Abstract
Since the introduction of modern dental implants in the 1980s, the number of inserted implants has steadily increased. Implant systems have become more sophisticated and have enormously enhanced patients’ quality of life. Although there has been tremendous development in implant materials and clinical methods, bacterial infections are still one of the major causes of implant failure. These infections involve the formation of sessile microbial communities, called biofilms. Biofilms possess unique physical and biochemical properties and are hard to treat conventionally. There is a great demand for innovative methods to functionalize surfaces antibacterially, which could be used as the basis of new implant technologies. Present, there are few test systems to evaluate bacterial growth on these surfaces under physiological flow conditions. We developed a flow chamber model optimized for the assessment of dental implant materials. As a result it could be shown that biofilms of the five important oral bacteria Streptococcus gordonii, Streptococcus oralis, Streptococcus salivarius, Porphyromonas gingivalis, and Aggregatibacter actinomycetemcomitans, can be reproducibly formed on the surface of titanium, a frequent implant material. This system can be run automatically in combination with an appropriate microscopic device and is a promising approach for testing the antibacterial effect of innovative dental materials.
Introduction
More than 700 bacterial species inhabit the human oral cavity [1]. While a large proportion of these bacteria are harmless commensals, opportunistic bacteria can trigger common oral diseases like caries, peri-implantitis and the chronic inflammatory disease periodontitis [1, 2]. Most bacteria within the oral cavity are sessile and form highly organised microbial communities, referred to as biofilm, on the surfaces of soft and hard tissues. Bacteria are embedded in a matrix of self-secreted extracellular polymeric substances (EPS) that determine the three dimensional structure of the biofilm [3]. As the EPS matrix shields cells in the biofilm from antimicrobials as well as from the host immune response, sessile bacteria can exhibit up to 5000-fold greater resistance to antibiotics than free floating (planktonic) cells [4]. There are three mechanisms that increase antibiotic resistance: First, the EPS acts as a potent diffusion barrier that antagonizes antibiotic penetration into the biofilm [5, 6]. Second, the sessile life cycle stage is accompanied by a metabolic activity change that reduces the antibiotic uptake [7–11]. Third, via horizontal gene transfer resistance genes can be exchanged between bacteria in biofilms as reported by Roberts et al. [12]. The high antibiotic tolerance makes biofilm infections hard to treat. In spite of intensive research, these bacterial communities still pose a severe medical complication. Therefore, the biofilm-related infections belong to the main reasons for early and late implant loss in dental implantology, as well as in other medical disciplines [13]. Therefore, studies are necessary investigating oral biofilm formation in order to develop implant surfaces that reduce biofilm formation and the risk of implant failure.
Even tough flow chamber systems have been used to study biofilm formation in oral implants research, most studies were conducted under static conditions [14–18]. These require a less sophisticated experimental set-up and show greater ease of handling [19]. However, the physiological flow conditions, as they are found at the dental implantation site, are not considered in these systems. Even though, the application of a static or dynamic system depends on the considered scientific question. The microenvironmental conditions have substantial impact on biofilm morphology and growth behavior as they influence: a) oxygen and nutrients transport processes through the biofilm [20–22], b) transport of signal molecules, e.g. quorum sensing messengers that alternates the biofilm morphology and physiology [23–25], c) heat and mass transfer that coordinates the biotransformation reaction and energy losses [21], d) gene expression and EPS content [26] and e) spreading of biofilms through increasing the mobility of pioneering bacterial cells [26].
Sternberg et al., Besemer et al., Purevdori et al., and Zhang et al. focused on a variety of features of biofilms, including the influence of flow velocity on biofilm morphology and the distribution of bacterial growth activity within a flow chamber system [23, 27–30]. Biofilm behavior was also tested under different chemical and physiological conditions as the pH or the different nutrient and oxygen concentrations in a flow environment [20, 21, 23, 27–30].
Clinical research with flow chamber systems has increased rapidly within the last decade. For example, Zhao et al. developed a model to investigate different implant materials for the suppression of biofilm formation and Chin et al. used a flow chamber system to test the effects of antibacterial agents on orthodontic binding materials [31, 32]. In 2013, da Silva Domingues et al. developed a parallel flow chamber system to elucidate the adherence mechanisms of Staphylococcus aureus and quantify phagocytosis by murine and human macrophages [33]. Even though, dynamic studies of oral biofilm formation and characterization have to be improved. These studies have to include the implant material, implant failure relevant bacteria, reproducible culture and flow conditions.
Biofilm architecture is greatly influenced by the colonising bacterial species or the species composition. The present study focused on a selection of oral biofilm formers that reside within the oral cavity during health and/or disease: S. gordonii, S. oralis, S. salivarius, P. ginigivalis, and A. actinomycetemcomitans. The gram-positive streptococci S. gordonii, S. oralis and S. salivarius are commensal bacteria that, as pioneer colonisers, provide adhesion sites to middle and late colonizing bacterial species [34, 35]. S. salivarius is an opportunistic pathogen that may occasionally cause infections such as caries in man and which is involved in the development of halitosis [36, 37]. One of the most virulent opportunistic pathogenic streptococci is S. oralis. This bacterium expresses a sialidase, an enzyme that hydrolyses the bonds between sialic acids residues and the hexose or hexosamine residues at the terminal side of the oligosaccharides in glycolipids and glycoproteins. In the bacterial host, the enzyme cleaves target structures and thus unfavourably influences cellular processes [38–41]. Moreover, S. oralis causes infective endocarditis and is a major pathogen in immunosuppressed patients [42–45].
P. gingivalis and A. actinomycetemcomitans are rod-shaped, anaerobic, gram-negative bacteria that can cause severe diseases, including periodontitis [46–48].
P. gingivalis secretes inflammatory compounds and toxins that attack host tissue and can lead dysbiosis of the microbial flora [49]. A. actinomycetemcomitans produces virulence factors that allow the migration and invasion of other bacteria, e.g. the expression of leukotoxins. As a result, host periodontal tissue is damaged and the immune response severely weakened [50], [51].
For oral implant materials testing a standardized in vitro biofilm model for a panel of relevant bacterial species is missing. The aim of the study was to establish and evaluate a flow chamber system for this purpose. The experimental parameters growth environment, cultivation duration and nutrient supply had to be optimized for the biofilm formers, S. gordonii, S. oralis, S. salivarius, P. gingivalis and A. actinomycetemcomitans to give a reproducible and robust biofilm formation in vitro.
Materials and methods
Bacterial strains
S. gordonii DSM 20568, S. salivarius DSM 20067 and P. gingivalis DSM 20709 were obtained from the German Collection of Microorganisms and Cell Cultures (DSMZ). The bacterial strains S. oralis ATCC 9811 and A. actinomycetemcomitans ATCC 2474 were purchased from the American Type Culture Collection (ATCC).
Bacterial cultivation and biofilm formation
S. gordonii, S. salivarius and S. oralis were routinely precultured in Tryptic Soy Broth (TSB; Oxoid, Unipath Ltd., Wesel, Germany), supplemented with 10% yeast extract (Roth, Karlsruhe, Germany), aerobically at 37°C in an incubator shaker (200 min-1, SM-30, Bühler, Utzwil, Switzerland) for 18 h (S. gordonii, S. salivarius) or 24 h (S. oralis). For the induction of biofilm formation, S. gordonii and S. salivarius cultures were adjusted to an optical density (OD600; BioPhotometer, Eppendorf, Hamburg, Germany) of 0.016 in TSB medium modified by addition of 50 mM glucose (Roth) and stirred (VMS-C7 advanced, VWR; Darmstadt, Germany) for 24 h at 37°C for biofilm formation. This OD600 corresponds to an inoculum of: 1.94 x 106 colony forming units CFU/mL for S. gordonii and 4.19 x 106 CFU/mL for S. salivarius, respectively. For S. oralis, the procedure was identical, except that an OD600 of 0.026 was chosen (3.5 x 106 CFU/mL) and that Brain Heart Infusion (BHI, Oxoid, Unipath Ltd., Wesel, Germany) medium supplemented with 5% sucrose (Roth) and 10 μg/mL vitamin K (Roth) was used as nutrient broth. S. oralis biofilm was also cultivated for 24 h.
P. gingivalis was cultured anaerobically (10% carbon dioxide, 10% hydrogen, 80% nitrogen) in BHI medium modified with 10 μg/mL of vitamin K for 24 h without agitation at 37°C, followed by culture in an incubator shaker (200 min-1) for 48 h. For biofilm formation experiments, the cultures were adjusted to an OD600 of 0.0375 (7.88 x 106 CFU/mL) in BHI medium supplemented with 5% sucrose and 10 μg/mL vitamin K. The bacterial suspension was anaerobically grown under continuous stirring for 48 h at 37°C for biofilm formation.
A. actinomycetemcomitans was routinely cultured anaerobically in Schaedler bouillon (Oxoid, Unipath Ltd., Wesel, Germany) supplemented with 10 μg/mL vitamin K at 37°C under agitation (200 min-1) for 48 h and was then transferred to an Erlenmeyer flask to be cultured for an additional 48 h under continuous stirring. To induce biofilm formation by A. actinomycetemcomitans, cultures were adjusted to an OD600 of 0.0319 (1.25 x106 CFU/mL) in Schaedler bouillon supplemented with 10 μg/mL vitamin K and anaerobically cultured for 72 h at 37°C under continuous stirring for biofilm formation. All experiments modified with vitamin K were protected from light to avoid the destruction of the compound by light. For the anaerobic cultivation of P. gingivalis and A. actinomycetemcomitans, the bioreactor and the flow chamber system were evacuated twice and subsequently flushed with anaerobic gas mix (10% hydrogen, 10% carbon dioxide, 80% nitrogen). The system was kept pressurized by connecting a gas filled balloon to the bioreactor. Thus the inflow of oxygen was inhibited throughout the experiment.
Flow chamber system
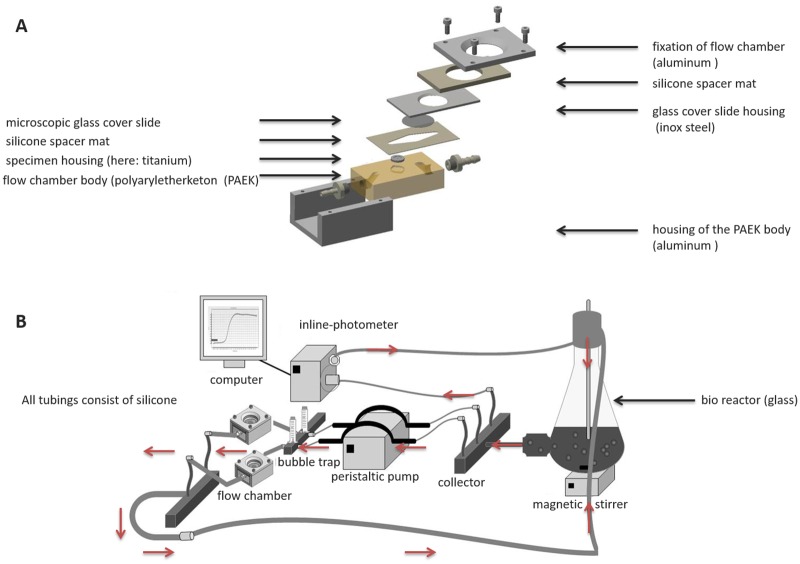
The flow chamber devices were 7.0 cm x 5.5 cm x 3.5 cm in size and were equipped with a 28 mm glass cover slip (Thermo Scientific, Waltham, USA) to allow direct macro- and microscopic analysis of biofilm formation on test surfaces (Fig 1). Titanium discs (grade 4) with a diameter of 12 mm were used as specimens. Each of these underwent surface treatment with a 45 μm diamond abrasives grinding disc to generate a uniform surface pattern for bacterial adhesion. The bacterial suspension was recirculated from the bioreactor to the flow chambers and back at a flow rate of 100 μL/min (IPC-16 peristaltic pump, Ismatec, Wertheim, Germany). The flow chamber system was kept air-free by use of bubble traps. To monitor bacterial growth in the bioreactor, the OD600 was continuously recorded during the experiment using an inline photometer (Elo-Check, biotronix, Hennigsdorf, Germany). Each flow chamber experiment was repeated independently five times with at least three technical replicates.
Fig 1. Sketch of the flow chamber and flow chamber system.
A) Assembly drawing with test specimen; B) General setup of the closed circuit system. The red arrows indicate the direction of the flow.
Biofilm imaging and analysis
The flow chambers were separated from the system by surgical clamps at the collector’s site. New sterile tubings were connected to the tubings of the peristaltic pump. Biofilms were washed by pumping phosphate buffered saline (PBS, Dulbecco`s Media, Sigma Aldrich, Hannover-Seelze, Germany) through the flow chambers to remove planktonic bacteria. The biofilms were specifically stained live/dead by flushing with a 1:1000 dilution of BacLight staining mix (Life Technologies, Darmstadt, Germany). Syto 9 is a green fluorescent dye that passes bacterial cell membranes by diffusion and intercalates unspecifically into bacterial DNA. Propidium iodide is a red fluorescent dye that, due to its size, cannot pass intact cell membranes. Dye intercalation is only observed in dead cells in which membrane integrity is impaired. The bacteria were stained for 15 min at a flow rate of 100 μL/min in the dark and subsequently examined by confocal laser scanning microscopy (CLSM, Leica-Upright MP microscope connected to a TCS SP2 AOBS scan head, Leica, Wetzlar, Germany). From each specimen, image stacks were acquired at five different positions; centre, right, left, top and bottom, using 10x magnification. The Imaris 3D image processing software (Version 6.2.1, Bitplane, Oxford instruments, Zurich, Switzerland) was used to calculate mean biofilm height.
Statistical analysis
The experiments were performed five times for each bacterial species, and the mean biofilm height and standard errors were calculated. The mean biofilm heights between independent biological replicates were compared using the univariate ANOVA test with a significance level of 0.05. All statistical analyses were conducted using the software package SPSS (v23.0.0, IBM, Armonk, USA).
Results
The flow chamber system
The flow chamber system was successfully manufactured according to the construction plans. An assembly drawing of the chamber is presented in Fig 1A. As test specimen titanium discs were used. To allow for macro-and microscopical specimen observation, the stainless steel cover plate had been manufactured with a circular recess, housing a microscopic coverglass. In Fig 1B, the complete setup of a flow chamber in a continuous flow circuit is depicted. The growth conditions were successfully adjusted under aerobic and anaerobic conditions as seen by the formation of stable, mature biofilms on the titanium specimens (Fig 2).
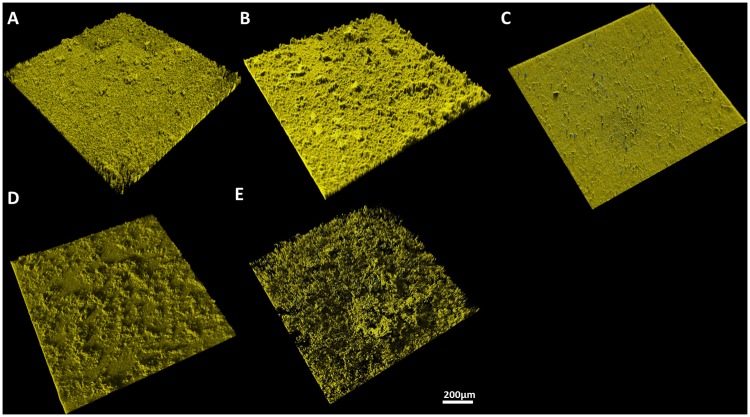
Fig 2. 3D reconstruction of biofilms in side view.
The cells were stained live/dead and analysed by CLSM. Vital cells are depicted in yellow, viable cells in blue. A) S. gordonii, B) S. oralis, C) S. salivarius, D) P. gingivalis, and E) A. actinomycetemcomitans.
Micro- and macroscopic evaluation of biofilm formation
Biofilm formation of S. gordonii, S. oralis, S. salivarius, P. gingivalis and A. actinomycetemcomitans was evaluated under constant flow conditions. A confluent biofilm formation was observed for all tested bacterial species. The bacterial cultures in the bioreactor showed normal growth behavior during the experiment and the resulting biofilms were structurally intact and composed of predominantly vital cells (Fig 2A–2E). For all species, biofilm formation was highly reproducible between independent experiments. However, the observed biofilm morphologies were unique to the individual species. In detail, S. gordonii (A) and S. salivarius (C) biofilms showed relatively homogenous surface pattern interspersed with a few tower-like structures. S. oralis (B) biofilms demonstrated an uneven surface pattern with numerous tower-like structures. The P. gingivalis (D) biofilm showed a rough biofilm surface with marcocolonies. The biofilm of A. actinomycetemcomitans (E) demonstrated an open and loose microbial biofilm structure.
Throughout all experiments, the biofilms exhibited high structural stability and no visible detachment was detected during sample preparation for microscopic analysis.
Calculation of mean biofilm height
Mean biofilm heights were determined for the five bacterial species tested. For each species, the reproducibility of biofilm formation between the independent experiments was statistically significant (Fig 3). The greatest mean biofilm height was observed for P. gingivalis with 38.85 μm (p = 5.2 x 10−13), followed by A. actinomycetemcomitans with 28.9 μm (p = 0.01), S. oralis with 28.5 μm (p = 0.003), S. salivarius with 26.5 μm (p = 0.008), and S. gordonii with 21.1 μm (p = 0.000009).
Fig 3. Biofilm heights on titanium substrata in the flow chamber system.
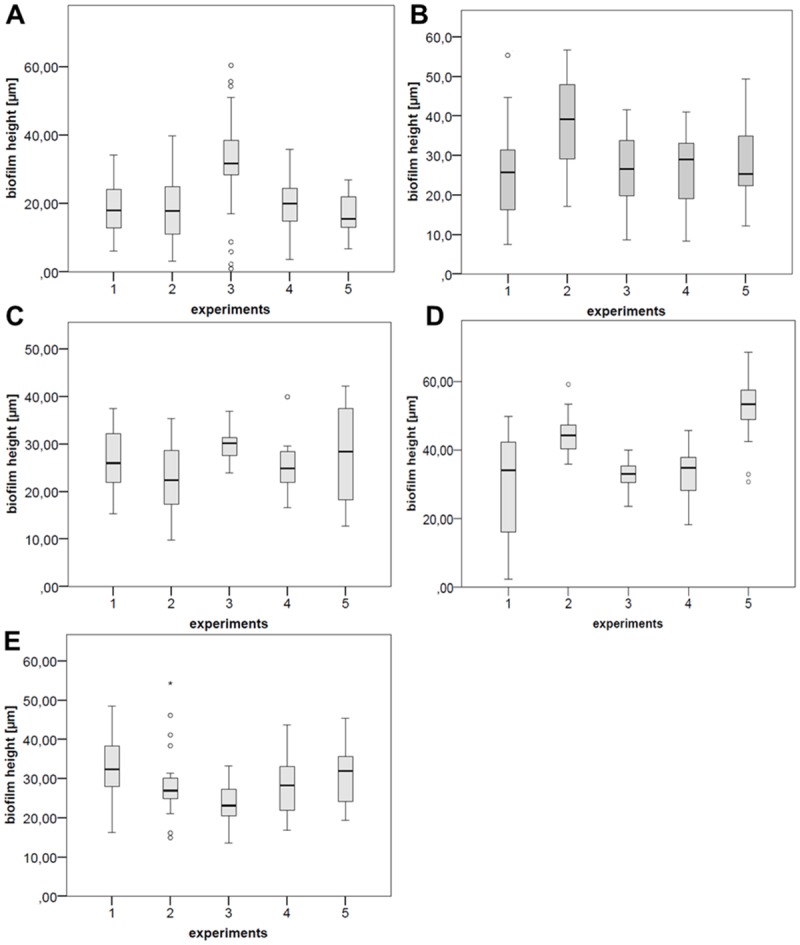
In each diagram, the mean biofilm heights for five independent experiments are shown. A = S. gordonii, B = S. oralis, C = S. salivarius, D = P. gingivalis, and E = A. actinomycetemcomitans.
Discussion
Many flow chamber systems have been developed within recent decades to analyse biofilm formation and dynamics in a fluid system. Our flow chamber system was optimized for the experimental investigation of dental relevant bacteria on the implant material titanium, as this is an important approach to prevent implant failure due to biofilm-associated infections. Within the presented flow chamber system, the replacement of the titanium disc with other disc-shaped sample materials of interest is applicable without time-consuming reconstruction measures. The complete flow chamber system is reusable and fully autoclavable. The cultivation setup can easily be modified according to the optimal growth conditions of the respective bacterial species under aerobic as well as anaerobic conditions.
We focused on a group of bacteria, consisting of oral commensal and periodontopathogenic bacteria: S. gordonii, S. oralis, S. salivarius, P. gingivalis, and A. actinomycetemcomitans. Previous studies have mostly described biofilm formation under static test conditions [52, 53], but in vivo the flow has great influence on the of the biofilm behavior. Therefore, fluid systems have been established to mimic the physiological flow conditions within the oral cavity [54]. To achieve a realistic experimental setup, we chose a flow speed of 100 μL/min, as it is described for the natural saliva flow in the hibernation mode [55–57]. The bacterial suspension was pumped continuously over the test specimens as in the oral cavity bacteria-contaminated saliva is permanently flooding the implant.
All bacterial species exhibited reproducible and homogenous growth behavior under the given flow conditions, as confirmed by 3D biofilm reconstruction from CLSM image stacks and statistical analyzes.
In contrast to the flow chamber systems of Weiger et al., Hauser-Gerspach et al., Meier et al., and Diaz et al., our test procedure allows direct investigation of biofilm formation for five bacterial species [56, 58–64]. The test specimens were not removed from the chambers for microscopic observation, so that detachment effects were minimised and biofilm quantification was highly precise. Only a few studies have analysed biofilm development for oral health relevant bacteria in a flow system [56, 58–64]. To the best of our knowledge, no other study has focused on the described group of bacteria for microbial adhesion testing in one flow chamber system.
The biofilm morphologies of the applied bacterial species have already been in other studies: In accordance to the study of Diaz et al., a homogeneously flat biofilm morphology with a few tower-shaped structures was observed for S. gordonii. However, contrasting to our study the bacteria were visualized by fluorescent in situ hybridization (FISH) [56].
For S. oralis Paramonova et al. designed a flow chamber system to analyse the influence of shear stress on biofilm formation [61]. The biofilm height of S. oralis was enhanced with increasing shear stress. The observed biofilm morphology of S. oralis showed a typical tower-like biofilm with a rough surface comparable to our study.
The S. salivarius biofilm morphology was homogenously flat, as also shown in the study of Gashti et al. [65]. However, they used microfluidic flow chambers, and focused primarily on the influence of pH on biofilm formation.
Davey et al. designed their flow chamber model for P. gingivalis according to Christensen et al. [64, 66]. They also observed a rough surface morphology with macrocolonies within the biofilm. Analogously to our experiments, the biofilm was grown under anaerobic conditions. However, the mean biofilm height was about five times higher compared to our study. These findings can be attributed to the different flow chamber design and the differing cultivation conditions. Davey et al. grew biofilms for 96 h compared to 48 h in our experiment [64]. Additionally, Davey et al. analyzed the living fraction of the biofilm by staining with SYTO9.
The biofilm morphology of A. actinomycetemcomitans showed an open and soft microbial architecture on the titanium substratum. The same findings were described by Sliepen et al. [63]. In contrast to our method, they tagged A. actinomycetemcomitans with a green fluorescent protein to analyze the biofilm formation by CLSM. However, it cannot be completely ruled out that this genetic modification may have influenced the adhesion behavior and biofilm formation.
Finally, in the present study, the reproducibility of the bacterial biofilm height was given in all our experiments.
Biofilm formation takes place throughout the whole oral cavity. However, daily oral hygiene measures reduce the amount of attached biofilms in the oral cavity. Especially at the interfaces gum/tooth or gum/implant bacteria begin to accumulate and form biofilms. If not removed, biofilms cause swelling and detachment of the gums from teeth or implants. Biofilms further proliferate in the formed periodontal pockets. As these are not isolated compartments but are connected to the oral cavity, biofilms are exposed to a low flow environment rather than static conditions. Therefore, the described flow chamber model approximates the in vivo situation, which is crucial for evaluation of surfaces intended to be used in the oral cavity. In conclusion, the here developed flow chamber system, in combination with CLSM-based biofilm quantification, proved to be a reliable instrument for the analysis of biofilm height and the formation of bacterial biofilms that are relevant in dentistry. Under the given experimental setting, the flow chambers can be used for evaluation of bacterial colonisation behavior of implant materials for the oral cavity. In further studies, the system will be optimised for studies of the formation oral multispecies biofilms, which is closer to the actual situation in the human mouth. Other interesting aspects will be the investigation of the influence of different flow velocities, nutrient concentrations and substrata on the biofilm formation.
Supporting information
Complementary information of all biofilm height data in μm are accessible. The tables are subdivided in live and dead bacterial biofilm populations.
(DOCX)
Acknowledgments
Henryke Rath was financially supported provided by the Ministry of Science and Culture of Lower Saxony, within the framework of the Doctoral Program MARIO (Multifunctional Active and Reactive Interfaces and Surfaces). Additionally, we thank Dr. Andeas Winkel, Dr. Sebastian Grade and Marly Felicia Dalton for statistical and technical support. The authors declare no conflicts of interest related to this study.
Data Availability
All biofilm files are available from the Zenodo database (DOI:10.5281/zenodo.259314).
Funding Statement
Henryke Rath was funded by Multifunktionale aktive und reaktive Interfaces und Oberflächen. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43(11):5721–32. 10.1128/JCM.43.11.5721-5732.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raj L S M, Jude J, I K, Krishna P S, Shankar K A S. Molecular Docking Study for Inhibitors of Aggregatibacter actinomycetamcomitans Toxins in Treatment of Aggressive Perioodontitis. J Clin Diagn Res. 2014;8(11):ZC48–51. 10.7860/JCDR/2014/10067.5133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costerton JW, Montanaro L, Arciola CR. Biofilm in implant infections: its production and regulation. Int J Artif Organs. 2005;28(11):1062–8. [DOI] [PubMed] [Google Scholar]
- 4.Costerton JW, Cheng KJ, Geesey GG, Ladd TI, Nickel JC, Dasgupta M, et al. Bacterial biofilms in nature and disease. Annu Rev Microbiol. 1987;41:435–64. Epub 1987/01/01. 10.1146/annurev.mi.41.100187.002251 [DOI] [PubMed] [Google Scholar]
- 5.Donlan RM. Biofilm formation: a clinically relevant microbiological process. Clin Infect Dis. 2001;33(8):1387–92. 10.1086/322972 [DOI] [PubMed] [Google Scholar]
- 6.Hoyle BD, Wong CK, Costerton JW. Disparate efficacy of tobramycin on Ca(2+)-, Mg(2+)-, and HEPES-treated Pseudomonas aeruginosa biofilms. Can J Microbiol. 1992;38(11):1214–8. [DOI] [PubMed] [Google Scholar]
- 7.Duguid IG, Evans E, Brown MR, Gilbert P. Effect of biofilm culture upon the susceptibility of Staphylococcus epidermidis to tobramycin. J Antimicrob Chemother. 1992;30(6):803–10. [DOI] [PubMed] [Google Scholar]
- 8.Duguid IG, Evans E, Brown MR, Gilbert P. Growth-rate-independent killing by ciprofloxacin of biofilm-derived Staphylococcus epidermidis; evidence for cell-cycle dependency. J Antimicrob Chemother. 1992;30(6):791–802. [DOI] [PubMed] [Google Scholar]
- 9.Stewart PS. Antimicrobial Tolerance in Biofilms. Microbiol Spectr. 2015;3(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stewart PS. Mechanisms of antibiotic resistance in bacterial biofilms. Int J Med Microbiol. 2002;292(2):107–13. 10.1078/1438-4221-00196 [DOI] [PubMed] [Google Scholar]
- 11.Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001;358(9276):135–8. [DOI] [PubMed] [Google Scholar]
- 12.Roberts AP, Pratten J, Wilson M, Mullany P. Transfer of a conjugative transposon, Tn5397 in a model oral biofilm. FEMS Microbiol Lett. 1999;177(1):63–6. [DOI] [PubMed] [Google Scholar]
- 13.Paquette DW, Brodala N, Williams RC. Risk factors for endosseous dental implant failure. Dent Clin North Am. 2006;50(3):361–74, vi 10.1016/j.cden.2006.05.002 [DOI] [PubMed] [Google Scholar]
- 14.Wolfaardt GM, Lawrence JR, Robarts RD, Caldwell SJ, Caldwell DE. Multicellular organization in a degradative biofilm community. Appl Environ Microbiol. 1994;60(2):434–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kroukamp O, Dumitrache RG, Wolfaardt GM. Pronounced effect of the nature of the inoculum on biofilm development in flow systems. Appl Environ Microbiol. 2010;76(18):6025–31. 10.1128/AEM.00070-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shemesh J, Jalilian I, Shi A, Heng Yeoh G, Knothe Tate ML, Ebrahimi Warkiani M. Flow-induced stress on adherent cells in microfluidic devices. Lab Chip. 2015;15(21):4114–27. 10.1039/c5lc00633c [DOI] [PubMed] [Google Scholar]
- 17.Fux CA, Costerton JW, Stewart PS, Stoodley P. Survival strategies of infectious biofilms. Trends Microbiol. 2005;13(1):34–40. 10.1016/j.tim.2004.11.010 [DOI] [PubMed] [Google Scholar]
- 18.Loo CY, Corliss DA, Ganeshkumar N. Streptococcus gordonii biofilm formation: identification of genes that code for biofilm phenotypes. J Bacteriol. 2000;182(5):1374–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilmore KS, Srinivas P, Akins DR, Hatter KL, Gilmore MS. Growth, development, and gene expression in a persistent Streptococcus gordonii biofilm. Infect Immun. 2003;71(8):4759–66. 10.1128/IAI.71.8.4759-4766.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Beer D, Stoodley P, Lewandowski Z. Liquid flow in heterogeneous biofilms. Biotechnol Bioeng. 1994;44(5):636–41. 10.1002/bit.260440510 [DOI] [PubMed] [Google Scholar]
- 21.Stoodley P, Dodds I, Boyle JD, Lappin-Scott HM. Influence of hydrodynamics and nutrients on biofilm structure. J Appl Microbiol. 1998;85 Suppl 1:19S–28S. [DOI] [PubMed] [Google Scholar]
- 22.Park, Joeong, Lee, Kim, Lee. Effect of shear stress on the formation of bacterial biofilm in a microfluidic channel. BioChip J.2011. p. 236–41. [Google Scholar]
- 23.Purevdorj B, Costerton JW, Stoodley P. Influence of hydrodynamics and cell signaling on the structure and behavior of Pseudomonas aeruginosa biofilms. Appl Environ Microbiol. 2002;68(9):4457–64. 10.1128/AEM.68.9.4457-4464.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Kievit TR, Iglewski BH. Bacterial quorum sensing in pathogenic relationships. Infect Immun. 2000;68(9):4839–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller MB, Bassler BL. Quorum sensing in bacteria. Annu Rev Microbiol. 2001;55:165–99. 10.1146/annurev.micro.55.1.165 [DOI] [PubMed] [Google Scholar]
- 26.Kim J, Park HD, Chung S. Microfluidic approaches to bacterial biofilm formation. Molecules. 2012;17(8):9818–34. 10.3390/molecules17089818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Besemer K, Singer G, Limberger R, Chlup AK, Hochedlinger G, Hödl I, et al. Biophysical controls on community succession in stream biofilms. Appl Environ Microbiol. 2007;73(15):4966–74. 10.1128/AEM.00588-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sternberg C, Christensen BB, Johansen T, Toftgaard Nielsen A, Andersen JB, Givskov M, et al. Distribution of bacterial growth activity in flow-chamber biofilms. Appl Environ Microbiol. 1999;65(9):4108–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang W, Sileika TS, Chen C, Liu Y, Lee J, Packman AI. A novel planar flow cell for studies of biofilm heterogeneity and flow-biofilm interactions. Biotechnol Bioeng. 2011;108(11):2571–82. 10.1002/bit.23234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Islam N, Kim Y, Ross JM, Marten MR. Proteomic analysis of Staphylococcus aureus biofilm cells grown under physiologically relevant fluid shear stress conditions. Proteome Sci. 2014;12:21 10.1186/1477-5956-12-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao B, van der Mei HC, Subbiahdoss G, de Vries J, Rustema-Abbing M, Kuijer R, et al. Soft tissue integration versus early biofilm formation on different dental implant materials. Dent Mater. 2014;30(7):716–27. 10.1016/j.dental.2014.04.001 [DOI] [PubMed] [Google Scholar]
- 32.Chin MY, Busscher HJ, Evans R, Noar J, Pratten J. Early biofilm formation and the effects of antimicrobial agents on orthodontic bonding materials in a parallel plate flow chamber. Eur J Orthod. 2006;28(1):1–7. 10.1093/ejo/cji094 [DOI] [PubMed] [Google Scholar]
- 33.da Silva Domingues JF, van der Mei HC, Busscher HJ, van Kooten TG. Phagocytosis of bacteria adhering to a biomaterial surface in a surface thermodynamic perspective. PLoS One. 2013;8(7):e70046 10.1371/journal.pone.0070046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.schonfeld ES. Contemporary oral microbiology and immunology. Slots J: Mosby Year. Book, St. Louis; 1992. p. 267–74. [Google Scholar]
- 35.Cook GS, Costerton JW, Lamont RJ. Biofilm formation by Porphyromonas gingivalis and Streptococcus gordonii. J Periodontal Res. 1998;33(6):323–7. [DOI] [PubMed] [Google Scholar]
- 36.Becker MR, Paster BJ, Leys EJ, Moeschberger ML, Kenyon SG, Galvin JL, et al. Molecular analysis of bacterial species associated with childhood caries. J Clin Microbiol. 2002;40(3):1001–9. Epub 2002/03/07. 10.1128/JCM.40.3.1001-1009.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sterer N, Rosenberg M. Streptococcus salivarius promotes mucin putrefaction and malodor production by Porphyromonas gingivalis. J Dent Res. 2006;85(10):910–4. [DOI] [PubMed] [Google Scholar]
- 38.Alam S, Brailsford SR, Adams S, Allison C, Sheehy E, Zoitopoulos L, et al. Genotypic heterogeneity of Streptococcus oralis and distinct aciduric subpopulations in human dental plaque. Appl Environ Microbiol. 2000;66(8):3330–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Byers HL, Tarelli E, Homer KA, Beighton D. Isolation and characterisation of sialidase from a strain of Streptococcus oralis. J Med Microbiol. 2000;49(3):235–44. 10.1099/0022-1317-49-3-235 [DOI] [PubMed] [Google Scholar]
- 40.Rothe B, Roggentin P, Schauer R. The sialidase gene from Clostridium septicum: cloning, sequencing, expression in Escherichia coli and identification of conserved sequences in sialidases and other proteins. Molecular & general genetics: MGG. 1991;226(1–2):190–7. Epub 1991/04/01. [DOI] [PubMed] [Google Scholar]
- 41.Kelm S, Schauer R. Sialic acids in molecular and cellular interactions. International review of cytology. 1997;175:137–240. Epub 1997/01/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Douglas CW, Heath J, Hampton KK, Preston FE. Identity of viridans streptococci isolated from cases of infective endocarditis. J Med Microbiol. 1993;39(3):179–82. 10.1099/00222615-39-3-179 [DOI] [PubMed] [Google Scholar]
- 43.Hardie JM. Recent developments in streptococcal taxonomy: their relation to infections. In: W RA, editor. Rev Med Microbiol.1994. p. 151–62. [Google Scholar]
- 44.Beighton D, Carr AD, Oppenheim BA. Identification of viridans streptococci associated with bacteraemia in neutropenic cancer patients. J Med Microbiol. 1994;40(3):202–4. 10.1099/00222615-40-3-202 [DOI] [PubMed] [Google Scholar]
- 45.West PW, Al-Sawan R, Foster HA, Electricwala Q, Alex A, Panigrahi D. Speciation of presumptive viridans streptococci from early onset neonatal sepsis. J Med Microbiol. 1998;47(10):923–8. 10.1099/00222615-47-10-923 [DOI] [PubMed] [Google Scholar]
- 46.Holt SC, Ebersole J, Felton J, Brunsvold M, Kornman KS. Implantation of Bacteroides gingivalis in nonhuman primates initiates progression of periodontitis. Science. 1988;239(4835):55–7. [DOI] [PubMed] [Google Scholar]
- 47.Haffajee AD, Socransky SS. Microbial etiological agents of destructive periodontal diseases. Periodontol 2000. 1994;5:78–111. [DOI] [PubMed] [Google Scholar]
- 48.Zambon JJ. Actinobacillus actinomycetemcomitans in human periodontal disease. J Clin Periodontol. 1985;12(1):1–20. [DOI] [PubMed] [Google Scholar]
- 49.Duran-Pinedo AE, Chen T, Teles R, Starr JR, Wang X, Krishnan K, et al. Community-wide transcriptome of the oral microbiome in subjects with and without periodontitis. Isme j. 2014;8(8):1659–72. Epub 2014/03/07. 10.1038/ismej.2014.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Henderson B, Nair SP, Ward JM, Wilson M. Molecular pathogenicity of the oral opportunistic pathogen Actinobacillus actinomycetemcomitans. Annu Rev Microbiol. 2003;57:29–55. 10.1146/annurev.micro.57.030502.090908 [DOI] [PubMed] [Google Scholar]
- 51.Lally ET, Kieba IR, Sato A, Green CL, Rosenbloom J, Korostoff J, et al. RTX toxins recognize a beta2 integrin on the surface of human target cells. The Journal of biological chemistry. 1997;272(48):30463–9. Epub 1997/12/31. [DOI] [PubMed] [Google Scholar]
- 52.Merritt JH, Kadouri DE, O'Toole GA. Growing and analyzing static biofilms. Current protocols in microbiology. 2005;Chapter 1:Unit 1B. Epub 2008/09/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Waters EM, McCarthy H, Hogan S, Zapotoczna M, O'Neill E, O'Gara JP. Rapid quantitative and qualitative analysis of biofilm production by Staphylococcus epidermidis under static growth conditions. Methods in molecular biology (Clifton, NJ). 2014;1106:157–66. Epub 2013/11/14. [DOI] [PubMed] [Google Scholar]
- 54.Rijnaarts HH, Norde W, Bouwer EJ, Lyklema J, Zehnder AJ. Bacterial Adhesion under Static and Dynamic Conditions. Appl Environ Microbiol. 1993;59(10):3255–65. Epub 1993/10/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chalmers NI. Multispecies Oral Biofilms Studied at the Single Community Level as a Model System for Spatiotemporal Development of Biofilms and Interspecies Interaction: University of Maryland Baltimore; 2008. [Google Scholar]
- 56.Diaz PI, Xie Z, Sobue T, Thompson A, Biyikoglu B, Ricker A, et al. Synergistic interaction between Candida albicans and commensal oral streptococci in a novel in vitro mucosal model. Infect Immun. 2012;80(2):620–32. 10.1128/IAI.05896-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cuadra-Saenz G, Rao DL, Underwood AJ, Belapure SA, Campagna SR, Sun Z, et al. Autoinducer-2 influences interactions amongst pioneer colonizing streptococci in oral biofilms. Microbiology. 2012;158(Pt 7):1783–95. Epub 2012/04/05. 10.1099/mic.0.057182-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weiger R, Decker EM, Krastl G, Brecx M. Deposition and retention of vital and dead Streptococcus sanguinis cells on glass surfaces in a flow-chamber system. Arch Oral Biol. 1999;44(8):621–8. [DOI] [PubMed] [Google Scholar]
- 59.Hauser-Gerspach I, Kulik EM, Weiger R, Decker EM, Von Ohle C, Meyer J. Adhesion of Streptococcus sanguinis to dental implant and restorative materials in vitro. Dental materials journal. 2007;26(3):361–6. Epub 2007/08/19. [DOI] [PubMed] [Google Scholar]
- 60.Meier R, Hauser-Gerspach I, Luthy H, Meyer J. Adhesion of oral streptococci to all-ceramics dental restorative materials in vitro. J Mater Sci Mater Med. 2008;19(10):3249–53. Epub 2008/05/13. 10.1007/s10856-008-3457-7 [DOI] [PubMed] [Google Scholar]
- 61.Paramonova E, Kalmykowa OJ, van der Mei HC, Busscher HJ, Sharma PK. Impact of hydrodynamics on oral biofilm strength. J Dent Res. 2009;88(10):922–6. 10.1177/0022034509344569 [DOI] [PubMed] [Google Scholar]
- 62.Gowrishankar, Kamaladevi, Ayyanar, Balamurugan, Pandian. Bacillus amyloliquefaciens-secreted cyclic dipeptide—cyclo(L-leucyl-L-prolyl) inhibits biofilm and virulence production in methicillin-resistant Staphylococcus aureus. RSC Adv.2015. p. 95788–804. [Google Scholar]
- 63.Sliepen I, Hofkens J, Van Essche M, Quirynen M, Teughels W. Aggregatibacter actinomycetemcomitans adhesion inhibited in a flow cell. Oral Microbiol Immunol. 2008;23(6):520–4. 10.1111/j.1399-302X.2008.00456.x [DOI] [PubMed] [Google Scholar]
- 64.Davey ME. Techniques for the growth of Porphyromonas gingivalis biofilms. Periodontol 2000. 2006;42:27–35. 10.1111/j.1600-0757.2006.00183.x [DOI] [PubMed] [Google Scholar]
- 65.Gashti MP, Asselin J, Barbeau J, Boudreau D, Greener J. A microfluidic platform with pH imaging for chemical and hydrodynamic stimulation of intact oral biofilms. Lab Chip. 2016;16(8):1412–9. 10.1039/c5lc01540e [DOI] [PubMed] [Google Scholar]
- 66.Christensen BB, Sternberg C, Andersen JB, Eberl L, Moller S, Givskov M, et al. Establishment of new genetic traits in a microbial biofilm community. Appl Environ Microbiol. 1998;64(6):2247–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Complementary information of all biofilm height data in μm are accessible. The tables are subdivided in live and dead bacterial biofilm populations.
(DOCX)
Data Availability Statement
All biofilm files are available from the Zenodo database (DOI:10.5281/zenodo.259314).