Abstract
Argininosuccinate synthetase 1 (ASS1), the rate-limiting enzyme for arginine biosynthesis, is expressed in many types of human malignancies. Recent studies showed that ASS1 may have tumor suppressor function and that ASS1 deficiency is associated with clinical aggressiveness in nasopharyngeal carcinoma, myxofibrosarcomas and bladder cancer. The goal of this study was to evaluate the prognostic impact of ASS1 expression in patients with pancreatic ductal adenocarcinoma (PDAC). Our study included two independent cohorts: untreated cohort, which was comprised of 135 patients with PDAC who underwent pancreatoduodenectomy (PD) without pre-operative neoadjuvant therapy, and treated cohort, which was comprised of 122 patients with PDAC who have completed neoadjuvant therapy and PD. The expression level of ASS1 was evaluated by immunohistochemistry and the results were correlated with clinicopathologic parameters and survival using SPSS statistics. Our study showed that 12% of PDAC in untreated cohort and 15% of PDAC in treated cohort has low expression of ASS1 (ASS1-low). ASS1-low was associated with higher recurrence (p = 0.045), shorter disease-free survival (DFS, 4.8 ± 1.6 months vs 15.3 ± 2.2 months, p = 0.001) and shorter overall survival (OS, 14.6 ± 6.4 months vs 26.5 ± 3.5 months, p = 0.005) in untreated cohort and shorter OS in treated cohort compared to ASS1-high tumors. In multivariate analysis, ASS1-low (HR: 0.45, 95% CI: 0.26–0.79, p = 0.005) was an independent prognostic factor for DFS in untreated cohort and an independent prognostic factor for OS (HR: 0.56, 95% CI: 0.32–0.97, p = 0.04) in treated cohort. Our results provide supporting evidence for future clinical trial using arginine deprivation agents either alone or in combination with conventional chemotherapy in treating pancreatic cancer.
Introduction
Pancreatic ductal adenocarcinoma (PDAC), the most common histological subtype of pancreatic cancer, is a highly aggressive disease with a 5-yr survival rate of 6% [1]. Surgical resection is the only possible way of curing PDAC. However, less than 20% of patients have resectable disease at the time of diagnosis [2]. Patients with surgically unresectable PDAC at diagnosis are often due to local invasion and/or distant metastasis and have a median survival of less than 6 months. Systemic chemotherapies or chemoradiation therapies remains the standard treatment approach for these patients. However, the benefits of these therapies are limited because PDAC is frequently resistant or develop resistance quickly to conventional therapeutic agents. Recent studies have been focused on identification of more effective therapeutic agents that target the tumor at the molecular level or modulate host immune response, the so-called cancer immunotherapy [3], or agents that can interfere with tumor metabolism [4].
Arginine deprivation therapy combined with the standard chemotherapy has been proposed to treat PDAC since some PDAC are argininosuccinate synthetase 1 (ASS1) deficient and their growth is arginine-dependent (a phenomenon described as “auxotrophy”) [5, 6]. In the susceptible tumors, ASS1 is not up-regulated even when arginine is deprived [7, 8]. In contrast, arginine is produced in normal human cells from citrulline via the urea cycle by the catalytic actions of ASS and argininosuccinate lyase, making it a non-essential amino acid [9]. Systemic deprivation of arginine can be used alone or in combination with other chemotherapy agents to induce tumor cell death in arginine-dependent PDAC. Recently two groups almost simultaneously reported that a novel anticancer enzyme degrading intracellular arginine, pegylated arginine deiminase (PEG-ADI), synergistically augments the cytotoxicity of gemcitabine on PDAC through induction of metabolic stress or inhibition of NF-κB signaling [5, 10]. Arginine deprivation thus may offer a therapeutic opportunity for PDAC deficient in arginine synthesis.
Interestingly, besides it role in arginine synthesis, Ass1 gene is recently identified as a novel tumor suppressor gene in myxofibrosarcomas [11]. ASS1 has been shown to play an important role in inhibition of tumor cell proliferation via induction of G1 arrest, as well as inhibition of tumor cell migration, invasion and tumor angiogenesis. Loss of ASS1 expression is associated with clinical aggressiveness of the disease [11]. Similar observations have been reported in bladder cancer [12] and nasopharyngeal carcinoma [13]. The prognostic significance of ASS1 in PDAC has not been reported. We therefore in this study examined if ASS1 expression has a prognostic impact in PDAC treated with PD, with or without neoadjuvant therapy. We also examined if neoadjuvant therapy affects ASS1 expression in these patients. Our study will help identify important prognostic factors affecting the survival in patients with PDAC, as well as patients who might benefit from arginine deprivation therapy.
Materials and methods
Study populations, patient characteristic, treatment sequencing and follow-up
This study was approved by the Institutional Review Board of the University of Texas M. D. Anderson Cancer Center, Houston, TX. Cases were retrieved from the surgical pathology files of the Department of Pathology, University of Texas M. D. Anderson Cancer Center. Our study population consisted of two independent cohorts: (1) Untreated cohort, which was comprised of 135 patients with stage II PDAC treated with upfront pancreaticoduodenectomy (PD) without pre-operative neoadjuvant therapy (58 women and 77 men with median age of 64.3 years). One patient with stage I disease and four patients with stage IV disease was excluded since the number of cases with either stage I or IV disease was too small to be representative. (2) Treated cohort, which was comprised of 122 patients with PDAC who have completed neoadjuvant therapy before PD at our institution (49 women and 73 men with median age of 62.7 years). Within the treated cohort, 18 patients (14.7%) received fluoropyrimidine-based chemoradiation, 39 patients (32.0%) received gemcitabine-based chemoradiation, 45 patients (36.9%) received gemcitabine followed by gemcitabine-based chemoradiation, 15 patients (12.3%) received gemcitabine followed by fluoropyrimidine -based chemoradiation, and the remaining 5 patients (4.1%) received neoadjuvant systemic chemotherapy alone. All patients in the treated cohort underwent restaging evaluation after completion of neoadjuvant therapy. Only patients, who had no disease progression or metastasis and had no contraindications to major abdominal surgery, were selected for PD.
All cases had confirmed diagnosis of PDAC by histology and were evaluated for clinical presentation, tumor size, differentiation, margins status, extrapancreatic tissue involvement, pathological stage, treatment and clinical outcome based on a standardized system established at our institute. Pathologic stages were grouped according to the AJCC staging manual, 7th edition [14]. Clinical follow-up information was extracted from a prospectively maintained database at the Department of Surgical Oncology at MD Anderson Cancer Center and subsequently verified by reviewing patient medical records and/or the US Social Security Death Index. Recurrence status was updated at each follow-up clinic visit.
Immunohistochemistry and grading for ASS1 expression
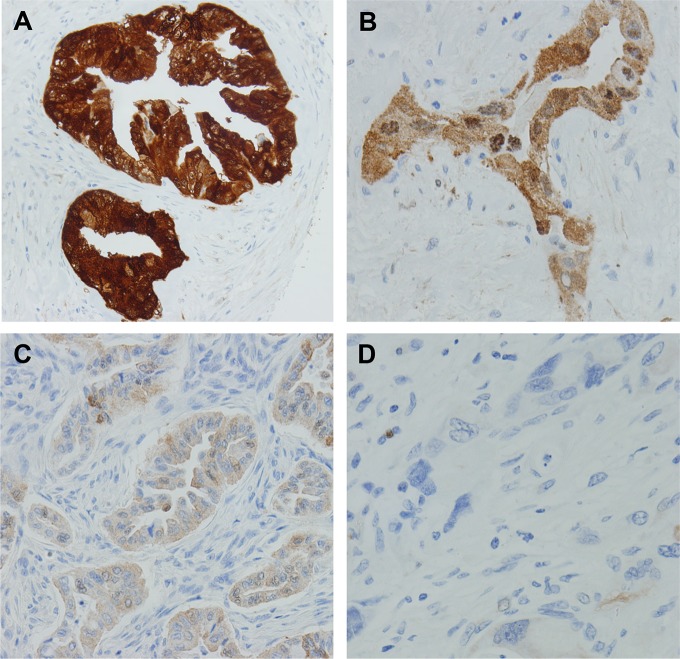
Immunohistochemical staining for ASS1 was performed on tissue microarray slides, which contain three representative 1.0 mm cores from each patient (two from tumor and one from benign pancreatic tissue). The slides were incubated with mouse anti-ASS1 antibody (1:400, Polaris Group, San Diego, CA). The staining intensity of ASS1 was quantified by visual scoring of staining. The staining results are graded by combined score of the intensity of cytoplasmic staining (0-negative, 1-weak, 2-moderate, and 3-strong, Fig 1) and the percentage of positive tumor cells. The formula for staining score was used: S = p1 x1 + p2 x 2 + p3 x 3 in which p1, p2 and p3 represented fractions of tumor cells representing each staining categories of 1, 2 and 3 respectively. The expression of ASS1 was categorized as ASS1-low and ASS1-high using the combined score 1.5 as a cutoff. The cutoff score of 1.5 was determined based on the previous operational observation that only tumors with an ASS1 immunohistochemical score of 1.5 or lower will respond to pegylated arginine deiminase (PEG-ADI) using the same immunohistochemical protocol and also as reported by other groups previously [15].
Fig 1. Representative micrographs showing the immunohistochemical (IHC) scores of ASS1 expression (on a scale of 0–3) in pancreatic ductal adenocarcinomas.
(A) IHC score 3, (B) IHC score 2, (C) IHC score 1 and (D) IHC score 0.
Statistics
The expression of ASS1 was correlated with clinicopathologic parameters and survival using Statistical Package for Social Sciences software (SPSS Inc. Chicago, IL). Categorical variables were compared using the Χ2 analysis, Fischer’ exact test or Likelihood ratio. The student’s t test was used to compare the expression of ASS1 in treated cohort to that in untreated cohort. Survival analyses were performed using the Kaplan-Meier method and the statistical significance of difference in survival was evaluated using the log-rank test. Disease-free survival (DFS) was calculated as the time from the date of surgery to the date of first recurrence after surgery in patients with recurrence or to the date of last follow-up in patients without recurrence. Overall survival (OS) was calculated as the time from the date of diagnosis to the date of death or the date of last follow-up if death did not occur. Univariate Cox regression analysis was used to determine the prognostic significance of ASS1 expression and other clinicopathologic characteristics. Cox proportional hazards models were fitted for multivariate analysis. After interactions between the variables were examined, a backward stepwise procedure was used to derive the best-fitting model. All tests are two-sided. P values less than 0.05 are considered statistically significant.
Results
ASS1 expression in benign pancreatic tissue, treated and untreated pancreatic cancer samples
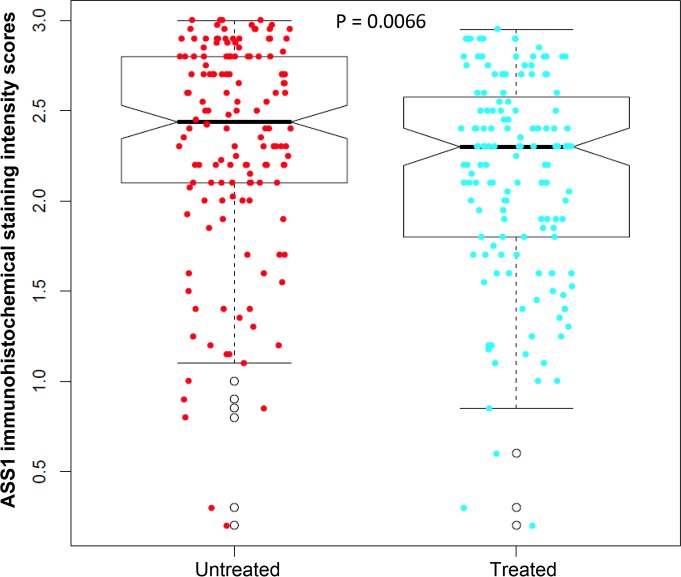
Representative micrographs showing different levels of ASS1 expression among the examined cases are shown in Fig 1. Immunohistochemical stain for ASS1 showed cytoplasmic staining in PDAC cells in cases that were positive for ASS1. The average score of ASS1 expression was 2.13 in treated cohort compared to 2.32 in untreated cohort (p = 0.007, Fig 2). There was either no or very low ASS1 expression in cancer associated fibroblasts in all cases.
Fig 2. The average immunohistochemical scores of ASS1 expression is significantly lower in treated cohort than that in untreated cohort (p = 0.007).
Among the 163 matched benign pancreatic tissue samples that were available for examination, 124 were histologically normal pancreas and 39 were chronic pancreatitis. In benign pancreas tissue, ASS1expression was detected mainly in pancreatic ductal cells, but not pancreatic islet cells. Pancreatic acinar cell showed negative, weak or moderate staining for ASS1, which may represent non-specific crossing reactions. The average scores of ASS1 expression were 2.31 in normal pancreatic ductal cells and 2.46 in the proliferating pancreatic ductules of chronic pancreatitis respectively. Representative micrographs showing the ASS1 expression in normal pancreas and chronic pancreatitis tissue are shown in Fig 3.
Fig 3.
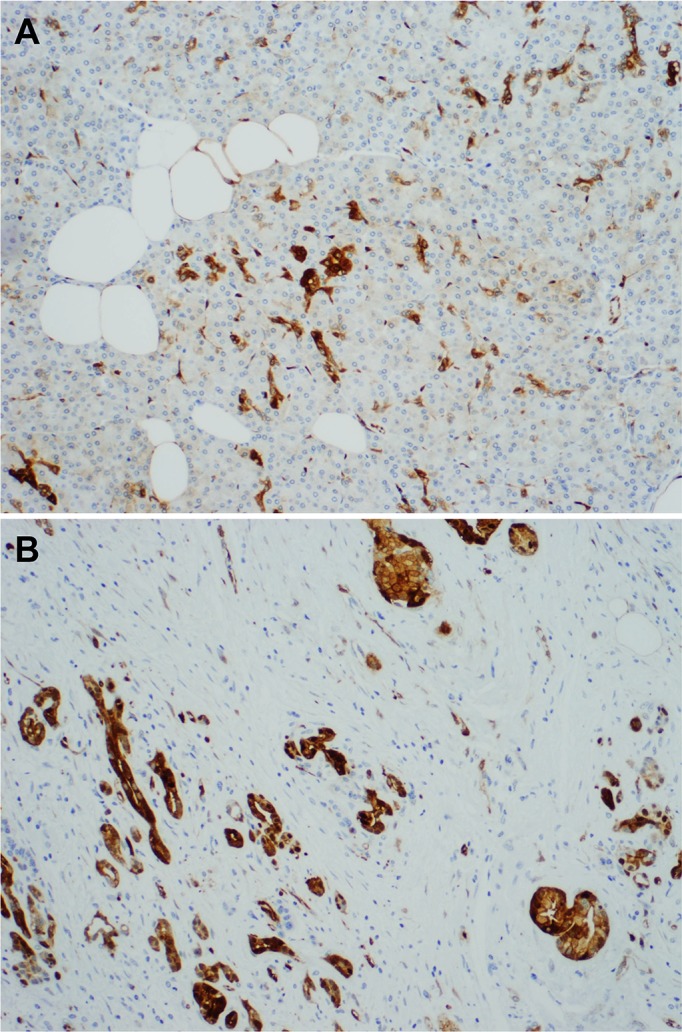
Representative micrographs showing ASS1 expression in normal pancreas (A) and chronic pancreatitis tissue (B).
Correlation of ASS1 expression with clinicopathologic parameters in untreated and treated cohorts
The correlations of ASS1 expression with clinicopathologic characteristics in the untreated and treated cohorts are summarized in Table 1. In the untreated cohort, ASS1 expression correlated significantly with tumor size and tumor recurrence after surgery (Likelihood ratio, p<0.05). All (100%) ASS1-low tumors were greater than 2.0 cm compared to 84% among the ASS1-high tumors (Likelihood ratio, p = 0.02). Local and distant recurrences were present in 37.5% (6/16) and 56.3% (9/16) respectively in ASS1-low tumors compared to 16.0 (19/119) local recurrence and 57.1% (68/119) distant recurrence respectively in ASS1-high tumors (Likelihood ratio, p = 0.045). These correlations, however, were not observed in treated cohort. There were no significant correlations between ASS1 expression and other clinicopathologic parameters including gender, age, tumor differentiation, AJCC stage, lymph node status or margin status in either the untreated or treated cohort (p > 0.05).
Table 1. Correlation of ASS1 Expression and Clinicopathologic Parameters in Untreated Group and Treated Groups.
| Untreated | Treated | |||||
|---|---|---|---|---|---|---|
| Characteristics | ASS1-low (%) (n = 16) | ASS1-high (%) (n = 119) | p value | ASS1-low (%) (n = 18)* | ASS1-high (%) (n = 104)* | p value |
| Gender | 0.64 | 0.91 | ||||
| Female | 6 (37.5) | 52 (43.7) | 7 (38.9) | 42 (40.4) | ||
| Male | 10 (62.5) | 67 (56.3) | 11 (61.1) | 62 (59.6) | ||
| Age | 0.55 | 0.42 | ||||
| <60 | 6 (37.5) | 33 (27.7) | 9 (50) | 41 (39.4) | ||
| 60–70 | 7 (43.8) | 49 (41.2) | 7 (38.9) | 37 (35.6) | ||
| >70 | 3 (18.7) | 37 (31.1) | 2 (11.1) | 26 (25) | ||
| Tumor differentiation | 0.07 | 0.06 | ||||
| Well-moderate | 8 (50) | 86 (72.3) | 8 (44.4) | 70 (67.3) | ||
| Poor | 8 (50) | 33 (27.3) | 10 (55.6) | 34 (32.7) | ||
| Tumor size | 0.02 | 0.8 | ||||
| ≤2.0 cm | 0 (0) | 19 (16.0) | 4 (22.2) | 26 (25.0) | ||
| >2.0 cm | 16 (100) | 100 (84.0) | 14 (77.8) | 78 (75.0) | ||
| AJCC stage | 0.24 | 0.84 | ||||
| Stage IIA | 2 (12.5) | 31 (26.1) | 6 (33.3) | 33 (31.7) | ||
| Stage IIB | 14 (87.5) | 88 (73.9) | 12 (66.7) | 69 (66.3) | ||
| Lymph node status | 0.24 | 0.98 | ||||
| Negative | 2 (12.5) | 31 (26.1) | 6 (33.3) | 35 (33.7) | ||
| Positive | 14 (87.5) | 88 (73.9) | 12 (66.7) | 69 (66.3) | ||
| Recurrence | 0.045 | 0.44 | ||||
| No recurrence | 1 (6.3) | 32 (26.9) | 3 (17.7) | 27 (26.2) | ||
| Local recurrence | 6 (37.5) | 19 (16.0) | 5 (29.4) | 23 (22.3) | ||
| Distant recurrence | 9 (56.2) | 68 (57.1) | 9 (52.9) | 53 (51.5) | ||
| Margin status | 0.78 | 0.06 | ||||
| Negative | 14 (87.5) | 101 (84.9) | 14 (77.8) | 96 (92.3) | ||
| Positive | 2 (12.5) | 18 (15.1) | 4 (22.2) | 8 (7.7) | ||
* Due to unavailability of recurrence data, one patient is taken out from ASS1-low and ASS-high groups, respectively.
ASS1 expression correlated with disease-free and overall survival in untreated cohort
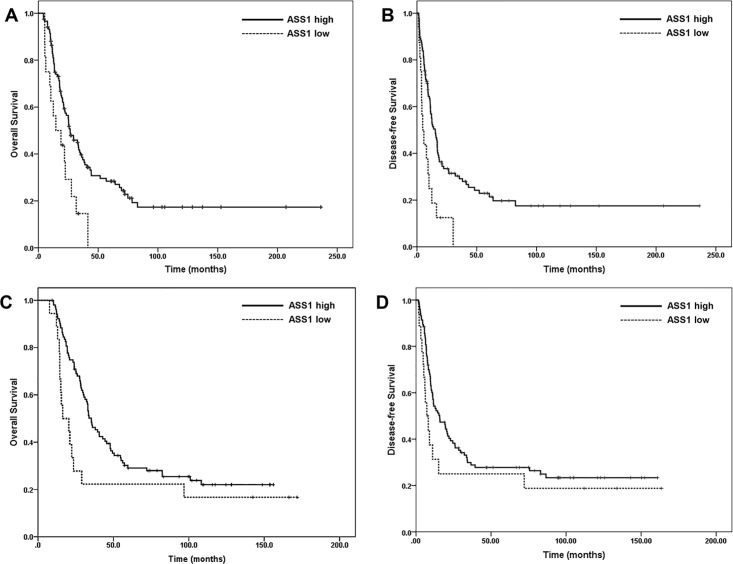
The median follow-up time was 21.8 months (range: 4.2–236.3 months) for the overall untreated cohort and 41.2 months (range: 4.7–236.3 months) for patients who did not die from disease. At the time of last follow-up, 94 (69.6%) patients died of PDAC, 3 (2.2%) died of other causes, 7 (5.2%) patients were alive with disease and 31 (23.0%) were alive with no clinical or radiographic evidence of disease. The median DFS and OS was 4.8 ± 1.6 months and 14.6 ± 6.4 months respectively in ASS1-low group compared to15.3 ± 2.2 months (p = 0.001) and 26.5 ± 3.5 months (p = 0.005) respectively in ASS-high group (Fig 4A and 4B). By univariate analysis, ASS1 expression, tumor size (greater than 2.0 cm), margin status, lymph node status and AJCC stage correlated significantly with both DFS and OS (p<0.05, Table 2). By multivariate analysis, ASS1 expression [hazard ratio (HR): 0.45, 95% confidence interval (CI): 0.26–0.78) was an independent prognostic factor for DFS (p = 0.005), but not OS (p = 0.06, Table 3).
Fig 4.
Kaplan–Meier survival curves showing that low ASS1 expression (ASS1-low) is associated reduced overall survival (p = 0.005, A) and disease-free survival (p = 0.001, B) compared to those whose tumors are ASS1-high in the untreated cohort. (C) and (D) Kaplan–Meier survival curves showing that ASS1-low is associated reduced overall survival (p = 0.04, C), but not disease-free survival (p = 0.13, D) compared to those whose tumors are ASS1-high in the treated cohort.
Table 2. Univariate Cox Regression Analysis of Disease-free and Overall Survival in Untreated Group.
| Characteristics | No. of patients | Disease-free Survival | Overall Survival | ||
|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | ||
| ASS1 expression | |||||
| Low (ref) | 16 | 1.00 | 1.00 | ||
| High | 119 | 0.39 (0.22–0.68) | 0.001 | 0.45 (0.25–0.80) | 0.007 |
| Age (years) | |||||
| ≤ 60 (ref) | 39 | 1.00 | 1.00 | ||
| 60–70 | 56 | 1.06 (0.66–1.71) | 0.81 | 1.30 (0.79–2.12) | 0.3 |
| ≥ 70 | 40 | 1.39 (0.83–2.34) | 0.21 | 1.64 (0.96–2.83) | 0.07 |
| Gender | |||||
| Female (ref) | 58 | 1.00 | 1.00 | ||
| Male | 77 | 1.19 (0.97–1.45) | 0.09 | 1.13 (0.82–1.56) | 0.46 |
| Tumor size | |||||
| ≤ 2cm (ref) | 19 | 1.00 | 1.00 | ||
| > 2 cm | 116 | 3.73 (1.71–8.13) | 0.001 | 3.25 (1.56–6.81) | 0.002 |
| Tumor differentiation | |||||
| Well-Moderate (ref) | 94 | 1.00 | 1.00 | ||
| Poor | 41 | 0.91 (0.59–1.41) | 0.12 | 0.89 (0.57–1.40) | 0.62 |
| Margins | |||||
| Negative (ref) | 115 | 1.00 | 1.00 | ||
| Positive | 20 | 1.78 (1.06–2.98) | 0.03 | 1.87 (1.10–3.19) | 0.02 |
| Lymph node status | |||||
| Negative (ref) | 33 | 1.00 | 1.00 | ||
| Positive | 102 | 2.60 (1.53–4.42) | <0.001 | 2.02 (1.18–3.45) | 0.01 |
Abbreviations: HR: hazard ratio; 95% CI: 95% confidence interval
Table 3. Multivariate Cox Regression Analysis of Disease-free and Overall Survival in Untreated Group.
| Characteristics | No. of patients | Disease-free Survival | Overall Survival | ||
|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | ||
| ASS1 expression | |||||
| Low (ref) | 16 | 1.00 | 1.00 | ||
| High | 119 | 0.45 (0.26–0.79) | 0.005 | 0.57 (0.32–1.03) | 0.06 |
| Tumor size | |||||
| ≤ 2.0 cm (ref) | 19 | 1.00 | 1.00 | ||
| > 2.0 cm | 116 | 2.48 (1.11–5.55) | 0.03 | 2.44 (1.12–5.28) | 0.02 |
| Margins | |||||
| Negative (ref) | 115 | 1.00 | 1.00 | ||
| Positive | 20 | 1.81 (1.07–3.07) | 0.03 | 1.65 (0.96–2.82) | 0.07 |
| Lymph node status | |||||
| Negative (ref) | 33 | 1.00 | 1.00 | ||
| Positive | 102 | 2.26 (1.31–3.89) | 0.003 | 1.58 (0.91–2.77) | 0.11 |
Abbreviations: HR: hazard ratio; 95% CI: 95% confidence interval; ref: reference
ASS1 expression correlated with overall survival in treated cohort
The median follow-up time was 33.0 months (range: 7.6–171.9 months) for the overall treated cohort and 107.1 months (range: 9.4–171.9 months) for patients who did not die from disease. At the time of last follow-up, 91 (74.6%) patients died of PDAC, 1 (0.8%) died of other causes, 1 (0.8%) patients were alive with disease and 29 (23.8%) were alive with no clinical or radiographic evidence of disease. The median OS was 16.5 ± 5.2 months in ASS1-low group compared to 35.3 ± 2.8 months in ASS-high group (p = 0.04, Fig 4C). The median DFS was 7.2 ± 2.0 months in ASS1-low group compared to 15.7 ± 3.7 months in ASS-high group (p = 0.13, Fig 4D). By univariate analysis, ASS1 expression, lymph node status and AJCC stage correlated significantly with OS (p<0.05, Table 4). By multivariate analysis, both ASS1 expression (HR: 0.56, 95% CI: 0.32–0.97, p = 0.04) and lymph node metastasis (HR: 1.57, 95% CI: 1.004–2.47, p = 0.048) were independent prognostic factors for OS (Table 5)
Table 4. Univariate Cox Regression Analysis of Overall Survival in Treated Group.
| Characteristics | No. of patients | Overall Survival | |
|---|---|---|---|
| HR (95% CI) | p value | ||
| ASS1 expression | |||
| Low (ref) | 18 | 1.00 | |
| High | 104 | 0.56 (0.32–0.98) | 0.04 |
| Age (years) | |||
| ≤ 60 (ref) | 50 | 1.00 | |
| 60–70 | 44 | 0.85 (0.53–1.34) | 0.48 |
| ≥ 70 | 28 | 0.76 (0.44–1.30) | 0.32 |
| Neoadjuvant regimens | |||
| Fluoropyrimidine-Rad | 18 | 1.00 | |
| Gem-Rad | 39 | 1.04 (0.53–2.07) | 0.90 |
| Gem-based with GemRad | 45 | 1.48 (0.77–2.85) | 0.24 |
| Gem-based-FPRad | 15 | 1.25 (0.56–2.78) | 0.59 |
| Chemo alone | 5 | 1.65 (0.53–5.14) | 0.39 |
| Gender | |||
| Female (ref) | 49 | 1.00 | |
| Male | 73 | 1.10 (0.90–1.36) | 0.35 |
| Tumor size | |||
| ≤ 2cm (ref) | 30 | 1.00 | |
| > 2 cm | 92 | 1.05 (0.64–1.72) | 0.85 |
| Tumor differentiation | |||
| Well-Moderate (ref) | 78 | 1.00 | |
| Poor | 44 | 1.24 (0.81–1.90) | 0.31 |
| Margins | |||
| Negative (ref) | 110 | 1.00 | |
| Positive | 12 | 1.61 (0.83–3.11) | 0.16 |
| Lymph node status | |||
| Negative (ref) | 41 | 1.00 | |
| Positive | 81 | 1.57 (1.00–2.45) | 0.05 |
Abbreviations: HR: hazard ratio; 95% CI: 95% confidence interval
Table 5. Multivariate Cox Regression Analysis of Overall Survival in Treated Group.
| Characteristics | No. of patients | Overall Survival | |
|---|---|---|---|
| HR (95% CI) | p value | ||
| ASS1 expression | |||
| Low (ref) | 18 | 1.00 | |
| High | 104 | 0.56 (0.32–0.97) | 0.04 |
| Tumor differentiation | |||
| Well-Moderate (ref) | 78 | 1.00 | |
| Poor | 44 | 1.17 (0.74–1.84) | 0.51 |
| Margins | |||
| Negative (ref) | 110 | 1.00 | |
| Positive | 12 | 1.38 (0.70–2.71) | 0.36 |
| Lymph node status | |||
| Negative (ref) | 41 | 1.00 | |
| Positive | 81 | 1.57 (1.00–2.47) | 0.048 |
Abbreviations: HR: hazard ratio; 95% CI: 95% confidence interval
Discussion
Pathologic factors that are known to confer a negative prognostic impact in patients with PDAC include high pathologic primary tumor (pT) stage [16–18], poor tumor differentiation [16, 19–21], positive lymph node status [16, 18, 20–24] etc. Our study identified ASS1 as a new prognostic factor in patients with PDAC in two large independent cohorts of patients: 135 patients who underwent upfront PD without neoadjuvant therapy (untreated cohort) and 122 patients received pre-operative neoadjuvant therapy and PD (treated cohort). We showed that low level of ASS1 expression (ASS1-low) is associated with higher recurrence after PD and is an independent negative prognostic factor for survival in both untreated cohort and treated cohort. Our data indicated that ASS1 might represent a valuable marker for early prognostic evaluation in patients with PDAC. ASS1 expression can be evaluated on surgical biopsy specimens or cell blocks for fine needle aspiration of pancreatic tumor. A low ASS1 combined score (less than 1.5) will help identify patients with poor prognosis, and patients more likely with better response to arginine deprivation therapy in the early course of the disease. Similar to what we reported here, a deficient expression of ASS1 has been previously described to be associated with clinical aggressiveness in myxofibrosarcomas, bladder cancer and nasopharyngeal carcinoma [11–13]. The negative prognostic impact associated with deficient ASS1 might be attributed to the newly identified tumor suppressor function of ASS1 besides its functions in arginine metabolism. Huang et al showed that expression of Ass1 gene inhibits tumoral angiogenesis, tumor growth, cell migration and invasion in myxofibrosarcomas, while knockdown of Ass1 gene confers tumor proliferative and metastatic capabilities [11].
ASS1 is present ubiquitously in mammals, making arginine a nonessential amino acid. However, expression of ASS1 could be quite different in various tissues, depending on the need of tissues for arginine. Similarly, ASS1 expression is greatly variable in human malignant tumors. Majority of the lung and colon carcinomas show ASS1 expression, while melanoma, hepatocellular carcinoma and prostate carcinomas are frequently ASS1-deficient [7]. ASS1 deficiency in the latter may make these tumors sensitive to external arginine depletion. Several regulatory mechanisms including hormones, nutrients, pro-inflammatory cytokines have been described to be involved in regulation of Ass1 gene expression [25]. Recently, Huang et al. and Lan et al. for the first time demonstrated at the molecular level that loss of ASS1 protein expression is strongly linked to Ass1 promotor hypermethylation [11, 13]. In our study, we found that low expression of ASS1 in 12% and 15% of the untreated and treated PDAC samples respectively. Whether the low expression of ASS1 in PDAC is attributed to Ass1 promotor hypermethylation needs to be examined in future studies.
In our study, ASS1 expression is significantly lower in patients treated with pre-operative neoadjuvant therapy (treated cohort) than that in untreated cohort, implicating a role of neoadjuvant therapy in regulation of ASS1 expression. This might occur through a direct regulation of ASS1 expression by neoadjuvant therapy at transcriptional, epigenetic e.g. hypermethylation of Ass1 promotor or translational level, or through altered pro-inflammatory cytokines induced by neoadjuvant therapy. Alternatively, neoadjuvant therapy might apply a selection pressure on tumor cells; with those express low ASS1 (more aggressive phenotype and more resistant to therapy) survive.
Our study showed that 12% of PDAC in untreated cohort and 15% of PDAC in treated cohort has low expression of ASS1 (ASS1-low). These patients may benefit from arginine depletion therapy using PEG-ADI either alone or in combination with conventional chemoradiation therapies. In addition, patients whose tumors have reduced ASS1 expression after neoadjuvant therapy may also be susceptible to arginine deprivation. Consistent with this notion, previous study has shown that five of seven pancreatic cancer cell lines lacked ASS1 expression and that arginine deprivation by treatment with PEG-ADI specifically inhibited the growth of pancreatic cancer cell lines that lack ASS1 expression both in vitro and in vivo [26]. However, our data indicated that majority of the PDAC patients might not benefit from arginine deprivation treatment as their tumor have sufficient ASS1 protein for endogenous arginine production. Interestingly, Daylami et al. showed that PEG-ADI synergistically increases the cytotoxicity of gemcitabine in human pancreatic cancer cell lines both in vitro and in vivo [5, 10]. The underlying mechanisms are unclear, and the authors proposed that arginine deprivation induces cellular changes that re-program cells allowing sensitization to traditional chemotherapy [5, 27]. This hypothesis, however, is only meaningful when majority of the tumor cells expressing deficient/low ASS1.
In summary, our study showed that a small percentage of PDAC has low expression of ASS1 and that ASS1 expression is reduced in patients who received neoadjuvant therapies. Low level ASS1 expression is associated with higher frequency of recurrence after surgery and is associated with shorter survival in both untreated and treated PDAC patients. Our findings provide supporting evidence for future clinical trial using arginine deprivation agents either alone or in combination with gemcitabine and other conventional chemotherapy agents in treating pancreatic cancer.
Data Availability
Some restrictions will apply. Data are available from the University of Texas MD Anderson Cancer Center Data Access / Ethics Committee for researchers who meet the criteria for access to confidential data. Researchers may contact Marion B. Olson (mbolson@mdanderson.org) and Dr. Huamin Wang (hmwang@mdanderson.org) for more information. The authors do not have special access privileges to the data underlying this study.
Funding Statement
Support was provided by the National Institutes of Health grant (1R01 CA196941-01A1) [https://www.nih.gov/grants-funding] and the Khalifa Bin Zayed Al Nahyan Foundation Institute for Pancreatic Cancer Research at The University of Texas M. D. Anderson Cancer Center [https://www.mdanderson.org/publications/conquest/fall-2011/khalifa-bin-zayed-al-nahyan-foundation.html].
References
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61(4):212–36. 10.3322/caac.20121 [DOI] [PubMed] [Google Scholar]
- 2.Wolfgang CL, Herman JM, Laheru DA, Klein AP, Erdek MA, Fishman EK, et al. Recent progress in pancreatic cancer. CA Cancer J Clin. 2013;63(5):318–48. PubMed Central PMCID: PMC3769458. 10.3322/caac.21190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paniccia A, Merkow J, Edil BH, Zhu Y. Immunotherapy for pancreatic ductal adenocarcinoma: an overview of clinical trials. Chin J Cancer Res. 2015;27(4):376–91. PubMed Central PMCID: PMC4560736. 10.3978/j.issn.1000-9604.2015.05.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perera RM, Bardeesy N. Pancreatic Cancer Metabolism: Breaking It Down to Build It Back Up. Cancer Discov. 2015;5(12):1247–61. PubMed Central PMCID: PMC4687899. 10.1158/2159-8290.CD-15-0671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daylami R, Muilenburg DJ, Virudachalam S, Bold RJ. Pegylated arginine deiminase synergistically increases the cytotoxicity of gemcitabine in human pancreatic cancer. J Exp Clin Cancer Res. 2014;33:102 PubMed Central PMCID: PMC4279680. 10.1186/s13046-014-0102-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glazer ES, Kaluarachchi WD, Massey KL, Zhu C, Curley SA. Bioengineered arginase I increases caspase-3 expression of hepatocellular and pancreatic carcinoma cells despite induction of argininosuccinate synthetase-1. Surgery. 2010;148(2):310–8. PubMed Central PMCID: PMC2905509. 10.1016/j.surg.2010.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dillon BJ, Prieto VG, Curley SA, Ensor CM, Holtsberg FW, Bomalaski JS, et al. Incidence and distribution of argininosuccinate synthetase deficiency in human cancers: a method for identifying cancers sensitive to arginine deprivation. Cancer. 2004;100(4):826–33. 10.1002/cncr.20057 [DOI] [PubMed] [Google Scholar]
- 8.Yoon CY, Shim YJ, Kim EH, Lee JH, Won NH, Kim JH, et al. Renal cell carcinoma does not express argininosuccinate synthetase and is highly sensitive to arginine deprivation via arginine deiminase. Int J Cancer. 2007;120(4):897–905. 10.1002/ijc.22322 [DOI] [PubMed] [Google Scholar]
- 9.Tapiero H, Mathe G, Couvreur P, Tew KD. I. Arginine. Biomed Pharmacother. 2002;56(9):439–45. [DOI] [PubMed] [Google Scholar]
- 10.Liu J, Ma J, Wu Z, Li W, Zhang D, Han L, et al. Arginine deiminase augments the chemosensitivity of argininosuccinate synthetase-deficient pancreatic cancer cells to gemcitabine via inhibition of NF-kappaB signaling. BMC Cancer. 2014;14:686 PubMed Central PMCID: PMC4189535. 10.1186/1471-2407-14-686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang HY, Wu WR, Wang YH, Wang JW, Fang FM, Tsai JW, et al. ASS1 as a novel tumor suppressor gene in myxofibrosarcomas: aberrant loss via epigenetic DNA methylation confers aggressive phenotypes, negative prognostic impact, and therapeutic relevance. Clin Cancer Res. 2013;19(11):2861–72. 10.1158/1078-0432.CCR-12-2641 [DOI] [PubMed] [Google Scholar]
- 12.Allen MD, Luong P, Hudson C, Leyton J, Delage B, Ghazaly E, et al. Prognostic and therapeutic impact of argininosuccinate synthetase 1 control in bladder cancer as monitored longitudinally by PET imaging. Cancer Res. 2014;74(3):896–907. 10.1158/0008-5472.CAN-13-1702 [DOI] [PubMed] [Google Scholar]
- 13.Lan J, Tai HC, Lee SW, Chen TJ, Huang HY, Li CF. Deficiency in expression and epigenetic DNA Methylation of ASS1 gene in nasopharyngeal carcinoma: negative prognostic impact and therapeutic relevance. Tumour Biol. 2014;35(1):161–9. 10.1007/s13277-013-1020-8 [DOI] [PubMed] [Google Scholar]
- 14.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–4. 10.1245/s10434-010-0985-4 [DOI] [PubMed] [Google Scholar]
- 15.Yang H, Lin M, Xiong FX, Yang Y, Nie X, Zhou RL. Reduced expression of ASS is closely related to clinicopathological features and post-resectional survival of hepatocellular carcinoma. Oncol Lett. 2010;1(1):31–6. PubMed Central PMCID: PMCPMC3436254. 10.3892/ol_00000005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kedra B, Popiela T, Sierzega M, Precht A. Prognostic factors of long-term survival after resective procedures for pancreatic cancer. Hepatogastroenterology. 2001;48(42):1762–6. [PubMed] [Google Scholar]
- 17.Meyer W, Jurowich C, Reichel M, Steinhauser B, Wunsch PH, Gebhardt C. Pathomorphological and histological prognostic factors in curatively resected ductal adenocarcinoma of the pancreas. Surg Today. 2000;30(7):582–7. 10.1007/s005950070096 [DOI] [PubMed] [Google Scholar]
- 18.Yeo CJ, Cameron JL, Lillemoe KD, Sitzmann JV, Hruban RH, Goodman SN, et al. Pancreaticoduodenectomy for cancer of the head of the pancreas. 201 patients. Ann Surg. 1995;221(6):721–31; discussion 31–3. PubMed Central PMCID: PMC1234702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4(6):567–79. [DOI] [PubMed] [Google Scholar]
- 20.Sperti C, Pasquali C, Piccoli A, Pedrazzoli S. Recurrence after resection for ductal adenocarcinoma of the pancreas. World J Surg. 1997;21(2):195–200. [DOI] [PubMed] [Google Scholar]
- 21.Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman J, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. J Gastrointest Surg. 2006;10(9):1199–210; discussion 210–1. 10.1016/j.gassur.2006.08.018 [DOI] [PubMed] [Google Scholar]
- 22.Benassai G, Mastrorilli M, Quarto G, Cappiello A, Giani U, Forestieri P, et al. Factors influencing survival after resection for ductal adenocarcinoma of the head of the pancreas. J Surg Oncol. 2000;73(4):212–8. [DOI] [PubMed] [Google Scholar]
- 23.Ozaki H, Hiraoka T, Mizumoto R, Matsuno S, Matsumoto Y, Nakayama T, et al. The prognostic significance of lymph node metastasis and intrapancreatic perineural invasion in pancreatic cancer after curative resection. Surg Today. 1999;29(1):16–22. 10.1007/BF02482964 [DOI] [PubMed] [Google Scholar]
- 24.Takai S, Satoi S, Toyokawa H, Yanagimoto H, Sugimoto N, Tsuji K, et al. Clinicopathologic evaluation after resection for ductal adenocarcinoma of the pancreas: a retrospective, single-institution experience. Pancreas. 2003;26(3):243–9. [DOI] [PubMed] [Google Scholar]
- 25.Husson A, Brasse-Lagnel C, Fairand A, Renouf S, Lavoinne A. Argininosuccinate synthetase from the urea cycle to the citrulline-NO cycle. Eur J Biochem. 2003;270(9):1887–99. [DOI] [PubMed] [Google Scholar]
- 26.Bowles TL, Kim R, Galante J, Parsons CM, Virudachalam S, Kung HJ, Bold RJ. Pancreatic cancer cell lines deficient in argininosuccinate synthetase are sensitive to arginine deprivation by arginine deiminase. Int J Cancer. 2008;123(8): 1950–51. 10.1002/ijc.23723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delage B, Fennell DA, Nicholson L, McNeish I, Lemoine NR, Crook T, et al. Arginine deprivation and argininosuccinate synthetase expression in the treatment of cancer. Int J Cancer. 2010;126(12):2762–72. 10.1002/ijc.25202 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some restrictions will apply. Data are available from the University of Texas MD Anderson Cancer Center Data Access / Ethics Committee for researchers who meet the criteria for access to confidential data. Researchers may contact Marion B. Olson (mbolson@mdanderson.org) and Dr. Huamin Wang (hmwang@mdanderson.org) for more information. The authors do not have special access privileges to the data underlying this study.