander | 38.96 | 1522 | 1518 | Fresh, aldehydic, citrus, grapefruit peel |
| Nerolidol | parsley | 38.97 | 1525 | 1528 | Floral, green, citrus, woody |
| Myristicin | dill, parsley | 39.97 | 1543 | 1532 | Spice, warm, balsam, woody |
| E-2-Tridecenal | coriander | 41.58 | 1582 | 1571 | Citrus, peel tangerine |
| 1-Tetradecanol | coriander | 42.81 | 1615 | 1618 | Fruity, coconut |
| Tetradecanal | coriander | 43.31 | 1632 | 1623 | Dairy, creamy, fishy with a fruity, pear nuance. |
† RT = retention time; Exp. = experimental and Lit. = Literature; ‡ All compounds were identified using retention indexes, mass spectra and retention time of standards; ¶ SAFC (2015); www.pherobase.com; www.thegoodscentscompany.com.
Table 2.
Volatile composition of dill essential oil at two commercial stages (mg·kg−1·fw).
| Compound | ANOVA † | D1 | D2 |
|---|---|---|---|
| Concentration, (mg·kg−1·fw) | |||
| trans-2-Hexenal | *** | 0.18 b ¥ | 1.81 a |
| α-Thujene | *** | 1.35 b | 1.81 a |
| α-Pinene | *** | 7.84 b | 8.70 a |
| Sabinene | *** | 0.35 b | 0.51 a |
| Myrcene | *** | 2.41 b | 3.20 a |
| β-Pinene | *** | 0.66 a | 0.31 b |
| α-Phellandrene | *** | 342 b | 474 a |
| p-Cymene | *** | 12.5 a | 3.92 b |
| Limonene | *** | 17.6 b | 21.4 a |
| β-Phellandrene | *** | 46.0 b | 60.0 a |
| trans-β-Ocimene | *** | 5.13 b | 7.50 a |
| Terpinolene | *** | 2.53 a | 0.33 b |
| Undecane | *** | 4.38 a | 1.11 b |
| Dill ether | *** | 46.2 b | 62.9 a |
| Carvone | NS | 0.02 a | 0.02 a |
| Tridecane | *** | 0.56 a | 0.20 b |
| Germacrene-D | *** | 4.19 a | 1.38 b |
| Myristicin | *** | 13.8 a | 0.02 b |
| TOTAL | *** | 508 b | 649 a |
† NS = not significant F ratio (p < 0.05); *** significant at p < 0.001. ‡ Treatment means of the ANOVA test (values are the mean value of 3 replications). ¥ Values followed by the same letter, within the same row, were not significant different (p < 0.05), Tukey’s multiple-range test.
Table 3.
Volatile composition of parsley essential oil at three commercial stages (mg·kg−1·fw).
| Compound | ANOVA † | P1 | P2 | P3 |
|---|---|---|---|---|
| Concentration, (mg·kg−1·fw) | ||||
| α-Pinene | *** | 7.07 c ¥ | 8.53 b | 9.65 a |
| Sabinene | *** | 0.30 b | 0.38 b | 0.55 a |
| Myrcene | *** | 27.0 a | 27.1 a | 24.3 b |
| β-Pinene | *** | 2.47 c | 4.14 a | 3.64 b |
| cis-3-Hexenyl acetate | *** | 1.73 a | 0.35 b | 0.33 b |
| α-Phellandrene | *** | 6.46 c | 11.0 a | 8.63 b |
| p-Cymene | *** | 1.40 b | 1.41 b | 1.83 a |
| Limonene | *** | 12.5 b | 11.3 b | 13.7 a |
| β-Phellandrene | *** | 101 c | 122 a | 110 b |
| Trans-β-Ocimene | *** | 2.89 b | 2.33 c | 3.69 a |
| γ-Terpinene | *** | 0.45 a | 0.29 b | 0.31 b |
| Terpinolene | *** | 22.8 a | 17.2 b | 18.5 b |
| 1,3,8-p-Menthatriene | *** | 222 a | 159 c | 192 b |
| α-Terpineol | *** | 0.26 c | 0.40 b | 0.82 a |
| trans-β-Caryophyllene | *** | 0.61 c | 1.64 b | 2.15 a |
| Germacrene-D | *** | 0.96 c | 1.63 a | 1.39 b |
| Nerolidol | *** | 0.15 b | 0.07 c | 0.23 a |
| Myristcin | *** | 45.1 a | 45.9 a | 25.9 b |
| TOTAL | *** | 455 a | 414 b | 418 b |
† NS = not significant F ratio (p < 0.05); *** significant at p < 0.001. ‡ Treatment means of the ANOVA test (values are the mean value of 3 replications). ¥ Values followed by the same letter, within the same row, were not significant different (p < 0.05), Tukey’s multiple-range test.
Table 4.
Volatile composition of coriander essential oil at three commercial stages (mg·kg−1·fw).
| Compound | ANOVA † | C1 | C2 | C3 |
|---|---|---|---|---|
| Concentration, (mg·kg−1·fw) | ||||
| Octane | *** | 16.9 c ¥ | 20.7 a | 18.2 b |
| cis-3-Hexenyl acetate | *** | 0.64 b | 1.17 a | 1.11 a |
| Limonene | *** | 1.06 a | 0.18 b | 0.00 c |
| β-Phellandrene | NS | 0.04 c | 0.14 b | 1.39 a |
| Undecane | *** | 0.45 b | 1.09 a | 0.36 b |
| Linalool | *** | 0.06 a | 0.14 a | 0.05 a |
| Nonanal | NS | 0.01 c | 0.92 a | 0.22 b |
| Decanal | *** | 30.3 b | 25.5 c | 36.4 a |
| Carvone | *** | 3.06 a | 0.01 c | 0.37 b |
| E-2-Decenal | *** | 0.27 b | 0.39 b | 1.47 a |
| 1-Decanol | *** | 3.70 c | 5.05 b | 6.64 a |
| Undecanal | *** | 2.23 c | 3.93 b | 6.70 a |
| E-2-Undecenal | NS | 0.01 c | 0.44 b | 0.75 a |
| 1-Undecanol | NS | 0.05 b | 0.11 a | 0.05 b |
| Decyl acetate | NS | 0.01 a | 0.03 a | 0.00 a |
| Dodecanal | *** | 24.1 a | 24.4 a | 17.6 b |
| Z-2-Dodecenal | *** | 0.12 b | 0.26 a | 0.10 b |
| E-2-Dodecenal | *** | 15.0 c | 39.9 a | 25.7 b |
| E-2-Dodecen-1-ol | *** | 1.90 a | 1.00 b | 0.04 c |
| 1-Dodecanol | *** | 1.80 a | 0.21 b | 0.01 c |
| Tridecanal | *** | 1.90 a | 1.18 b | 1.22 b |
| E-2-Tridecenal | *** | 1.33 b | 4.69 a | 4.59 a |
| 1-Tetradecanol | *** | 0.12 b | 0.27 a | 0.08 b |
| Tetradecanal | *** | 1.61 b | 1.98 a | 0.88 c |
| TOTAL | *** | 107 c | 134 a | 124 b |
† NS = not significant F ratio (p < 0.05); *** significant at p < 0.001. ‡ Treatment means of the ANOVA test (values are the mean value of 3 replications). ¥ Values followed by the same letter, within the same row, were not significant different (p < 0.05), Tukey’s multiple-range test.
Table 5.
Volatile composition of mint essential oil at two commercial stages (mg·kg−1·fw).
| Compound | ANOVA † | M1 | M2 |
|---|---|---|---|
| Concentration, (mg·kg−1·fw) | |||
| Santene | *** | 22.5 a ¥ | 24.05 a |
| Camphene | NS | 2.17 b | 3.50 a |
| β-Pinene | *** | 12.3 a | 13.1 a |
| Myrcene | *** | 23.6 b | 28.3 a |
| cis-3-Hexenyl acetate | NS | 0.56 a | 0.62 a |
| p-Cymene | NS | 1.29 b | 2.55 a |
| α-Terpinene | NS | 0.54 b | 2.90 a |
| Limonene | *** | 590 b | 735 a |
| trans-β-Ocimene | *** | 19.1 a | 18.2 a |
| γ-Terpinene | NS | 1.31 b | 5.17 a |
| trans-Sabinene hydrate | *** | 34.3 b | 73.6 a |
| Nonanal | *** | 10.6 a | 12.0 a |
| Linalool | NS | 1.29 b | 2.23 a |
| cis-Limonene oxide | NS | 1.28 a | 1.23 a |
| trans-Limonene oxide | NS | 2.84 a | 1.79 b |
| cis-p-Mentha-2,8-dien-1-ol | NS | 6.43 a | 6.39 a |
| trans-p-Mentha-2,8-dien-1-ol | NS | 4.45 b | 11.4 a |
| cis-Carveol | *** | 65.3 b | 85.8 a |
| trans-Carveol | NS | 8.12 a | 9.73 a |
| Carvone | *** | 2462 a | 1854 b |
| Bornyl acetate | NS | 0.51 a | 0.43 a |
| Carvomenthyl acetate | *** | 12.0 b | 14.7 a |
| β-Bourbonene | *** | 14.6 a | 14.0 a |
| β-Caryophyllene | NS | 2.30 b | 3.59 a |
| trans-Caryophyllene | *** | 22.1 b | 35.5 a |
| Alloaromadendrene | NS | 1.74 b | 2.57 a |
| α-Humulene | NS | 3.58 b | 5.11 a |
| TOTAL | *** | 3326 a | 2968 b |
† NS = not significant F ratio (p < 0.05); *** significant at p < 0.001. ‡ Treatment means of the ANOVA test (values are the mean value of 3 replications). ¥ Values followed by the same letter, within the same row, were not significant different (p < 0.05), Tukey’s multiple-range test.
The semi-quantification of the volatile compounds was performed on a gas chromatograph, Shimadzu 2010, with a flame ionization detector (FID). The column and chromatographic conditions were those previously reported for the GC-MS analysis. The injector temperature was 200 °C and nitrogen was used as carrier gas (1 mL·min−1). The quantification was obtained from electronic integration measurements using flame ionization detection (FID). Benzyl acetate (1000 mg·L−1) was added as internal standard at the beginning of the distillation procedure to simulate the behavior of volatile compounds; this chemical was used as an internal standard after checking that it was absent in herbs, it separates well from other volatiles, it possesses similar FID and MS response factors to most of the volatiles in the aromatic herb essential oil, it is stable at high temperatures, and does not react with water. Calibration curves were performed with the following compounds (Sigma-Aldrich, Madrid, Spain) as representative of each chemical family: α-phellandrene (monoterpenes), α-terpineol (terpenoids), trans-β-caryophyllene (sesquiterpenes), dill ether (terpene ethers), nonanal (aldehydes), myristicin (phenylpropanoids), bornyl acetate (esters), 1-decanol (alcohols), undecane (alkanes); the correlation coefficients (R2) for all compounds were >0.995, and results were expressed as mg·kg−1 fresh weight, fw.
2.4. Sensory Evaluation with a Trained Panel
A trained panel was used to evaluate the intensity of the main aroma attributes of fresh herbs. Samples were evaluated by seven panelists (five males and two females), with ages between 23 and 56 years old. Panelists belonged to the Food Quality and Safety research group of the Universidad Miguel Hernández de Elche and had over 1000 h of evaluation experience; they had been trained in descriptive evaluation of aromatic herbs [2,8,9].
An odor profile method was used to describe the dill samples. During two preliminary orientation sessions of 90 min, panelists discussed about the main odor characteristics of the herbs and agreed on their use of odor attributes. During these orientation experiments, panelists evaluated different coded samples of Spanish fresh aromatic herbs together with samples from field. Panelists agreed that the odor of the samples could be described using seven attributes: aromatic herb(dill/coriander/mint/parsley)-ID (clean fresh green, bitter, pungent aromatics associated with fresh dill/coriander/mint/parsley; reference: dill/coriander/mint/parsley water; preparation: 25 g chopped fresh dill/coriander/mint/parsley soaked in 300 mL room temperature deionized water for 15 min, filtered), green grass (green aromatics associated with newly cut-grass and leafy plants; reference: hexanal in propylene glycol, 10 g·L−1 = 6), citrus (aromatics associated with commonly known citrus fruits, such as lemons, limes, oranges, which could also contain a peely note; reference: McCormick lemon grass = 3.0; preparation: take 0.1 g of lemon grass and place it in a medium snifter together with 100 mL of deionized water, and cover it), pine (aromatics reminiscent of resinous pine tree; can be medicinal or disinfectant in character; reference: El Corte Inglés raw pine nuts = 3.0), spicy (sharp aromatics with a physically penetrating sensation in the nose reminiscent of radish and horseradish; reference: fresh radish = 3.0), earthy (humus-like aromatics that may or may not include damp soil, decaying vegetation or cellar-like characteristics; reference: hexanal in propylene glycol, 5 g·L−1 = 1.5), and woody (brown, musty aromatics associated with very fibrous plants and bark; reference: Hacendado dried parsley = 4.5). The key sensory descriptors (high intensities being related to high quality products) are: herb-ID, green grass, citrus, spicy, and pine; earthy and woody are not positively correlated with the herb quality. Reference products of these attributes with different intensity, were prepared and provided to the panel.
Individual booths with controlled illumination and temperature were used in this study. Three digit numbers were used to code samples, and they were randomly offered to panelists in plastic beakers of 100 mL with lids; samples were left 15 min at room temperature prior to analyses. The intensity of the seven odor attributes was scored using scale from 0 to 10, where 0 = none or not perceptible intensity, and 10 = extremely high intensity.
2.5. Statistical Analysis
To compare the experimental data two consecutive tests were performed: (i) one-way analysis of variance (ANOVA), and (ii) Tukey’s multiple range. Homogenous groups and the least significant difference (LSD) were determined at significance level of p ≤ 0.05. Statgraphics Plus 5.0 software (Manugistics, Inc., Rockville, MD, USA) was the program used for the statistical analyses.
3. Results and Discussion
3.1. Volatile Composition of Essential Oil of Dill
Dill was planted on 24 September 2014 and the first harvest date for commercial purpose was on 26 November 2014 (D1) while the second harvest date was on 11 February 2015 (D2). After isolation of the essential oil of dill shoots, 18 compounds were identified by GC-MS (Table 1). The identified volatile compounds can be grouped in eight main chemical groups: monoterpenes (110 compounds), followed by alkanes (two compounds), terpenoids (one compound), sesquiterpenes (one compound), aldehydes (one compound), phenylpropanoids (one compound), terpenes (one compound), and monoterpene ethers (one compound). The eight main compounds were: α-phellandrene, dill ether, β-phellandrene, limonene, p-cymene, α-pinene, trans-β-ocimene, and myristicin. In several studies, α-phellandrene was predominating in the “leaf” essential oil of dill from Romania, Egypt, and Finland [3,16,17,18]. The extraction of the essential oils can be done using different isolation techniques, and this analytical methodology can affect the volatile profile. For example, Huopalahti and Linko [3] found 22 compounds in dill by using a modified Soxhlet technique; however, α-phellandrene, dill ether, β-phellandrene were reported as the major compounds.
Table 2 shows that 3 compounds (α-phellandrene, dill ether, and β-phellandrene) clearly dominated the dill shoots essential oil, representing 85%–92% of the total concentration of volatile compounds. This experimental finding is supported as well by previous studies by Vokk et al. and Radulescu et al. [16,19] who reported that α-phellandrene, β-phellandrene, and dill ether were the main compounds of dill essential oil.
Volatile composition of dill essential oil at two commercial stages were investigated in the current study and the total concentration of volatile compounds was higher in commercial stage of D2 as compared to D1 stage; these trend was also true for the three main components (α-phellandrene, dill ether, and β-phellandrene). For α-phellandrene the concentration changed from 342 mg·kg−1 in D1 to 474 mg·kg−1 in D2 (an increase of 38.6 %), where the concentration in dill ether changed from 46.2 mg·kg−1 in D1 to 62.9 mg·kg−1 in D2 (an increase of 36.1 %) and from 46 mg·kg−1 (D1) to 60 mg·kg−1 (D2) for β-phellandrene (an increase of 30.4 %). Zlatev [20] and El-Gengaihi and Hornok [21] found that the essential oil content in dill increased continuously during the growing period. During the growth of dill, the contents of limonene, 3,6-dimethyl-2,3,3a,4,5,7a-hexahydrobenzofuran, and carvone increased, while those of α-phellandrene, β-pellandrene, myristicin, and apiol decreased. The total amount of aroma compounds varied widely during the growth [3].
3.2. Volatile Composition of Essential Oil of Parsley
Parsley was planted on 24 September 2014, the first harvest date for commercial purposes was on 19 November 2014 (P1), the second harvest date was on 5 January 2015 (P2), and the third harvest date was on 25 February 2015 (P3). Eighteen compounds were identified in the essential oil of parsley and the chemical classification of these 18 compounds was as follows: monoterpenes (nine compounds), sesquiterpenes (three compounds), terpenes (two compounds), monoterpenoids (one compound), monoterpene alcohol (one compound), esters (one compound), and phenylpropanoids (one compound). The main eight compounds of the essential oil of parsley shoots were: 1,3,8-p-menthatriene (38.4%–48.8%), β-phellandrene (22.2%–29.5%), myristcin (6.2%–11.1%), myrcene (5.8%–6.5%), terpinolene (4.2%–5.0%), limonene (2.7%–3.3%), α-pinene (1.6%–2.3%), and α-phellandrene (1.4%–2.7%). Vokk et al. [19] used Clevenger distillation method for essential oil isolation and gas chromatography for identifying the extracts and the major constituents of essential oil of parsley leaves were as following: myristicin (30.7%–42.7%), β-phellandrene (21.8%–35.9%), 1,3,8-p-menthatriene (5.4%–10.0%), and β-myrcene (4.5%–8.7%). Essential oils obtained by simultaneous distillation–extraction (SDE) from leaves of parsley plants and the main components were β-phellandrene, 1,3,8-p-menthatriene, α-p-dimethylstyrene, myristicin, β-myrcene, and apiole [15]. The results by Vokk et al. [19] and Petropoulos et al. [15] agree quite well with the results of this study.
Volatile composition of parsley essential oil at three commercial stages were investigated in the current study and the highest value of total concentration of essential oil was that of the commercial stage P1, 455 mg·kg−1, as compared to 414 and 418 mg·kg−1 at P2 and P3 stages, respectively. The main two components were 1,3,8-p-menthatriene and β-phellandrene, which represented 68%–72% of the total concentration of essential oil of parsley. 1,3,8-p-Menthatriene had the highest concentration, 222 mg·kg−1, at the P1 stage, while the highest concentration of β-phellandrene was found at 122 mg·kg−1 P2 harvest. For myristicin and myrcene, the highest concentration was in found at P2 stage, while for limonene was P3 and for terpinolene P1 (Table 3). For the essential oil of parsley plant Petropoulos et al. [15] recorded that, comparing the relative concentrations of the components for the two sowing dates, important differences were found for β-phellandrene (39.0%–22.0%) and 1,3,8-p-menthatriene (17.4%–45.7%) at the first growth stage and between 1,3,8-p-menthatriene (15.7%–29.0%) and myristicin (27.2%–4.6%) at the second stage.
3.3. Volatile Composition of Essential Oil of Coriander
The essential oil of three commercial samples of coriander was analyzed by GC-MS after extracted by hydrodistillation technique. The first crop (C1) was planted on 30 September 2014 and the harvest date was 19 November 2014, the second crop (C2) was planted on 22 October 2014 and harvested on 5 January 2015 and the third crop (C3) was planted on 29 December 2014 and harvested on 25 February 2015.
GC-MS was used to identify the chemical composition of essential oil of coriander plant. Twenty-four compounds were identified (Table 1) and the chemical classification of these 24 compounds were as follows: aldehydes (11 compounds), followed by alcohols (five compounds), esters (two compounds), alkanes (two compounds), monoterpenes (two compounds), terpenoids (one compound), and terpenes alcohol (one compound). The main seven compounds of the essential oil of coriander shoots were: decanal (30.7 mg·kg−1, mean of all treatments), E-2-dodecenal (mean of 26.9 mg·kg−1), dodecanal (22.0 mg·kg−1), octane (18.6 mg·kg−1), 1-decanol (5.13 mg·kg−1), undecanal (4.30 mg·kg−1), and E-2-tridecenal (3.54 mg·kg−1). Given the results in Table 4, decanal, E-2-dodecenal, dodecanal, and octane represented a big percentage of the total concentration of volatile compounds in the essential oil of coriander shoots (70.9%–82.5%). The highest concentrations of E-2-dodecenal (39.9 mg·kg−1), dodecanal (24.4 mg·kg−1), and octane (20.7 mg·kg−1) were found at C2 harvest, while the maximum value of decanal was at C3 with 36.4 mg·kg−1 (Table 4). Nurzyńska-Wierdak [22] reported that the essential oil of the coriander herb contained the highest amount of aliphatic aldehydes, with decanal, E-2-dodecanol, and E-2-decenol having the highest contents. In coriander (Coriandrum sativum L.) the most abundant compounds are E-2-decenal, E-2-dodecenal, decanal, dodecanal, E-2-tridecenal and tetradecenal; these compounds have characteristic green, soapy, and cilantro-like aromas and are particularly important in the overall aroma of the C. sativum herb [23,24]. These results are in concordance to a large extent with the current results.
3.4. Volatile Composition of Essential Oil of Mint
Peppermint (Mentha piperita L.) was planted on 6 October 2014 and the first harvest date was on 11 December 2014 (M1) while the second harvest date was on 5 February 2015 (M2). After isolation of the essential oil of mint shoots, 27 compounds were identified by GC-MS (Table 1). The identified volatile compounds can be grouped in eight main chemical groups: monoterpenes (six compounds), terpenoid alcohols (three compounds), sesquiterpenes (two compounds), aldehydes (one compound), terpenes (three compounds), esters (two compounds), aldehyde (one compound), terpene alcohols (one compound), and polycyclic alkenes (one compound). The eight main compounds were: carvone, limonene, cis-carveol, trans-sabinene hydrate, trans-caryophyllene, myrcene, santene, and trans-β-ocimene.
Volatile composition of mint essential oil at two commercial stages was investigated in the current study and the total concentration of volatile compounds in the essential oil was higher in plants of the commercial stage M1 as compared to those of M2. The contents of the main compound, carvone, also followed this trend (M1 > M2), while the concentrations of limonene and cis-carveol were higher in M2 as compared to M1. For carvone the concentration decreased from 2462 mg·kg−1 in M1 to 1854 mg·kg−1 in (M2) (a decrease of 24.7 %), while the concentration of limonene increased from 590 mg·kg−1 in M1 to 735 mg·kg−1 in M2 (an increase of 24.6 %) (Table 5).
According the previous studies, the harvest date and harvest time can affect the water content, the concentrations of menthol and menthofuran and the yield of limonene, menthol, and menthofuran in Mentha canadensis [25,26]. Besides, in Japanese mint (Mentha arvensis L.) the content of menthol was not affected by the planting date or harvesting schedule but menthone signficantly decreased with the delay in harvesting [27].
The major components of peppermint essential oil were menthol (30.35 %), menthone (21.12 %), and trans-carane (10.99 %) according to previous studies [5]. These results did not agree with the results obtained in the current study. Mentha piperita (peppermint) showed in the composition of its essential oil a higher content of monoterpenes D-carvone (58.79 %) and limonene (28.29 %) [28]. Rohloff [29] found increased levels of other oxygenated monoterpenes and limonene in their work compared to Mentha piperita grown in Norway [28]. These results correspond to a large extent with the results in the current study.
3.5. Descriptive Sensory Evaluation
Volatiles directly affect the sensory quality of fresh fruits, vegetables and aromatic herbs. Within the sensory quality, the odor (perception of volatile compounds with the food outside the mouth) [30] plays an important role, especially in essential oils of aromatic herbs; the aroma is formed by a complex group of chemical substances, which includes aldehydes, alcohols, ketones, esters, lactones, terpenes, among other volatile compounds. The concentration of these volatile compounds is generally low (mg· kg-1) and can be affected by a number of agronomic (variety, climatological conditions, ripening stage) [31,32] and technological (harvest, post-harvest treatments, storage and processing conditions) factors [33]. The quality of the vegetal products can be affected by both too high or too low concentrations of the volatile compounds; thus an equilibrium among them is necessary. Thus, all the information related to descriptive sensory evaluation (DSA), which is going to be shown, corresponds to those samples with the highest concentrations of the volatile compounds present in each essential oil (Table 2, Table 3, Table 4 and Table 5); of course, this is a first step that will need further research but it is a very important step, which is done by the first time and will provide very practical information for farmers. According to this statement, the DSA was carried out with the following samples: D2 (dill harvested at the second commercial stage); P1 (parsley harvested at the first commercial stage); C2 (coriander harvested at the second commercial stage); and M1 (mint harvested at the third commercial stage).
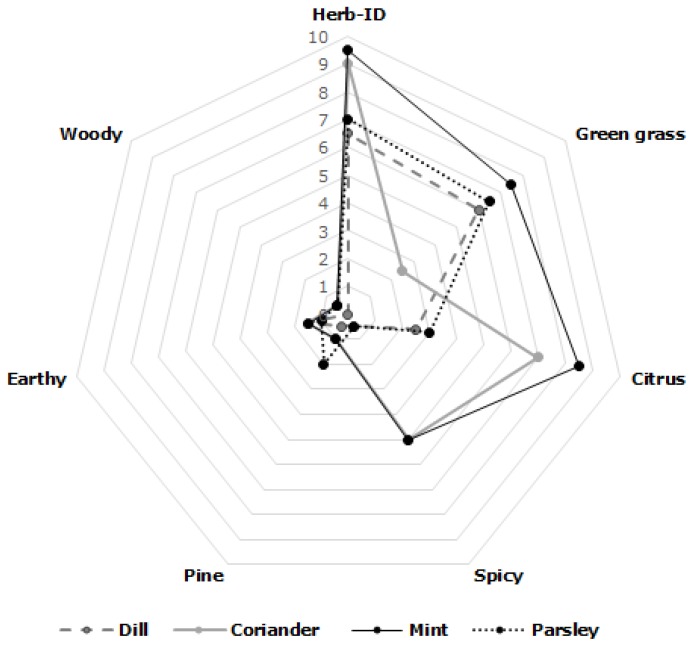
Figure 1 shows the DSA profiles of different aromatic herb samples. The descriptors selected for the DSA were successfully used by this research group in previous studies [2,8,9].
Figure 1.
Descriptive sensory analysis of Spanish aromatic herbs.
Dill samples (D2), with a total number of 18 volatile compounds accounting a total concentration of 649 mg·kg−1 of its essential oil, were characterized by high intensity of dill-ID (herb-ID in Figure 1) (6.5), green grass (6.0), and citrus (2.5) notes (Figure 1). On the other hand, dill samples scored low values of attributes such as spicy (0.5), earthy (1.5), pine (0.5), or woody (0) (Figure 1).
Parsley samples (P1), with total number of 18 volatile compounds accounting a total concentration of 455 mg·kg−1 of its essential oil, were characterized by high intensity of parsley-ID (herb-ID in Figure 1) (7), citrus (3), and green grass (6.5) notes (Figure 1), while undesirable parsley attributes scored low values, for instance spicy (0.5), earthy (1.0), pine (2.0), or woody (0.5) (Figure 1).
Coriander samples (C2), with a total number of 24 volatile compounds accounting a total concentration of 134 mg·kg−1 of its essential oil, were characterized by high intensity of coriander-ID (herb-ID in Figure 1) (9), citrus (7), and spicy (5) and low values of green grass (2.5), earthy (1.5), pine (1.0), or woody (0.5) (Figure 1).
Finally, mint samples (M1), with the highest number of volatile compounds (27) and the higher concentration of these compounds in its essential oils (3326 mg·kg−1), were characterized by high intensity of mint-ID (herb-ID in Figure 1) (9.5), green grass (7.5), citrus (8.5), and spicy (5).
The present study was the first one reporting data on descriptive sensory analysis of aromatic herbs at their optimal harvest time according to the content of volatile compounds of their essential oils. This information is valuable for farmers because the reported data shows the optimal date according to the highest productions of essential oils and high sensory quality.
4. Conclusions
Considering all the data generated in this study, the final recommendation according to the essential oil content and sensory quality is to harvest at the following dates: for dill (11 February 2015) however, for parsley (19 November 2014) while, for coriander (5 January 2015), and for mint (11 December 2014), there are relevant aspects which must subjected to further studies such as, different irrigation treatments, different plant densities, or fertilization conditions. In addition, the effect of pre-harvest treatments with organic compounds may be employed.
Acknowledgments
The author Hussein El-Zaeddi has a scholarship from the Higher Education Ministry of Libya (resolution number 293/2013). Authors acknowledge financial support by Rambla de Los Molinos S.A and Cooperativa Agrícola Católica de Orihuela Sociedad Cooperativa, through research contract UMH 84/2014.
Author Contributions
H.E.Z., J.M.M., F.B., Á.A.C.B., and Á.C.S. planned and designed the experiments; H.E.Z. and Á.C.S. performed the experiments; H.E.Z., J.M.M., F.B., Á.A.C.B., and Á.C.S. analyzed the data; H.E.Z., J.M.M., F.B., Á.A.C.B., and Á.C.S. wrote the manuscript and H.E.Z., Á.A.C.B., and Á.C.S. edited the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Bakkali F., Averbeck S., Averbeck D., Idaomar M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008;46:446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 2.Calín-Sánchez A., Figiel A., Lech K., Szumny A., Martínez-Tomé J., Carbonell-Barrachina A.A. Drying methods affect the aroma of (Origanum majorana L.), analyzed by GC–MS and descriptive sensory analysis. Ind. Crops Prod. 2015;74:218–227. [Google Scholar]
- 3.Huopalahti R., Linko R. Composition and content of aroma compounds in dill, Anethum graveolens L., at three different growth stages. J. Agric. Food Chem. 1983;31:331–333. doi: 10.1021/jf00116a036. [DOI] [Google Scholar]
- 4.Wong P.Y.Y., Kitts D.D. Studies on the dual antioxidant and antibacterial properties of parsley (Petroselinum crispum) and cilantro (Coriandrum sativum) extracts. Food Chem. 2006;97:505–515. doi: 10.1016/j.foodchem.2005.05.031. [DOI] [Google Scholar]
- 5.Tsai M.L., Wu C.T., Lin T.F., Lin W.C., Huang Y.C., Yang C.H. Chemical composition and biological properties of essential oils of two mint species. Trop. J. Pharmac. Res. 2013;12:577–582. doi: 10.4314/tjpr.v12i4.20. [DOI] [Google Scholar]
- 6.Costa S.S., Gariepy Y., Sandra C.S., Rocha C.S.S., Raghavan V. Microwave extraction of mint essential oil—Temperature calibration for the oven. J. Food Eng. 2014;126:1–6. doi: 10.1016/j.jfoodeng.2013.10.033. [DOI] [Google Scholar]
- 7.Viuda-Martos M., Rúiz-Navajas Y., Fernández-López J., Pérez-Álvarez J.A. Spices as functional foods. Crit. Rev. Food Sci. Nutr. 2011;51:13–28. doi: 10.1080/10408390903044271. [DOI] [PubMed] [Google Scholar]
- 8.Calín-Sánchez A., Lech K., Szumny A., Figiel A., Carbonell-Barrachina A.A. Volatile composition of sweet basil essential oil (Ocimum basilicum L.) as affected by drying method. Food Res. Int. 2012;48:217–225. [Google Scholar]
- 9.Calín-Sánchez A., Figiel A., Lech K., Szumny A., Carbonell-Barrachina A.A. Effects of drying methods on the composition of thyme (Thymus vulgaris L.) essential oil. Drying Technol. 2013;31:224–235. [Google Scholar]
- 10.Díaz-Maroto M.C., Pérez-Coello M.S., Sánchez-Palomo E., González-Viñas M.A. Impact of drying and storage time on sensory characteristics of rosemary (Rosmarinus officinalis L.) J. Sens. Stud. 2007;22:34–48. [Google Scholar]
- 11.Lee S.J., Umano K., Shibamoto T., Lee K.G. Identification of volatile components in basil (Ocimum basilicum L.) and thyme leaves (Thymus vulgaris L.) and their antioxidant properties. Food Chem. 2005;91:131–137. doi: 10.1016/j.foodchem.2004.05.056. [DOI] [Google Scholar]
- 12.Angioni A., Barra A., Cereti E., Barile D., Coisson J.D., Arlorio M., Dessi S., Coroneo V., Cabras P. Chemical composition, plant genetic differences, antimicrobial and antifungal activity investigation of the essential of Rosmarinus officinalis L. J. Agric. Food Chem. 2004;52:3530–3535. doi: 10.1021/jf049913t. [DOI] [PubMed] [Google Scholar]
- 13.Khazaie H.R., Nadjafib F., Bannayana M. Effect of irrigation frequency and planting density on herbage biomass and oil production of thyme (Thymus vulgaris) and hyssop (Hyssopus officinalis) Ind. Crops Prod. 2008;27:315–321. doi: 10.1016/j.indcrop.2007.11.007. [DOI] [Google Scholar]
- 14.Callan N.W., Johnson D.L., Westcott M.P., Welty L.E. Herb and oil composition of dill (Anethum graveolens L.): Effects of crop maturity and plant density. Ind. Crops Prod. 2007;25:282–287. doi: 10.1016/j.indcrop.2006.12.007. [DOI] [Google Scholar]
- 15.Petropoulos S.A., Daferera D., Akoumianakis C.A., Passam H.C., Polissiou M.G. The effect of sowing date and growth stage on the essential oil composition of three types of parsley (Petroselinum crispum) J. Sci. Food Agric. 2004;84:1606–1610. doi: 10.1002/jsfa.1846. [DOI] [Google Scholar]
- 16.Radulescu V., Popescu M.L., Ilies D.C. Chemical composition of the volatile oil from different plant parts of Anethum graveolens L. (Umbelliferae) cultivated in Romania. Farmacia. 2010;58:594–600. [Google Scholar]
- 17.Orhan I., Senol F.S., Ozturk N., Celik S.A., Pulur A., Kan Y. Phytochemical contents and enzyme inhibitory and antioxidant properties of Anethum graveolens L. (dill) samples cultivated under organic and conventional agricultural conditions. Food Chem. Toxicol. 2013;59:96–103. doi: 10.1016/j.fct.2013.05.053. [DOI] [PubMed] [Google Scholar]
- 18.Amin W.M.A., Sleem A.A. Chemical and biological study of aerial parts of dill (Anethum graveolens L.) Egyptian J. Biomed. Sci. 2007;23:1–18. doi: 10.4314/ejbs2.v23i1.40296. [DOI] [Google Scholar]
- 19.Vokk R., Lõugas T., Mets K., Kravets M. Dill (Anethum graveolens L.) and Parsley (Petroselinum crispum (Mill.) Fuss) from Estonia: Seasonal Differences in Essential Oil Composition. Agron Res. 2011;9:515–520. [Google Scholar]
- 20.Zlatev S.K. Dynamics of accumulation of the essential oil in the dill (Anethum graveolens Linnaeus) during its ontogenical development. Riv. Ital. Essenze Profumi Piante Off. Aromi Saponi Cosmet. Aerosol. 1975;57:203–209. [Google Scholar]
- 21.El-Gengaihi S.E., Hornok L. The effect of plant age on content and composition of dill essential oil Anethum graveolens L. Acta Hortic. 1978;73:213. doi: 10.17660/ActaHortic.1978.73.26. [DOI] [Google Scholar]
- 22.Nurzyńska-Wierdak R. Essential oil composition of the coriander (Coriandrum sativum L.) Herb depending on the development stage. Acta Agrobotanica. 2013;66:53–60. [Google Scholar]
- 23.Cadwallader K.R., Benitez D., Pajjanapimol S., Suriyaphan O., Singh T. Characteristic aroma components of the cilantro mimics. Nat. Flavors Fragr. 2005;117:128. [Google Scholar]
- 24.Donega M.A., Mello S.C., Moraes R.M., Cantrell C.L. Nutrient uptake, biomass yield and quantitative analysis of aliphatic aldehydes in cilantro plants. Ind. Crops Prod. 2013;44:127–131. doi: 10.1016/j.indcrop.2012.11.004. [DOI] [Google Scholar]
- 25.Shiwakoti S., Sintim H.Y., Poudyal S., Bufalo J., Cantrell C.L., Astatkie T., Jeliazkova E., Ciampa L., Zheljazkov V.D. Diurnal effects of Mentha canadensis oil concentration and composition at two different havests. HortScience. 2015;50:85–89. [Google Scholar]
- 26.Zheljazkov V.D., Cantrell C.L., Astatkie T., Jeliazkova E. Mentha Canadensis L, a subtropical plant, can withstand first few fall frosts when grown in northern climate. Ind. Crops Prod. 2013;49:521–525. doi: 10.1016/j.indcrop.2013.05.034. [DOI] [Google Scholar]
- 27.Brar S.K., Gill B.S., Brar A.S., Kaur T. Planting date and Straw mulch affect biomass yield, oil yield and oil quality of Japanese mint (Mentha arvensis L.) harvested at successive intervals. J. Essent. Oil Bear. Pl. 2014;17:676–695. doi: 10.1080/0972060X.2014.958549. [DOI] [Google Scholar]
- 28.De sousa barros A., De morais S.M., Ferreira P.A.T., Vieira Í.G.P., Craveiro A.A., Dos santos fontenelle R.O., De menezes J.E.S.A., Da Silva F.W.F., De Sousa H.A. Chemical composition and functional properties of essential oils from Mentha species. Ind. Crops Prod. 2015;76:557–564. doi: 10.1016/j.indcrop.2015.07.004. [DOI] [Google Scholar]
- 29.Rohloff J. Monoterpene composition of essential oil from peppermint (Mentha piperita L.) with regard to leaf position using solid-phase microextraction and gas chromatography/mass spectrometry analysis. J. Agric. Food Chem. 1999;47:3782–3786. doi: 10.1021/jf981310s. [DOI] [PubMed] [Google Scholar]
- 30.Alonso A., Vázquez-Araújo L., García-Martínez S., Ruiz J.J., Carbonell-Barrachina A.A. Volatile compounds of traditional and virus-resistant breeding lines of Muchamiel tomatoes. Eur. Food Res. Technol. 2009;230:315–323. doi: 10.1007/s00217-009-1173-2. [DOI] [Google Scholar]
- 31.Melgarejo P., Calín-Sánchez A., Hernández F., Szumny A., Martínez J.J., Legua P., Martínez R., Carbonell-Barrachina A.A. Chemical, functional and quality properties of Japanese plum (Prunus salicina Lindl.) as affected by mulching. Sci. Hort. 2012;134:114–120. doi: 10.1016/j.scienta.2011.11.014. [DOI] [Google Scholar]
- 32.Vendramini A.L., Trugo L.C. Chemical composition of acerola fruit (Malpighia punicifolia L.) at three stages of maturity. Food Chem. 2000;71:195–198. [Google Scholar]
- 33.Gironés-Vilaplana A., Calín-Sánchez Á., Moreno D.A., Carbonell-Barrachina Á.A., García-Viguera C. Novel maqui liquor using traditional pacharán processing. Food Chem. 2015;173:1228–1235. doi: 10.1016/j.foodchem.2014.10.062. [DOI] [PubMed] [Google Scholar]