Abstract
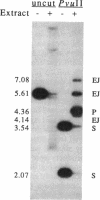
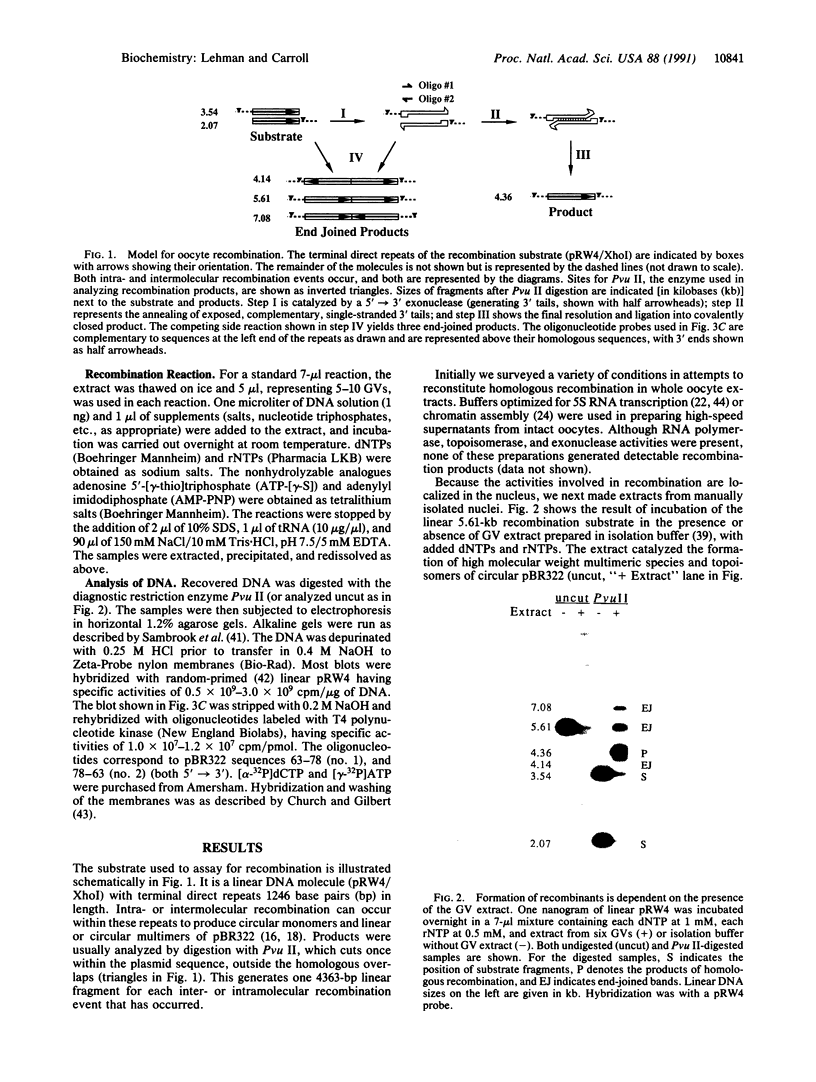
Xenopus laevis oocytes efficiently recombine linear DNA injected into their nuclei (germinal vesicles). This process requires homologous sequences at or near the molecular ends. Here we report that a cell-free extract made from germinal vesicles is capable of accomplishing the complete recombination reaction in vitro. Like the in vivo process, the extract converts the overlapping ends of linear substrate molecules into covalently closed products. Establishment of this cell-free system has allowed examination of the cofactors required for recombination. The first step involves a 5'----3' exonuclease activity that requires a divalent cation but not NTPs. Completion of recombination requires a hydrolyzable NTP; maximal product formation occurs in the presence of millimolar levels of ATP or dATP. At submillimolar levels of all four dNTPs, homologous recombination is inefficient, and a side reaction produces end-joined products. This cell-free system should facilitate a step-by-step understanding of an homologous recombination pathway that operates not only in Xenopus laevis oocytes but also in cells from a wide variety of organisms.
Full text
PDF
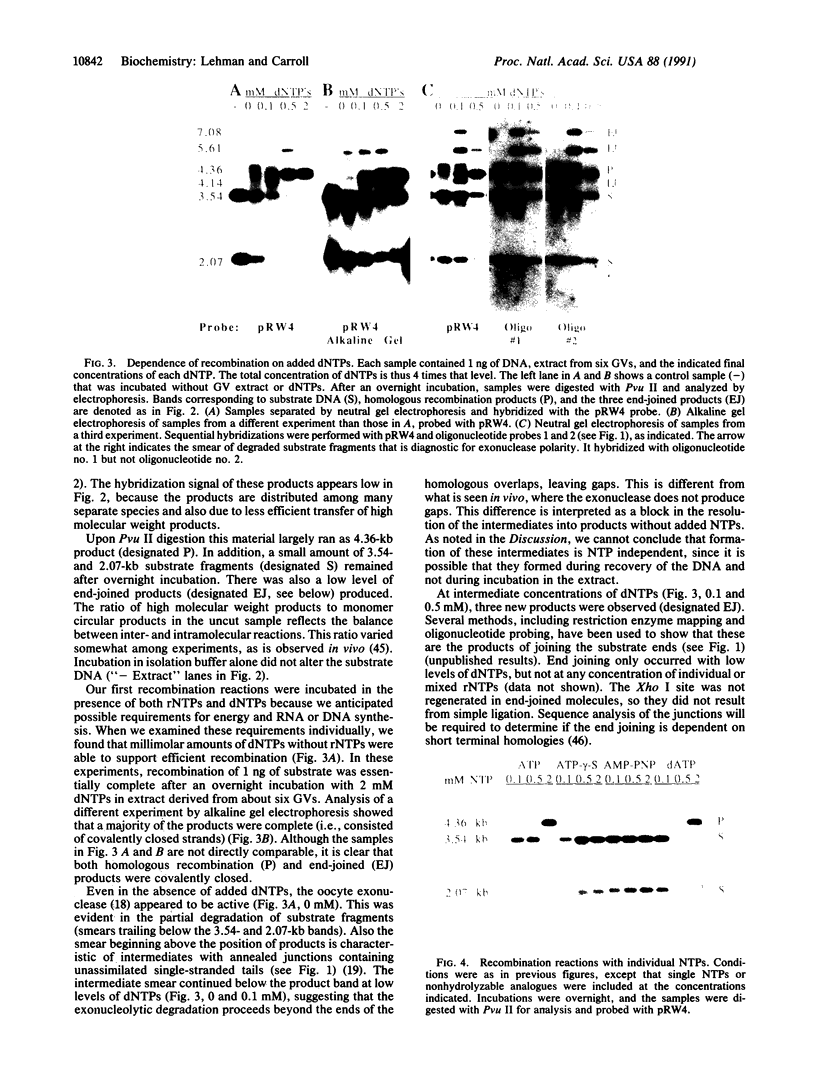
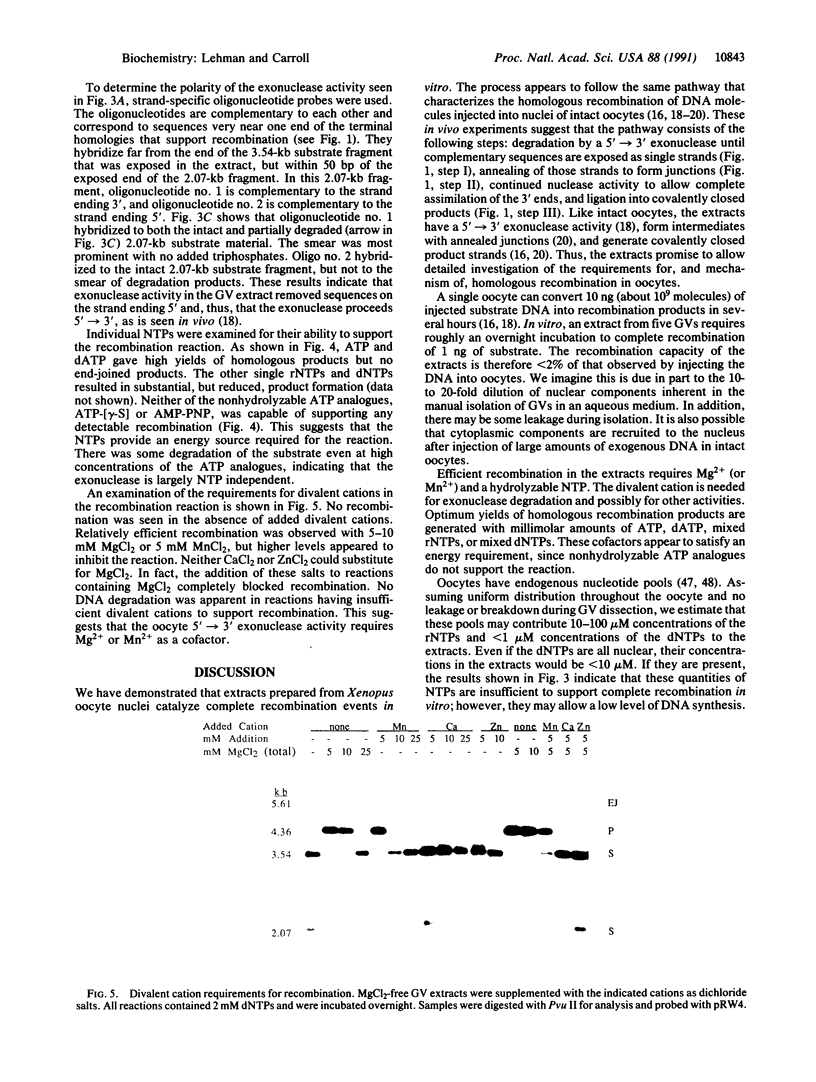

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. A., Eliason S. L. Recombination of homologous DNA fragments transfected into mammalian cells occurs predominantly by terminal pairing. Mol Cell Biol. 1986 Sep;6(9):3246–3252. doi: 10.1128/mcb.6.9.3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass B. L., Weintraub H. A developmentally regulated activity that unwinds RNA duplexes. Cell. 1987 Feb 27;48(4):607–613. doi: 10.1016/0092-8674(87)90239-x. [DOI] [PubMed] [Google Scholar]
- Baur M., Potrykus I., Paszkowski J. Intermolecular homologous recombination in plants. Mol Cell Biol. 1990 Feb;10(2):492–500. doi: 10.1128/mcb.10.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayne M. L., Alexander R. F., Benbow R. M. DNA binding protein from ovaries of the frog, Xenopus laevis which promotes concatenation of linear DNA. J Mol Biol. 1984 Jan 5;172(1):87–108. doi: 10.1016/0022-2836(84)90416-9. [DOI] [PubMed] [Google Scholar]
- Benbow R. M., Krauss M. R. Recombinant DNA formation in a cell-free system from Xenopus laevis eggs. Cell. 1977 Sep;12(1):191–204. doi: 10.1016/0092-8674(77)90197-0. [DOI] [PubMed] [Google Scholar]
- Birkenmeier E. H., Brown D. D., Jordan E. A nuclear extract of Xenopus laevis oocytes that accurately transcribes 5S RNA genes. Cell. 1978 Nov;15(3):1077–1086. doi: 10.1016/0092-8674(78)90291-x. [DOI] [PubMed] [Google Scholar]
- Blow J. J., Laskey R. A. Initiation of DNA replication in nuclei and purified DNA by a cell-free extract of Xenopus eggs. Cell. 1986 Nov 21;47(4):577–587. doi: 10.1016/0092-8674(86)90622-7. [DOI] [PubMed] [Google Scholar]
- Brooks P., Dohet C., Almouzni G., Méchali M., Radman M. Mismatch repair involving localized DNA synthesis in extracts of Xenopus eggs. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4425–4429. doi: 10.1073/pnas.86.12.4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buczynski G., Potter R. L. Nucleoside diphosphate kinase from Xenopus oocytes; partial purification and characterization. Biochim Biophys Acta. 1990 Dec 5;1041(3):296–304. doi: 10.1016/0167-4838(90)90288-q. [DOI] [PubMed] [Google Scholar]
- Carroll D. Genetic recombination of bacteriophage lambda DNAs in Xenopus oocytes. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6902–6906. doi: 10.1073/pnas.80.22.6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll D., Wright S. H., Wolff R. K., Grzesiuk E., Maryon E. B. Efficient homologous recombination of linear DNA substrates after injection into Xenopus laevis oocytes. Mol Cell Biol. 1986 Jun;6(6):2053–2061. doi: 10.1128/mcb.6.6.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont J. N. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory maintained animals. J Morphol. 1972 Feb;136(2):153–179. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
- Edelmann W., Kröger B., Goller M., Horak I. A recombination hotspot in the LTR of a mouse retrotransposon identified in an in vitro system. Cell. 1989 Jun 16;57(6):937–946. doi: 10.1016/0092-8674(89)90332-2. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fishel R. A., Detmer K., Rich A. Identification of homologous pairing and strand-exchange activity from a human tumor cell line based on Z-DNA affinity chromatography. Proc Natl Acad Sci U S A. 1988 Jan;85(1):36–40. doi: 10.1073/pnas.85.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folger K. R., Thomas K., Capecchi M. R. Nonreciprocal exchanges of information between DNA duplexes coinjected into mammalian cell nuclei. Mol Cell Biol. 1985 Jan;5(1):59–69. doi: 10.1128/mcb.5.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandini Attardi D., Martini G., Mattoccia E., Tocchini-Valentini G. P. Effect of Xenopus laevis oocyte extract on supercoiled simian virus 40 DNA: formation of complex DNA. Proc Natl Acad Sci U S A. 1976 Feb;73(2):554–558. doi: 10.1073/pnas.73.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandini Attardi D., Mattoccia E., Tocchini-Valentini G. P. Formation of branched DNA structures by Xenopus laevis oocyte extract. Nature. 1977 Dec 22;270(5639):754–756. doi: 10.1038/270754a0. [DOI] [PubMed] [Google Scholar]
- Glikin G. C., Ruberti I., Worcel A. Chromatin assembly in Xenopus oocytes: in vitro studies. Cell. 1984 May;37(1):33–41. doi: 10.1016/0092-8674(84)90298-8. [DOI] [PubMed] [Google Scholar]
- Grzesiuk E., Carroll D. Recombination of DNAs in Xenopus oocytes based on short homologous overlaps. Nucleic Acids Res. 1987 Feb 11;15(3):971–985. doi: 10.1093/nar/15.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurdon J. B., Wickens M. P. The use of Xenopus oocytes for the expression of cloned genes. Methods Enzymol. 1983;101:370–386. doi: 10.1016/0076-6879(83)01028-9. [DOI] [PubMed] [Google Scholar]
- Hardy S., Aoufouchi S., Thiebaud P., Prigent C. DNA ligase I from Xenopus laevis eggs. Nucleic Acids Res. 1991 Feb 25;19(4):701–705. doi: 10.1093/nar/19.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison C. J., Cox R., Drepaul R. S., Gomperts M., Ford C. C. Periodic DNA synthesis in cell-free extracts of Xenopus eggs. EMBO J. 1987 Jul;6(7):2003–2010. doi: 10.1002/j.1460-2075.1987.tb02464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger R., Berg P. Repair of deletions and double-strand gaps by homologous recombination in a mammalian in vitro system. Mol Cell Biol. 1991 Jan;11(1):445–457. doi: 10.1128/mcb.11.1.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschmidt J. A., Seiter A., Zentgraf H. Nucleosome assembly in vitro: separate histone transfer and synergistic interaction of native histone complexes purified from nuclei of Xenopus laevis oocytes. EMBO J. 1990 Apr;9(4):1309–1318. doi: 10.1002/j.1460-2075.1990.tb08240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmiec E. B., Holloman W. K. Heteroduplex formation and polarity during strand transfer promoted by Ustilago rec 1 protein. Cell. 1983 Jul;33(3):857–864. doi: 10.1016/0092-8674(83)90028-4. [DOI] [PubMed] [Google Scholar]
- Kohne D. E., Levison S. A., Byers M. J. Room temperature method for increasing the rate of DNA reassociation by many thousandfold: the phenol emulsion reassociation technique. Biochemistry. 1977 Nov 29;16(24):5329–5341. doi: 10.1021/bi00643a026. [DOI] [PubMed] [Google Scholar]
- Kolodner R. Genetic recombination of bacterial plasmid DNA: electron microscopic analysis of in vitro intramolecular recombination. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4847–4851. doi: 10.1073/pnas.77.8.4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucherlapati R. S., Ayares D., Hanneken A., Noonan K., Rauth S., Spencer J. M., Wallace L., Moore P. D. Homologous recombination in monkey cells and human cell-free extracts. Cold Spring Harb Symp Quant Biol. 1984;49:191–197. doi: 10.1101/sqb.1984.049.01.022. [DOI] [PubMed] [Google Scholar]
- Lin F. L., Sperle K., Sternberg N. Intermolecular recombination between DNAs introduced into mouse L cells is mediated by a nonconservative pathway that leads to crossover products. Mol Cell Biol. 1990 Jan;10(1):103–112. doi: 10.1128/mcb.10.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F. L., Sperle K., Sternberg N. Model for homologous recombination during transfer of DNA into mouse L cells: role for DNA ends in the recombination process. Mol Cell Biol. 1984 Jun;4(6):1020–1034. doi: 10.1128/mcb.4.6.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund E., Dahlberg J. E. In vitro synthesis of vertebrate U1 snRNA. EMBO J. 1989 Jan;8(1):287–292. doi: 10.1002/j.1460-2075.1989.tb03375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maller J., Wu M., Gerhart J. C. Changes in protein phosphorylation accompanying maturation of Xenopus laevis oocytes. Dev Biol. 1977 Jul 15;58(2):295–312. doi: 10.1016/0012-1606(77)90093-8. [DOI] [PubMed] [Google Scholar]
- Maryon E., Carroll D. Characterization of recombination intermediates from DNA injected into Xenopus laevis oocytes: evidence for a nonconservative mechanism of homologous recombination. Mol Cell Biol. 1991 Jun;11(6):3278–3287. doi: 10.1128/mcb.11.6.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maryon E., Carroll D. Degradation of linear DNA by a strand-specific exonuclease activity in Xenopus laevis oocytes. Mol Cell Biol. 1989 Nov;9(11):4862–4871. doi: 10.1128/mcb.9.11.4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maryon E., Carroll D. Involvement of single-stranded tails in homologous recombination of DNA injected into Xenopus laevis oocyte nuclei. Mol Cell Biol. 1991 Jun;11(6):3268–3277. doi: 10.1128/mcb.11.6.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y., Bogenhagen D. F. Repair of a synthetic abasic site in DNA in a Xenopus laevis oocyte extract. Mol Cell Biol. 1989 Sep;9(9):3750–3757. doi: 10.1128/mcb.9.9.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng S. Y., Parker C. S., Roeder R. G. Transcription of cloned Xenopus 5S RNA genes by X. laevis RNA polymerase III in reconstituted systems. Proc Natl Acad Sci U S A. 1979 Jan;76(1):136–140. doi: 10.1073/pnas.76.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr-Weaver T. L., Szostak J. W. Fungal recombination. Microbiol Rev. 1985 Mar;49(1):33–58. doi: 10.1128/mr.49.1.33-58.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozenberger B. A., Roeder G. S. A unique pathway of double-strand break repair operates in tandemly repeated genes. Mol Cell Biol. 1991 Mar;11(3):1222–1231. doi: 10.1128/mcb.11.3.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck L. J., Millstein L., Eversole-Cire P., Gottesfeld J. M., Varshavsky A. Transcriptionally inactive oocyte-type 5S RNA genes of Xenopus laevis are complexed with TFIIIA in vitro. Mol Cell Biol. 1987 Oct;7(10):3503–3510. doi: 10.1128/mcb.7.10.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer P., Vielmetter W. Joining of nonhomologous DNA double strand breaks in vitro. Nucleic Acids Res. 1988 Feb 11;16(3):907–924. doi: 10.1093/nar/16.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth D. B., Wilson J. H. Nonhomologous recombination in mammalian cells: role for short sequence homologies in the joining reaction. Mol Cell Biol. 1986 Dec;6(12):4295–4304. doi: 10.1128/mcb.6.12.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruberti I., Beccari E., Bianchi E., Carnevali F. Large scale isolation of nuclei from oocytes of Xenopus laevis. Anal Biochem. 1989 Jul;180(1):177–180. doi: 10.1016/0003-2697(89)90108-5. [DOI] [PubMed] [Google Scholar]
- Rudin N., Sugarman E., Haber J. E. Genetic and physical analysis of double-strand break repair and recombination in Saccharomyces cerevisiae. Genetics. 1989 Jul;122(3):519–534. doi: 10.1093/genetics/122.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalenghe F., Buscaglia M., Steinheil C., Crippa M. Large scale isolation of nuclei and nucleoli from vitellogenic oocytes of Xenopus laevis. Chromosoma. 1978 May 16;66(4):299–308. doi: 10.1007/BF00328531. [DOI] [PubMed] [Google Scholar]
- Seidman M. M. Intermolecular homologous recombination between transfected sequences in mammalian cells is primarily nonconservative. Mol Cell Biol. 1987 Oct;7(10):3561–3565. doi: 10.1128/mcb.7.10.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. R. Homologous recombination in procaryotes. Microbiol Rev. 1988 Mar;52(1):1–28. doi: 10.1128/mr.52.1.1-28.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]
- Symington L. S. Double-strand-break repair and recombination catalyzed by a nuclear extract of Saccharomyces cerevisiae. EMBO J. 1991 Apr;10(4):987–996. doi: 10.1002/j.1460-2075.1991.tb08033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symington L. S., Fogarty L. M., Kolodner R. Genetic recombination of homologous plasmids catalyzed by cell-free extracts of Saccharomyces cerevisiae. Cell. 1983 Dec;35(3 Pt 2):805–813. doi: 10.1016/0092-8674(83)90113-7. [DOI] [PubMed] [Google Scholar]
- Thaler D. S., Stahl F. W. DNA double-chain breaks in recombination of phage lambda and of yeast. Annu Rev Genet. 1988;22:169–197. doi: 10.1146/annurev.ge.22.120188.001125. [DOI] [PubMed] [Google Scholar]
- Wallace R. A., Jared D. W., Dumont J. N., Sega M. W. Protein incorporation by isolated amphibian oocytes. 3. Optimum incubation conditions. J Exp Zool. 1973 Jun;184(3):321–333. doi: 10.1002/jez.1401840305. [DOI] [PubMed] [Google Scholar]
- West S. C., Cassuto E., Howard-Flanders P. recA protein promotes homologous-pairing and strand-exchange reactions between duplex DNA molecules. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2100–2104. doi: 10.1073/pnas.78.4.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodland H. R., Pestell R. Q. Determination of the nucleoside triphosphate contents of eggs and oocytes of Xenopus laevis. Biochem J. 1972 Apr;127(3):597–605. doi: 10.1042/bj1270597. [DOI] [PMC free article] [PubMed] [Google Scholar]